Abstract
Background
Coinfection of human immunodeficiency virus (HIV) and hepatitis C virus (HCV) is a substantial medical and public health concern due to its increasing prevalence and complex patient management. Alcohol use may worsen HCV-related liver disease and interfere with adherence to antiretroviral therapy (ART) and medical care. We therefore studied the association between HCV infection and markers of HIV disease progression in adults with alcohol problems.
Methods
This is a longitudinal study of 396 HIV-infected persons with alcohol problems, 199 (50%) of whom were coinfected with HCV (positive HCV RNA test). CD4 cell counts and HIV RNA levels were assessed at baseline and then every 6 months for up to 42 months. Hepatitis C virus RNA status was determined at study enrollment. We examined the relationship between HCV infection and laboratory markers of HIV progression (CD4 cell count and log10 HIV RNA) by fitting multivariable longitudinal regression models for each outcome.
Results
Among subjects who were adherent to ART, the presence of HCV infection was associated with a lower CD4 cell count (adjusted mean difference -46.0 cells/μL, p = 0.03). There was no association observed between HCV infection and CD4 cell count among those not adherent to ART or those not taking ART. No significant association was observed between HCV infection and HIV RNA regardless of ART status.
Conclusions
Hepatitis C virus infection has an adverse effect on CD4 cell count in patients with alcohol problems who are adherent to ART. Addressing HCV coinfection among these patients may confer additional immunologic benefit for this patient population.
Keywords: Hepatitis C Virus, HIV, Coinfection, Disease Progression, Alcohol Abuse
Coinfection with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) is a substantial medical and public health concern because of its increasing prevalence and problematic patient management. With improved treatment of HIV infection, coinfection with HCV is responsible for substantial morbidity and mortality in this patient population (Sulkowski et al., 2000). An estimated 15 to 30% of HIV-infected persons in the United States and Europe are coinfected with HCV (Sulkowski et al., 2002). In certain subgroups of HIV-infected patients, the prevalence of HCV coinfection is even higher. For example, 60 to 90% of HIV-infected persons with a history of injection drug use are coinfected with HCV (Bonacini and Puoti, 2000; Sulkowski and Thomas, 2003).
The impact of HCV on HIV disease progression has been an area of active investigation with conflicting conclusions (Braitstein et al., 2006; De Luca et al., 2002; Dorrucci et al., 1995; Greub et al., 2000; Klein et al., 2003; Macias et al., 1998; Piroth et al., 1998; Rancinan et al., 2002; Staples et al., 1999; Sulkowski et al., 2000; Sulkowski and Thomas, 2003; Weis et al., 2006). For example, the results of the 2 largest studies to date addressing this issue are inconsistent. Greub et al. (2000) reported that HCV infection was associated with decreased survival and more rapid progression to acquired immunodeficiency syndrome (AIDS) in 3,111 HIV-infected persons enrolled in the Swiss HIV Cohort Study. However, Sulkowski et al. (2002) found no association between HCV infection and survival, progression to AIDS, or response to highly active antiretroviral therapy (ART) in a prospective cohort study of 1,955 subjects from an urban HIV clinic in the United States.
Recommendations for management of HCV infection in HIV-infected patients continue to evolve (Alberti et al., 2005; Soriano et al., 2005), although HCV treatment is often the exception rather than the rule (Fleming et al., 2003). Patient management is complex because of the limited effectiveness of and numerous contraindications to HCV therapy, its duration and adverse effects, potential hepatotoxicity of ART, and lack of clarity concerning the effect of HCV infection on HIV disease progression (Chung et al., 2004; Sulkowski and Thomas, 2003; Torriani et al., 2004).
The objective of this study was to test the hypothesis that HCV infection has an adverse effect on markers of HIV disease progression in adults with alcohol problems. Previous studies of patients starting ART have suggested that HCV infection is associated with a blunted CD4 cell recovery (Greub et al., 2000). The biological mechanism of such an effect is unclear, but HCV is known to replicate in mononuclear cells and recent studies have suggested that both HCV infection and HCV proteins may enhance lymphocyte apoptosis (Nunez et al., 2006; Thoren et al., 2004).
It is important to study whether HCV impacts HIV disease progression in subjects with alcohol problems as alcohol use can be hepatotoxic and its use is prevalent among HIV-infected persons (Cook et al., 1997; Krupitsky et al., 2005; Samet et al., 2004b). Alcohol use has also been shown to worsen HCV-related liver disease (Schiff, 1999) and interfere with patient adherence to ART and medical care (Kresina et al., 2002; Samet et al., 2004a). The impact of alcohol on both HCV and HIV-related outcomes makes it imperative to understand the effect of HCV on HIV disease progression among subjects with alcohol problems to inform the medical management of this patient population.
METHODS
Study Design and Participant Recruitment
Subjects were participants in HIV-Longitudinal Interrelationships of Viruses and Ethanol (HIV-LIVE), a prospective, observational cohort study of HIV-infected patients with alcohol problems. The study sample included a spectrum of alcohol problems that was not limited to a diagnosis of current alcohol abuse or dependence. Data were collected at baseline and every 6 months thereafter for up to 42 months.
Of the 400 subjects, 38% (n = 154) were recruited from the HIV-ALC (HIV-Alcohol Longitudinal Cohort) study, a previous cohort study at Boston Medical Center (BMC). Inclusion and exclusion criteria for HIV-ALC and HIV-LIVE were identical. Subjects also were recruited from the Diagnostic Evaluation Unit (n = 88, 22%) (Samet et al., 1995), an intake clinic for HIV-infected patients at BMC, and the HIV Primary Care and Specialty Clinics at Beth Israel Deaconess Medical Center (BIDMC) (n = 31, 8%). Remaining subjects (n = 127, 32%) were recruited through flyers distributed in health care centers, homeless shelters, and drug treatment programs, advertisements in newspapers and referrals from other HIV-LIVE subjects. Figure 1 illustrates the distribution of subject recruitment across various sources.
Fig. 1.
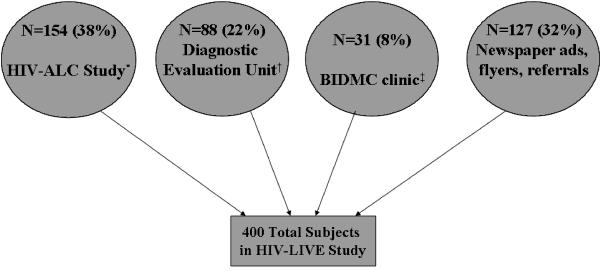
Distribution of subject recruitment.
*Previous cohort study of HIV-infected subjects with alcohol problems conducted by study investigators
†Clinic for patients newly diagnosed with HIV
‡Beth Israel Deaconess Medical Center Primary Care Clinic
Eligibility criteria were (1) documented HIV antibody test by ELISA confirmed by Western blot; (2) ≥2 affirmative responses to the CAGE alcohol screening questionnaire (Buchsbaum et al., 1991; Mayfield et al., 1974) or diagnosis of lifetime alcohol abuse or dependence based on a study physician investigator clinical assessment; (3) ability to speak English or Spanish; and (4) at least 1 contact person. Exclusion criteria were: (1) score of <21 on the 30-item Folstein Mini-Mental State Examination (Folstein et al., 1975; Smith et al., 2006); or (2) trained interviewer assessment that the patient could not comprehend informed consent or answer the interview questions. Eligible subjects who wished to participate provided written informed consent before enrollment. Enrollment began August 2001 and ended July 2003. Most interviews took place at the clinical research units. The Institutional Review Boards of Boston University Medical Center and BIDMC approved this study. Additional privacy protection was secured with a Certificate of Confidentiality from the Department of Health and Human Services to protect subjects from release of research data under court order or subpoena.
Subject Assessment
Subjects received an interviewer-administered assessment, including questions on demographics, HIV risk behaviors, alcohol consumption, and ART use in the past 30 days. Past month alcohol consumption was assessed using a validated calendar method (Sobell and Sobell, 1992). The Composite International Diagnostic Interview (CIDI) Alcohol Module (Robins et al., 1988) was administered following study enrollment to determine current (past 6 months) and lifetime diagnoses of alcohol abuse and dependence. Recent drug use and current (past 12 months) diagnosis of drug dependence was assessed at enrollment using the CIDI. Drug use and drug dependence diagnosis (past 6 months) were assessed at each follow-up using the CIDI Short Form (Kessler et al., 1991). Interviews were conducted in English or Spanish.
We recorded CD4 cell counts and HIV RNA levels at each interview. Values were obtained by phlebotomy if not available from clinical records within 4 months of the interview. Human immunodeficiency virus RNA testing was performed using either a branched-chain DNA assay or polymerase chain reaction (PCR) (Pachl et al., 1995). The lower threshold of detection was between 50 and 75 copies/mL over the course of the study. All subjects were tested for HCV infection by measurement of HCV antibody. Antibody-positive subjects were tested for HCV RNA if this was unavailable from medical records. Hepatitis C virus RNA was measured using commercially available assays, either by branched chain DNA or PCR-based assays. The lower level of detection of the assays was 615 IU/mL. HCV antibody-negative subjects were assumed to be HCV RNA negative (Thio et al., 2000).
Outcomes
The primary outcomes were CD4 cell count per microliter and log10 HIV RNA copies per milliliter, 2 laboratory markers of HIV disease progression. For analysis purposes, undetectable HIV RNA levels were assigned half the value of the lower limit of detection. CD4 count/total lymphocyte count percentage was analyzed as a secondary endpoint. This value may be more reflective of immune status than absolute CD4 count in the setting of asplenia or advanced liver disease (Zurlo et al., 1995).
Primary Independent Variable
The main independent variable was HCV infection status, defined as HCV RNA positive versus HCV RNA negative.
Potential Confounding Factors
Adherence to antiretroviral medications was determined using the AIDS Clinical Trials Group Questionnaire for Adherence to Anti-Retroviral Medications (Chesney et al., 2000). Patients reported their current antiretroviral medications, number of daily doses, and pills. Patients who reported being <100% adherent during the previous 3 days were considered not adherent; the 100% cut-off was used due to the brief timeframe of assessment. We used information concerning current receipt of ART, defined as receiving at least one antiretroviral medication, and adherence to create a 3-category variable representing ART status: receiving ART and adherent, receiving ART and not adherent, or not receiving ART.
Other potential covariates were gender, age, race (black, white, other), whether the subject was recruited from a previous cohort study of HIV-infected subjects with alcohol problems, homelessness, alcohol consumption, drug dependence diagnosis, injection drug use, time since starting HIV ART, and time since study enrollment. Homelessness was defined as at least 1 night in a shelter or on the street in the past 6 months (Kertesz et al., 2005). Alcohol use was categorized as heavy, moderate, or abstinent. Heavy alcohol use was defined as >14 drinks/wk or ≥5 drinks on 1 occasion for men <66 years old and >7 drinks/wk or ≥4 drinks on 1 occasion for men ≥66 years old and all women (NIAAA, 1995). Moderate alcohol use was defined as any drinking less than heavy amounts but not abstinent. Self-reported information was available on whether subjects injected drugs in the 6 months before enrollment and lifetime injection behaviors. The latter variable was highly correlated with HCV infection, whereas the former was not. Thus, current injection drug use was selected as the covariate to avoid collinearity and because current injection drug use would be more likely to affect HIV disease progression.
Statistical Analysis
Descriptive statistics were used to characterize the study sample at enrollment overall and by HCV RNA status. Continuous variables were compared between HCV RNA groups using t-tests or the Wilcoxon rank sum test. Chi-square and Fisher’s exact tests were used as appropriate to compare categorical variables. We examined the relationship between HCV infection and HIV progression by fitting separate multivariable longitudinal regression models for each outcome. Generalized linear mixed effects models (Laird and Ware, 1982) were used to account for the correlation from using repeated observations on the same subject. Because the effect of HCV infection on HIV progression was expected to differ based on ART status, interactions between HCV RNA status and ART status were included in all models. The estimated effect of HCV infection is reported separately by ART status. Potential confounding factors were included in regression models. Alcohol use, homelessness, and ART status were included in regression models as time-varying covariates. Baseline CD4 cell count was included as a covariate in analyses of CD4 cell count. Analyses of HIV RNA did not adjust for baseline values as it was expected that subjects had achieved their viral “set points,” and thus HIV RNA would remain somewhat constant over time. We evaluated whether alcohol use was a confounder of the relationship between HCV infection and HIV progression by excluding alcohol use and refitting the multivariable regression models. The regression coefficients for HCV infection for the models with and without alcohol use were then compared to assess whether there was confounding by alcohol use. A change in estimate of >10% was used to identify confounding. Secondary analyses assessed whether time since starting HIV ART confounded the relationship between HCV infection and markers of HIV disease progression by including it as an additional covariate in regression models. To minimize the potential for collinearity, we assessed correlation between pairs of independent variables and verified that no pair of variables included in the same regression model was highly correlated (i.e., >0.40). All analyses were conducted using 2-sided tests and a significance level of 0.05. Analyses were performed using SAS software (version 9.1; SAS Institute, Cary, NC).
Sample Size Considerations
Of the 396 subjects with known HCV RNA status, 185 were adherent to their HIV medications (99 HCV RNA positive; 86 HCV RNA negative), 61 were not adherent to their HIV medications (29 HCV RNA positive; 32 HCV RNA negative), and 150 were not on HIV medications (71 HCV RNA positive; 79 HCV RNA negative) at study enrollment. These sample sizes allow our study 80% power to detect minimum differences in CD4 cell count of 134, 233, and 149 between HCV RNA positive versus negative among subjects who were adherent, not adherent, and not on HIV medications, respectively. For the outcome log HIV RNA, our study has 80% power to detect differences as small as 0.54, 0.93, and 0.60 between HCV RNA positive versus negative subjects who were adherent, not adherent, and not on HIV medications, respectively. Power calculations were based on 2-sided 2-sample t-tests using a significance level of 0.05. Standard deviations of 325 and 1.3 were assumed for CD4 cell count and log HIV RNA, respectively. Power calculations were based on a simple setting that utilizes a single time point, however the data were analyzed using longitudinal regression methods that allow for repeated observations from subjects, therefore we expected to be able to detect smaller differences with equal power.
RESULTS
Study Subjects
Of the 400 HIV-LIVE subjects, 235 (59%) were HCV antibody-positive. Of the 231 HCV antibody-positive subjects who had an HCV RNA test, 199 (86%) were HCV RNA positive. One HCV-infected subject received interferon therapy during the study. Analyses were conducted on 396 subjects with known HCV RNA status.
Baseline characteristics of the study subjects are displayed in Table 1. The majority were male (75%), nonwhite race (67%), and housed (75%). The average age of the subjects was 42.5 years. At enrollment, 14% reported injection drug use (past 6 months), 43% had a current diagnosis (past 12 months) of drug dependence, 70% had a lifetime diagnosis of alcohol dependence and 18% alcohol abuse; 10% had a current diagnosis (past 6 months) of alcohol dependence. Thirty-one percent reported heavy alcohol consumption, 11% moderate consumption, and 58% were abstinent in the past 30 days. Median CD4 cell count was 403 cells/μL. Median log10 HIV RNA was 2.95 and 31% of subjects had undetectable HIV RNA. Sixty-two percent were on ART (93% of these subjects were taking at least 3 HIV medications).
Table 1.
Baseline Characteristics of the 396 HIV-Infected Subjects in the Study Cohort, Overall and by HCV RNA Status
| Total sample (N = 396) | HCV RNA+ (N = 199) | HCV RNA- (N = 197) | p-Value | |
|---|---|---|---|---|
| Age (y), mean (SD) | 42.5 (7.4) | 43.9 (7.0) | 40.9 (7.5) | <0.0001 |
| Gender | ||||
| Female | 25% | 27% | 23% | 0.42 |
| Male | 75% | 73% | 77% | |
| Race | ||||
| Black | 41% | 38% | 44% | 0.46 |
| White | 33% | 34% | 32% | |
| Other | 26% | 28% | 24% | |
| Median CD4 counta (IQR) | 403 (238, 624) | 356 (231, 542) | 424 (257, 655) | 0.02 |
| Median log10 HIV RNAb (IQR) | 2.95 (1.57, 4.14) | 2.92 (1.57, 4.14) | 3.03 (1.40, 4.18) | 0.94 |
| Undetectable HIV RNA | ||||
| Yes | 31% | 29% | 33% | 0.49 |
| No | 69% | 71% | 67% | |
| HIV ART statusc | ||||
| Adherent | 47% | 50% | 44% | 0.48 |
| Not adherent | 15% | 15% | 16% | |
| Not on meds | 38% | 36% | 40% | |
| Injection drug use everd | ||||
| Yes | 55% | 89% | 20% | <.0001 |
| No | 45% | 11% | 80% | |
| Injection drug use, past 6 mod | ||||
| Yes | 14% | 23% | 5% | <.0001 |
| No | 86% | 77% | 95% | |
| Drug dependence, past 12 mo | ||||
| Yes | 43% | 44% | 40% | 0.42 |
| No | 57% | 56% | 60% | |
| Homeless | ||||
| Yes | 25% | 29% | 20% | 0.06 |
| No | 75% | 71% | 80% | |
| Lifetime alcohol dependence | ||||
| Dependence | 70% | 74% | 66% | 0.14 |
| Abuse | 18% | 17% | 19% | |
| No diagnosis | 12% | 9% | 15% | |
| Alcohol dependence, past 6 mo | ||||
| Dependence | 10% | 11% | 9% | 0.70 |
| Abuse | 2% | 2% | 2% | |
| No diagnosis | 88% | 87% | 89% | |
| Alcohol consumption, past mod | ||||
| None | 58% | 63% | 53% | 0.09 |
| Moderate | 11% | 8% | 13% | |
| Heavy | 31% | 29% | 34% | |
| Participated in previous HIV studye | ||||
| Yes | 38% | 43% | 33% | 0.04 |
| No | 62% | 57% | 67% | |
| Time since diagnosis of HIV (y), mean (SD) | 9.2 (4.9) | 9.8 (4.9) | 8.6 (4.8) | 0.02 |
| Time since starting HIV ART (y), mean (SD) | 6.9 (4.1) | 7.1 (4.2) | 6.7 (4.0) | 0.30 |
N = 375, 187, and 188, respectively.
N = 361, 180, and 181, respectively.
Adherent: N = 185, 99, and 86, respectively; Not adherent: N = 61, 29, and 32, respectively; Not on meds: N = 150, 71, and 79, respectively.
N = 396, 200, and 196, respectively.
“Yes” indicates subjects were previously enrolled in an observational cohort study conducted by the study investigators.
HIV, human immunodeficiency virus; ART, antiretroviral therapy.
Hepatitis C virus RNA-positive subjects were older (mean age 44 vs 41 years), more likely to have used injection drugs in the past 6 months (23 vs 5%), and had a lower median CD4 cell count (356 vs 424 cells/μL) compared with HCV RNA-negative subjects.
Multivariable Regression Results
The primary analyses used data from 396 subjects contributing 1,171 observations to examine CD4 cell count and 1,542 observations for log HIV RNA. Human immunodeficiency virus RNA analyses included a larger number of observations as log HIV RNA at enrollment was modeled as part of the longitudinal outcome whereas baseline CD4 cell count was a covariate in CD4 cell count analyses. The number of subjects included in CD4 cell count analyses (n = 344) was less than that in HIV RNA analyses as only those with a follow-up interview (87%) were included in analyses of CD4 cell count. The median number of observations per subject was 4 (interquartile range: 3-5) for CD4 cell count and HIV RNA. Table 2 presents results of the longitudinal regression analyses. Among subjects adherent to ART, those with detectable HCV RNA had lower CD4 cell counts (adjusted mean difference -46.0 in CD4 cells/μL, p-value = 0.03). No association was observed between HCV infection and CD4 cell count for subjects not adherent to ART or not taking ART. No significant association was observed between HCV RNA status and log HIV RNA regardless of ART status (Table 2).
Table 2.
Adjusted Mean Differences in CD4 Cell Count, Log10 HIV RNA, and Percent CD4 Cells from Longitudinal Regression Models Comparing HCV RNA-Positive Subjects Versus HCV RNA-Negative Subjects According to ART Status
| CD4 cell counta |
Log10 HIV RNAb |
Percent CD4 cellsc |
|||||
|---|---|---|---|---|---|---|---|
| ART status | HCV status | Adjusted mean difference (SE)d | p-Value | Adjusted mean difference (SE)d | p-Value | Adjusted mean difference (SE)d | p-Value |
| Adherent to ART | HCV RNA+ | -46.0 (21) | 0.03 | 0.086 (.12) | 0.46 | -2.3 (.76) | <0.01 |
| HCV RNA- | — | — | — | ||||
| Not adherent to ART | HCV RNA+ | -4.5 (28) | 0.88 | -0.010 (0.16) | 0.96 | -1.3 (1.0) | 0.18 |
| HCV RNA- | — | — | — | ||||
| Not on ART | HCV RNA+ | -16.3 (23) | 0.48 | 0.23 (0.13) | 0.08 | -0.48 (0.84) | 0.57 |
| HCV RNA- | — | — | — | ||||
Bold font indicates significant differences (p<0.05).
Analyses based on 344 subjects and 1,171 observations.
Analyses based on 395 subjects and 1,542 observations.
Analyses based on 341 subjects and 1,160 observations.
All analyses adjusted for: ART status, interaction between HCV status and ART status, gender, age, race, homelessness, participation in previous HIV cohort study, alcohol consumption, drug dependence diagnosis, injection drug use past 6 mo, time, and baseline value of the outcome (CD4 cell count analyses only).
HIV, human immunodeficiency virus; ART, antiretroviral therapy.
For the secondary endpoint percent CD4 cells, HCV RNA positivity was associated with a lower percentage of CD4 cells (adjusted mean difference -2.3%, p-value <0.01) among those adherent to ART. No association was observed between HCV infection and percentages of CD4 cells among those not adherent to ART and those not on ART (Table 2).
We also evaluated whether alcohol use was a confounder of the relationship between HCV infection and HIV disease progression. In the multivariable regression models that did not adjust for alcohol use, the estimated effects of HCV infection were similar to the primary models that adjusted for alcohol use, suggesting that alcohol use was not a confounder. Among subjects adherent to ART, those with detectable HCV RNA had lower CD4 cell counts (adjusted mean difference -44.9 in CD4 cells/μL, p-value = 0.03) and lower percentage of CD4 cells (adjusted mean difference -2.2%, p-value <0.01).
The results were similar after adjusting for time since starting HIV ART in the regression models. Among subjects adherent to ART, subjects with detectable HCV RNA had significantly lower CD4 cell counts (adjusted mean difference 44.5 in CD4 cells/μL, p-value = 0.03) and percentage of CD4 cells (adjusted mean difference 2.2%, p-value <0.01).
DISCUSSION
Coinfection with HCV is common in HIV-infected patients, especially among those with injection drug use. Determining whether HCV coinfection affects HIV disease progression is important for understanding the optimal timing and sequencing of therapies for these infections. This is a particularly key issue for coinfected persons with alcohol problems due to several complicating factors: alcohol exposure can result in more rapid progression of liver disease (Schiff, 1999); heavy alcohol use is a contraindication for interferon therapy (Soriano et al., 2005); and alcohol use is associated with worse adherence to ART (Kresina et al., 2002). Analysis of this prospective cohort of HIV-infected persons with alcohol problems demonstrated a significant association between coinfection with HCV and lower CD4 cell counts among patients adherent to ART. Reductions were present in both CD4 cell count and percentage, suggesting these effects were probably not related to hypersplenism secondary to the underlying liver disease. Analyses controlled for multiple characteristics associated with HIV disease progression.
The observation of a lower CD4 cell count in patients with HCV infection that were receiving and adherent to ART is of particular interest. These findings are consistent with those described by Greub et al. (2000) in the Swiss HIV Cohort Study in which initiation of ART in HCV coinfected persons was associated with an attenuated recovery in CD4 cell count compared with those without HCV. Similar to subjects adherent to ART in the current study, Greub and colleagues found a decreased response of CD4 cell count to ART among coinfected persons despite no observed differences in HIV RNA. Interestingly, a follow-up report from the Swiss HIV Cohort Study investigators showed that differences in CD4 cell count between these groups did not persist after 4 years of follow-up (Kaufmann et al., 2003). This “muting” effect of HCV infection on CD4 cell count response to ART has been shown in other studies (De Luca et al., 2002; Law et al., 2004), but differs from the recently published EUROSIDA study and a smaller prospective U.S. study which showed no effect of HCV on CD4 cell recovery (Chung et al., 2002; Rockstroh et al., 2005).
The reason for discrepancies across study findings is unclear. They may be related in part to the timepoints at which CD4 cell recovery was measured, particularly if HCV infection delays but does not prevent immunologic reconstitution. Why HCV should affect CD4 cell count recovery in the face of similar HIV viral suppression is unknown, though it may indicate that HCV infection has a direct effect on CD4 cells rather than being mediated through HIV viral replication. This study’s finding that coinfection of HCV and HIV is associated with lower CD4 cell counts among those adherent to ART is consistent with other observations suggesting a direct effect of HCV on the immune system (Bare et al., 2003, 2005; El-Serag et al., 2002; Pawlotsky et al., 1995). Recent data have shown that HCV proteins and HCV infection of mononuclear cells are associated with enhanced lymphocyte apoptosis (Nunez et al., 2006; Thoren et al., 2004). It is unclear why we observed an effect of HCV only in those individuals receiving ART. However it is possible that exposure to antiretroviral medications enhances the oxidative or apoptotic “stress” within these cells. It is also possible that HCV induces alterations in CD4 cells through an alternative but poorly defined mechanism as HCV infection is associated with a number of lymphoproliferative and autoimmune conditions including cryogloblinemia, lymphoma, and autoimmune phenomena.
Whether HCV infection has an effect on mortality in HIV-infected patients is controversial. Several studies have shown increased mortality in those with HCV coinfection (Anderson et al., 2004; Backus et al., 2005; Braitstein et al., 2005; Weis et al., 2006), while others have observed similar rates (El-Serag et al., 2005). Although we did not examine the effect of HCV on this clinical endpoint, a major strength of this study was the ability to control for factors such as presence of detectable HCV RNA, active drug and alcohol use, medication adherence and social factors such as homelessness. These factors were not addressed in several larger cohort studies (Backus et al., 2005; De Luca et al., 2002; El-Serag et al., 2005; Jaggy et al., 2003; Keiser et al., 2004; Law et al., 2004; Quintana et al., 2003; Rockstroh et al., 2005).
Although alcohol use may worsen HCV-related liver disease and interfere with medical care, we did not find evidence in our data to suggest that alcohol use is a confounder of the association between HCV status and HIV disease progression. Our findings that HCV infection has an adverse effect on CD4 cell count and percent CD4 cells in patients adherent to ART persisted regardless of whether alcohol use was accounted for in the analyses.
This study’s findings have important implications for understanding the optimal management approach for coinfected patients. Recent work has shown that effective treatment of HIV infection can significantly reduce the risk of HCV-related liver disease progression (Qurishi et al., 2003). However, our results suggest that HCV infection may adversely impact HIV-related immune reconstitution. If this is true, additional improvement in HIV outcomes may be achieved by effective treatment of HCV infection in patients who are adherent to ART. Corroboration of these findings would add effective HCV treatment to the multidimensional approach to address HIV infection and strengthen the argument to initiate HCV therapy early in the course of HIV disease. These findings may also support the earlier introduction of ART in HCV coinfected individuals to both offset the negative impact of HIV on HCV disease progression and the potentially negative impact of HCV on CD4 cell recovery.
The limitations of this study merit explanation. As HCV coinfection is more common in injection drug users, this group’s delayed presentation for medical care, compared with noninjection drug users, may account for lower CD4 cell counts among coinfected individuals (Samet et al., 2001). However, adjustment for CD4 cell count at study enrollment in the multivariable analysis should mitigate this argument. Also, HCV-negative subjects were not diagnosed with HIV earlier and had not been on ART longer than HCV-positive subjects, suggesting our results are not explained by the groups being in care for different lengths of time. It is possible that we did not adequately control for all potential contributing factors to HIV disease progression. Nonetheless, multiple characteristics known to be associated with HIV disease progression were included as covariates in the multivariable analyses. This study may have been underpowered to detect a statistically significant effect of HCV infection. The observed differences in CD4 cell count and log HIV RNA between the HCV RNA-positive and negative subjects were smaller than anticipated, thus it is likely that the study was not adequately powered to detect associations of the observed magnitude.
In summary, in this cohort of subjects with alcohol problems, HCV infection is associated with lower CD4 cell counts in patients adherent to ART. Addressing HCV coinfection in HIV-infected patients who have maximized ART may confer additional immunologic benefit for this patient population.
ACKNOWLEDGMENTS
We appreciate the contributions of the research associates and data managers. Research was conducted in part in the General Clinical Research Centers at Boston University School of Medicine, USPHS Grant MO1 RR00533 and Beth Israel Deaconess Medical Center, USPHS Grant M01 RR01032.
This work was supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) of the NIH: R01-AA13216 (Clinical Impact of HCV and Alcohol in HIV-Infected Persons), R01-AA11785 (Medication Adherence in Alcohol Abusing HIV Patients); R01-AA10870 (Enhanced Linkage of Alcohol Abusers to Primary Care); and K24-AA015674 (Impact of Alcohol Use on HIV Infection—In US and Russia).
REFERENCES
- Alberti A, Clumeck N, Collins S, Gerlich W, Lundgren J, Palu G, Reiss P, Thiebaut R, Weiland O, Yazdanpanah Y, Zeuzem S. Short statement of the first European Consensus Conference on the treatment of chronic hepatitis B and C in HIV co-infected patients. J Hepatol. 2005;42:615–624. doi: 10.1016/j.jhep.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Anderson KB, Guest JL, Rimland D. Hepatitis C virus coinfection increases mortality in HIV-infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta VA Cohort Study. Clin Infect Dis. 2004;39:1507–1513. doi: 10.1086/425360. [DOI] [PubMed] [Google Scholar]
- Backus LI, Phillips BR, Boothroyd DB, Mole LA, Burgess J, Rigsby MO, Chang SW. Effects of hepatitis C virus coinfection on survival in veterans with HIV treated with highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;39:613–619. [PubMed] [Google Scholar]
- Bare P, Massud I, Belmonte L, Corti M, Villafane M, Perez Bianco R, de Tezanos-pinto M, de Bracco MM, Ruibal-Ares B. HCV recovery from peripheral blood mononuclear cell culture supernatants derived from HCV-HIV co-infected haemophilic patients with undetectable HCV viraemia. Haemophilia. 2003;9:598–604. doi: 10.1046/j.1365-2516.2003.00808.x. [DOI] [PubMed] [Google Scholar]
- Bare P, Massud I, Parodi C, Belmonte L, Garcia G, Nebel MC, Corti M, Pinto MT, Bianco RP, Bracco MM, Campos R, Ares BR. Continuous release of hepatitis C virus (HCV) by peripheral blood mononuclear cells and B-lymphoblastoid cell-line cultures derived from HCV-infected patients. J Gen Virol. 2005;86:1717–1727. doi: 10.1099/vir.0.80882-0. [DOI] [PubMed] [Google Scholar]
- Bonacini M, Puoti M. Hepatitis C in patients with human immunodeficiency virus infection: diagnosis, natural history, meta-analysis of sexual and vertical transmission, and therapeutic issues. Arch Intern Med. 2000;160:3365–3373. doi: 10.1001/archinte.160.22.3365. [DOI] [PubMed] [Google Scholar]
- Braitstein P, Yip B, Montessori V, Moore D, Montaner JS, Hogg RS. Effect of serostatus for hepatitis C virus on mortality among antiretrovirally naive HIV-positive patients. Can Med Assoc J. 2005;173:160–164. doi: 10.1503/cmaj.045202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitstein P, Zala C, Yip B, Brinkhof MW, Moore D, Hogg RS, Montaner JS. Immunologic response to antiretroviral therapy in hepatitis C Virus-coinfected adults in a population-based HIV/AIDS treatment program. J Infect Dis. 2006;193:259–268. doi: 10.1086/498908. [DOI] [PubMed] [Google Scholar]
- Buchsbaum DG, Buchanan RG, Centor RM, Schnoll SH, Lawton MJ. Screening for alcohol abuse using CAGE scores and likelihood ratios. Ann Intern Med. 1991;115:774–777. doi: 10.7326/0003-4819-115-10-774. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, Peters MG, Koziel MJ, Bhan AK, Alston B, Colquhoun D, Nevin T, Harb G, van der Horst C. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung RT, Evans SR, Yang Y, Theodore D, Valdez H, Clark R, Shikuma C, Nevin T, Sherman KE. Immune recovery is associated with persistent rise in hepatitis C virus RNA, infrequent liver test flares, and is not impaired by hepatitis C virus in co-infected subjects. AIDS. 2002;16:1915–1923. doi: 10.1097/00002030-200209270-00008. [DOI] [PubMed] [Google Scholar]
- Cook RT, Stapleton JT, Ballas ZK, Klinzman D. Effect of a single ethanol exposure on HIV replication in human lymphocytes. J Invest Med. 1997;45:265–271. [PubMed] [Google Scholar]
- De Luca A, Bugarini R, Lepri AC, Puoti M, Girardi E, Antinori A, Poggio A, Pagano G, Tositti G, Cadeo G, Macor A, Toti M, D’Arminio Monforte A. Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med. 2002;162:2125–2132. doi: 10.1001/archinte.162.18.2125. [DOI] [PubMed] [Google Scholar]
- Dorrucci M, Pezzotti P, Phillips AN, Lepri AC, Rezza G. Coinfection of hepatitis C virus with human immunodeficiency virus and progression to AIDS. Italian Seroconversion Study. J Infect Dis. 1995;172:1503–1508. doi: 10.1093/infdis/172.6.1503. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Giordano TP, Kramer J, Richardson P, Souchek J. Survival in hepatitis C and HIV co-infection: a cohort study of hospitalized veterans. Clin Gastroenterol Hepatol. 2005;3:175–183. doi: 10.1016/s1542-3565(04)00620-2. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Hampel H, Yeh C, Rabeneck L. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439–1445. doi: 10.1053/jhep.2002.37191. [DOI] [PubMed] [Google Scholar]
- Fleming CA, Craven DE, Thornton D, Tumilty S, Nunes D. Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis. 2003;36:97–100. doi: 10.1086/344907. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Greub G, Ledergerber B, Battegay M, Grob P, Perrin L, Furrer H, Burgisser P, Erb P, Boggian K, Piffaretti JC, Hirschel B, Janin P, Francioli P, Flepp M, Telenti A. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV cohort study. Lancet. 2000;356:1800–1805. doi: 10.1016/s0140-6736(00)03232-3. [DOI] [PubMed] [Google Scholar]
- Jaggy C, von Overbeck J, Ledergerber B, Schwarz C, Egger M, Rickenbach M, Furrer HJ, Telenti A, Battegay M, Flepp M, Vernazza P, Bernasconi E, Hirschel B. Mortality in the Swiss HIV cohort study (SHCS) and the Swiss general population. Lancet. 2003;362:877–878. doi: 10.1016/S0140-6736(03)14307-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann GR, Perrin L, Pantaleo G, Opravil M, Furrer H, Telenti A, Hirschel B, Ledergerber B, Vernazza P, Bernasconi E, Rickenbach M, Egger M, Battegay M. CD4 T-lymphocyte recovery in individuals with advanced HIV-1 infection receiving potent antiretroviral therapy for 4 years: the Swiss HIV Cohort Study. Arch Intern Med. 2003;163:2187–2195. doi: 10.1001/archinte.163.18.2187. [DOI] [PubMed] [Google Scholar]
- Keiser O, Taffe P, Zwahlen M, Battegay M, Bernasconi E, Weber R, Rickenbach M. All cause mortality in the Swiss HIV Cohort Study from 1990 to 2001 in comparison with the Swiss population. AIDS. 2004;18:1835–1843. doi: 10.1097/00002030-200409030-00013. [DOI] [PubMed] [Google Scholar]
- Kertesz SG, Larson MJ, Horton NJ, Winter M, Saitz R, Samet JH. Homeless chronicity and health-related quality of life trajectories among adults with addictions. Med Care. 2005;43:574–585. doi: 10.1097/01.mlr.0000163652.91463.b4. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen H. The world health organization composite international diagnostic interview short form (CIDI-SF) Int J Methods Psychiatr Res. 1991;7:171–185. [Google Scholar]
- Klein MB, Lalonde RG, Suissa S. The impact of hepatitis C virus coinfection on HIV progression before and after highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2003;33:365–372. doi: 10.1097/00126334-200307010-00011. [DOI] [PubMed] [Google Scholar]
- Kresina TF, Flexner CW, Sinclair J, Correia MA, Stapleton JT, Adeniyi-Jones S, Cargill V, Cheever LW. Alcohol use and HIV pharmacotherapy. AIDS Res Hum Retrov. 2002;18:757–770. doi: 10.1089/08892220260139495. [DOI] [PubMed] [Google Scholar]
- Krupitsky EM, Horton NJ, Williams EC, Lioznov D, Kuznetsova M, Zvartau E, Samet JH. Alcohol use and HIV risk behaviors among HIV-infected hospitalized patients in St. Petersburg, Russia. Drug Alcohol Depend. 2005;79:251–256. doi: 10.1016/j.drugalcdep.2005.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Law WP, Duncombe CJ, Mahanontharit A, Boyd MA, Ruxrungtham K, Lange JM, Phanuphak P, Cooper DA, Dore GJ. Impact of viral hepatitis co-infection on response to antiretroviral therapy and HIV disease progression in the HIV-NAT cohort. AIDS. 2004;18:1169–1177. doi: 10.1097/00002030-200405210-00010. [DOI] [PubMed] [Google Scholar]
- Macias J, Pineda JA, Leal M, Abad MA, Garcia-Pesquera F, Delgado J, Gallardo JA, Sanchez-Quijano A, Lissen E. Influence of hepatitis C virus infection on the mortality of antiretroviral-treated patients with HIV disease. Eur J Clin Microbiol Infect Dis. 1998;17:167–170. doi: 10.1007/BF01691112. [DOI] [PubMed] [Google Scholar]
- Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121–1123. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism . The Physician’s Guide to Helping Patients With Alcohol Problems. National Institute on Alcohol Abuse and Alcoholism; Washington, DC.: 1995. [Google Scholar]
- Nunez M, Soriano V, Lopez M, Ballesteros C, Cascajero A, Gonzalez-Lahoz J, Benito JM. Coinfection with hepatitis C virus increases lymphocyte apoptosis in HIV-infected patients. Clin Infect Dis. 2006;43:1209–1212. doi: 10.1086/508355. [DOI] [PubMed] [Google Scholar]
- Pachl C, Todd JA, Kern DG, Sheridan PJ, Fong SJ, Stempien M, Hoo B, Besemer D, Yeghiazarian T, Irvine B, Kolberg J, Kokka R, Neuwald P, Urdea MS. Rapid and precise quantification of HIV-1 RNA in plasma using a branched DNA signal amplification assay. J Acq Immun Def Synd Hum Retrov. 1995;8:446–454. doi: 10.1097/00042560-199504120-00003. [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM, Bouvier M, Fromont P, Deforges L, Duval J, Dhumeaux D, Bierling P. Hepatitis C virus infection and autoimmune Thrombocytopenic purpura. J Hepatol. 1995;23:635–639. doi: 10.1016/0168-8278(95)80027-1. [DOI] [PubMed] [Google Scholar]
- Piroth L, Duong M, Quantin C, Abrahamowicz M, Michardiere R, Aho LS, Grappin M, Buisson M, Waldner A, Portier H, Chavanet P. Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS. 1998;12:381–388. doi: 10.1097/00002030-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Quintana M, del Amo J, Barrasa A, Perez-Hoyos S, Ferreros I, Hernandez F, Villar A, Jimenez V, Bolumar F. Progression of HIV infection and mortality by hepatitis C infection in patients with haemophilia over 20 years. Haemophilia. 2003;9:605–612. doi: 10.1046/j.1365-2516.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- Qurishi N, Kreuzberg C, Luchters G, Effenberger W, Kupfer B, Sauerbruch T, Rockstroh JK, Spengler U. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708–1713. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- Rancinan C, Neau D, Saves M, Lawson-Ayayi S, Bonnet F, Mercie P, Dupon M, Couzigou P, Dabis F, Chene G. Is hepatitis C virus co-infection associated with survival in HIV-infected patients treated by combination antiretroviral therapy? AIDS. 2002;16:1357–1362. doi: 10.1097/00002030-200207050-00007. [DOI] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, Farmer A, Jablenski A, Pickens R, Regier DA, Sartorius N, Towle LH. The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–1077. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- Rockstroh JK, Mocroft A, Soriano V, Tural C, Losso MH, Horban A, Kirk O, Phillips A, Ledergerber B, Lundgren J. Influence of hepatitis C virus infection on HIV-1 disease progression and response to highly active antiretroviral therapy. J Infect Dis. 2005;192:992–1002. doi: 10.1086/432762. [DOI] [PubMed] [Google Scholar]
- Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol Clin Exp Res. 2004;28(a):572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Samet JH, Libman H, LaBelle C, Steger K, Lewis R, Craven DE, Freedberg KA. A model clinic for the initial evaluation and establishment of primary care for persons infected with human immunodeficiency virus. Arch Intern Med. 1995;155:1629–1633. [PubMed] [Google Scholar]
- Samet JH, Phillips SJ, Horton NJ, Traphagen ET, Freedberg KA. Detecting alcohol problems in HIV-infected patients: use of the CAGE questionnaire. AIDS Res Hum Retroviruses. 2004;20(b):151–155. doi: 10.1089/088922204773004860. [DOI] [PubMed] [Google Scholar]
- Schiff ER. The alcoholic patient with hepatitis C virus infection. Am J Med. 1999;107:95S–99S. doi: 10.1016/s0002-9343(99)00393-9. [DOI] [PubMed] [Google Scholar]
- Smith KL, Horton NJ, Saitz R, Samet JH. The use of the mini-mental state examination in recruitment for substance abuse research studies. Drug Alcohol Depend. 2006;82:231–237. doi: 10.1016/j.drugalcdep.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ.: 1992. pp. 41–69. [Google Scholar]
- Soriano V, Puoti M, Bonacini M, Brook G, Cargnel A, Rockstroh J, Thio C, Benhamou Y. Care of patients with chronic hepatitis B and HIV co-infection: recommendations from an HIV-HBV International Panel. AIDS. 2005;19:221–240. [PubMed] [Google Scholar]
- Staples CT, Jr., Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29:150–154. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Moore RD, Mehta SH, Chaisson RE, Thomas DL. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected person. Ann Intern Med. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- Thio CL, Nolt KR, Astemborski J, Vlahov D, Nelson KE, Thomas DL. Screening for hepatitis C virus in human immunodeficiency virus-infected individuals. J Clin Microbiol. 2000;38:575–577. doi: 10.1128/jcm.38.2.575-577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoren F, Romero A, Lindh M, Dahlgren C, Hellstrand K. A hepatitis C virus-encoded, nonstructural protein (NS3) triggers dysfunction and apoptosis in lymphocytes: role of NADPH oxidase-derived oxygen radicals. J Leukoc Biol. 2004;76:1180–1186. doi: 10.1189/jlb.0704387. [DOI] [PubMed] [Google Scholar]
- Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-Garcia J, Lazzarin A, Carosi G, Sasadeusz J, Katlama C, Montaner J, Sette H, Jr., Passe S, De Pamphilis J, Duff F, Schrenk UM, Dieterich DT. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- Weis N, Lindhardt BO, Kronborg G, Hansen AB, Laursen AL, Christensen PB, Nielsen H, Moller A, Sorensen HT, Obel N. Impact of hepatitis C virus coinfection on response to highly active antiretroviral therapy and outcome in HIV-infected individuals: a nationwide cohort study. Clin Infect Dis. 2006;42:1481–1487. doi: 10.1086/503569. [DOI] [PubMed] [Google Scholar]
- Zurlo JJ, Wood L, Gaglione MM, Polis MA. Effect of splenectomy on T lymphocyte subsets in patients infected with the human immunodeficiency virus. Clin Infect Dis. 1995;20:768–771. doi: 10.1093/clinids/20.4.768. [DOI] [PubMed] [Google Scholar]


