Abstract
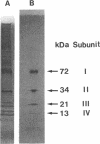
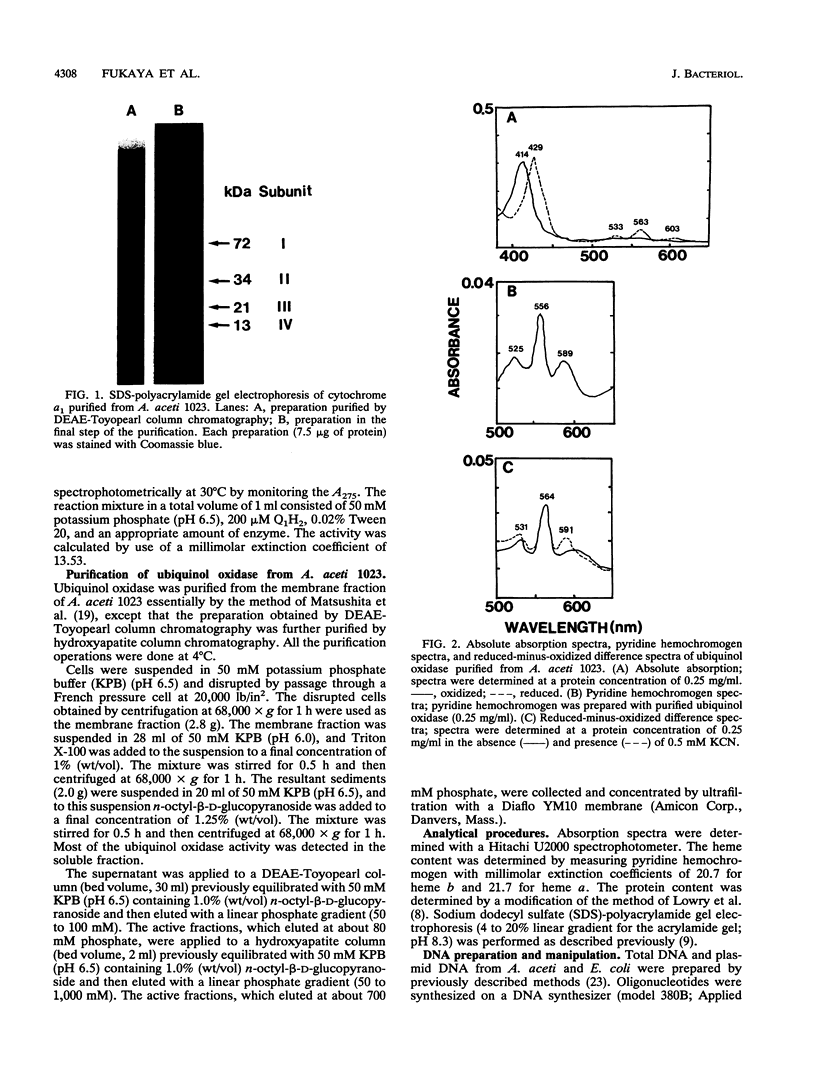
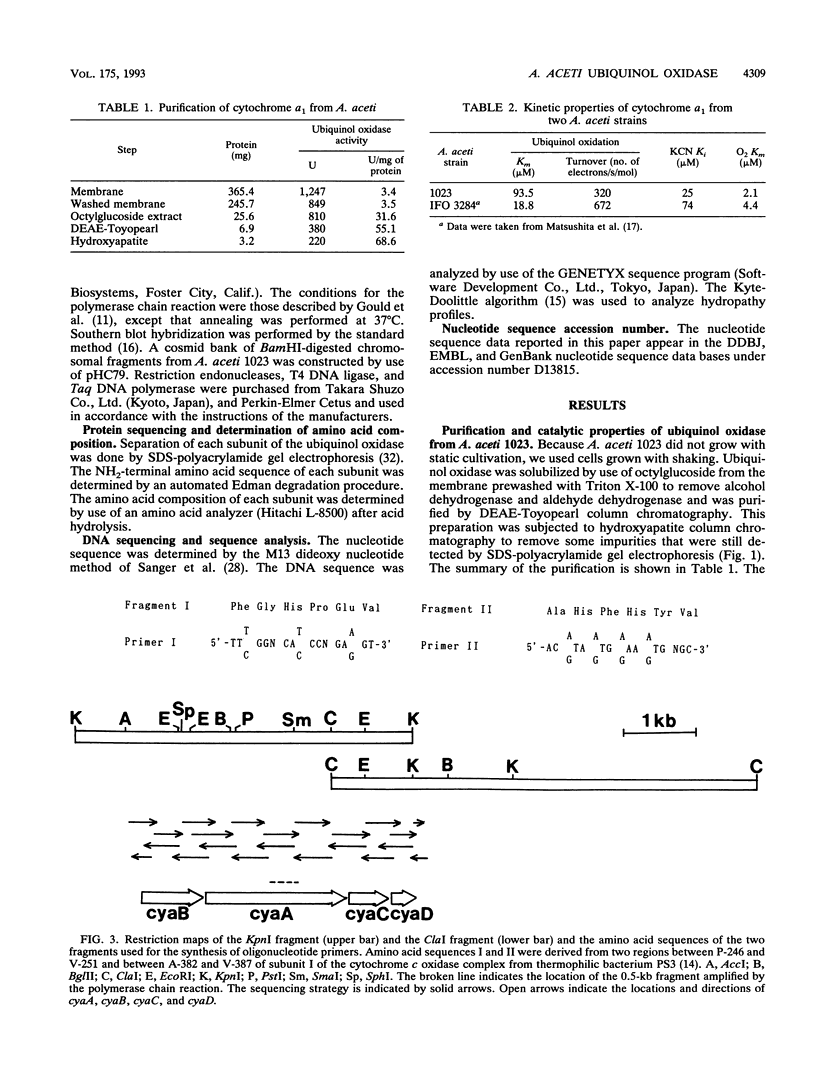
The terminal oxidase for ethanol oxidation in Acetobacter aceti was purified as a complex consisting of four subunits (subunits I, II, III, and IV) with molecular masses of 72, 34, 21, and 13 kDa, respectively. Spectrophotometric analysis and catalytic properties determined with the purified enzyme showed that it belonged to a family of cytochrome a1 (ba)-type ubiquinol oxidases. A polymerase chain reaction with two oligonucleotides designed for amino acid sequences that are conserved in subunit I of the aa3-type cytochrome c oxidases from various origins and of an Escherichia coli o (bo)-type ubiquinol oxidase was used for cloning the cytochrome a1 gene. A 0.5-kb fragment thus amplified was used as the probe to clone a 4.5-kb KpnI fragment that contained a putative open reading frame for the whole subunit I gene. The molecular weight and amino acid composition of the product of this open reading frame (cyaA) were the same as those of the purified protein from A. aceti. The amino acid sequence of CyaA was homologous to that of subunit I of the E. coli o-type ubiquinol oxidase. Nucleotide sequence analysis of the region neighboring the cyaA gene revealed that the genes (cyaB, cyaC, and cyaD) encoding the other three subunits (subunits II, III, and IV) were clustered upstream and downstream of the cyaA gene in the order cyaB, cyaA, cyaC, and cyaD and with the same transcription polarity, forming an operon. As expected from the enzymatic properties, CyaB, CyaC, and CyaD showed great similarity in amino acid sequence to the corresponding sununits of the E. coli o-type ubiquinol oxidase and as(3)-type cytochrome c oxidases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chepuri V., Lemieux L., Au D. C., Gennis R. B. The sequence of the cyo operon indicates substantial structural similarities between the cytochrome o ubiquinol oxidase of Escherichia coli and the aa3-type family of cytochrome c oxidases. J Biol Chem. 1990 Jul 5;265(19):11185–11192. [PubMed] [Google Scholar]
- Covello P. S., Gray M. W. RNA sequence and the nature of the CuA-binding site in cytochrome c oxidase. FEBS Lett. 1990 Jul 30;268(1):5–7. doi: 10.1016/0014-5793(90)80958-l. [DOI] [PubMed] [Google Scholar]
- Denda K., Fujiwara T., Seki M., Yoshida M., Fukumori Y., Yamanaka T. Molecular cloning of the cytochrome aa3 gene from the archaeon (Archaebacterium) Halobacterium halobium. Biochem Biophys Res Commun. 1991 Nov 27;181(1):316–322. doi: 10.1016/s0006-291x(05)81420-2. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Fukaya M., Tayama K., Tamaki T., Tagami H., Okumura H., Kawamura Y., Beppu T. Cloning of the Membrane-Bound Aldehyde Dehydrogenase Gene of Acetobacter polyoxogenes and Improvement of Acetic Acid Production by Use of the Cloned Gene. Appl Environ Microbiol. 1989 Jan;55(1):171–176. doi: 10.1128/aem.55.1.171-176.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Subramani S., Scheffler I. E. Use of the DNA polymerase chain reaction for homology probing: isolation of partial cDNA or genomic clones encoding the iron-sulfur protein of succinate dehydrogenase from several species. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1934–1938. doi: 10.1073/pnas.86.6.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Holm L., Saraste M., Wikström M. Structural models of the redox centres in cytochrome oxidase. EMBO J. 1987 Sep;6(9):2819–2823. doi: 10.1002/j.1460-2075.1987.tb02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka M., Machida K., Shimada S., Mogi A., Tsuchiya T., Ohmori T., Souma Y., Gonda M., Sone N. Nucleotide sequence of the gene coding for four subunits of cytochrome c oxidase from the thermophilic bacterium PS3. J Biochem. 1990 Nov;108(5):866–873. doi: 10.1093/oxfordjournals.jbchem.a123294. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Ebisuya H., Adachi O. Homology in the structure and the prosthetic groups between two different terminal ubiquinol oxidases, cytochrome a1 and cytochrome o, of Acetobacter aceti. J Biol Chem. 1992 Dec 5;267(34):24748–24753. [PubMed] [Google Scholar]
- Matsushita K., Ebisuya H., Ameyama M., Adachi O. Change of the terminal oxidase from cytochrome a1 in shaking cultures to cytochrome o in static cultures of Acetobacter aceti. J Bacteriol. 1992 Jan;174(1):122–129. doi: 10.1128/jb.174.1.122-129.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Shinagawa E., Adachi O., Ameyama M. Cytochrome a1 of acetobacter aceti is a cytochrome ba functioning as ubiquinol oxidase. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9863–9867. doi: 10.1073/pnas.87.24.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa J., Nakamura H., Yamato I., Mogi T., Anraku Y. Transcriptional regulation of the cytochrome b562-o complex in Escherichia coli. Gene expression and molecular characterization of the promoter. J Biol Chem. 1990 Jul 5;265(19):11198–11203. [PubMed] [Google Scholar]
- Minghetti K. C., Goswitz V. C., Gabriel N. E., Hill J. J., Barassi C. A., Georgiou C. D., Chan S. I., Gennis R. B. Modified, large-scale purification of the cytochrome o complex (bo-type oxidase) of Escherichia coli yields a two heme/one copper terminal oxidase with high specific activity. Biochemistry. 1992 Aug 4;31(30):6917–6924. doi: 10.1021/bi00145a008. [DOI] [PubMed] [Google Scholar]
- Puustinen A., Wikström M. The heme groups of cytochrome o from Escherichia coli. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6122–6126. doi: 10.1073/pnas.88.14.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitio M., Jalli T., Saraste M. Isolation and analysis of the genes for cytochrome c oxidase in Paracoccus denitrificans. EMBO J. 1987 Sep;6(9):2825–2833. doi: 10.1002/j.1460-2075.1987.tb02579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Metso T., Nakari T., Jalli T., Lauraeus M., Van der Oost J. The Bacillus subtilis cytochrome-c oxidase. Variations on a conserved protein theme. Eur J Biochem. 1991 Jan 30;195(2):517–525. doi: 10.1111/j.1432-1033.1991.tb15732.x. [DOI] [PubMed] [Google Scholar]
- Sone N., Yokoi F., Fu T., Ohta S., Metso T., Raitio M., Saraste M. Nucleotide sequence of the gene coding for cytochrome oxidase subunit I from the thermophilic bacterium PS3. J Biochem. 1988 Apr;103(4):606–610. doi: 10.1093/oxfordjournals.jbchem.a122314. [DOI] [PubMed] [Google Scholar]
- Steinrücke P., Steffens G. C., Panskus G., Buse G., Ludwig B. Subunit II of cytochrome c oxidase from Paracoccus denitrificans. DNA sequence, gene expression and the protein. Eur J Biochem. 1987 Sep 15;167(3):431–439. doi: 10.1111/j.1432-1033.1987.tb13356.x. [DOI] [PubMed] [Google Scholar]
- Tamaki T., Fukaya M., Takemura H., Tayama K., Okumura H., Kawamura Y., Nishiyama M., Horinouchi S., Beppu T. Cloning and sequencing of the gene cluster encoding two subunits of membrane-bound alcohol dehydrogenase from Acetobacter polyoxogenes. Biochim Biophys Acta. 1991 Feb 16;1088(2):292–300. doi: 10.1016/0167-4781(91)90066-u. [DOI] [PubMed] [Google Scholar]
- Tamaki T., Horinouchi S., Fukaya M., Okumura H., Kawamura Y., Beppu T. Nucleotide sequence of the membrane-bound aldehyde dehydrogenase gene from Acetobacter polyoxogenes. J Biochem. 1989 Oct;106(4):541–544. doi: 10.1093/oxfordjournals.jbchem.a122889. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]