Abstract
Cell type–specific gene expression patterns are maintained by the stable inheritance of transcriptional states through mitosis, requiring the action of multiprotein complexes that remodel chromatin structure. Genetic and molecular interactions between chromatin remodeling factors and components of the DNA replication machinery have been identified in Schizosaccharomyces pombe, indicating that some epigenetic marks are replicated simultaneously to DNA with the participation of the DNA replication complexes. This model of epigenetic inheritance might be extended to the plant kingdom, as we report here with the positional cloning and characterization of INCURVATA2 (ICU2), which encodes the putative catalytic subunit of the DNA polymerase α of Arabidopsis thaliana. The strong icu2-2 and icu2-3 insertional alleles caused fully penetrant zygotic lethality when homozygous and incompletely penetrant gametophytic lethality, probably because of loss of DNA polymerase activity. The weak icu2-1 allele carried a point mutation and caused early flowering, leaf incurvature, and homeotic transformations of sepals into carpels and of petals into stamens. Further genetic analyses indicated that ICU2 interacts with TERMINAL FLOWER2, the ortholog of HETEROCHROMATIN PROTEIN1 of animals and yeasts, and with the Polycomb group (PcG) gene CURLY LEAF. Another PcG gene, EMBRYONIC FLOWER2, was found to be epistatic to ICU2. Quantitative RT-PCR analyses indicated that a number of regulatory genes were derepressed in the icu2-1 mutant, including genes associated with flowering time, floral meristem, and floral organ identity.
INTRODUCTION
Organogenesis depends on cell fate decisions that must be maintained through the cell divisions that lead to the adult body of multicellular organisms. Such maintenance requires the stable inheritance of gene expression states and behaves as a cellular memory that allows the determined cells to retain their developmental identity through successive mitotic cycles (Schubert et al., 2005). Loss of such cellular memory causes diverse developmental aberrations and diseases, including cancer (Rountree et al., 2001; Gil et al., 2005).
Certain small RNAs are involved in initiating the heterochromatinization of specific chromosome regions (Grewal and Moazed, 2003; Zilberman et al., 2003; Craig, 2004; Lippman and Martienssen, 2004; Mathieu and Bender, 2004). After initiation, protein complexes are recruited that introduce epigenetic marks in histones. Methylation of H3K9 (the Lys-9 residue of the N-terminal tail of the H3 histone) is the most widely studied of these epigenetic marks and seems to be an essential element in the heterochromatinization process (Soppe et al., 2002; Elgin and Grewal, 2003; Craig, 2004). Methylated H3K9 is recognized by the Heterochromatin protein 1 (HP1) of Drosophila melanogaster (Bannister et al., 2001) and its orthologs, Switching 6 (Swi6) of Schizosaccharomyces pombe and the human HP1 (Lachner et al., 2001). After its binding to methylated histones, HP1 mediates the recruitment of other proteins to assemble multiprotein complexes with histone methyltransferase activity (Fransz and de Jong, 2002; Craig, 2004), which repress gene expression through the formation of higher-order chromatin structures (Grewal and Elgin, 2002). During S phase, these structures need to be removed to allow DNA replication and then quickly reassembled to maintain the gene expression states.
The Polycomb group (PcG) of repressors includes crucial components of cellular memory mechanisms that rely on the inheritance of modifications in specific histone tails. They were first discovered in D. melanogaster and found to be responsible for the maintenance of early determined transcription patterns throughout the life cycle of the fly. Loss-of-function mutations in PcG genes result in ectopic expression of homeotic genes (Paro et al., 1998; Francis and Kingston, 2001; Cunliffe, 2003). PcG genes encode components of multiprotein complexes that catalyze methylation of the N-terminal tail of the H3 and H4 histones (Peterson and Laniel, 2004).
Chromatin-mediated gene repression systems exist in plants (He and Amasino, 2005), where an HP1 homolog and several PcG genes have been described. The only protein of Arabidopsis thaliana with overall sequence similarity to HP1 is TERMINAL FLOWER2 (TFL2; also known as LIKE HETEROCHROMATIN PROTEIN1; Larsson et al., 1998; Gaudin et al., 2001; Turck et al., 2007). TFL2 acts as a repressor of genes involved in flowering time, floral organ identity, meiosis, and seed maturation (Nakahigashi et al., 2005). TFL2 is a euchromatic protein that mainly associates with regions evenly distributed along euchromatin, each covering one or two genes marked by the trimethylated H3K27 repressive mark (Turck et al., 2007).
The PcG genes identified in Arabidopsis include, among others, CURLY LEAF (CLF; Goodrich et al., 1997), which represses the floral organ identity gene AGAMOUS (AG; Yanofsky et al., 1990) throughout plant development, VERNALIZATION2 (VRN2; Gendall et al., 2001), which is required for the maintenance of the repression of FLOWERING LOCUS C (FLC; Bastow et al., 2004) in response to vernalization, and EMBRYONIC FLOWER2 (EMF2; Yoshida et al., 2001), which is required to maintain vegetative development and represses flower development (Moon et al., 2003). It has been proposed that different PcG complexes exist in plants, which would include common components, such as EMF2, and specific components, such as CLF and VRN2 (Otte and Kwaks, 2003; Reyes and Grossniklaus, 2003; Chanvivattana et al., 2004). Loss-of-function alleles of the CLF and EMF2 PcG genes share a pleiotropic phenotype that include leaf curling, early flowering, and the misexpression of many genes, some of which encode transcription factors (reviewed in Reyes, 2006). Some of these phenotypic traits are also caused by mutations in genes that encode putative chromatin remodeling factors, such as TFL2, EARLY BOLTING IN SHORT DAYS (Piñeiro et al., 2003), and SPLAYED (Wagner and Meyerowitz, 2002), and by the silencing of BRAHMA (Farrona et al., 2004).
We have already studied at a preliminary level the incurvata2-1 (icu2-1) mutant, which displayed mildly incurved leaves and early flowering. It was identified in a study of 152 Arabidopsis mutants with abnormally shaped leaves, which belong to the Arabidopsis Information Service Form Mutants collection, 13 of which had been isolated by G. Röbbelen and displayed curled, involute leaves, a phenotype that we named Incurvata (Serrano-Cartagena et al., 1999). We found the icu mutations to belong to five complementation groups, one of which, ICU2, was represented by a single recessive allele, icu2-1, which caused the ectopic derepression of several floral organ identity genes in the leaves. In addition, the icu2-1 clf double mutants displayed a synergistic phenotype. Taken together, our results suggested a functional relationship between the ICU2 gene and PcG-mediated gene repression (Serrano-Cartagena et al., 2000).
Here, we report the positional cloning of the ICU2 gene, which was found to encode the putative catalytic subunit of DNA polymerase α, and the isolation of lethal icu2 alleles. The morphological and molecular phenotypes caused by the icu2 mutations, the molecular nature of the icu2-1 allele, and its genetic interactions with mutations in TFL2 and several PcG genes suggest a role for the replication machinery in epigenetic inheritance and plant chromatin packaging. Our results suggest the existence in plants of a cellular memory mechanism that involves DNA polymerase α, as proposed by previous authors for the fission yeast (Nakayama et al., 2000, 2001; Ahmed et al., 2001; Vermaak et al., 2003).
RESULTS
The Recessive icu2-1 Mutation Causes Leaf Incurvature, Early Flowering, and Homeotic Floral Transformations
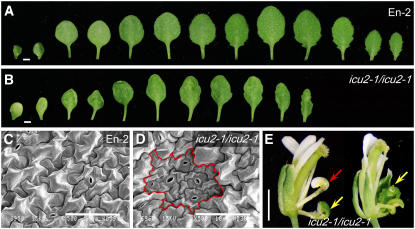
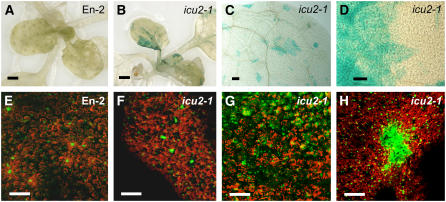
The phenotype of the icu2-1 mutant is pleiotropic. Its vegetative leaves were of slightly reduced size and displayed some bilateral asymmetry and a variable degree of incurvature (Figures 1A and 1B). The surface of these leaves was uneven, which correlated with the presence of patches of small epidermal cells (Figures 1C and 1D). The icu2-1 mutant showed reduced apical dominance, developing an increased number of secondary stems, and its flowers displayed partial homeotic transformations of sepals into carpels and of petals into stamens (Figure 1E). These flower aberrations are similar to those shown by mutants carrying loss-of-function apetala2 alleles, which derepress AG in the first and second floral whorls (Bowman et al., 1989). The icu2-1 mutant was early flowering, bolting 24.43 ± 2.10 d after sowing (the wild-type Enkheim-2 [En-2] bolted 37.80 ± 2.90 d after sowing) and produced less vegetative leaves (9.36 ± 0.81) than En-2 (12.40 ± 0.52).
Figure 1.
Leaf and Flower Phenotypic Traits of the icu2-1 Mutant.
(A) and (B) Excised cotyledons (left) and leaves.
(C) and (D) Scanning electron micrographs of the central region of the adaxial surface of third node leaf laminae (obtained as described in Serrano-Cartagena et al., 2000).
(E) Flowers.
(A) and (C) The En-2 wild type.
(B), (D), and (E) icu2-1/icu2-1 plants.
A patch of epidermal cells with reduced size is highlighted in (D). Arrows in (E) indicate partial homeotic transformations of sepals into carpels (yellow) and petals into stamens (red). Bars = 1 mm in (A), (B), and (E) and 100 μm in (C) and (D).
The ICU2 Gene Encodes the Catalytic Subunit of DNA Polymerase α
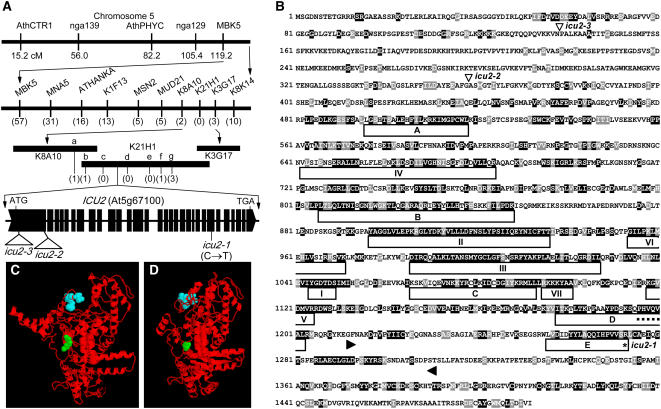
We previously mapped the icu2-1 mutation at a low-resolution level to the lower arm of chromosome 5, linked to the nga129 and MBK5 microsatellites (Serrano-Cartagena et al., 2000). Further linkage analyses allowed us to define a 50-kb candidate region (Figure 2A), which encompassed 15 annotated genes. All the putative transcription units within the candidate region were sequenced in icu2-1/icu2-1 and En-2 plants. A missense mutation was found in the At5g67100 gene (Figures 2A and 2B), which encodes the catalytic subunit of the DNA polymerase α of Arabidopsis.
Figure 2.
Positional Cloning and Structural Analysis of the ICU2 Gene.
(A) Map-based strategy followed for the cloning of the icu2-1 mutation. A mapping population of 1000 F2 plants, derived from icu2-1/icu2-1 × Landsberg erecta (Ler) and icu2-1/icu2-1 × Columbia (Col-0) crosses (the icu2-1 mutation was in an En-2 genetic background) was used to delimit ICU2 to a region of 90 kb, which encompassed three overlapping transformation-competent artificial chromosome (Liu et al., 1999) clones. Several of the candidate genes were amplified and sequenced, allowing us to develop and use for further analyses seven previously unidentified single nucleotide polymorphism (SNP) markers (SNPk8a10-a and SNPk21h1-b to SNPk21h1-g; see Table 2), which were polymorphic between En-2 and either Ler or Col-0. This reduced the candidate region to 50 kb, all whose putative transcription units (http://mips.gsf.de/proj/thal/db/index.html) were PCR amplified and sequenced. Only the At5g67100 gene, which encodes the catalytic subunit of DNA polymerase α, was found to carry a point mutation in the icu2-1 mutant. The number of informative recombinants identified is indicated in parentheses. In the representation of the structure of the ICU2 gene, exons are indicated by boxes, introns by lines between boxes, and T-DNA (icu2-2) and Ds (icu2-3) insertions by triangles. cM, centimorgan.
(B) Predicted amino acid sequence of the catalytic subunit of DNA polymerase α in Arabidopsis. The domains named as I to VII in Wang et al. (1989) and A to E in Miyazawa et al. (1993) are boxed. The region assumed to interact with primase is bordered by arrowheads (Biswas et al., 2003). The putative HP1 binding pentamere (MIR domain) is indicated by a dotted line, the position of the icu2-1 mutation by an asterisk, and the icu2-2 (T-DNA) and icu2-3 (Ds) insertions by triangles. Residues shaded in black and gray indicate the identities and similarities, respectively, found after the alignment of the sequence of the Arabidopsis DNA polymerase α catalytic subunit (GI 15240200) with those of Homo sapiens (8393995), Mus musculus (6679409), D. melanogaster (217344), Caenorhabditis elegans (32565317), O. sativa (6015010), S. pombe (6018683), and Neurospora crassa (32416196). The sequences were aligned using the ClustalX 1.5b program (Thompson et al., 1997) and shaded with BOX SHADE 3.21 (http://www.ch.embnet.org/software/BOX_form.html). The gaps generated by the alignment have been removed.
(C) and (D) Models for the three-dimensional structure of the protein products of the En-2 wild-type (C) and icu2-1 (D) alleles of the ICU2 gene. The residue affected by the mutation is in green, and the HP1 binding domain is in blue. The three-dimensional models were obtained using the 3D-JIGSAW (Bates et al., 2001; http://www.bmm.icnet.uk/∼3djigsaw/) and RasMol 2.7 (Sayle and Milner-White, 1995; http://www.umass.edu/microbio/rasmol/) programs.
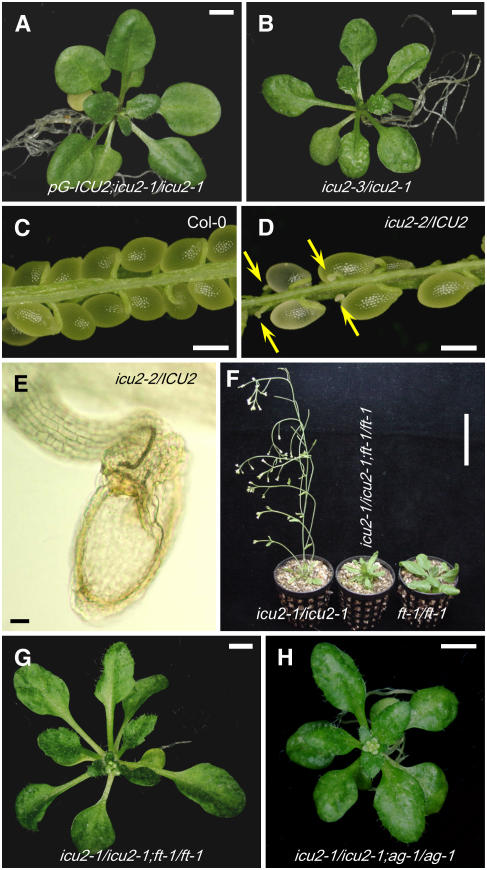
To confirm that loss of function of the At5g67100 gene causes the mutant phenotype of icu2-1/icu2-1 plants, a transgene-mediated complementation experiment was performed. A wild-type (En-2) genomic fragment of 9467 bp encompassing the entire region between the At5g67090 and At5g67110 genes was cloned into the pGreenII0179 vector. The resulting construct was named pG-ICU2 and transferred into icu2-1/icu2-1 plants (see Methods). Nine transformant lines were obtained, all of which displayed wild-type morphology (Figure 3A).
Figure 3.
Other Phenotypic Traits of icu2 Alleles and Suppression of Some of Them by ag and ft Mutations.
(A) and (B) Rosettes from a phenotypically wild-type transgenic line carrying the pG-ICU2 wild-type transgene in an icu2-1/icu2-1 background, demonstrating the phenotypic rescue of icu2-1 by the wild-type allele of At5g67100 (A) and an icu2-3/icu2-1 heterozygote (B), which is indistinguishable to the icu2-1/icu2-1 homozygotes.
(C) to (E) Dissected siliques from selfed Col-0 (C) and heterozygous icu2-2/ICU2 ([D]; in a Col-0 genetic background) plants. Arrows in (D) indicate abnormal seeds that are likely to be aborted or unfertilized ovules. One of the latter is magnified in (E) as seen with Nomarski optics and did not display any embryonic structure, which was clearly visible in the morphologically normal seeds.
(F) to (H) Suppression of some of the phenotypic traits of the icu2-1 mutation by loss-of-function mutations in FT and AG.
(F) and (G) Early flowering is suppressed in icu2-1/icu2-1;ft-1/ft-1.
(H) Leaf incurvature is suppressed in icu2-1/icu2-1;ag-1/ag-1 double mutant plants.
Images were taken 21 ([A], [B], [G], and [H]), 40 ([C] to [E]), and 35 (F) d after sowing. Bars = 2 mm in (A), (B), (G), and (H), 1 mm in (C) and (D), 0.1 mm in (E), and 5 cm in (F).
At5g67100 is a large, single-copy gene that contains 30 exons, coding for a 1492–amino acid protein (Figure 2B). The icu2-1 mutation consisted of a C→T transition in the 24th exon of At5g67100, in nucleotide position 6762 of the transcription unit (numbering from the initiation codon), which substituted Arg by Cys in amino acid position 1273 (Figure 2B), a residue that is outside the predicted polymerase catalytic domains of the enzyme (see the II, III, and V domains in Figure 2B; Wong et al., 1998) and other conserved regions. The promoter of this gene includes the E2F binding site motif TTTCCCGC, which is characteristic of cell cycle–regulated genes (Fry and Farnham, 1999; Chaboute et al., 2000).
Proteins interacting with HP1 in D. melanogaster share the PXVXL consensus sequence, named the MOD1-interacting region (MIR) domain (Murzina et al., 1999; Smothers and Henikoff, 2000). A similar pentameric sequence (PFVQV) is present in Swi7, the DNA polymerase α of S. pombe, and is required for its binding to the HP1 homolog Swi6 (Nakayama et al., 2001). This sequence is also shared by other DNA polymerase α proteins, including ICU2 (PHVQV; Figure 2B) and those of D. melanogaster (PHVQV) and mammals (PHVHV). A computer-based prediction of protein structure suggested that the amino acid change caused by the icu2-1 mutation (Figures 2C and 2D) would reduce or abolish access of some proteins to the MIR domain of ICU2.
The icu2-2 and icu2-3 Recessive Alleles Cause Early Lethality
A search for insertional alleles of ICU2 in public collections allowed us to find the GK-731A12 line in the GABI-Kat database (Li et al., 2003; Rosso et al., 2003), which was confirmed as carrying a T-DNA (Sulr) insertion in position 1075 bp (numbering from the initiation codon) in the first exon of the ICU2 gene, and named icu2-2 (Figures 2A and 2B). An enhancer trap line (N175161) that had been generated by J. Clarke and M. Bevan was confirmed as carrying a Ds (Kanr) transposable element in the first exon of the ICU2 gene and named icu2-3 (Figures 2A and 2B). The presence of Ds- or T-DNA–tagged read-through transcripts was tested by nonquantitative RT-PCR in ICU2/icu2-2 and ICU2/icu2-3 heterozygotes, with only the icu2-3 chimeric transcript being detected.
The ICU2/icu2-2 and ICU2/icu2-3 heterozygotes were phenotypically wild-type. No homozygous mutants were found in their progeny, which suggested recessive lethality. We did not find icu2-1/icu2-2 or icu2-2/icu2-3 heterozygous plants in the progeny of icu2-1/icu2-1 × ICU2/icu2-2 or ICU2/icu2-2 × ICU2/icu2-3 crosses, respectively. On the contrary, the icu2-1/icu2-3 heterozygotes were viable and displayed a morphological phenotype indistinguishable from that of the icu2-1/icu2-1 homozygotes (Figure 3B). Moreover, the icu2-2 allele was not transmitted to the viable progeny of reciprocal icu2-1/icu2-1 × ICU2/icu2-2 crosses (Table 1 ).
Table 1.
Transmission of icu2 Alleles
| Seed Phenotype as Shown in Opened Siliques
|
Phenotypes of Plants Grown on
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic-Supplemented Medium
|
Nonsupplemented Medium
|
||||||||||||
| Crosses (Female × Male) | Aborted or Unfertilized Ovules | Aborted Seeds | Mature Seeds | Ungerminated Seeds | Sulr Wild Type | Sulr Icu2 | Suls | Kanr Wild Type | Kanr Icu2 | Kans | Ungerminated Seeds | Wild Type | Icu2 |
| ICU2/ICU2 × ICU2/ICU2 | 2 | 0 | 726 | 0 | 175 | ||||||||
| icu2-1/icu2-1 × icu2-1/icu2-1 | 102 | 0 | 327 | 0 | 175 | ||||||||
| ICU2/icu2-2 × ICU2/icu2-2 | 413 | 0 | 424 | 0 | 84 | 467 | |||||||
| ICU2/ICU2 × ICU2/icu2-2 | 63 | 0 | 118 | 9 | 6 | 38 | |||||||
| ICU2/icu2-2 × ICU2/ICU2 | 99 | 0 | 90 | 22 | 11 | 126 | |||||||
| ICU2/icu2-3 × ICU2/icu2-3 | 160 | 45 | 389 | 0 | 275 | 273 | |||||||
| ICU2/ICU2 × ICU2/icu2-3 | 40 | 9 | 62 | 7 | 27 | 30 | |||||||
| ICU2/icu2-3 × ICU2/ICU2 | 17 | 4 | 100 | 8 | 92 | 104 | |||||||
| icu2-1/icu2-1 × ICU2/icu2-2 | 41 | 2 | 15 | 8 | 0 | 0 | 2 | 9 | 22 | 0 | |||
| ICU2/icu2-2 × icu2-1/icu2-1 | 106 | 0 | 65 | 26 | 0 | 0 | 84 | 14 | 71 | 0 | |||
| icu2-1/icu2-1 × ICU2/icu2-3 | 24 | 11 | 36 | 10 | 0 | 9 | 12 | 0 | 10 | 10 | |||
| ICU2/icu2-3 × icu2-1/icu2-1 | 54 | 18 | 175 | 1 | 0 | 44 | 50 | 3 | 68 | 53 | |||
| icu2-1/icu2-3 × icu2-1/icu2-3 | 99 | 100 | 172 | 7 | 0 | 87 | 46 | 2 | 0 | 36 | |||
| ICU2/icu2-2 × ICU2/icu2-3 | 28 | 1 | 5 | 2 | 3 | 9 | 4 | 12 | |||||
Sulr, sulfadiazine resistant; Suls, sulfadiazine sensitive; Kanr, kanamycin resistant; Kans, kanamycin sensitive; Icu2, mutant morphology indistinguishable from that of icu2-1/icu2-1 plants.
When developing siliques were dissected in selfed ICU2/icu2-2 plants, half of the seeds were found to be abnormal, small, and white and seemed to be aborted or unfertilized ovules without any visible embryonic structure (Figures 3C to 3E), whereas all the remaining seeds were morphologically normal, and only 15% of which carried the icu2-2 allele (Table 1). The siliques of ICU2/ICU2 × ICU2/icu2-2 reciprocal crosses displayed aborted or unfertilized ovules and mature seeds, some of which gave morphologically wild-type ICU2/icu2-2 plants. Our results indicate that most of the female icu2-2 gametes are not viable and that some wild-type ovules yield lethal zygotes after fertilization by icu2-2 pollen.
The icu2-3 allele also caused gametic lethality with incomplete penetrance, although weaker than that of icu2-2. This conclusion was reached from analyses of the progeny of selfed ICU2/icu2-3 plants and those of ICU2/ICU2 × ICU2/icu2-3 reciprocal crosses. The corresponding siliques displayed a higher proportion of normal seeds, together with aborted or unfertilized ovules as well as brown and shrivelled seeds that seemed to have arrested their development in a later stage of maturation. The proportion of ICU2/icu2-3 plants was in all cases higher than that of ICU2/icu2-2, further indicating that the deleterious effects of icu2-2 are stronger than those of icu2-3. Similar conclusions were reached from the analysis of reciprocal icu2-1/icu2-1 × ICU2/icu2-2 and icu2-1/icu2-1 × ICU2/icu2-3 crosses. Although gametogenesis seemed to be affected also by the weak icu2-1 allele, the aborted or unfertilized ovules found in the siliques of nonmanipulated, naturally selfed icu2-1/icu2-1 plants were absent from icu2-1/icu2-1 siliques pollinated by hand with icu2-1 pollen. This suggests that the early lethality observed in icu2-1/icu2-1 plants is due to insufficient pollen quantity rather than poor pollen quality. Taken together, these results indicate that the icu2-2 and icu2-3 alleles, but not icu2-1, affect both ovule and pollen formation.
The Phenotype of the icu2-1 Mutant Is Largely Due to the Derepression of AG and FT
Given that the early flowering phenotype of the tfl2 mutants is suppressed (Kotake et al., 2003) by mutations at the flowering promoter gene FLOWERING LOCUS T (FT; Kobayashi et al., 1999), and due to the resemblance between the phenotypes of tfl2-2, icu2-1, and clf-2, we crossed the ft-1 mutant to either icu2-1 or clf-2. Given that the double mutants displayed the leaf morphology of their icu2-1 or clf-2 parentals and late flowering, such as that of ft-1 (Figures 3F and 3G), we concluded that the early flowering of icu2-1 requires FT. We already found that the severe loss-of-function ag-1 mutation (Chen and Meyerowitz, 1999) suppressed the mutant phenotype of icu2-1/icu2-1 leaves (Figure 3H), where not only AG, but also APETALA1 (AP1; Mandel et al., 1992), AP3 (Jack et al., 1992), and PISTILLATA (PI; Bowman et al., 1989) were ectopically derepressed (Serrano-Cartagena et al., 2000). We obtained in this work double mutant combinations of icu2-1 with the strong loss-of-function ap1-1 (Bowman et al., 1993), ap3-4 (Jack et al., 1992; Irish and Yamamoto, 1995), and pi-1 (Bowman et al., 1991) mutations and found that both leaf morphology and flowering time of all the double mutants were similar to those of the icu2-1 single mutant. These results indicate that both AG and FT are epistatic to ICU2 and that their derepressions (see below) cause leaf incurvature and early flowering, respectively, in the icu2-1 mutant; the results also show that FT is repressed not only by TFL2 but also by CLF and ICU2.
ICU2 Genetically Interacts with CLF, TFL2, EMF2, FAS1, and FAS2
Ectopic expression of AG and other MADS box genes is known to be caused by loss of function of TFL2, CLF, or EMF2, among others (reviewed in Schubert et al., 2005). The mutants affected in these genes share several phenotypic traits with icu2-1, including leaf curvature and early flowering. Therefore, we decided to analyze the genetic interactions between icu2, tfl2, clf, and emf2. We obtained double mutants involving icu2-1 (Figure 4B) and the null clf-2 (Goodrich et al., 1997) and tfl2-2 (Kotake et al., 2003) mutations (Figures 4C and 4D). The phenotypes of the icu2-1 clf-2, icu2-1 tfl2-2, and clf-2 tfl2-2 double mutants (Figures 4E to 4G) were very similar and much more severe than those of either parental, displaying strongly incurved and narrow leaves arranged in an extremely small rosette. These synergistic genetic interactions suggested that the ICU2, CLF, and TFL2 genes are functionally related.
Figure 4.
Genetic Interactions between Mutations Affecting ICU2 and Genes Involved in Chromatin-Mediated Cellular Memory.
Rosettes are shown for the wild-type En-2 (A) and single ([B] to [D] and [H]) and double mutants ([E] to [G] and [I]). All plants shown were homozygous for the indicated mutations. Images were taken 21 d after sowing. Bars = 2 mm.
The emf2-5 mutant carries a weak hypomorphic allele of the EMF2 gene (Yang et al., 1995; Chen et al., 1997), is extremely early flowering, and develops a tiny rosette composed of small, narrow, and involute leaves (Yoshida et al., 2001; Figure 4H). Its phenotype is similar to those of the icu2-1 clf-2, icu2-1 tfl2-2, and clf-2 tfl2-2 double mutants. We found emf2-5 to be epistatic to icu2-1, as deduced from a comparison of the icu2-1/icu2-1;emf2-5/emf2-5 plants (Figure 4I) with their ICU2/-;emf2-5/emf2-5 siblings, which were phenotypically indistinguishable in the F2 progeny (427 plants) of an emf2-5/emf2-5 × icu2-1/icu2-1 cross. Taken together with the previously described epistasis of emf2 to clf (Chanvivattana et al., 2004), our results suggested a functional relationship among ICU2, CLF, TFL2, and EMF2 genes.
We also crossed the icu2-1 mutant to viable mutants defective in Chromatin Assembling Factor-1 (CAF-1) function, which is required for histone deposition in Arabidopsis (Schönrock et al., 2006). CAF-1 is a heterotrimeric complex that includes the CAC1, CAC2, and CAC3 proteins in yeast, p150, p60, and p48 in mammals, and FAS1, FAS2, and MSI1 in plants, loss of function of which results in transcription of some silenced genes. Mammalian CAF-1 is necessary for coupling chromatin assembly with DNA replication (Hoek and Stillman, 2003), and its p150 subunit is known to interact with HP1 (Murzina et al., 1999; Quivy et al., 2004). The fas1 and fas2 mutants of Arabidopsis are characterized by fasciated and flat stems, disrupted phyllotaxy, altered floral organ structure and number, leaf serration, and inhibition of root elongation (Leyser and Furner, 1992; Figures 5B and 5E). We crossed icu2-1 to the fas mutants and found that the icu2-1 fas1-1 double mutant displayed a synergistic phenotype consisting of small, narrow, and involute leaves (Figures 5C, 5D, and 5F), similar to those of the icu2-1 clf-2 and icu2-1 tfl2-2 double mutants obtained in this work. In addition, the icu2-1 fas1-1 floral organs had much more severe alterations than those of either parental (Figure 5F), displaying disrupted phyllotaxy and strongly curly siliques, which only contained aborted or unfertilized ovules. In addition, we performed an icu2-1/icu2-1 × fas2-1/fas2-1 cross, finding in its F2 progeny no icu2-1/icu2-1;fas2-1/fas2-1 plants, as well as a reduced frequency of the icu2-1/icu2-1;FAS2/fas2-1 and ICU2/icu2-1;fas2-1/fas2-1 genotypes (expected, 2/15 each; observed, 1/100 each), which were phenotypically indistinguishable to their icu2-1/icu2-1;FAS2/FAS2 and ICU2/ICU2;fas2-1/fas2-1 siblings, respectively.
Figure 5.
Genetic Interactions between Mutations Affecting ICU2 and FAS1.
Plants are shown for single ([A], [B], and [E]) and double ([C], [D], and [F]) mutants. A magnification of the lateral view of (C) is highlighted in (D). All plants shown were homozygous for the indicated mutations, and all the mutants are in an En-2 background. Images were taken 10 d ([A] to [D]) and 2 months ([E] and [F]) after sowing. Bars = 1 mm.
A Number of Genes Encoding Transcription Factors Are Upregulated in the icu2-1 Mutant
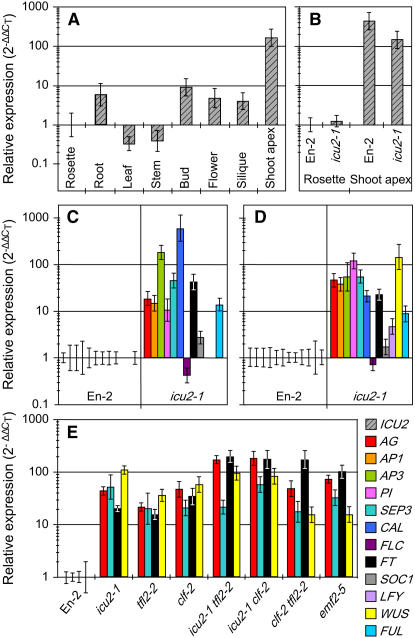
When ICU2 transcript levels were analyzed in assorted tissues of the wild-type En-2 by means of quantitative RT-PCR (QRT-PCR; Figure 6A), we found ∼10-fold more expression in shoot apices (where meristematic cells are more abundant) than in roots, flower buds, open flowers, and siliques, which, in turn, displayed 10-fold more expression than the whole rosettes that were used as a reference. The lowest levels of ICU2 activity were found in leaves and stems. The icu2-1 point mutation did not significantly change the transcriptional activity of the ICU2 gene in rosettes or in shoot apices (Figure 6B). The same conclusion can also be reached by examining the expression profiles of ICU2 obtained from Genevestigator (http://www.genevestigator.ethz.ch; Zimmermann et al., 2004).
Figure 6.
QRT-PCR Analyses.
Quantifications are shown for the transcript levels of ICU2 ([A] and [B]) in assorted tissues of the wild-type En-2 (A) and rosettes and shoot apices of En-2 and the icu2-1 mutant (B), as well as for those of the indicated genes in leaves (C) or whole rosettes ([D] and [E]) of the icu2-1 mutant ([C] and [D]) and some other single and double mutants (E). All plants were homozygous for the mutations indicated. Plant material was collected 21 d after sowing, with the roots being removed before the extraction of rosette RNA. All data were referred (using the 2−ΔΔCT method) to those obtained for the En-2 wild type, to which a value of 1 was given.
Using a nonquantitative method, we have already demonstrated the ectopic expression of the AG, AP1, AP3, and PI genes in the leaves of the icu2-1 mutant (Serrano-Cartagena et al., 2000). In this work, we used QRT-PCR to analyze the expression of these genes and of other genes, which included several members of the MADS box family (SEPALLATA3 [SEP3; Pelaz et al., 2000], CAULIFLOWER [CAL; Kempin et al., 1995], FRUITFULL [FUL; Gu et al., 1998], FLC [Michaels and Amasino, 1999], and SUPRESSOR OF OVEREXPRESSION OF CONSTANS1 [SOC1; Lee et al., 2000]), as well as meristem identity and flowering time genes (FT, LEAFY [LFY; Weigel et al., 1992], and WUSCHEL [WUS; Laux et al., 1996]). We found strongly upregulated the AG, AP1, AP3, PI, SEP3, CAL, FUL, SOC1, and FT genes both in excised leaves and whole rosettes of the icu2-1 mutant (Figures 5C and 5D), as well as LFY and, particularly, WUS only in whole rosettes (Figure 5D). To ascertain whether derepression of the AG, AP1, AP3, or PI floral organ identity genes might in turn be the cause of the derepression of the rest of the studied genes, we obtained the icu2-1 ag-1, icu2-1 ap1-1, icu2-1 ap3-4, and icu2-1 pi-1 double mutants and found no significant differences between their QRT-PCR analyses and those of the icu2-1 single mutant. These results indicated that derepression of SEP3, SEP1, SEP2, CAL, FUL, SOC1, FT, LFY, and WUS is not an indirect consequence of AG, AP1, AP3, or PI deregulation in the icu2-1 mutant.
Given the pivotal contribution of AG and FT derepression to the phenotype of the icu2-1, clf-2, and tfl2-2 mutants, and due to the important role of SEP3 and WUS in flower organogenesis and meristem function, respectively, we decided to quantify the expression of these four genes in the icu2-1, clf-2, tfl2-2, and emf2-5 single mutants as well as in the icu2-1 tfl2-2, icu2-1 clf-2, and clf-2 tfl2-2 double mutants. We found the AG, SEP3, FT, and WUS genes to be upregulated (Figure 5E). AG and FT transcript levels were similar in the double mutants and the emf2-5 single mutant and clearly higher than those observed in the icu2-1, clf-2, and tfl2-2 single mutants. The highest WUS transcript levels were observed in the icu2-1 single mutant and the icu2-1 tfl2-2 and icu2-1 clf-2 double mutants, suggesting a role for ICU2 in the repression of this meristematic gene.
The AG Locus Is Enriched in the H3ac Active Mark in the icu2-1 Mutant
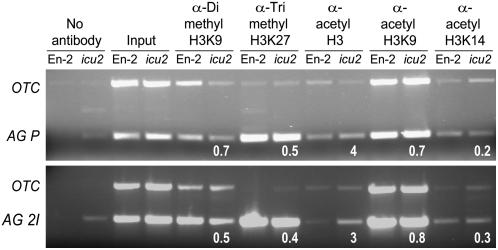
The genetic interactions among ICU2, CLF, TFL2, EMF2, FAS1, and FAS2 described here and the derepression of regulatory genes that we observed in the icu2-1 mutant suggested a role for ICU2 in epigenetic inheritance and plant chromatin packaging. In addition, it is known that expression of AG and STM, which are direct CLF targets, depends on their histone methylation pattern (Schubert et al., 2006). This prompted us to perform a chromatin immunoprecipitation (ChIP) analysis to test whether the icu2-1 mutation affects epigenetic marks in AG, which is derepressed in the icu2-1 mutant.
We tested the dimethylation of Lys-9 of histone H3 (H3K9me2), which in Arabidopsis is localized within silent heterochromatin (Lindroth et al., 2004), and the trimethylaltion of Lys-27 of H3 (H3K27me3), which is preferentially associated with open euchromatin regions (reviewed in Henikoff, 2005). These two epigenetic marks were not significantly changed in the promoter and second intron of the AG gene (Figure 7). We also tested H3 acetylation and found a noticeable enrichment of H3ac in the AG promoter in the icu2-1 mutant (Figure 7). To determine whether or not these histone modifications were associated with changes in DNA methylation, we analyzed by bisulfite genomic sequencing the AG promoter and found that cytosine methylation was not significantly different in the icu2-1 mutant (0.38%; 15 clones sequenced) and the En-2 wild type (1.32%; 10 clones).
Figure 7.
ChIP Assay.
The histone methylation and acetylation patterns of the promoter (AG P) and second intron (AG 2I) of AG in the En-2 wild type and the icu2-1 mutant (lanes headed as icu2) were tested. ChIP duplex PCR was used to amplify the ORNITHINE TRANSCARBAMILASE (OTC) gene and regions of the AG gene. Numbers below gel lanes indicate the ratio of the intensity of AG products compared with that of OTC products after normalization with input product intensity (Schubert et al., 2006).
ICU2 Interacts with TFL2 in Vitro
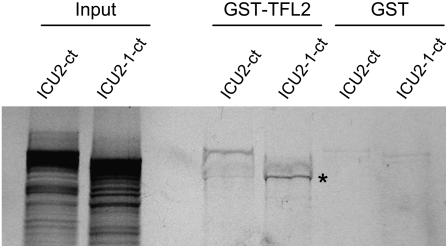
To analyze the physical interaction between ICU2 and TFL2, we expressed in bacteria TFL2 fused to the glutathione S-transferase (GST) protein. Both GST and GST-TFL2 proteins bound to sepharose beads were incubated with the carboxylic half of the wild-type ICU2 protein (ICU2-ct) and that of its mutated version (ICU2-1-ct), which had been translated in vitro in the presence of radiolabeled Met. GST-TFL2 interacted with the full-length ICU2-ct but not with the full-length ICU2-1-ct protein (Figure 8). Interestingly, a smaller subproduct of the synthesis of the ICU2-1-ct protein, which according to the molecular weight deducted from its migration in the gel contains the HP1 binding domain but not the amino acid changed by the icu2-1 mutation, was able to interact with GST-TFL2. This result indicated that the wild-type ICU2 protein interacts with TFL2 and suggested that the icu2-1 mutation disturbs somehow such interaction.
Figure 8.
GST Pull-Down Assay Demonstrating Direct Interaction between the TFL2 and ICU2 Proteins.
The carboxylic half of the wild-type ICU2 protein (ICU2-ct) and that of its mutated version (ICU2-1-ct) were translated in vitro in the presence of 35S-Met. GST-TFL2, but not GST, was able to interact with the full-length ICU2-ct as well as with a truncated ICU2-1-ct protein (indicated by an asterisk). GST-TFL2 did not interact with the full-length ICU2-1-ct. The input lane shows the signal from 10% of the amount of in vitro–translated ICU2-ct and ICU2-1-ct proteins present in each of the remaining samples.
The Spatial Expression Patterns of AG and TFL2 Are Patchy in the icu2-1 Mutant
To visualize the spatial expression pattern of AG in icu2-1/icu2-1 leaves, a transgenic line carrying the pAG-I:β-glucuronidase (GUS) reporter construct (Sieburth and Meyerowitz, 1997) was crossed to icu2-1/icu2-1 and En-2 plants. No GUS staining was observed in the leaves of the F2 plants carrying the transgene in a wild-type background (Figure 9A), whereas patches of GUS-stained cells were frequent in the pAG-I:GUS;icu2-1/icu2-1 leaves (Figures 9B to 9D). This patchy ectopic AG expression was displayed by mesophyll, epidermal, and vascular cells of icu2-1/icu2-1 leaves. The expression of pAG-I:GUS was also studied in a tfl2-2/tfl2-2 mutant background, with GUS staining only being found in vascular tissues.
Figure 9.
AG and TFL2 Spatial Expression Patterns in the icu2-1 Mutant.
(A) to (D) GUS staining of leaves of transgenic plants carrying the pAG-I:GUS construct in En-2 (A) and icu2-1/icu2-1 ([B] to [D]) backgrounds.
(E) to (H) Confocal micrographs of leaves from transgenic plants carrying the gTFL2:GFP construct in En-2 (E) and icu2-1/icu2-1 ([F] to [H]) backgrounds. All plants shown were homozygous for the indicated mutations. Images were taken 21 d after sowing. Bars = 1 mm in (A) and (B) and 100 μm in (C) to (H).
The spatial distribution of the TFL2 protein was studied in En-2 and icu2-1/icu2-1 leaves using the gTFL2:green fluorescent protein (GFP) transgene (Kotake et al., 2003), which contains the whole coding sequence and the promoter of TFL2. All wild-type leaf cells displayed a nuclear GFP signal (Figure 9E), as reported previously (Gaudin et al., 2001; Kotake et al., 2003). A similar GFP subcellular localization was observed in many transgenic icu2-1/icu2-1 leaves (Figure 9F), which displayed in addition patches of cells in which the signal was both nuclear and cytoplasmic (Figure 9G) and extremely high (Figure 9H). Although the size and distribution of the patches showing ectopic AG expression were very similar to those showing perturbation of TFL2 subcellular distribution, we have no proof of their colocalization.
DISCUSSION
Mutant Alleles of ICU2, a Plant Gene Encoding a DNA Polymerase α Subunit
Five eukaryotic DNA polymerases have been classically described (α, β, δ, ε, and γ; Kornberg and Baker, 1991), to which at least another 10 have been added in the last years (Hubscher et al., 2002). The β and ε polymerases have been associated with repair and the α and δ with replication, all of them in the nucleus, whereas the γ polymerase is required for mitochondrial DNA replication. The Arabidopsis gene encoding DNA polymerase ε is the only plant DNA polymerase gene that has been studied at a mutational level so far, with all its described mutant alleles causing gametic or embryonic lethality (Jenik et al., 2005; Ronceret et al., 2005).
We positionally cloned ICU2 (At5g67100), a single-copy gene encoding the putative catalytic subunit of DNA polymerase α, and identified and characterized three icu2 mutant alleles. DNA polymerase α is an essential piece in the cell cycle, where it is required for the initiation of replication; in all eukaryotes, it consists of a catalytic core, two primases, and the so-called B-subunit (of 170, 50, 60 and 70 to 75 kD, respectively; Uchiyama and Wang, 2004). These proteins are encoded in Arabidopsis by the At5g67100, At5g41880, At1g67320, and At1g67630 genes, respectively. Consistent with its important role in the initiation of DNA replication, transcription of the gene encoding the DNA polymerase α catalytic subunit is known to be cell cycle regulated in D. melanogaster, S. pombe, and human cells (Wahl et al., 1988; Hirose et al., 1991; Bouvier et al., 1992). In plants, its activity has been seen to peak before the S phase in rice (Oryza sativa) and maize (Zea mays; Yokoi et al., 1997; Gómez Roig and Vázquez-Ramos, 2003). As expected, the ICU2 promoter included the consensus sequence characterizing cell cycle–regulated genes, and its expression was higher in the tissues showing higher cell division rates, such as the shoot apex.
As expected from the essential role played by replication machinery elements, including DNA polymerase α, the strong icu2-2 and icu2-3 insertional alleles caused fully penetrant zygotic lethality when homozygous and incompletely penetrant gametophytic lethality, probably because of loss of DNA polymerase activity. The early lethality of these alleles is likely to be due to their inability to contribute to the initiation of replication in gametogenesis or early postfertilization. Although no phenotypic evidence of perturbation in DNA replication was observed in the icu2-1 mutant, we cannot exclude that it also lacks some degree of polymerase α activity. The phenotypic effects of the icu2-2 allele were the most severe, and the lethality of the icu2-1/icu2-2 genotype versus the viability of icu2-1/icu2-3 suggested that icu2-2 might not only be an extreme loss-of-function allele but also antimorphic. Although our icu2 alleles might shed light on plant DNA replication, further molecular research on their effects on the polymerase activity of ICU2 will be needed, which is beyond the scope of this work.
Genetic and Physical Interactions between ICU2 and TFL2
Like many other DNA polymerases, the structure of the catalytic DNA polymerase α subunit has been compared with a right hand and divided into three canonical domains termed palm, fingers, and thumb (Wang et al., 1997; Rothwell and Waksman, 2005). In Saccharomyces cerevisiae, single mutations in the palm region affect replication fidelity and genomic stability (Limsirichaikul et al., 2003; Niimi et al., 2004), whereas those damaging the fingers reduce processability or the replication rate (Ogawa et al., 2003). In S. pombe, only a few weak alleles of Swi7, the gene encoding DNA polymerase α, have been isolated, and null alleles are recessive lethal (Singh and Klar, 1993). The swi7-H4 allele carries a mutation in the conserved box VI and uncouples mitosis and DNA synthesis (Murakami and Okayama, 1995). The swi7-1 allele causes a Gly-to-Glu change in position 1116 of the thumb domain (12 residues upstream to the MIR domain) and perturbs mating-type switching. These results suggest a role for DNA replication in the organization of cellular differentiation programs (Singh and Klar, 1993). The icu2-1 mutation described here affects the thumb, changing the three-dimensional structure of the ICU2 protein, which probably affects accessibility to its MIR domain.
Both the presence of a MIR domain in ICU2 and the genetic interaction described here between the icu2-1 and tfl2-2 mutations pointed to the physical interaction of ICU2 and TFL2. In addition, a computer-based prediction of protein structure suggested that the icu2-1 mutation collapses the ICU2 thumb, which in turn could reduce or abolish the access of TFL2 to the MIR domain. We confirmed this genetic evidence by demonstrating that TFL2 binds to wild-type ICU2, a protein–protein interaction that is likely to be impaired by the amino acid substitution caused by the icu2-1 mutation.
We found in the icu2-1 mutant an abnormal subcellular distribution of TFL2, which was restricted to the nucleus in most leaf cells, but appeared in both the nucleus and the cytoplasm in other cells, which were often clustered. This suggested that reduced or abolished interaction between ICU2 and TFL2 perturbs the nucleocytoplasmic distribution of TFL2. However, we cannot rule out the possibility of position effect variegation, due to the characteristics of the genomic region flanking the gTFL2:GFP transgene, as the cause of the patchy redistribution and increase of the TFL2:GFP protein.
A Link between DNA Replication and Chromatin-Mediated Gene Repression in the Plant Kingdom
Several lines of evidence indicate a link between the DNA replication machinery and cellular memory (McNairn and Gilbert, 2003; Vermaak et al., 2003; Craig, 2004). On the one hand, mutations in the origin recognition complexes affect the repression state of different genes in yeast and perturb position effect variegation in D. melanogaster (Pak et al., 1997). On the other hand, some chromatin-associated proteins, such as HP1 or those of the CAF complexes, are known to physically interact with elements of the replication machinery, such as proliferating cell nuclear antigen (Zhang et al., 2000; Ehrenhofer-Murray, 2004; Sarraf and Stancheva, 2004). In addition, the DNA polymerase α Swi7 binds to the HP1 homolog Swi6 in S. pombe, and the swi7-1 and swi7-H4 mutants, which lack such interaction, display deregulation of the mating-type region and an abnormal chromosomal localization of Swi6. These latter observations indicate that some epigenetic marks are replicated simultaneously to DNA with the participation of the DNA replication complexes, at least in the fission yeast (Ahmed et al., 2001; Nakayama et al., 2001).
We hypothesize that the ICU2 DNA polymerase α plays a role in epigenetic inheritance in Arabidopsis by facilitating the interaction of TFL2 with histones, which results in propagation of epigenetic marks. In the icu2-1 mutant, this reheterochromatinization polymerase-mediated process would be impaired, making unstable the repression of many genes. Given that the icu2-1 mutation does not strongly affect viability or fertility, the interaction between ICU2 and TFL2 is probably not essential in Arabidopsis or not completely abolished in the icu2-1 mutant, since the MIR domain remains intact. In fact, in S. pombe mutants lacking Swi6–Swi7 interaction, DNA replication activity is almost normal and only the silencing of specific chromosomal regions is perturbed.
Chromatin-mediated gene repression is reduced by the icu2-1 mutation but not completely abolished, as suggested by the morphological aberrations and gene deregulation levels of the icu2-1 and tfl2-2 mutants, which are weaker than those of the icu2-1 tfl2-2 double mutant. The weak phenotype of the tfl2-2 mutant may be due to redundancy with other genes of the chromodomain family. In the icu2-1 mutant, chromatin-mediated cellular memory might fail for some genes in some cells and their progeny as a consequence of impaired TFL2–ICU2 interaction, which in turn would reduce or abolish TFL2–histone interaction, making the repression of TFL2-targeted genes unstable. This clonal behavior might explain the patchy pattern of epidermal cell size, AG ectopic expression, and TFL2 intracellular distribution found in the leaves of the icu2-1 mutant.
The dose level of HP1 is known to affect position effect variegation in D. melanogaster (Ayyanathan et al., 2003), which is consistent with a dynamic structure of silent chromatin, as proposed in the site exposure model (Ahmad and Henikoff, 2002). In the icu2-1 mutant, silent chromatin might lack epigenetic marks after replication longer than in the wild type, allowing misexpression of some genes. It is worth mentioning that ectopic expression of AG does not show a patchy pattern in the tfl2-2 and clf-2 mutants, in which it is uniform, which correlates with their not patchy morphological phenotype, consisting of leaf epidermal cells of reduced size (Larsson et al., 1998; Serrano-Cartagena et al., 2000). The patchy phenotype caused by the icu2-1 mutation might be a useful trait for analyzing the mechanism by which the epigenetic marks are transmitted through mitosis after DNA replication.
Other Genetic Interactions of ICU2
We found the emf2-5 mutation to be epistatic to icu2-1, clf-2, and tfl2-2. In addition, the morphological and molecular phenotypes of the icu2-1 tfl2-2, icu2-1 clf-2, and tfl2-2 clf-2 double mutants are very similar to those of the emf2-5 single mutant. Taken together, these results suggest that EMF2 acts in the same pathway or plays a more general role than that of ICU2, TFL2, and CLF. It is worth noting that the phenotypes of weak emf2 alleles are reminiscent of those of strong clf alleles (Chanvivattana et al., 2004), whereas those of the weak clf-9 mutation and icu2-1 are very similar. It seems that all these genes affect with different strengths the same biological process, which would be specifically related to the repression of FT and AG, among others.
The phenotype of the clf-2 mutant is weaker than those of the icu2-1 clf-2 and tfl2 clf-2 double mutants, suggesting that other PcG proteins or complexes participate in the repression mechanism, as has been proposed based on the partial redundancy found between CLF and SWINGER (Chanvivattana et al., 2004). The synergistic phenotype of the icu2-1/icu2-1;fas1-1/fas1-1 double mutants, together with the lethality of the icu2-1/icu2-1;fas2-1/fas2-1 genotype, and the reduced frequency of the icu2-1/icu2-1;FAS2/fas2-1 and ICU2/icu2-1;fas2-1/fas2-1 plants clearly indicate a functional relationship between ICU2 and the CAF-1 histone deposition complex. These observations reinforce the notion of a functional relationship between chromatin remodeling machinery and the ICU2 DNA polymerase α.
Several Genes Derepressed by the icu2-1 Mutation Are TFL2 Targets
Quantitative RT-PCR analyses in the icu2-1 mutant allowed us to identify the upregulation of several closely related MADS box genes of the MIKC group (Parenicova et al., 2003) mainly responsible for floral organ identity, including SEP1, SEP2, SEP3, AG, AP1, AP3, PI, CAL, and FUL. In addition, the flowering promoter gene FT was found upregulated, while the flowering repressor gene FLC was downregulated. Derepression of these genes could be an indirect effect of the derepression of the direct targets of ICU2, as suggested by the demonstration of the regulation of SEP3 and FUL by FT (Teper-Bamnolker and Samach, 2005).
Some of the genes that we found derepressed in the icu2-1 mutant have been found upregulated in a microarray analysis performed on the tfl2-3 mutant (AG, AP3, FLC, PI, and SEP3; Nakahigashi et al., 2005), and some are targeted by the TFL2 protein and enriched in the H3K27me3 repressive mark in the wild type (AG, AP3, FLC, and LFY; Turck et al., 2007).
Another gene found derepressed in the icu2-1 mutant, WUS, is known to be regulated by the FAS1 and FAS2 chromatin assembling factors, which are related with chromatin packaging and DNA replication (Kaya et al., 2001). This suggests that WUS, an important meristematic gene, might also be regulated by chromatin-mediated gene silencing. Indeed, WUS is overexpressed as a consequence of loss of function of the GCN5 gene, which encodes a histone acetyltransferase in Arabidopsis (Bertrand et al., 2003). Unexpectedly, we did not find the phenotypic traits characteristic of WUS overexpression, perhaps because the overexpression of other genes suppressed its effects. Our results bring to mind those obtained by other authors on the repression of homeotic genes in Drosophila and mammals and that of mating-type genes in yeasts and reinforce previous results indicating that floral transition, the major developmental switch in plant life, is controlled mainly by chromatin-mediated gene silencing (reviewed in He and Amasino, 2005; Reyes, 2006).
METHODS
Plant Material and Culture Conditions
Unless otherwise stated, all the mutations used in this work were loss of function and recessive. The icu2-1 (N329) mutant (which we initially named icu2 in Serrano-Cartagena et al., 2000) and its corresponding wild type, En-2 (N1138), as well as the ag-1 (NW25; in the Ler genetic background), tfl2-2 (N3797; Col), clf-2 (N290; Ler), ft-1 (N56; Ler), fas1-1 (N265; En-2), and fas2-1 (N266; Ler) lines, were provided by the Nottingham Arabidopsis Stock Centre. The icu2-2 (in the Col genetic background), icu2-3 (Ler) and emf2-5 (Ler) mutants, and the pAG-I:GUS (No-0) and gTFL2:GFP (Col) transgenic lines were provided by GABI-Kat (http://www.mpiz-koeln.mpg.de/GABI-Kat/), Jonathan Clarke, Nobumasa Yoshida, Justin Goodrich, and Koji Goto, respectively.
Plants were grown in sterile (in 150-mm diameter Petri plates containing 100 mL of Murashige and Skoog agar medium, including 1% sucrose) or nonsterile (in pots containing a 2:2:1 mixture of perlite, vermiculite, and sphagnum moss) conditions at 20 ± 1°C, 60 to 70% relative humidity, and continuous illumination of 7000 lx, as described by Ponce et al. (1998). Sterile cultures were performed at a density of 100 regularly spaced seeds per plate in Conviron TC16 tissue culture chambers, and the plants were either collected to be studied or transferred to soil 21 d after sowing. When required, culture media were supplemented with 25 μg/mL of kanamycin or 10 μg/mL of sulfadiazine.
Sequencing
The synthetic oligonucleotides used for cycle sequencing (Table 2) were bought from Applied Biosystems or from Sigma-Genosys. The sequences of the wild-type En-2 and mutant alleles of the ICU2 gene were obtained from both strands of the products of PCR amplifications of overlapping segments of the At5g67100 gene, whose wild-type Col-0 sequence was already available. Sequencing reactions were performed with ABI PRISM BigDye Terminator Cycle Sequencing kits in 5-μL reaction volumes, and sequencing electrophoreses were performed on an ABI PRISM 3100 Genetic Analyzer as described by Pérez-Pérez et al. (2004).
Table 2.
Primer Sets Used in This Work
| Oligonucleotide Sequences (5′→3′)
|
PCR Product Size (bp) and Polymorphismsa | |||
|---|---|---|---|---|
| Purpose | Template | Forward Primer | Reverse Primer | |
| Linkage analysis | SNPk8a10-a | TTTTGCTCTTCTCGTCGGTGAA | TCAGTTAAAACCTACCTTAGACC | 961; A324 (Col-0), G (En-2, Ler) |
| SNPk21h1-b | AGAAATACTATGCTGCATTGTCC | TTCTTCTCCATAAATTCACCAAAC | 714; G145 (Col-0, En-2), C (Ler) | |
| SNPk21h1-c | ACTTCAGTGCGAAGCACCCC | AGAACTTTATCAGACAACTCTCC | 253 (Col-0, En-2), 246 (Ler) | |
| SNPk21h1-d | TTTGAATGGAAGAGTTAAATGGTG | ATGATTACGTAAAATCACGGAATG | 686; G460 (Col-0), C (En-2), A (Ler) | |
| SNPk21h1-e | CAGCGTCTTTCCCATTACACTT | ATACTCCCATCTACATCCTACAT | 1739; G205 (Col-0, En-2), C (Ler); C812 (Col-0, En-2), G (Ler) | |
| SNPk21h1-f | TTAACTTCCCTGAGCTTCTTCC | GGCGCTAATGATTCGTAGAAGC | 406; T165 (Col-0, En-2), A (Ler) | |
| SNPk21h1-g | TCTACGATTATCATCCTAATGGC | TCTCCTTTCTCTAAATTAACCCC | 1331; A627 (Col-0, Ler), C (En-2) | |
| Genomic DNA amplification and cycle sequencing | ICU2 | TTGCAGTTTAAGGATGGGAAGC | TGATTTCACCTCATCAGGATGC | 871 |
| ICU2 | ATAGAGAACAATGATTTTGGATATG | TAACTGGAACAGTACCTGGTAG | 1053 | |
| ICU2 | AGGTTACTCCAGATGAGTCGG | ACTCGCCTTTAAGATCTTCTGG | 1275 | |
| ICU2 | AAATCGTCTGTTTTCTTACATCTC | TGTGAAGGATCACAAACACAGC | 1106 | |
| ICU2 | AAATTCAAAGAACGGCTGCAATG | AGTAAGCTGGAGAGTGAGAGG | 1236 | |
| ICU2 | TCTTTTCTTTCAGATTGAATGTGG | TTAGACGATTATGTCAGAATTAGG | 990 | |
| ICU2 | AGTATTGTATTGGTGACTGCTGC | ATTTTCTGCAAGAAAGGGTATGC | 1089 | |
| ICU2 | TCTGTGCAGGGTAATGCTAGC | GTGCTTGCATGACTCGTCTTC | 1024 | |
| FAS1 | ACTATGGTAGCTGTGAAGAGTG | TGAGCTGTTCTTCTGCATCATG | 567 | |
| FAS2 | TATTTCTGGCTCAGTGGACAAC | GATCCATCAGGTGACCATGAC | 502 | |
| Confirmation of insertions | ICU2 and Ds | ATAGAGAACAATGATTTTGGATATG | ACCCGACCGGATCGTATCGGTb | 854 |
| Ds and ICU2 | GAAACGGTCGGGAAACTAGCTCTACc | ACTCGCCTTTAAGATCTTCTGG | 1405 | |
| T-DNA | CCACACGTGGATCGATCCGTCGd | GAACCCTAATTCCCTTATCTGGGe | 1181 | |
| ICU2 and T-DNA | ATAGAGAACAATGATTTTGGATATG | ATATTGACCATCATACTCATTGCf | 1590 | |
| Rescue | ICU2 | ATAGCGGCCGCGTCTGAGCAAGTTTTTTTCTCTg | ATAGTCGACGCAGAAACTCATTCCCAATTCCh | 9467 |
| pGreenII0179 | TCTTCGCTATTACGCCAGCTG | GCGGATAACAATTTCACACAGG | 332 | |
| QRT-PCR | AG | CCGATCCAAGAAGAATGAGCTCTT | CATTTTCAGCTATCTTTGCACGAA | 110 |
| AP1 | CGACGTCAATACAAACTGGTCGAT | CTTTAGGGCTCATTGCTTGCA | 116 | |
| AP3 | CCCTAACACCACAACGAAGGAGAT | GTTTCCTCTTGGTTTCTTGCATTC | 103 | |
| CAL | CTCACGTTAATGCACAGACGAA | AGATCCTTGAGGCTCATTGGTT | 126 | |
| FLC | TTGAACTTGTGGATAGCAAGCTT | CGGTCTTCTTGGCTCTAGTCA | 121 | |
| FT | GAACAACCTTTGGCAATGAGATT | CACCCTGGTGCATACACTGTT | 120 | |
| FUL | CGACTCTTGCATGGAGAGGAT | CTTGAGCTTAGCATGTTCTAGAA | 120 | |
| ICU2 | TGTTGAAGGAGGTCAGTTATTCT | CACAAGTGTTTTGGATGACTGAA | 118 | |
| LFY | CCCACCAAGGTGACGAACCA | ACAGTGAACGTAGTGTCGCATT | 93 | |
| OTC | TGAAGGGACAAAGGTTGTGTATGTT | CGCAGACAAAGTGGAATGGA | 94 | |
| PI | TTCAAATGCCTGAGCTCCAGTT | GCTAAGCATGAGAACCTTAGCA | 83 | |
| SEP3 | TTAGCAGTTGAACTTAGTAGCCA | CCAAGATCTTCTCCCAACAGAT | 100 | |
| SOC1 | GCCAGCTCCAATATGCAAGATA | CTTCATATTTCAAATGCTGCATATT | 108 | |
| WUS | TGGATCTATGGAACAAGACTGTT | GGCTTTGCTCTATCGAAGAAGT | 113 | |
| Bisulfite sequencingi | AG promoter | AAGAAGAAGATTGATATTTGTTGTAAT | CTTATTAAAAACACCCCCAAATTAAA | 281 |
| AG 2nd intron | AGATTTAGTTTTGTAGAATTAAGATTT | TCAAATATATAATATAATATAATTCATAAA | 378 | |
| ChIP | AG promoter | GAAGAAGATCGATATTTGTTGTAAC | ATTAAGGACACCCCCAAATTGAG | 276 |
| AG 3′ 2nd intron | TTCTTCTTCTCGTGCTCTGTTC | ATCTAAATCTTCAAGTACTTGTTAG | 606 | |
| AG 3′ 2nd intron | ATTCAGTTTTGTAGAACTAAGATTC | AATATATGATATGATATAATTCATGAG | 373 | |
| AG 3′ 2nd intron | TTACTTTCCTTTCTTATCTCTAGC | TACTAGTTTGAGTAATGTAGTTCG | 485 | |
| AG 5′ 2nd intron | CTCTCTCATTATGGGTACTGAG | TCAAATCGACCACTTGCACAGT | 742 | |
| AG 5′ 2nd intron | TGGAACGTTGTGATGTTACTCG | TCAAATCGACCACTTGCACAGT | 478 | |
Polymorphisms are indicated by numbers that correspond to positions within the PCR amplification product, starting from the 5′ end of the forward primer.
The sequences of Ds3-1b and Ds5-1c were obtained from the Arabidopsis Transposon Insertion Service.
The sequences of Sulf-9525,d Sulf-10706,e and Lb-8409f were obtained from http://www.gabi-kat.de/General_Information/GABI-Kat-pAC161T-DNAmapPr.html.
The 5′ tails of these oligonucleotides include restriction sites for NotIg and SalIh.
We also used M13 forward and reverse standard primers.
For bisulfite sequencing, genomic DNA was extracted from 21-d-old plants and bisulfite treated using the EZ DNA Methylation-Gold kit (Zymo Research; D5005) and the following reaction conditions: 10 min at 98°C, 10 min at 53°C, eight cycles consisting of 6 min at 53°C, and 30 min at 37°C, and a final step of 4°C. A 2-μL aliquot of bisulfite-treated DNA was used for each PCR amplification. Primer design (Table 2) and PCR conditions were similar to those previously described (Clark et al., 1994). PCR products were cloned using the TOPO TA cloning kit (Invitrogen). Ten to fifteen individual clones were sequenced for each experiment.
Complementation of the icu2-1 Mutation
A 9467-bp DNA molecule, encompassing the whole ICU2 transcription unit, was PCR amplified in 50-μL reaction mixes using K21H1 transformation-competent artificial chromosome as a template and the primers shown in Table 2, whose 5′ tails included NotI and SalI restriction sites. The Expand Long Template PCR system (Roche) was used following the indications of the manufacturer and a polymerization time of 11 min. The PCR product obtained was digested with NotI and SalI and cloned into the pGreenII0179 vector (Hellens et al., 2000). Competent Escherichia coli DH5α cells were transformed, and transformants were isolated on Luria-Bertani plates supplemented with 25 μg/mL of kanamycin, 0.25 mg/mL of X-Gal, and 0.04 mg/mL of isopropylthio-β-galactoside and tested by PCR for the presence of the construct. Plasmid DNA was isolated from positive clones and used to transform competent Agrobacterium tumefaciens C58C1 cells carrying the pSoup helper plasmid (Hellens et al., 2000). Agrobacterium transformants were selected on Luria-Bertani medium supplemented with 25 μg/mL of kanamycin and 5 μg/mL of tetracycline. Liquid cultures of the positives clones were used for in planta transformations of Arabidopsis thaliana wild-type and mutant plants (Bechtold and Pelletier, 1998). For the isolation of transformant plants, T2 seeds were sown in agar plates supplemented with 40 μg/mL of hygromycin. The presence of the transgene in the putative transformants was verified by PCR.
GUS Staining and GFP Visualization
Homozygotes for either the gTFL2:GFP or pAG-I:GUS transgene were crossed to mutant icu2-1/icu2-1 and wild-type En-2 plants and their F1 allowed to selfpollinate. F2 plants carrying the transgene were selected in 25 μg/mL of kanamycin-supplemented plates. For GUS analyses, 21-d-old plants carrying the pAG-I:GUS transgene in wild-type and icu2-1/icu2-1 backgrounds were collected and treated with 90% acetone for 15 min on ice. Samples were then washed with 100 mM sodium phosphate buffer, pH 7.0, containing 3 mM K4Fe(CN)6, 3 mM K3Fe(CN)6, and 0.1% Triton X-100 for 2 min on ice and then covered with 1 mM 5-bromo-4-chloro-3-indolyl β-d-glucuronide in the same buffer and incubated overnight at 37°C. After staining, leaves were cleared in an ethanol series. For GFP visualization, vegetative leaves of the 4th and 5th nodes were excised from 21-d-old plants carrying the gTFL2:GFP construct, mounted in water on slides, and directly observed by confocal laser scanning microscopy in a Leica TCS-NT microscope equipped with FITC/TRITC filters.
Real-Time QRT-PCR
QRT-PCR amplifications and measurements were performed as described by Pérez-Pérez et al. (2004) in an ABI PRISM 7000 sequence detection system. For each of the genes under study, a primer pair was designed (Table 2) to amplify a product of ∼100 bp. To avoid amplification of genomic DNA, the 5′ and 3′ ends from each pair matched the sequences of two adjacent exons.
Plants were collected 21 d after sowing, frozen in liquid N2, and ground in RNase-free conditions. RNA was extracted with the Qiagen RNeasy plant mini kit, and finally resuspended in 88 μL of RNase-free water and treated with DNaseI at 37°C for 30 min. RNA was then precipitated and resuspended in 40 μL of RNase-free water. Three to five micrograms of the RNA solution obtained were reverse transcribed using SuperScript II reverse transcriptase (Gibco BRL) following the manufacturer's instructions. One microliter of the resulting cDNA solution was used as template in a 25-μL QRT-PCR reaction mix, which included 12.5 μL of the SYBR-Green PCR master mix (Applied Biosystems) and 0.4 μM of primers. Relative quantification of gene expression was performed using the 2−ΔΔCT or comparative CT method (Livak and Schmittgen, 2001). Reactions were made in triplicate, and the expression levels were normalized using the CT values obtained for the OTC housekeeping gene (Quesada et al., 1999), which was used as an internal reference gene (Cnops et al., 2004; Pérez-Pérez et al., 2004).
Pull-Down Assays
TFL2 cDNA was amplified from 5-d-old seedling mRNA using the Superscript III one-step RT system (Invitrogen) and specific primers spanning from the ATG to the stop codons. The cDNA was cloned in the Gateway-compatible vector D221 (Invitrogen). Afterwards, the cDNA was cloned into the pGEX gateway–adapted vector using the Gateway recombination protocol and introduced into the BL21 bacterial strain to express the recombinant GST or GST-TFL2 proteins under standard conditions. The recombinant proteins were bound to Glutathione sepharose beads (Amersham) and washed extensively with PBS-0.1% Tween 20. The amount of protein bound to beads was quantified by resolving the proteins in SDS-PAGE and staining the gel with SYPRO Ruby (Invitrogen).
The ICU2 wild-type (En-2) and icu2-1 mutant alleles were cloned as described for TFL2, the only exception being that only a segment of the full-length cDNA from nucleotide position 2041 after the ATG to the stop codon was cloned. The wild-type and mutant fragments were named ICU2-ct and icu2-1-ct, respectively. Both of them contained the region encoding the putative TFL2 binding domain, as well as that including the icu2-1 mutation (in the icu2-1-ct fragment), and were cloned into pDEST17 (Invitrogen) and used for in vitro translation with the Wheat Germ system (Promega) in the presence of 35S-Met following the instructions of the manufacturer.
For the pull-down assays, 1 μg of GST or GST-TFL2 bound to Glutathione sepharose beads (GE Healthcare) was incubated with the 35S-labeled protein in PBS-0.1% Tween 20 at 4°C for 3 h. Afterwards, the beads were washed four times during 15 min with 1.5 mL of PBS-0.2% Tween 20. Proteins were resolved on an SDS-PAGE/8% bis-acrylamide gel, and the 35S-products were detected by autoradiography.
ChIP Assay
ChIP was performed according to the protocol in the EpiQuik Plant ChIP kit manual (Epigentek) using 2-week-old En-2 and icu2-1/icu2-1 seedlings and the following antibodies: anti-dimethyl H3K9 (Upstate Biotechnology), anti-trimethyl-H3K27 (Lindroth et al., 2004), anti-acetyl-H3, anti-acetyl-H3K9, and anti-acetyl-H3K14. We used the OTC housekeeping gene for normalization (Quesada et al., 1999). Semiquantitative PCR values were obtained as described by Schubert et al. (2006).
Accession Numbers
The Arabidopsis Genome Initiative locus identifiers for the genes identified and/or analyzed in this study are as follows: AP1, At1g69120; AP3, At3g54340; CAL, At1g26310; CLF, At2g23380; EMF2, At5g51230; FAS1, At1g65470; FAS2, At5g64630; FLC, At5g10140; FUL, At5g60910; ICU2, At5g67100; LFY, At5g61850; OTC, At1g75330; PI, At5g20240; SEP3, At1g24260; SOC1, At2g45660; and WUS, At2g17950.
Acknowledgments
We thank the Nottingham Arabidopsis Stock Centre, GABI-Kat, J. Clarke, N. Yoshida, J. Goodrich, and K. Goto for providing seeds; C. Gutiérrez, H. Candela, J.M. Pérez-Pérez, V. Quesada, P. Robles, and three anonymous referees for comments on the manuscript; J.M. Serrano, V. García, and T. Trujillo for technical assistance; and A. Martínez-Laborda for his help in the low-resolution mapping of ICU2 in the MBK5-K8K14 interval. This work was supported by fellowships (to J.M.B. and R.G.-B.) and grants (BMC2002-02840 and BMC2005-01031 to J.L.M., BMC2003-09763 to M.R.P., and BIO2004-01749 to J.C.P.) from the Ministerio de Educación y Ciencia of Spain.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: José Luis Micol.
References
- Ahmad, K., and Henikoff, S. (2002). Epigenetic consequences of nucleosome dynamics. Cell 111 281–284. [DOI] [PubMed] [Google Scholar]
- Ahmed, S., Saini, S., Arora, S., and Singh, J. (2001). Chromodomain protein Swi6-mediated role of DNA polymerase alpha in establishment of silencing in fission yeast. J. Biol. Chem. 276 47814–47821. [DOI] [PubMed] [Google Scholar]
- Ayyanathan, K., Lechner, M.S., Bell, P., Maul, G.G., Schultz, D.C., Yamada, Y., Tanaka, K., Torigoe, K., and Rauscher III, F.J. (2003). Regulated recruitment of HP1 to a euchromatic gene induces mitotically heritable, epigenetic gene silencing: A mammalian cell culture model of gene variegation. Genes Dev. 17 1855–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister, A.J., Zegerman, P., Partridge, J.F., Miska, E.A., Thomas, J.O., Allshire, R.C., and Kouzarides, T. (2001). Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410 120–124. [DOI] [PubMed] [Google Scholar]
- Bastow, R., Mylne, J.S., Lister, C., Lippman, Z., Martienssen, R.A., and Dean, C. (2004). Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427 164–167. [DOI] [PubMed] [Google Scholar]
- Bates, P.A., Kelley, L.A., MacCallum, R.M., and Sternberg, M.J.E. (2001). Enhancement of protein modelling by human intervention in applying the automatic programs 3D-JIGSAW and 3D-PSSM. Proteins 5 39–46. [DOI] [PubMed] [Google Scholar]
- Bechtold, N., and Pelletier, G. (1998). In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vacuum infiltration. Methods Mol. Biol. 82 259–266. [DOI] [PubMed] [Google Scholar]
- Bertrand, C., Bergounioux, C., Domenichini, S., Delarue, M., and Zhou, D.X. (2003). Arabidopsis histone acetyltransferase AtGCN5 regulates the floral meristem activity through the WUSCHEL/AGAMOUS pathway. J. Biol. Chem. 278 28246–28251. [DOI] [PubMed] [Google Scholar]
- Biswas, S.B., Khopde, S.M., Zhu, F.X., and Biswas, E.E. (2003). Subunit interactions in the assembly of Saccharomyces cerevisiae DNA polymerase alpha. Nucleic Acids Res. 31 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier, D., Pignede, G., Damagnez, V., Tillit, J., de Recondo, A.M., and Baldacci, G. (1992). DNA polymerase alpha in the fission yeast Schizosaccharomyces pombe: Identification and tracing of the catalytic subunit during the cell cycle. Exp. Cell Res. 198 183–190. [DOI] [PubMed] [Google Scholar]
- Bowman, J.L., Alvarez, J., Weigel, D., Meyerowitz, E.M., and Smyth, D.R. (1993). Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 119 721–743. [Google Scholar]
- Bowman, J.L., Smyth, D.R., and Meyerowitz, E.M. (1989). Genes directing flower development in Arabidopsis. Plant Cell 1 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman, J.L., Smyth, D.R., and Meyerowitz, E.M. (1991). Genetic interactions among floral homeotic genes of Arabidopsis. Development 112 1–20. [DOI] [PubMed] [Google Scholar]
- Chaboute, M.E., Clement, B., Sekine, M., Philipps, G., and Chaubet-Gigot, N. (2000). Cell cycle regulation of the tobacco ribonucleotide reductase small subunit gene is mediated by E2F-like elements. Plant Cell 12 1987–2000. [PMC free article] [PubMed] [Google Scholar]
- Chanvivattana, Y., Bishopp, A., Schubert, D., Stock, C., Moon, Y.H., Sung, Z.R., and Goodrich, J. (2004). Interaction of Polycomb-group proteins controlling flowering in Arabidopsis. Development 131 5263–5276. [DOI] [PubMed] [Google Scholar]
- Chen, L., Cheng, J.C., Castle, L., and Sung, Z.R. (1997). EMF genes regulate Arabidopsis inflorescence development. Plant Cell 9 2011–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X., and Meyerowitz, E.M. (1999). HUA1 and HUA2 are two members of the floral homeotic AGAMOUS pathway. Mol. Cell 3 349–360. [DOI] [PubMed] [Google Scholar]
- Clark, S., Harrison, J., Paul, C.L., and Frommer, M.R. (1994). High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 22 2990–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnops, G., Jover-Gil, S., Peters, J.L., Neyt, P., De Block, S., Robles, P., Ponce, M.R., Gerats, T., Micol, J.L., and Van Lijsebettens, M. (2004). The rotunda2 mutants identify a role for the LEUNIG gene in vegetative leaf morphogenesis. J. Exp. Bot. 55 1529–1539. [DOI] [PubMed] [Google Scholar]
- Craig, J.M. (2004). Heterochromatin–many flavours, common themes. Bioessays 27 17–28. [DOI] [PubMed] [Google Scholar]
- Cunliffe, V.T. (2003). Memory by modification: The influence of chromatin structure on gene expression during vertebrate development. Gene 305 141–150. [DOI] [PubMed] [Google Scholar]
- Ehrenhofer-Murray, A.E. (2004). Chromatin dynamics at DNA replication, transcription and repair. Eur. J. Biochem. 271 2335–2349. [DOI] [PubMed] [Google Scholar]
- Elgin, S.C., and Grewal, S.I. (2003). Heterochromatin: Silence is golden. Curr. Biol. 13 R895–R898. [DOI] [PubMed] [Google Scholar]
- Farrona, S., Hurtado, L., Bowman, J.L., and Reyes, J.C. (2004). The Arabidopsis thaliana SNF2 homolog AtBRM controls shoot development and flowering. Development 131 4965–4975. [DOI] [PubMed] [Google Scholar]
- Francis, N.J., and Kingston, R.E. (2001). Mechanisms of transcriptional memory. Nat. Rev. Mol. Cell Biol. 2 409–421. [DOI] [PubMed] [Google Scholar]
- Fransz, P.F., and de Jong, J.H. (2002). Chromatin dynamics in plants. Curr. Opin. Plant Biol. 5 560–567. [DOI] [PubMed] [Google Scholar]
- Fry, C.J., and Farnham, P.J. (1999). Context-dependent transcriptional regulation. J. Biol. Chem. 274 29583–29586. [DOI] [PubMed] [Google Scholar]
- Gaudin, V., Libault, M., Pouteau, S., Juul, T., Zhao, G., Lefebvre, D., and Grandjean, O. (2001). Mutations in LIKE HETEROCHROMATIN PROTEIN 1 affect flowering time and plant architecture in Arabidopsis. Development 128 4847–4858. [DOI] [PubMed] [Google Scholar]
- Gendall, A.R., Levy, Y.Y., Wilson, A., and Dean, C. (2001). The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell 107 525–535. [DOI] [PubMed] [Google Scholar]
- Gil, J., Bernard, D., and Peters, G. (2005). Role of polycomb group proteins in stem cell self-renewal and cancer. DNA Cell Biol. 24 117–125. [DOI] [PubMed] [Google Scholar]
- Gómez Roig, E., and Vázquez-Ramos, J.M. (2003). Maize DNA polymerase alpha is phosphorylated by a PCNA-associated cyclin/Cdk complex: Effect of benzyladenine. J. Plant Physiol. 160 983–990. [DOI] [PubMed] [Google Scholar]
- Goodrich, J., Puangsomlee, P., Martin, M., Long, D., Meyerowitz, E.M., and Coupland, G. (1997). A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature 386 44–51. [DOI] [PubMed] [Google Scholar]
- Grewal, S.I., and Elgin, S.C. (2002). Heterochromatin: New possibilities for the inheritance of structure. Curr. Opin. Genet. Dev. 12 178–187. [DOI] [PubMed] [Google Scholar]
- Grewal, S.I., and Moazed, D. (2003). Heterochromatin and epigenetic control of gene expression. Science 301 798–802. [DOI] [PubMed] [Google Scholar]
- Gu, Q., Ferrandiz, C., Yanofsky, M.F., and Martienssen, R. (1998). The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 125 1509–1517. [DOI] [PubMed] [Google Scholar]
- He, Y., and Amasino, R.M. (2005). Role of chromatin modification in flowering-time control. Trends Plant Sci. 10 30–35. [DOI] [PubMed] [Google Scholar]
- Hellens, R.P., Edwards, E.A., Leyland, N.R., Bean, S., and Mullineaux, P.M. (2000). pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol. Biol. 42 819–832. [DOI] [PubMed] [Google Scholar]
- Henikoff, S. (2005). Histone modifications: Combinatorial complexity or cumulative simplicity? Proc. Natl. Acad. Sci. USA 102 5308–5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose, F., Yamaguchi, M., Nishida, Y., Masutani, M., Miyazawa, H., Hanaoka, F., and Matsukage, A. (1991). Structure and expression during development of Drosophila melanogaster gene for DNA polymerase alpha. Nucleic Acids Res. 19 4991–4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek, M., and Stillman, B. (2003). Chromatin assembly factor 1 is essential and couples chromatin assembly to DNA replication in vivo. Proc. Natl. Acad. Sci. USA 100 12183–12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubscher, U., Maga, G., and Spadari, S. (2002). Eukaryotic DNA polymerases. Annu. Rev. Biochem. 71 133–163. [DOI] [PubMed] [Google Scholar]
- Irish, V.F., and Yamamoto, Y.T. (1995). Conservation of floral homeotic gene function between Arabidopsis and Antirrhinum. Plant Cell 7 1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack, T., Brockman, L.L., and Meyerowitz, E.M. (1992). The homeotic gene APETALA3 of Arabidopsis thaliana encodes a MADS box and is expressed in petals and stamens. Cell 68 683–697. [DOI] [PubMed] [Google Scholar]
- Jenik, P.D., Jurkuta, R.E., and Barton, M.K. (2005). Interactions between the cell cycle and embryonic patterning in Arabidopsis uncovered by a mutation in DNA polymerase epsilon. Plant Cell 17 3362–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya, H., Shibahara, K.I., Taoka, K.I., Iwabuchi, M., Stillman, B., and Araki, T. (2001). FASCIATA genes for chromatin assembly factor-1 in Arabidopsis maintain the cellular organization of apical meristems. Cell 104 131–142. [DOI] [PubMed] [Google Scholar]
- Kempin, S.A., Savidge, B., and Yanofsky, M.F. (1995). Molecular basis of the cauliflower phenotype in Arabidopsis. Science 267 522–525. [DOI] [PubMed] [Google Scholar]
- Kobayashi, Y., Kaya, H., Goto, K., Iwabuchi, M., and Araki, T. (1999). A pair of related genes with antagonistic roles in mediating flowering signals. Science 286 1960–1962. [DOI] [PubMed] [Google Scholar]
- Kornberg, A., and Baker, T. (1991). DNA Replication, 2nd ed. (New York: Freeman and Company).
- Kotake, T., Takada, S., Nakahigashi, K., Ohto, M., and Goto, K. (2003). Arabidopsis TERMINAL FLOWER 2 gene encodes a heterochromatin protein 1 homolog and represses both FLOWERING LOCUS T to regulate flowering time and several floral homeotic genes. Plant Cell Physiol. 44 555–564. [DOI] [PubMed] [Google Scholar]
- Lachner, M., O'Carroll, D., Rea, S., Mechtler, K., and Jenuwein, T. (2001). Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410 116–120. [DOI] [PubMed] [Google Scholar]
- Larsson, A.S., Landberg, K., and Meeks-Wagner, D.R. (1998). The TERMINAL FLOWER2 (TFL2) gene controls the reproductive transition and meristem identity in Arabidopsis thaliana. Genetics 149 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux, T., Mayer, K.F., Berger, J., and Jurgens, G. (1996). The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 122 87–96. [DOI] [PubMed] [Google Scholar]
- Lee, H., Suh, S., Park, E., Cho, E., Ahn, J.H., Kim, S., Lee, J.S., Kwon, Y.M., and Lee, I. (2000). The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 14 2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyser, H.M., and Furner, I.J. (1992). Characterization of three shoot apical meristem mutants of Arabidopsis thaliana. Development 116 397–403. [Google Scholar]
- Li, Y., Rosso, M.G., Strizhov, N., Viehoever, P., and Weisshaar, B. (2003). GABI-Kat SimpleSearch: A flanking sequence tag (FST) database for the identification of T-DNA insertion mutants in Arabidopsis thaliana. Bioinformatics 19 1441–1442. [DOI] [PubMed] [Google Scholar]
- Limsirichaikul, S., Ogawa, M., Niimi, A., Iwai, S., Murate, T., Yoshida, S., and Suzuki, M. (2003). The Gly-952 residue of Saccharomyces cerevisiae DNA polymerase alpha is important in discriminating correct deoxyribonucleotides from incorrect ones. J. Biol. Chem. 278 19079–19086. [DOI] [PubMed] [Google Scholar]
- Lindroth, A.M., et al. (2004). Dual histone H3 methylation marks at lysines 9 and 27 required for interaction with CHROMOMETHYLASE3. EMBO J. 23 4286–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman, Z., and Martienssen, R. (2004). The role of RNA interference in heterochromatic silencing. Nature 431 364–370. [DOI] [PubMed] [Google Scholar]
- Liu, Y.G., Shirano, Y., Fukaki, H., Yanai, Y., Tasaka, M., Tabata, S., and Shibata, D. (1999). Complementation of plant mutants with large genomic DNA fragments by a transformation-competent artificial chromosome vector accelerates positional cloning. Proc. Natl. Acad. Sci. USA 96 6535–6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K.J., and Schmittgen, T.D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25 402–408. [DOI] [PubMed] [Google Scholar]
- Mandel, M.A., Gustafson-Brown, C., Savidge, B., and Yanofsky, M.F. (1992). Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360 273–277. [DOI] [PubMed] [Google Scholar]
- Mathieu, O., and Bender, J. (2004). RNA-directed DNA methylation. J. Cell Sci. 117 4881–4888. [DOI] [PubMed] [Google Scholar]
- McNairn, A.J., and Gilbert, D.M. (2003). Epigenomic replication: Linking epigenetics to DNA replication. Bioessays 25 647–656. [DOI] [PubMed] [Google Scholar]
- Michaels, S.D., and Amasino, R.M. (1999). FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11 949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa, H., Izumi, M., Tada, S., Takada, R., Masutani, M., Ui, M., and Hanaoka, F. (1993). Molecular cloning of the cDNAs for the four subunits of mouse DNA polymerase alpha-primase complex and their gene expression during cell proliferation and the cell cycle. J. Biol. Chem. 268 8111–8122. [PubMed] [Google Scholar]
- Moon, Y.H., Chen, L., Pan, R.L., Chang, H.S., Zhu, T., Maffeo, D.M., and Sung, Z.R. (2003). EMF genes maintain vegetative development by repressing the flower program in Arabidopsis. Plant Cell 15 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, H., and Okayama, H. (1995). A kinase from fission yeast responsible for blocking mitosis in S phase. Nature 374 817–819. [DOI] [PubMed] [Google Scholar]
- Murzina, N., Verreault, A., Laue, E., and Stillman, B. (1999). Heterochromatin dynamics in mouse cells: Interaction between chromatin assembly factor 1 and HP1 proteins. Mol. Cell 4 529–540. [DOI] [PubMed] [Google Scholar]
- Nakahigashi, K., Jasencakova, Z., Schubert, I., and Goto, K. (2005). The Arabidopsis HETEROCHROMATIN PROTEIN1 homolog (TERMINAL FLOWER2) silences genes within the euchromatic region but not genes positioned in heterochromatin. Plant Cell Physiol. 46 1747–1756. [DOI] [PubMed] [Google Scholar]
- Nakayama, J., Allshire, R.C., Klar, A.J., and Grewal, S.I. (2001). A role for DNA polymerase α in epigenetic control of transcriptional silencing in fission yeast. EMBO J. 20 2857–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, J., Klar, A.J., and Grewal, S.I. (2000). A chromodomain protein, Swi6, performs imprinting functions in fission yeast during mitosis and meiosis. Cell 101 307–317. [DOI] [PubMed] [Google Scholar]
- Niimi, A., Limsirichaikul, S., Yoshida, S., Iwai, S., Masutani, C., Hanaoka, F., Kool, E.T., Nishiyama, Y., and Suzuki, M. (2004). Palm mutants in DNA polymerases alpha and eta alter DNA replication fidelity and translesion activity. Mol. Cell. Biol. 24 2734–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, M., Limsirichaikul, S., Niimi, A., Iwai, S., Yoshida, S., and Suzuki, M. (2003). Distinct function of conserved amino acids in the fingers of Saccharomyces cerevisiae DNA polymerase alpha. J. Biol. Chem. 278 19071–19078. [DOI] [PubMed] [Google Scholar]
- Otte, A.P., and Kwaks, T.H. (2003). Gene repression by Polycomb group protein complexes: A distinct complex for every occasion? Curr. Opin. Genet. Dev. 13 448–454. [DOI] [PubMed] [Google Scholar]
- Pak, D.T., Pflumm, M., Chesnokov, I., Huang, D.W., Kellum, R., Marr, J., Romanowski, P., and Botchan, M.R. (1997). Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell 91 311–323. [DOI] [PubMed] [Google Scholar]
- Parenicova, L., de Folter, S., Kieffer, M., Horner, D.S., Favalli, C., Busscher, J., Cook, H.E., Ingram, R.M., Kater, M.M., Davies, B., Angenent, G.C., and Colombo, L. (2003). Molecular and phylogenetic analyses of the complete MADS-box transcription factor family in Arabidopsis: new openings to the MADS world. Plant Cell 15 1538–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paro, R., Strutt, H., and Cavalli, G. (1998). Heritable chromatin states induced by the Polycomb and trithorax group genes. Novartis Found. Symp. 214 51–61. [DOI] [PubMed] [Google Scholar]
- Pelaz, S., Ditta, G.S., Baumann, E., Wisman, E., and Yanofsky, M.F. (2000). B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405 200–203. [DOI] [PubMed] [Google Scholar]
- Pérez-Pérez, J.M., Ponce, M.R., and Micol, J.L. (2004). The ULTRACURVATA2 gene of Arabidopsis encodes an FK506-binding protein involved in auxin and brassinosteroid signaling. Plant Physiol. 134 101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, C.L., and Laniel, M.A. (2004). Histones and histone modifications. Curr. Biol. 14 R546–R551. [DOI] [PubMed] [Google Scholar]
- Piñeiro, M., Gomez-Mena, C., Schaffer, R., Martinez-Zapater, J.M., and Coupland, G. (2003). EARLY BOLTING IN SHORT DAYS is related to chromatin remodeling factors and regulates flowering in Arabidopsis by repressing FT. Plant Cell 15 1552–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce, M.R., Quesada, V., and Micol, J.L. (1998). Rapid discrimination of sequences flanking and within T-DNA insertions in the Arabidopsis genome. Plant J. 14 497–501. [DOI] [PubMed] [Google Scholar]
- Quesada, V., Ponce, M.R., and Micol, J.L. (1999). OTC and AUL1, two convergent and overlapping genes in the nuclear genome of Arabidopsis thaliana. FEBS Lett. 461 101–106. [DOI] [PubMed] [Google Scholar]
- Quivy, J.P., Roche, D., Kirschner, D., Tagami, H., Nakatani, Y., and Almouzni, G. (2004). A CAF-1 dependent pool of HP1 during heterochromatin duplication. EMBO J. 23 3516–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes, J.C. (2006). Chromatin modifiers that control plant development. Curr. Opin. Plant Biol. 9 21–27. [DOI] [PubMed] [Google Scholar]
- Reyes, J.C., and Grossniklaus, U. (2003). Diverse functions of Polycomb group proteins during plant development. Semin. Cell Dev. Biol. 14 77–84. [DOI] [PubMed] [Google Scholar]
- Ronceret, A., Guilleminot, J., Lincker, F., Gadea-Vacas, J., Delorme, V., Bechtold, N., Pelletier, G., Delseny, M., Chaboute, M.E., and Devic, M. (2005). Genetic analysis of two Arabidopsis DNA polymerase epsilon subunits during early embryogenesis. Plant J. 44 23–36. [DOI] [PubMed] [Google Scholar]
- Rosso, M.G., Li, Y., Strizhov, N., Reiss, B., Dekker, K., and Weisshaar, B. (2003). An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Mol. Biol. 53 247–259. [DOI] [PubMed] [Google Scholar]
- Rothwell, P.J., and Waksman, G. (2005). Structure and mechanism of DNA polymerases. Adv. Protein Chem. 71 401–440. [DOI] [PubMed] [Google Scholar]
- Rountree, M.R., Bachman, K.E., Herman, J.G., and Baylin, S.B. (2001). DNA methylation, chromatin inheritance, and cancer. Oncogene 20 3156–3165. [DOI] [PubMed] [Google Scholar]
- Sarraf, S.A., and Stancheva, I. (2004). Methyl-CpG binding protein MBD1 couples histone H3 methylation at lysine 9 by SETDB1 to DNA replication and chromatin assembly. Mol. Cell 15 595–605. [DOI] [PubMed] [Google Scholar]
- Sayle, R.A., and Milner-White, E.J. (1995). RASMOL: Biomolecular graphics for all. Trends Biochem. Sci. 20 374. [DOI] [PubMed] [Google Scholar]
- Schönrock, N., Exner, V., Probst, A., Gruissem, W., and Hennig, L. (2006). Functional genomic analysis of CAF-1 mutants in Arabidopsis thaliana. J. Biol. Chem. 281 9560–9568. [DOI] [PubMed] [Google Scholar]
- Schubert, D., Clarenz, O., and Goodrich, J. (2005). Epigenetic control of plant development by Polycomb-group proteins. Curr. Opin. Plant Biol. 8 553–561. [DOI] [PubMed] [Google Scholar]
- Schubert, D., Primavesi, L., Bishopp, A., Roberts, G., Doonan, J., Jenuwein, T., and Goodrich, J. (2006). Silencing by plant Polycomb-group genes requires dispersed trimethylation of histone H3 at lysine 27. EMBO J. 25 4638–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Cartagena, J., Candela, H., Robles, P., Ponce, M.R., Pérez-Pérez, J.M., Piqueras, P., and Micol, J.L. (2000). Genetic analysis of incurvata mutants reveals three independent genetic operations at work in Arabidopsis leaf morphogenesis. Genetics 156 1363–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Cartagena, J., Robles, P., Ponce, M.R., and Micol, J.L. (1999). Genetic analysis of leaf form mutants from the Arabidopsis Information Service collection. Mol. Gen. Genet. 261 725–739. [DOI] [PubMed] [Google Scholar]
- Sieburth, L.E., and Meyerowitz, E.M. (1997). Molecular dissection of the AGAMOUS control region shows that cis elements for spatial regulation are located intragenically. Plant Cell 9 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, J., and Klar, A.J. (1993). DNA polymerase-alpha is essential for mating-type switching in fission yeast. Nature 361 271–273. [DOI] [PubMed] [Google Scholar]
- Smothers, J.F., and Henikoff, S. (2000). The HP1 chromo shadow domain binds a consensus peptide pentamer. Curr. Biol. 10 27–30. [DOI] [PubMed] [Google Scholar]
- Soppe, W.J., Jasencakova, Z., Houben, A., Kakutani, T., Meister, A., Huang, M.S., Jacobsen, S.E., Schubert, I., and Fransz, P.F. (2002). DNA methylation controls histone H3 lysine 9 methylation and heterochromatin assembly in Arabidopsis. EMBO J. 21 6549–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper-Bamnolker, P., and Samach, A. (2005). The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. Plant Cell 17 2661–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F., and Higgins, D.G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck, F., Roudier, F., Farrona, S., Martin-Magniette, M.L., Guillaume, E., Buisine, N., Gagnot, S., Martienssen, R.A., Coupland, G., and Colot, V. (2007). Arabidopsis TFL2/LHP1 specifically associates with genes marked by trimethylation of histone H3 Lysine 27. PLoS Genet. 3: e86. [DOI] [PMC free article] [PubMed]
- Uchiyama, M., and Wang, T.S. (2004). The B-subunit of DNA polymerase alpha-primase associates with the origin recognition complex for initiation of DNA replication. Mol. Cell. Biol. 24 7419–7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaak, D., Ahmad, K., and Henikoff, S. (2003). Maintenance of chromatin states: An open-and-shut case. Curr. Opin. Cell Biol. 15 266–274. [DOI] [PubMed] [Google Scholar]
- Wagner, D., and Meyerowitz, E.M. (2002). SPLAYED, a novel SWI/SNF ATPase homolog, controls reproductive development in Arabidopsis. Curr. Biol. 12 85–94. [DOI] [PubMed] [Google Scholar]
- Wahl, A.F., Geis, A.M., Spain, B.H., Wong, S.W., Korn, D., and Wang, T.S. (1988). Gene expression of human DNA polymerase alpha during cell proliferation and the cell cycle. Mol. Cell. Biol. 8 5016–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Sattar, A.K., Wang, C.C., Karam, J.D., Konigsberg, W.H., and Steitz, T.A. (1997). Crystal structure of a pol alpha family replication DNA polymerase from bacteriophage RB69. Cell 89 1087–1099. [DOI] [PubMed] [Google Scholar]
- Wang, T.S., Wong, S.W., and Korn, D. (1989). Human DNA polymerase alpha: Prredicted functional domains and relationships with viral DNA polymerases. FASEB J. 3 14–21. [DOI] [PubMed] [Google Scholar]
- Weigel, D., Alvarez, J., Smyth, D.R., Yanofsky, M.F., and Meyerowitz, E.M. (1992). LEAFY controls floral meristem identity in Arabidopsis. Cell 69 843–859. [DOI] [PubMed] [Google Scholar]
- Wong, S.W., Wahl, A.F., Yuan, P.M., Arai, N., Pearson, B.E., Arai, K., Korn, D., Hunkapiller, M.W., and Wang, T.S. (1998). Human DNA polymerase alpha gene expression is cell proliferation dependent and its primary structure is similar to both prokaryotic and eukaryotic replicative DNA polymerases. EMBO J. 7 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, C.H., Chen, L.J., and Sung, Z.R. (1995). Genetic regulation of shoot development in Arabidopsis: Role of the EMF genes. Dev. Biol. 169 421–435. [DOI] [PubMed] [Google Scholar]
- Yanofsky, M.F., Ma, H., Bowman, J.L., Drews, G.N., Feldmann, K.A., and Meyerowitz, E.M. (1990). The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346 35–39. [DOI] [PubMed] [Google Scholar]
- Yokoi, M., Ito, M., Izumi, M., Miyazawa, H., Nakai, H., and Hanaoka, F. (1997). Molecular cloning of the cDNA for the catalytic subunit of plant DNA polymerase alpha and its cell-cycle dependent expression. Genes Cells 2 695–709. [DOI] [PubMed] [Google Scholar]
- Yoshida, N., Yanai, Y., Chen, L., Kato, Y., Hiratsuka, J., Miwa, T., Sung, Z.R., and Takahashi, S. (2001). EMBRYONIC FLOWER2, a novel polycomb group protein homolog, mediates shoot development and flowering in Arabidopsis. Plant Cell 13 2471–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z., Shibahara, K., and Stillman, B. (2000). PCNA connects DNA replication to epigenetic inheritance in yeast. Nature 408 221–225. [DOI] [PubMed] [Google Scholar]
- Zilberman, D., Cao, X., and Jacobsen, S.E. (2003). ARGONAUTE4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 299 716–719. [DOI] [PubMed] [Google Scholar]
- Zimmermann, P., Hirsch-Hoffmann, M., Hennig, L., and Gruissem, W. (2004). GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol. 136 2621–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]