Abstract
The hypothesis, that a decrease in metabolic rate mediates the life span prolonging effect of caloric restriction (CR), was tested using two strains of mice, one of which, C57BL/6, exhibits life span extension as a result of CR, while the other, DBA/2, shows little or no effect. Comparisons of the rate of resting oxygen consumption and body temperature were made between the strains after they were fed ad libitum (AL) or maintained under 40% CR, from 4 to 16 months of age. Ad libitum-fed mice of the two strains weighed the same when young and consumed similar amounts of food throughout the experiment; however, the C57BL/6 mice weighed 25% more than DBA/2 mice at 15 months of age. The rate of oxygen consumption was normalized as per gram body weight, lean body mass or organ weight as well as per animal. The body temperature and the rate of oxygen consumption, expressed according to all of the four criteria, were decreased in the DBA/2 mice following CR. The C57BL/6 mice also showed a CR-related decrease in body temperature and in the rate of oxygen consumption per animal and when normalized according to lean body mass or organ weight. The results of this study indicate that CR indeed lowers the rate of metabolism; however, this effect by CR does not necessarily entail the prolongation of the life span of mice.
Keywords: caloric restriction, metabolic rate, aging, life span, energy balance, obesity, inbred mice, C57BL/6, DBA/2
1. Introduction
Although it has been repeatedly demonstrated that the life spans of laboratory rodents can be extended by long-term reduction in food intake, or caloric restriction, the nature of the underlying mechanisms is presently unclear. It was initially hoped that comparisons of ad libitum (AL) fed and calorically restricted (CR) animals at different ages would reveal physiological features and/or mechanisms that are associated with prolongation of life span; however, the results of numerous studies have collectively indicated that most of the manifestations of aging are attenuated or retarded by CR (for reviews see Weindruch and Walford, 1988; Yu, 1994; Masoro, 2002). Instead, what may be reasonably inferred from such studies is that those age-related changes that are not affected by CR are unlikely to be the primary determinants of longevity. One approach to overcome this limitation and to also broaden the scope of the CR model may be the inclusion of a ‘negative control’, i.e., a relevant strain of laboratory rodents in which CR does not lengthen the life span. For instance, comparisons between AL and CR mice that do or do not respond to CR with an extension of life span should distinguish the physiological effects that are associated with the extension of life span, from among those produced by CR.
It is often generalized that CR prolongs the life spans of most species; however, several groups have demonstrated that this is not a universal phenomenon (Fernandes et al., 1976; Harrison and Archer, 1987; Willott et al., 1995; Forster et al., 2003; Cooper et al., 2004; Mockett et al., 2006). Indeed, our studies have shown that while the AL-fed DBA/2 and C57BL/6 mice have similar mean and maximum life spans, CR by 40% causes a 25% increase in the mean and maximum life span in C57BL/6 mice, but has no life span prolongation effect in DBA/2 mice (Forster et al., 2003). This finding is considered here to qualify this mouse strain as a possible negative control for a strain whose life span is extended by CR.
One school of thought concerning the nature of the possible mechanisms by which CR extends life span is that CR lowers the rate of metabolism (Sacher, 1977), thereby decreasing the production of reactive oxygen species (ROS) and accrual of oxidative damage (for discussion see Sohal and Weindruch, 1996; Ramsey et al., 2000). Whereas it is widely agreed that CR indeed lowers the generation of ROS and the steady-state levels of oxidative damage (Sohal and Weindruch, 1996), there is an intense controversy about its effect on the rate of metabolism (oxygen consumption), with different studies reporting an increase, decrease, or no effect (for discussion see Masoro, 2002; Selman et al., 2005; Hunt et al., 2006; Ramsey and Hagopian, 2006).
An acknowledged source of such discrepancies has been the use of different criteria or estimates of body or metabolic mass for standardizing the rate of oxygen consumption. The AL and CR animals greatly differ in the relative amounts of fat-laden, metabolically inert, white adipose tissue (reviewed in Weindruch and Sohal, 1997). Thus, in comparisons between AL and CR mice, units of total body mass alone would be inappropriate for standardizing the rate of oxygen consumption. The problem with the use of lean body mass is that it is primarily constituted by skeletal muscle tissue, which accounts for only 20−33% of the resting metabolic rate. Furthermore, lean body mass may not necessarily reflect CR-related changes that occur in the weights of tissues such as brain, heart, kidney and liver, which consume significant amounts of oxygen (for discussion see Ramsey et al., 2000; Selman et al., 2005). Therefore, it has been suggested that organ weights may provide a more valid criterion than the lean body mass for normalizing the rate of oxygen consumption (Greenberg and Boozer, 2000). The aforementioned complications highlight the complexities facing the comparisons of metabolic rate between AL and CR animals.
The main purpose of the present study was to investigate whether extension of life span in response to CR is linked to a decrease in the rate of oxygen consumption, and/or a decrease in body temperature, using multiple criteria for standardizing the data as well as a negative control. Specifically, comparisons were made between AL and CR groups of C57BL/6 mice, whose life span is extended by CR, and DBA/2 mice, which exhibit little or no prolongation of life span by CR. Rates of resting oxygen consumption and core body temperature were used as indicators of metabolic rate. Four different criteria were used to normalize the rate of oxygen consumption, namely the whole mouse, body weight, lean body mass, or organ weight (liver + kidney + heart + brain).
2. Materials and methods
2.1. Experimental animals
Male DBA/2 and C57BL/6 male mice were obtained from The Jackson Laboratory (Bar Harbor, ME) at 8−9 weeks of age. Immediately upon arrival at the University of Southern California (USC) vivarium facilities, the mice were housed individually in polycarbonate cages with mesh tops, connected to an automatic watering system. Prior to the initiation of caloric restriction, all mice were fed standard NIH-31 diet ad libitum and kept on a 12 h light/dark cycle, with the light phase beginning at 0600 h.
At 14 weeks of age, one half of the mice from each strain were put on the caloric restriction (CR) regimen described previously by Turturro et al. (1999). The CR was introduced over a 3-week period, starting with a 10% reduction in the first week, 20% in the second, and 40% in the third and thereafter. The CR animals were fed a special NIH-31 formulation to equalize the intake of essential nutrients. The amount of food consumed by the AL mice was monitored throughout the study, and used to ensure that the intake of CR animals was 60% of the amount eaten by the AL mice. The rate of oxygen consumption during rest was measured in the AL and CR groups between 14 and 16 months of age.
For studies on colonic temperature, 16-month-old male DBA/2 and C57BL/6 mice, kept on the same AL or CR regimens described above, were obtained from the National Institute on Aging (NIA) and housed in the University of North Texas Health Science Center (UNTHSC) vivarium facilities under conditions similar to those at USC. They were fed at 2200 h, which is approximately 2-h prior to the acrophase of the normal circadian feeding cycle of the AL mice. The purpose of the night feeding regimen was to synchronize the circadian cycles of the AL and CR mice. Colonic temperature was measured at 17−18 months of age.
2.2. Measurement of oxygen consumption
Mice were placed in a polycarbonate cylindrical respiration chamber (713 cm3) and desiccated air, with carbon dioxide removed, was passed through the chamber at a flow rate of 200 ml•min−1 . Changes in oxygen concentration were determined at 1-s intervals thereafter using an Oxzilla Dual Absolute and Differential Oxygen Analyzer (Sable Systems International, Las Vegas, NV). The rate of oxygen consumption was calculated over a 10−30 min period when the mouse was asleep, as indicated by inactivity, decreased oxygen consumption, and stabilization of the recording line.
For studies on oxygen consumption, the feeding regimen of the CR mice was phase-shifted from 0800−1000 h to 1600−1700 h, to preclude any effect on oxygen consumption (e.g. temperature spikes prior to feeding or postprandial energy expenditure). Two mice, one of each strain, were tested per day and the sequence of testing was alternated.
Body weights were measured immediately before the mice were placed in the respiration chamber. Oxygen consumption, lean body mass and organ weight could not be determined from the same mice, due to the longitudinal nature of this study. Instead, lean body mass and organ weight were calculated by correcting the total body mass according to percentage estimates from the same mouse strains in comparable studies of CR. The percent body fat content was reported by Brochmann et al. (Brochmann et al., 2003) and organ weight (the combined weight of the liver, kidney, heart, and brain) was determined by us in separate cohorts of 14-month-old DBA/2 and C57BL/6 mice.
2.3 Colonic temperature
Measurements of colonic temperature were made at 4-h intervals over a 24-h period, using a Sensortech rectal probe (Thermalert model TH-6, Bailey Instr., Inc., Saddle Break, NJ). To minimize discomfort and stress, measurements were made such that only two of the time points (at least 12-h apart) occurred within a given 24-h period.
2.4 Statistical Analysis
Respiration rates were analyzed using separate two-way Analyses of Variance, with Strain and Diet as the factors. Planned comparisons of AL and CR groups of each strain were made using single degree of freedom F-tests within the overall analyses. Body weight and colonic temperature were considered in three-way analyses, with Strain and Diet as between-groups factors, and Time (age or time of day) as a within groups factor. These results were confirmed using a regression-based adjustment of oxygen consumption that included body weight, lean body mass, or organ weight as covariates in the analysis. Statistical significance was defined as P < 0.05.
3. Results
3.1 Effect of strain and CR on food intake and body weight
Food consumption and body weight of the mice were monitored between 2 and 16 months of age. The food intake by the AL groups showed little variation as a function of age, and throughout the experiment the amounts of food consumed by DBA/2 and C57BL/6 mice did not differ significantly (average food intake = 3.99 ± 0.1 gm/day). Thus, for the duration of the experiment, both strains of mice on the CR regimen were provided with the same amount of food (2.4 ± 0.05 gm) daily, effecting a 40% reduction in caloric intake.
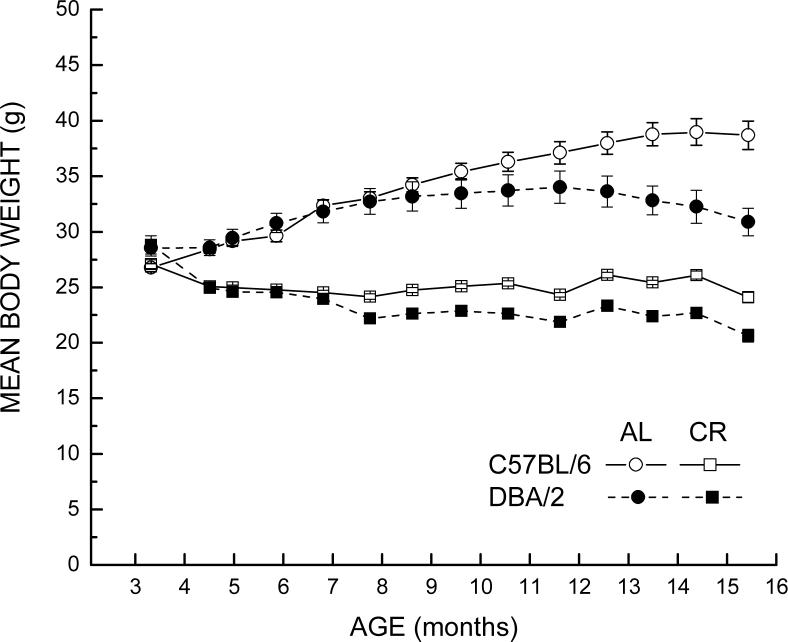
Prior to the initiation of CR, the body weights of the mice of the two strains were similar. The AL-fed mice of both strains showed a similar increase in body weight from 3 to 8 months of age (Fig. 1). Thereafter, the C57BL/6 mice continued to gain weight until the age of 15 months, whereas the weights of the DBA/2 mice remained stable until 13 months of age and then gradually declined. Notably, at 15 months of age, the C57BL/6 mice on the AL regimen were 25% heavier than the corresponding DBA/2 mice.
Fig. 1.
Effect of caloric restriction on body weight (± SEM) of surviving DBA/2 and C57BL/6 mice as a function of age. Mice of each strain (n = 19−20) were maintained under the ab libitum (AL) or caloric restriction (CR) feeding regimen from 14 weeks until the rate of resting oxygen consumption was measured at 14−16 months of age. A 10% CR was initiated at 14 weeks (leftmost data point), which was increased in a stepwise fashion to 40% by 4 months of age. One mouse (a DBA/2 maintained on CR) died after 8.2 months.
Caloric restriction was imposed incrementally starting at 14 weeks of age and the reduction of caloric intake by 40% was fully implemented when the mice reached 4 months of age. There was a similar decrease in body weight in the two strains following the initiation of CR, and their body weights remained nearly equivalent from 5 to 7 months. Thereafter, small differences began to develop between the two strains, paralleling the divergence in body weights of the corresponding AL-fed mice. After 15 months of age, which is approximately 12 months after the onset of 40 % CR, the DBA/2 mice weighed 14% less than the corresponding C57BL/6 mice. It is important to note that the percent differences in body weights between AL and CR groups of each strain at this age were quite similar (33% for DBA/2 and 38% for C57BL/6), although the absolute difference in weights of AL and CR mice (i.e., the weight differential in grams) was 43% greater in the C57BL/6 mice (−10.2 g for DBA/2 vs −14.6 g for C57BL/6). Analysis of variance of body weights yielded a significant 3-way interaction between Strain, Diet, and Age (P<0.001). This interaction was driven primarily by the different effect of age on body weight of the AL-fed C57BL/6 mice, when compared with the AL-fed DBA/2 mice.
3.2 Effect of strain and CR on rate of resting oxygen consumption
The rate of resting oxygen consumption was measured in groups of 14- to 16-month-old mice that had been maintained on AL or CR regimens. Four different criteria were used for standardizing the rate of oxygen consumption: (i) whole mouse, (ii) body weight, (iii) lean body mass and (iv) organ weight (Fig. 2).
Fig. 2.
Rate of resting oxygen consumption in 14−16-month-old C57BL/6 (top panel) and DBA/2 mice (bottom panel) maintained under ad libitum (AL) or 40% caloric restriction (CR) regimens since 4 months of age. Resting oxygen consumption of each mouse was determined during a 10−30 min period of sleep and standardized using different criteria as shown in the panels from left to right: The whole mouse, body weight, lean body mass and organ weight (liver + kidney + heart + brain) (see METHODS). Data are represented as the mean ± SEM (n = 10). * P < 0.025 when compared with AL group.
In general, the rate of oxygen consumption was lower in the CR compared to the AL mice of the same strain. According to all four of the criteria used for standardization, CR significantly decreased the rate of oxygen consumption in DBA/2 mice. The magnitude of the decrease ranged from 24% (per gram body weight) to 50% (per mouse). In the C57BL/6 mice, CR had a comparable effect on the rate of oxygen consumption per mouse (50%) and per gram lean body mass (37%), but the difference between AL and CR groups of this strain was smaller than that exhibited by DBA/2 mice, when expressed in units of organ weight (13% in C57BL/6 vs 32% in DBA/2). Caloric restriction had little or no effect on oxygen consumption when calculated on the basis of per gram body weight in the C57BL/6 mice. Separate two-way analyses of variance performed on rates of oxygen consumption, and expressed as units of body weight or organ weight, suggested a significant Diet × Strain interaction (P< 0.016), which is consistent with the observed relatively smaller effect of CR on C57BL/6 versus DBA/2 for these measures. However, when considered per mouse or per gram lean body mass, there was no indication of a significant main effect of Strain, nor a Strain × Diet interaction (all P>0.063). A significant main effect of Diet was noted regardless of the criteria used to standardize the rate of oxygen consumption (all P < 0.01).
3.3 Effect of strain and CR on colonic temperature
Colonic temperature of 17- to 18- month-old AL and CR mice was measured at 4-h intervals, over a 24-h period (Fig 3). Circadian variations in body temperature occurred in both strains of mice and under both dietary regimens, with a peak between 2200 to 0200 h and an apparent nadir at 1400 h. Overall, the DBA/2 mice on the AL regimen maintained a higher colonic temperature than the C57BL/6 mice on the same regimen.
Fig. 3.
Colonic temperatures of 17−18-month-old C57BL/6 (left panel) and DBA/2 mice (right panel) maintained under ad libitum (AL) or 40% caloric restriction (CR) regimens since 4 months of age. Colonic temperatures were sampled at 4-h intervals, over a 24-h period, while the mice were on a 12-h light:dark cycle (indicated by grey/white bars). The CR groups were fed at 2200 h to mimic the feeding cycle of the AL mice. Data are represented as the mean ± SEM (n = 12−13).
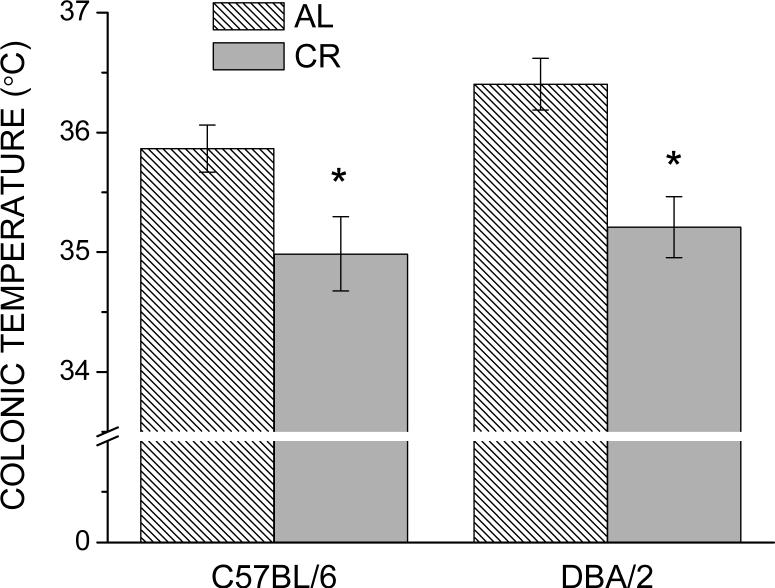
During the light segment of the light:dark cycle (0600 to 1800 h), the colonic temperature of the CR group was lower than the AL group in both strains of mice. This effect of CR on daytime colonic temperature was slightly greater in the DBA/2 when compared with C57BL/6 mice. Notwithstanding, a 3-way analysis of variance on the temperature data did not indicate significant 2- or 3-way interactions of Strain with Diet or Time (all P>0.42), but revealed significant main effects of these factors (all P<0.002), as well as a Diet × Time interaction (P<0.001). When average daytime temperature was considered separately (Fig. 4), there was a significant difference in the temperature of the CR mice in both strains.
Fig. 4.
Average colonic temperature of 17−18-month-old C57BL/6 and DBA/2 mice under AL or CR regimens. Average temperature was calculated from measurements made during the light portion of the light:dark cycle (0600, 1000, and 1400 h). Data are represented as the mean ± SEM (n = 12−13). * P < 0.025 when compared with strain-matched AL group.
4. Discussion
The question, whether or not long-term reduction in caloric intake results in a chronic lowering of metabolic rate, has been vigorously debated in the literature, but still remains unresolved (for discussion see Greenberg and Boozer, 2000; Masoro, 2002; Blanc et al., 2003). In our view, there are two separate issues in this debate: one is whether CR lowers metabolic rate, and the other is whether CR-associated life span extension is linked to a decrease in metabolic rate, and vice versa.
The present study addresses these issues by employing a ‘negative control’, the DBA/2 mice, in which the standard CR regimen fails to prolong the median or maximum life span (Fernandes et al., 1976; Forster et al., 2003). It was reasoned here that if the life span prolongation-effect of CR depends on its ability to decrease the metabolic rate, then an obvious prediction would be that such an effect is attenuated or absent in the DBA/2 mice, but present in the C57BL/6 mice, a genotype that responds to the CR regimen with an increase in median and maximum life span (Turturro et al., 1999; Forster et al., 2003). In this context, a key finding of this study was that CR decreased the rate of oxygen consumption and body temperature in both strains of mice, suggesting that a reduction in metabolic rate by itself is insufficient to account for the longevity extension effect of CR.
At 14−16 months of age, which is approximately 12 months after the initiation of 40% CR, the rate of resting oxygen consumption tended to be relatively lower in both strains of mice. When considered per mouse, the decrease relative to AL groups was nearly equal in the two strains, suggesting that the overall impact of CR on resting energy expenditure was similar. A decrease in oxygen consumption following CR was also evident in both strains of mice, when normalized to metabolically-significant body mass, such as the lean body mass and organ weight, i.e., the combined weight of liver, kidney, heart, and brain (Greenberg and Boozer, 2000).
Notably, in C57BL/6 mice, the effect of CR on the rate of oxygen consumption per gram body weight was smaller than that calculated on the basis of per gram lean body mass. This difference could be attributed to a relatively higher amount of body fat in mature AL C57BL/6 mice (Brochmann et al., 2003; Funkat et al., 2004). In contrast, there was a robust CR-induced decrease in metabolic rate, normalized as per gram body weight, in DBA/2 mice. The latter are leaner than C57BL/6 mice under the AL condition, but are reported to have a similar percentage of body fat as the C57BL/6 mice when maintained under CR (Brochmann et al., 2003; Funkat et al., 2004). Given the strain-related difference in the relative amount of body fat under the AL condition, total body weight would obviously be a less desirable criterion than lean body mass or organ weight for the normalization of metabolic rate. Indeed, when the latter two measures were used to standardize the rate of resting oxygen consumption, there was little or no difference between the strains in the effect of CR.
It has been argued that metabolic rate may decrease only transiently following initiation of CR (e.g., Masoro, 2002). Results of the present study indicate that in two genetically divergent strains of mice, a relatively lower metabolic rate is evident 12 months after the initiation of 40% CR. Although it may be necessary to test mice of younger and older ages to determine the exact period over which the decreased metabolic rate is sustained, the current results accord with the conclusions of Blanc et al. (2003), that CR may indeed result in a sustained decrease in the metabolic rate in a number of other species, including rats, humans and monkeys (Blanc et al., 2003; Raman et al., 2007). In humans and monkeys, these decreases are associated with a decline in the levels of triiodothyronine (T3) (Roth et al., 2002a; Fontana et al., 2006; Heilbronn et al., 2006). It is not yet clear to what extent this may be the case in C57BL/6 or DBA/2 mice.
While there is considerable disagreement about whether or not CR affects the rate of oxygen consumption, there is a virtual consensus that CR decreases the body temperature in rodents and primates, an effect that has been postulated to be linked to the life span-prolongation effect of CR (Lane et al., 1996; Duffy et al., 1997; Roth et al., 2002b; Rikke et al., 2003; Rikke and Johnson, 2004; Heilbronn et al., 2006). Since body temperature in homeotherms is largely dependent upon oxidative phosphorylation, reduction in body temperature in the context of CR is quite likely reflective of a corresponding decrease in the rate of oxygen consumption. In this study, under a long-term CR regimen, both C57BL/6 and DBA/2 mice exhibited a decrease in colonic temperature, which accords with the finding of Rikke et al (Rikke et al., 2003; Rikke and Johnson, 2004), who tested the same strains of mice at younger ages. The CR-related decreases in body temperature in the current studies are consistent with the diminished rates of oxygen consumption in the DBA/2 and C57BL/6 mice. While studies of transgenic mice have suggested that reduction of body temperature may increase median life span independently of CR (Conti et al., 2006), the current results do not support the hypothesis that this effect, in the context of CR, necessarily entails a prolongation of life span.
In this context, the question arises why CR and the resultant lowering of the rate of metabolism and body temperature do not extend the life span of DBA/ mice. One notable difference between the two strains of mice was the age-related pattern of changes in the body weight. Although both mouse strains consumed similar amounts of food throughout the study, the body weight of the AL-fed C57BL/6 mice showed a steady increase between 8 and 15 months of age, whereas the AL-fed DBA/2 mice maintained relatively stable weights after 8 months of age, followed by a decrease beginning at approximately 13 months. These results suggest that under AL feeding conditions, the DBA/2 and C57BL/6 strains may differ in some physiological or behavioral factors influencing energy expenditure that are not reflected in the resting metabolic rate, but result in an apparent negative energy balance in the AL-fed DBA/2 mice over a significant portion of their later life span. Indeed, the results of this study show that AL-fed DBA/2 mice maintain a higher body temperature than C57BL/6 mice, in agreement with reports for mice at younger ages (Rikke et al., 2003; Rikke and Johnson, 2004). Although spontaneous or voluntary motor activity of AL mice of the two strains was found to be similar at young ages (Forster and Lal, 1991; Rocha et al., 1998; Funkat et al., 2004), studies have found that the DBA/2 mice are more active than C57BL/6 following CR (Gelegen et al., 2006; Gelegen et al., 2007). In addition, the DBA/2 mice have been reported to secrete more insulin in response to glucose stimulation than C57BL/6 or 129T2 mice (Andrikopoulos et al., 2005), which may be indicative of strain differences in nutrient absorption and/or utilization. Regardless of the mechanisms involved, the maintenance of a neutral or negative energy balance in the AL-fed DBA/2 mice, may confer upon them a diminished ability for CR to increase longevity.
It is widely hypothesized that life span extension by CR may be due to an attenuation of oxidative stress (Sohal and Weindruch, 1996). Indeed CR diminishes the rate of mitochondrial generation of ROS and oxidative damage to proteins and DNA (Sohal et al., 1994a; Sohal et al., 1994b), as well as causes an elevation in glutathione redox potential in C57BL/6 mice (Rebrin et al., 2003). It was therefore of interest to determine whether or not CR also lowers the level of oxidative stress in the DBA/2 mice. Studies on different regions of the brain of DBA/2 mice indicated that CR decreased the level of oxidative stress, as indicated by the elevation of GSH:GSSG ratio and glutathione redox potential, and a decrease in the amount of protein mixed disulfides (Rebrin et al., 2007). These findings suggest that adaptations besides the lowering of metabolic rate, body temperature, or oxidative stress, may be required for effecting life span prolongation by CR. It can be speculated that CR-mediated extension of life span requires more than one or two physiological modulations, and the DBA/2 mice are resistant to some of them.
In summary, results suggest that although rates of oxygen consumption and body temperature are lowered by CR, they do not necessarily associate with life span prolongation.
Acknowledgement
This work was supported by the grant R01 AG13563 from the National Institutes of Health-National Institute on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrikopoulos S, Massa CM, Aston-Mourney K, Funkat A, Fam BC, Hull RL, Kahn SE, Proietto J. Differential effect of inbred mouse strain (C57BL/6, DBA/2, 129T2) on insulin secretory function in response to a high fat diet. J. Endocrinol. 2005;187:45–53. doi: 10.1677/joe.1.06333. [DOI] [PubMed] [Google Scholar]
- Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, Newton W, Wink K, Baum S, Ramsey J. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J. Clin. Endocrinol. Metab. 2003;88:16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- Brochmann EJ, Duarte ME, Zaidi HA, Murray SS. Effects of dietary restriction on total body, femoral, and vertebral bone in SENCAR, C57BL/6, and DBA/2 mice. Metabolism. 2003;52:1265–1273. doi: 10.1016/s0026-0495(03)00194-x. [DOI] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Cooper TM, Mockett RJ, Sohal BH, Sohal RS, Orr WC. Effect of caloric restriction on life span of the housefly, Musca domestica. FASEB J. 2004;18:1591–1593. doi: 10.1096/fj.03-1464fje. [DOI] [PubMed] [Google Scholar]
- Duffy PH, Leakey JEA, Pipkin JL, Turturro A, Hart RW. The physiologic, neurologic, and behavioral effects of caloric restriction related to aging, disease, and environmental factors. Environ. Res. 1997;73:242–248. doi: 10.1006/enrs.1997.3714. [DOI] [PubMed] [Google Scholar]
- Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc. Natl. Acad. Sci. USA. 1976;73:1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J. Clin. Endocrinol. Metab. 2006;91:3232–3235. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Neurobehavioral biomarkers of aging: Influence of genotype and dietary restriction. Biomed. Environ. Sci. 1991;4:144–165. [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funkat A, Massa CM, Jovanovska V, Proietto J, Andrikopoulos S. Metabolic adaptations of three inbred strains of mice (C57BL/6, DBA/2, and 129T2) in response to a high-fat diet. J. Nutr. 2004;134:3264–3269. doi: 10.1093/jn/134.12.3264. [DOI] [PubMed] [Google Scholar]
- Gelegen C, Collier DA, Campbell IC, Oppelaar H, Kas MJH. Behavioral, physiological, and molecular differences in response to dietary restriction in three inbred mouse strains. Am. J. Physiol. Endocrinol. Metab. 2006;291:E574–581. doi: 10.1152/ajpendo.00068.2006. [DOI] [PubMed] [Google Scholar]
- Gelegen C, Collier DA, Campbell IC, Oppelaar H, van den Heuvel J, Adan RAH, Kas MJH. Difference in susceptibility to activity-based anorexia in two inbred strains of mice. Eur. Neuropsychopharmacol. 2007;17:199–205. doi: 10.1016/j.euroneuro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Greenberg JA, Boozer CN. Metabolic mass, metabolic rate, caloric restriction, and aging in male Fischer 344 rats. Mech. Ageing Dev. 2000;113:37–48. doi: 10.1016/s0047-6374(99)00094-9. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Archer JR. Genetic effects on responses to food restriction in aging mice. J. Nutr. 1987;117:376–382. doi: 10.1093/jn/117.2.376. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E, Pennington CT. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt ND, Hyun D-H, Allard JS, Minor RK, Mattson MP, Ingram DK, de Cabo R. Bioenergetics of aging and calorie restriction. Ageing Res. Rev. 2006;5:125–143. doi: 10.1016/j.arr.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc. Natl. Acad. Sci. USA. 1996;93:4159–4164. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Caloric Restriction: A Key to Understanding and Modulating Aging. first ed. Elsevier; Amsterdam: 2002. [Google Scholar]
- Mockett RJ, Cooper TM, Orr WC, Sohal RS. Effects of caloric restriction are species-specific. Biogerontology. 2006;7:157–160. doi: 10.1007/s10522-006-9004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman A, Ramsey JJ, Kemnitz JW, Baum ST, Newton W, Colman RJ, Weindruch R, Beasley MT, Schoeller DA. Influences of calorie restriction and age on energy expenditure in the rhesus monkey. Am. J. Physiol. Endocrinol. Metab. 2007;292:E101–106. doi: 10.1152/ajpendo.00127.2006. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Hagopian K. Energy expenditure and restriction of energy intake: could energy restriction alter energy expenditure in companion animals? J. Nutr. 2006;136:1958S–1966S. doi: 10.1093/jn/136.7.1958S. [DOI] [PubMed] [Google Scholar]
- Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free. Radic. Biol. Med. 2000;29:946–968. doi: 10.1016/s0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Effects of age and caloric restriction on gluathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain Res. 2007;1127:10–18. doi: 10.1016/j.brainres.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free. Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikke BA, Johnson TE. Lower body temperature as a potential mechanism of life extension in homeotherms. Exp. Gerontol. 2004;39:927–930. doi: 10.1016/j.exger.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Yerg JE,, 3rd, Battaglia ME, Nagy TR, Allison DB, Johnson TE. Strain variation in the response of body temperature to dietary restriction. Mech. Ageing Dev. 2003;124:663–678. doi: 10.1016/s0047-6374(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Odom LA, Barron BA, Ator R, Wild SA, Forster MJ. Differential responsiveness to cocaine in C57BL/6J and DBA/2J mice. Psychopharmacology. 1998;138:82–88. doi: 10.1007/s002130050648. [DOI] [PubMed] [Google Scholar]
- Roth GS, Handy AM, Mattison JA, Tilmont EM, Ingram DK, Lane MA. Effects of dietary caloric restriction and aging on thyroid hormones of rhesus monkeys. Horm. Metab. Res. 2002a;34:378–382. doi: 10.1055/s-2002-33469. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter JE. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002b;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Sacher GA. Life table modifications and life prolongation. In: Finch E, Hayflick L, editors. Handbook of the Biology of Aging. Van Nostrand; New York: 1977. pp. 582–638. [Google Scholar]
- Selman C, Phillips T, Staib JL, Duncan JS, Leeuwenburgh C, Speakman JR. Energy expenditure of calorically restricted rats is higher than predicted from their altered body composition. Mech. Ageing Dev. 2005;126:783–793. doi: 10.1016/j.mad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Candas M, Forster M, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech. Ageing Dev. 1994a;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku H-H, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994b;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J. Gerontol. A. Biol. Sci. Med. Sci. 1999;54:B492–501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Caloric intake and aging. N. Engl. J. Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL. The Retardation of Aging and Disease by Dietary Restriction. Charles C Thomas Pub. Ltd.; Springfield, IL: 1988. [Google Scholar]
- Willott JF, Erway LC, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice. II. Strain differences and effects of caloric restriction on cochlear pathology and evoked response thresholds. Hear. Res. 1995;88:143–155. doi: 10.1016/0378-5955(95)00107-f. [DOI] [PubMed] [Google Scholar]
- Yu BP. Modulation of Aging Processes by Dietary Restriction. CRC Press; Boca Raton: 1994. [Google Scholar]