Abstract
Flavonoids are synthesized in response to developmental and environmental signals and perform many functions in plants. Arabidopsis (Arabidopsis thaliana) roots grown in complete darkness do not accumulate flavonoids since the expression of genes encoding enzymes of flavonoid biosynthesis is light dependent. Yet, flavonoids accumulate in root tips of plants with light-grown shoots and light-shielded roots, consistent with shoot-to-root flavonoid movement. Using fluorescence microscopy, a selective flavonoid stain, and localized aglycone application to transparent testa mutants, we showed that flavonoids accumulated in tissues distal to the application site, indicating uptake and movement systems. This was confirmed by time-course fluorescence experiments and high-performance liquid chromatography. Flavonoid applications to root tips resulted in basipetal movement in epidermal layers, with subsequent fluorescence detected 1 cm from application sites after 1 h. Flavonoid application to midroot or cotyledons showed movement of flavonoids toward the root tip mainly in vascular tissue. Naringenin, dihydrokaempferol, and dihydroquercetin were taken up at the root tip, midroot, or cotyledons and traveled long distances via cell-to-cell movement to distal tissues, followed by conversion to quercetin and kaempferol. In contrast, kaempferol and quercetin were only taken up at the root tip. Using ATP-binding cassette (ABC) transporter and H+-ATPase inhibitors suggested that a multidrug resistance-associated protein ABCC transporter facilitated flavonoid movement away from the application site.
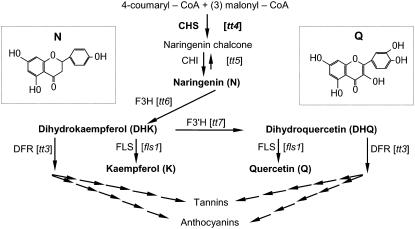
Flavonoids are plant secondary metabolites that have important developmental and physiological functions (Taylor and Grotewold, 2005). Flavonoids are implicated in the control of auxin transport (Jacobs and Rubery, 1988; Mathesius et al., 1998; Murphy et al., 2000; Brown et al., 2001; Buer and Muday, 2004; Peer et al., 2004), allelopathy (Bais et al., 2004), pollen function in some species (Coe et al., 1981; Mo et al., 1992; Taylor and Jorgensen, 1992), signaling to symbiotic microorganisms (Redmond et al., 1986; Djordjevic et al., 1987; Harrison, 2005; Wasson et al., 2006), somatic embryogenesis (Imin et al., 2006), UV protection (Li et al., 1993), defense (for review, see Treutter, 2005), flower coloring (Mol et al., 1998), and seed dispersal (Debeaujon et al., 2000), and appear to accumulate in progenitor cells of root organs (Morris and Djordjevic, 2006). The biosynthetic pathway is known and several mutants with defects in genes encoding these enzymes occur in Arabidopsis (Arabidopsis thaliana; Fig. 1). Flavonoids are likely synthesized by a cytoplasmic metabolic complex (Winkel-Shirley, 2001), and the expression of genes encoding flavonoid biosynthetic enzymes is regulated developmentally (Winkel-Shirley, 2002) and by light (Pelletier and Shirley, 1996; Kubasek et al., 1998; Jenkins et al., 2001).
Figure 1.
The Arabidopsis flavonoid biosynthetic pathway. The Arabidopsis mutants in the early part of the pathway are bracketed. The mutants used in these experiments are in bold type. The insets show the chemical structures of N (a flavanone) and Q (a flavonol). Enzyme abbreviations: CHI, chalcone isomerase; CHS, chalcone synthase; DFR, dihydroflavonol reductase; F3H, flavonol 3-hydroxylase; FLS, flavonol synthase. The figure is modified from Buer and Muday (2004).
Long-distance movement of secondary metabolites is largely unexplored but potentially has profound developmental effects. Grafting experiments conducted in the early 1900s suggested that alkaloids move from the site of manufacture (the root) to the aerial tissue (for review, see Waller and Nowacki, 1978). More recent grafting experiments showed that root-synthesized metabolites, perhaps carotenoids, regulate shoot development (Sorefan et al., 2003; Van Norman et al., 2004). The current view is that flavonoids are synthesized in the cells in which they accumulate and serve local functions (Peer et al., 2001). Flavonoids are also sequestered in vacuoles or secreted to the environment (Grotewold, 2004). Several studies hypothesized that flavonoids may move from their site of synthesis (Djordjevic et al., 1997; Saslowsky and Winkel-Shirley, 2001; Buer and Muday, 2004). For example, light is required for flavonoid synthesis in Arabidopsis seedlings (Pelletier and Shirley, 1996; Kubasek et al., 1998; Jenkins et al., 2001), and, when seedlings are grown with roots exposed to light, the presence of flavonoids regulates root branching (Brown et al., 2001) and gravitropism (Buer and Muday, 2004). Yet, for these regulatory roles of flavonoids to be relevant when plants are grown in light with roots in the dark, it may be necessary for either light signals or flavonoids themselves be transported to the roots. Therefore, flavonoid movement could have important physiological functions.
Fluorescence microscopy of seedlings stained with diphenylboric acid 2-aminoethyl ester (DPBA; fluoresces upon binding flavonoids) provides a powerful tool to examine the accumulation, localization, and movement of flavonoids in living plants (Sheahan et al., 1998; Murphy et al., 2000; Peer et al., 2001; Wasson et al., 2006). Arabidopsis transparent testa4 (tt4) mutants treated with DPBA have only dim green fluorescence (Peer et al., 2001), as they lack flavonoids (Sheahan and Rechnitz, 1993; Burbulis et al., 1996; Sheahan et al., 1998). DPBA generates compound-specific fluorescence (Peer et al., 2001; Wuyts et al., 2006). DPBA-conjugated kaempferol (K) emits a yellow-green fluorescence and quercetin (Q) yellow-gold (Peer et al., 2001; Wuyts et al., 2006). This relationship between DPBA-flavonoid fluorescence and specific flavonoid compounds was verified with gas chromatography-mass spectrometry analysis (Peer et al., 2001). Therefore, DPBA-enhanced flavonoid fluorescence provides a practical method of following flavonoid synthesis in situ.
The Arabidopsis tt4 mutants are helpful in demonstrating the function of flavonoids using the mutant phenotype and chemical complementation with flavonoid precursors. Multiple tt4 alleles have altered auxin transport and auxin-dependent physiological processes, such as gravity responses and root development (Brown et al., 2001; Buer and Muday, 2004; Peer et al., 2004; Buer et al., 2006). Adding the flavonoid precursor naringenin (N) to tt4 mutants restores flavonoid synthesis (Shirley et al., 1995) and complements the growth and gravitropism phenotypes of tt4 alleles (Buer and Muday, 2004; Buer et al., 2006). These results suggest a role for flavonoids as auxin transport regulators.
To test the hypothesis that flavonoids move long distances in Arabidopsis, we applied flavonoid aglycones locally to various tissues of the tt4 mutants. We found that DPBA staining combined with epifluorescence and confocal microscopy provided a sensitive and versatile method to follow flavonoid entry, conversion to downstream compounds, and rapid movement from the localized application sites. We used confocal microscopy to examine which tissues harbored the DPBA fluorescence and determined which tissues facilitated long-distance flavonoid movement. To verify that flavonoids were capable of root-to-shoot and shoot-to-root movement, we used HPLC analysis of tissues distal to the application site to show that downstream products of the flavonoid pathway were present in these tissues. Finally, to determine how this movement was facilitated, we tested various inhibitors of ATP-binding cassette (ABC) transporters and H+-ATPase and described a putative mechanism for this movement. Together, these results showed that flavonoids can move in Arabidopsis and suggest that this movement may be mediated by an ABC-type transporter.
RESULTS
A Physiological Necessity for a Flavonoid Movement System
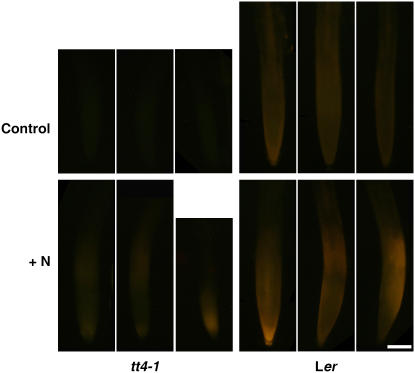
Etiolated wild-type Arabidopsis seedlings accumulate no flavonoids in roots (Buer and Muday, 2004) since flavonoid biosynthetic enzyme synthesis is light dependent (Pelletier and Shirley, 1996; Kubasek et al., 1998; Jenkins et al., 2001). We examined root tips of wild-type plants with light-grown shoots and light-shielded roots for the presence of flavonoids, which would be consistent with shoot-to-root flavonoid movement. Figure 2 shows that wild-type root tips possessed DPBA-flavonoid fluorescence that was enhanced by supplying N to cotyledons for 24 h. In tt4 mutants, there was no flavonoid fluorescence in the root tips unless N was added to the cotyledons for 24 h. These results are consistent with flavonoid movement from cotyledons to root tips.
Figure 2.
Flavonoids move from shoots to roots. Top, Although the expression of flavonoid pathway enzymes is light dependent, wild-type Arabidopsis roots grown in the dark fluoresce from flavonoid accumulation when the shoots are light grown, whereas tt4 root tips are flavonoid free. Bottom, The addition of N to cotyledons for 24 h leads to flavonoid fluorescence in tt4 root tips and enhances it in wild-type root tips. Scale bar = 100 μm.
DPBA Binds to Flavonoids, Generating Intense Fluorescence in Vitro and in Planta
DPBA is a molecule that binds to flavonoids and fluoresces in vitro and in vivo. The specificity of this dye for flavonoids was previously demonstrated, as tt4 mutants make no flavonoids and have no fluorescence upon DPBA treatment (Sheahan and Rechnitz, 1993; Peer et al., 2001; Buer and Muday, 2004). To determine which aglycones generated fluorescence when complexed with DPBA, we assessed DPBA-conjugated fluorescence with N, dihydrokaempferol (DHK), dihydroquercetin (DHQ), K, and Q using spectrofluorometry (Supplemental Fig. S1). The signal for K and Q was much greater than the signal for N, DHK, and DHQ. The area under the curves in these graphs indicated that relative to the weakest signal in N, there was 4- and 7-fold greater DHQ and DHK fluorescence and 244- and 433-fold greater fluorescence with K and Q, respectively. This strong differential fluorescence of the flavonoid intermediates suggested that the strongest DPBA fluorescence observable in plant tissues is due to DPBA complexes with K and Q (Murphy et al., 2000), and that fluorescence in response to the application of flavonoid precursors is likely due to conversion to downstream products. Furthermore, the color of the fluorescence differed between K and Q, with K having green fluorescence and Q having gold fluorescence, thereby allowing these flavonoids to be differentiated in vivo (Peer et al., 2001).
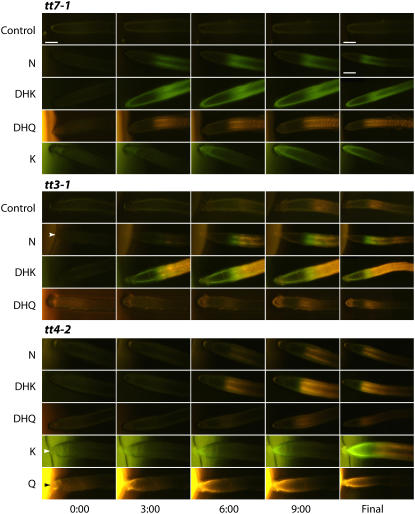
To verify this observation in planta, aglycones were supplied to tt7 and tt3 mutant root tips, which have lesions in various genes encoding the flavonoid pathway enzymes (Fig. 1; Shirley et al., 1995; Peer et al., 2001; Winkel-Shirley, 2001) and thus have altered flavonoid accumulation. The tt7 mutation is defective in flavonol 3′-hydroxylase (F3′H) and cannot produce DHQ or Q, thus accumulating DHK and K. As expected, these plants show green fluorescence consistent with the DPBA complex with K, within minutes of application (Fig. 3, top). The tt3 mutation in dihydroflavonol reductase prevents conversion of DHK and DHQ to other intermediates and accumulates K and Q (Shirley et al., 1995). Therefore, if DPBA is binding to K and Q and not intermediates after DHK and DHQ, enhanced fluorescence should be evident in tt3. Consistent with this prediction, there are greater levels of green and gold fluorescence (Fig. 3, middle) consistent with green fluorescence from K and golden fluorescence from Q.
Figure 3.
Adding aglycones to root tips of the tt mutants tt3, tt7, and tt4 resulted in flavonoid movement away from the application site. The time in minutes after aglycone application is indicated. The final column shows the extent of flavonoid fluorescence at the end of the timed sequence. DPBA was added to the agar cylinders to allow visualization of the subsequent flavonoid fluorescence. The controls are DPBA/DMSO agar cylinders. For K and Q generated images, the brighter green and gold fluorescence of DPBA in the agar is evident, at the left of the images, whereas DMSO, N, DHK, and DHQ exhibit little detectable fluorescence in combination with DPBA. All sequences proceeded for 10 min, except the tt4-K final image is after 25 min. The arrowheads indicate the instances where the root tip is in contact with the agar cylinder. Scale bars = 100 μm. The final images are generally smaller as they are montages of several images to show more of the root. The smaller scale bar corresponds to these images.
Exogenous Aglycone Applications to tt4 Root Tips Revealed Rapid Movement to Distal Tissues
To provide temporal and spatial information on how flavonoids move within plants, two alleles of the flavonoid-deficient tt4 mutant seedlings [tt4(2YY6) and tt4-1] were treated with the aglycones N, DHK, DHQ, K, and Q. These compounds were applied to the root tip in an agar cylinder also containing DPBA, and fluorescence micrographs were captured every 15 s (Fig. 3, bottom). The position of flavonoid fluorescence at 0, 3, 6, and 9 min postapplication is shown. These images were combined into movies showing the fluorescence as a function of time and are available online as Supplemental Movies S1 to S5. N, DHK, and DHQ were not visualized until converted to downstream flavonoid products, and the major product formed was consistent with the fluorescence from Q. The accumulation of Q from these precursors was not present at the root tip, but was restricted to the distal elongation zone and more basal positions. DPBA staining was detected as far as 1 mm from the root tip after 10 min. In contrast, K was taken up and fluoresced green in combination with DPBA at the root tip and was converted to Q after movement away from the tip. This reaction was slower than when applying N, DHK, or DHQ. The K images were collected every 1 min, and the final image was taken 25 min postapplication. Finally, bright golden Q fluorescence was evident in the agar and Q was taken up, resulting in fluorescence at the root tip after 10 min. The overnight application of flavonoid compounds to the root tip resulted in DPBA fluorescence in the leaf tissues from all compounds except K and Q (not shown).
In separate experiments, we also examined the movement of flavonoid intermediates, when DPBA was applied uniformly to tt4 seedlings by incubation in a solution containing this dye. This analysis was performed with microscopy at 5 and 60 min after aglycone application, and the subsequent distal fluorescence accumulation is shown in Supplemental Figure S2. Measurements were obtained for the flavonoid movement with this method of visualization to determine the distance of movement after 1 h, and these results are presented in Table I (under basipetal movement).
Table I.
Flavonoids move 2 times further from the application site when applied at the root tip than at midroot
No statistical differences exist between the mutant plants possessing the two alleles or the compounds in the basipetal direction. There is also no statistical difference in the acropetal direction for N, DHK, and DHQ and between the mutant plants possessing the two alleles.
| Compound |
tt4-1
|
tt4(2YY6)
|
||
|---|---|---|---|---|
| Basipetala | Acropetalb | Basipetala | Acropetalb | |
| mm | ||||
| N | 9.4 ± 0.4 | 4.2 ± 0.2 | 11.1 ± 0.8 | 4.2 ± 0.3 |
| DHK | 8.5 ± 0.5 | 4.3 ± 0.3 | 8.7 ± 0.4 | 3.9 ± 0.2 |
| DHQ | 8.4 ± 0.5 | 4.5 ± 0.2 | 7.6 ± 0.3 | 4.0 ± 0.3 |
| K | 8.3 ± 0.4 | n.d.c | 8.7 ± 0.6 | n.d.c |
| Q | 8.0 ± 0.4 | n.d.c | 7.3 ± 0.4 | n.d.c |
Average and se from three combined independent experiments (n ≥ 17). Compounds were applied in an agar cylinder at the root tip for 1 h and DPBA stained, and fluorescence distance was measured from the application site.
Average and se from three combined independent experiments (n ≥ 17). Compounds were applied in an agar cylinder 0.5 cm from root tip for 1 h, and roots were excised below agar line, DPBA stained, and fluorescence distance measured from the application site.
n.d., Not detected.
Control experiments with solvents, Suc, and other chemicals used in the aglycone applications were tested for possible effects on flavonoid movement. The presence or absence of these compounds did not significantly change the experimental outcomes (Supplemental Table S1). In particular, the similar movement of flavonoids in the absence of Suc suggested that flavonoid movement was not the result of Suc-driven loading.
Exogenous Flavonoid Applications to Midroot or Cotyledon Tissues Showed Differential Uptake and Movement Systems
To test the direction of flavonoid movement after localized applications to different sites, we applied the aglycones 5 mm above the root tip (termed midroot) and measured the distance that flavonoid fluorescence moved. N, DHK, and DHQ only moved toward the root tip (acropetal direction). There was no significant differences between N, DHK, and DHQ movements in either tt4-1 or tt4(2YY6) within these categories. We did not find evidence for acropetal or basipetal movement of K and Q when applied midroot. We rarely detected florescence in this tissue upon K or Q application, and in no cases was flavonoid fluorescence found at locations distant from the point of application, consistent with limited uptake and movement of K and Q.
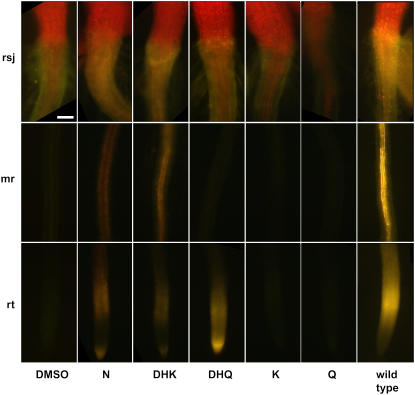
The overnight application of N, DHK, and DHQ to the cotyledons of Arabidopsis tt4 seedlings resulted in DPBA fluorescence in the root/shoot junction, midroot, and at the root tip (rsj, mr, and rt, respectively; Fig. 4). As with midroot applications, K and Q applications did not result in flavonoid movement from the application site. These results support the idea that precursors to K and Q probably are the molecules that move with conversion to K and Q occurring at the sites of DPBA fluorescence.
Figure 4.
Flavonoid compounds applied to cotyledons travel differentially to the root tip. The indicated compounds were applied to cotyledons and incubated overnight, and the resulting fluorescence was visualized following DPBA staining. The addition of N, DHK, and DHQ resulted in DPBA-flavonoid fluorescence in distal tissues, while K and Q did not result in fluorescence in any root tissue. The far right column shows a representative wild-type seedling for comparison. The red fluorescence in the tissues above the root/shoot junction is chlorophyll autofluorescence. Scale bar = 100 μm. Abbreviations: rsj, root/shoot junction; mr, midroot; rt, root tip.
HPLC Analyses Confirmed Flavonoid Compounds Are in Tissues Distal to Application Sites
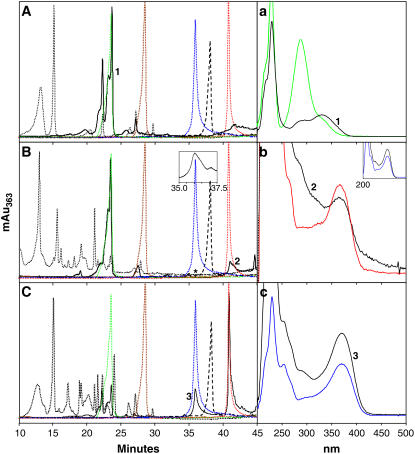
The accumulation of K and Q in tissues distal to the application of N treatment was confirmed using qualitative and quantitative HPLC. N was applied either to the root tip or to the cotyledons for 24 h and extracts from roots and aerial tissues were analyzed. The HPLC profiles of the aglycone standards were compared to crude extracts from untreated tt4-1, tt4-1 treated with N, and wild-type root and shoot extracts. These extracts were analyzed directly and after hydrolysis to remove any glycosides from flavonoid compounds, and representative chromatograms are presented in Figure 5. The absorption spectra maxima are shown in Supplemental Figure S3 and summarized in Supplemental Table S1, and selected spectra are compared to eluted peaks in Figure 5, a to c. A series of injections was also made using K as an internal standard. These elution times remained consistent with the crude extracts and hydrolyzed samples, confirming the quality of the chromatography. The control tt4-1 mutant had no detectable flavonoid compounds as reported previously (Fig. 5A; Burbulis et al., 1996; Pelletier et al., 1999; Saslowsky et al., 2000; Peer et al., 2001), consistent with the chalcone synthase lesion. Interestingly, a peak that eluted in the same fraction as DHQ had an entirely different absorption spectrum; thus, it is not DHQ (Fig. 5a). Adding N to tt4-1 roots produced subsequent downstream products in the aerial tissues (Fig. 5B). The non-DHQ peak was also present in the chromatograms from plants treated with N. Flavonoids extracted from aerial tissue after N application to tt4-1 root tips are shown in Figure 5B and are compared to flavonoids in extracts from wild-type aerial tissue (compare Fig. 5C). The chromatograms from tt4-1 root tip extracts following N application to cotyledons for 24 h were similar (not shown).
Figure 5.
The addition of N to tt4-1 root tips produced flavonoid compounds in aerial tissue after 24 h. Representative HPLC chromatograms are from crude extracts (dotted black lines) and hydrolyzed crude extracts (solid black lines) from tt4-1 negative controls (A); tt4-1 aerial tissue extracts after 100 μm N was applied at root tips for 24 h (B); and wild-type aerial tissue (C). The standards and their retention times are indicated by the overlaid colored peaks in A to C (brown: DHK, 29.7 min; green: DHQ, 23.4 min; blue: Q, 35.8 min; black dashes: N, 38.4 min; red: K, 40.8 min). a to c show the absorption spectra of the numbered peaks confirming their identity. The black curve is from the crude extract and the colored line is the standard with the same color scheme as above. The absorption quantities on the y axes have been removed for clarity. Generally, hydrolysis reduced the absorption by an order of magnitude. Extracts from root tissue after the application of N to the cotyledons produced similar chromatograms. The inset in B is an enlargement of the hydrolysis chromatogram between 35 and 37.5 min to show the small Q peak located at the asterisk. The inset in b shows the absorption profile for the same peak (black line) compared to that of the standard (blue line).
To quantitatively determine flavonoid compound amounts in the exogenously fed seedlings compared to the wild type, standard curves were generated using serially diluted authentic flavonoid standards and these results are presented in Table II. The wild type had higher concentrations of the glycosylated compounds than found in N-treated tt4-1 extracts as quantified in the hydrolyzed extract. This difference may reflect the very local application, the small amounts of flavonoids that are taken up, or the limited time for flavonoid synthesis after N treatment in tt4-1 compared to the wild type. N was not detected in any of our assays, suggesting immediate conversion to downstream products or the presence of extremely low concentrations. These results indicated that local application of N to the root tip or cotyledons of tt4-1 led to the accumulation of downstream flavonoids in distant locations in the plant.
Table II.
HPLC analysis of flavonoid intermediates at sites distal to their application
N was applied at the root tip or on cotyledons for 24 h. Seedlings were divided into aerial or root tissue at the root/shoot junction. tt4-1 control seedlings had no detectable flavonoid compounds, even after hydrolysis. The crude extracts or hydrolyzed crude extracts were injected into the HPLC. Statistical analyses compare wild type with the mutants with N applications within the hydrolyzed or unhydrolyzed category using two-tailed Student's t tests with equal or unequal variance depending on F test for variance. Data are the average and se from at least three combined independent experiments.
| Seedlings and Conditions | DHK
|
DHQ
|
K
|
Q
|
DHK
|
DHQ
|
K
|
Q
|
|---|---|---|---|---|---|---|---|---|
| Root Tissue | Aerial Tissue | |||||||
| Unhydrolyzed crude extracts, μg mg−1 fresh weight | ||||||||
| Ler | n.d.a | n.d.a | n.d.a | n.d.a | 91.8 ± 5.7 | 152.1 ± 68.3 | 0.2 ± 0.002 | n.d.a |
| tt4-1 + N root tips | n.d.a | 5.9 ± 0.6 | n.d.a | n.d.a | 204.4 ± 19.4b | 287.3 ± 115.9b | 2.0 ± 0.19 | 0.01 ± 0.005 |
| tt4-1 + N leaves | n.d.a | n.d.a | n.d.a | n.d.a | 39.1 ± 14.6 | 128.5 ± 59.5 | 0.12 ± 0.09 | 0.002 ± 0.014 |
| Hydrolyzed crude extracts, μg mg−1 fresh weight | ||||||||
| Ler | n.d.a | n.d.a | 51.4 ± 24.2 | 48.2 ± 15.0 | 29.5 ± 15.5 | 241.7 ± 69.8 | 27.8 ± 7.9 | 11.7 ± 4.0 |
| tt4-1 + N root tips | n.d.a | 7.8 ± 0.8 | 0.3 ± 0.025 | n.d.a | 23.4 ± 10.5 | 466.9 ± 27.2b | 4.3 ± 0.09b | 0.031 ± 0.006b |
| tt4-1 + N leaves | 0.7 ± 0.05 | n.d.a | 0.23 ± 0.017 | 0.029 ± 0.005b | 13.1 ± 8.1 | 386.4 ± 27.8 | 0.78 ± 0.056b | 0.06 ± 0.03b |
n.d., Not detected.
P < 0.05.
Flavonoids Localized in Tissues Where Auxin Transport Occurs and Moved Symplastically
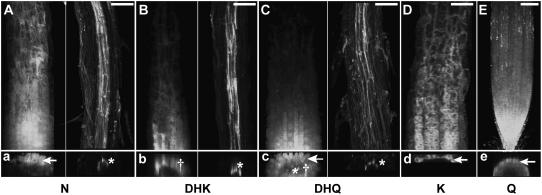
Confocal laser scanning microscopy (CLSM) was used to differentiate the tissue and cellular localization of flavonoid compounds after the exogenous application of flavonoid pathway intermediates (Fig. 6). In general, DPBA fluorescence localized in the epidermal and cortical cell layers when compounds were applied at the root tip (Fig. 6, A, C, E, G, and H), consistent with the localization of the fluorescence in the videos (Supplemental Movies S1–S5). In contrast, when N, DHK, and DHQ were applied midroot, DPBA fluorescence was detected in the vascular tissue (Fig. 6, B, D, and F). DHQ application led to the most widely distributed DPBA fluorescence pattern, with fluorescence found across the entire root, although the brightest fluorescence was in the epidermal layer. All the other compounds tended to localize specifically in the outer cell layers after root tip applications. These localization patterns are summarized in Supplemental Table S2.
Figure 6.
Flavonoid fluorescence localizes differentially following local aglycone applications. CLSM was used to optically section living tt4-1 Arabidopsis roots and to generate cross sections. The indicated aglycones were applied at the root tip (A, C, E, G, and H) or midroot (B, D, and F) of tt4-1 seedlings, incubated for 1 h, DPBA stained, and micrographs collected. The computer-generated cross sections through the brightest zone of fluorescence are directly below the corresponding localization image. Flavonoid fluorescence localized to the pericycle (*), cortex (†), or epidermis (arrows). Scale bars = 50 μm.
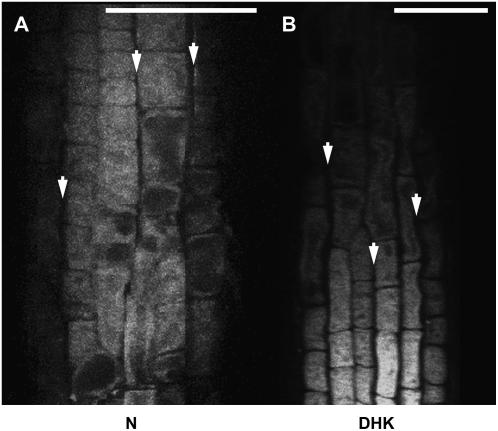
To determine if the transported flavonoids localized apoplastically or symplastically, 0.4 m NaCl was added to the mounting medium to induce slight plasmolysis. The results after applying N or DHQ at the root tip are shown in Figure 7. Plasmolysis suggested the flavonoid fluorescence was localized within cells, as the fluorescence retracted from the cell walls within the plasma membrane. Similar results occurred with the other exogenously applied aglycones (not shown). If the flavonoid compounds were localized at the plasma membrane, the fluorescence would be brighter at the cell edges owing to the stacking in the z-direction of fluorescence down the side of the cell. The fluorescence in this case was uniform across the cell, supporting intracellular localization and cell-to-cell movement via the symplasm.
Figure 7.
Flavonoids localize intracellularly in the root distal elongation zone after applying aglycones at the root tip. Confocal laser scanning micrographs of a tt4-1 root incubated for 1 h with the indicated compounds applied at the root tip are shown. NaCl at 0.4 m was added to the mounting medium to incipiently plasmolyze the cells. The flavonoid fluorescence shrank with the plasma membrane. The arrows indicate areas where the plasma membrane has receded from the cell wall and trapped the flavonoid fluorescence. The same result occurred for all exogenously applied flavonoid compounds. Scale bars = 50 μm.
Glybenclamide and Glutathione Inhibited Flavonoid Movement
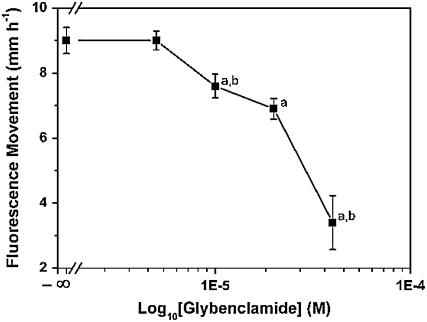
ABC transporters are implicated in the movement of plant secondary metabolites into vacuoles (Yazaki, 2006) and as auxin transport proteins (Geisler et al., 2005), and various inhibitors are used to decipher this movement (Rea, 1999; Klein et al., 2000; Sugiyama et al., 2007). We also asked whether inhibitors of proton ATPases blocked flavonoid movement. To determine if ABC transporters were responsible for the movement of flavonoids, we transferred tt4 seedlings to media containing the various inhibitors for 24 h. The seedlings were then transferred to slides coated with the same inhibitor, N was applied to the root tip, and the distance of flavonoid movement was measured to determine the apparent rate of flavonoid movement after 10 min (Table III). The only inhibitor that affected the movement of flavonoids was the ABCC transport inhibitor that acts on the multidrug resistance- associated protein class, glybenclamide, with a significant reduction in flavonoid movement at 10 μm and higher concentrations (Fig. 8). At 40 μm, root elongation was significantly compromised. The redox-affecting compound glutathione also significantly inhibited flavonoid movement (the reduced and oxidized forms, GSH and GSSG, respectively; Table III).
Table III.
Fluorescence movement after using various inhibitors
Inhibitors were supplied to seedlings in the media for 24 h. Seedlings were then transplanted to slides coated with the compounds and supplied with N plus DPBA for 10 min at the root tip. Distances of subsequent flavonoid movement was then tabulated.
| Inhibitor and Concentration | Site of Action and Reference | Fluorescence Movement |
|---|---|---|
| mm | ||
| Control (+N; 100 μm) | – | 9.0 ± 0.4 |
| Bafilomycin A1 (0.3 μm) | Vacuolar H+-ATPase (Scott and Allen, 1999) | 8.2 ± 0.8 |
| Cyclosporine A (0.3 μm) | ABCB transporters (p-glycoproteins; Loyola-Vargas et al., 2007) | 9.9 ± 1.0 |
| Glybenclamide (10 μm) | ABCC transporters (MRP proteins; Forestier et al., 2003) | 7.6 ± 0.4a |
| GSH (250 μm) | Redox (Espunya et al., 2006) | 5.6 ± 0.2a |
| GSSG (250 μm) | Redox (Espunya et al., 2006) | 7.5 ± 0.4a |
| Sodium o-vanadate (100 μm) | ABC transporters (Loyola-Vargas et al., 2007) | 8.0 ± 0.5 |
| NPAb (10 μm) | Auxin transport (Jacobs and Rubery, 1988) | 9.3 ± 0.6 |
| Nigericin (50 nm) | Membrane potentials (Casolo et al., 2003) | 9.9 ± 2.0 |
| Valinomycin (1 μm) | Membrane potentials (Casolo et al., 2003) | 7.3 ± 0.6 |
| Verapamil (100 μm) | ABCB transporters (p-glycoproteins; Loyola-Vargas et al., 2007) | 10.8 ± 2.0 |
Statistical analyses compared to control; tested with equal or unequal variance two-tailed Student's t tests depending on F test for variance; P < 0.05, n ≥ 6 replicates.
NPA, 1-N-naphthylphthalamic acid.
Figure 8.
Glybenclamide inhibited the movement of flavonoids in a dose-dependent manner. Seedlings of tt4-1 were incubated on increasing concentrations of glybenclamide for 24 h, transferred to slides coated with the same concentration, and N plus DPBA was applied at the root tips. The subsequent flavonoid synthesis and movement was measured after 10 min. Presented are the average and se of at least six replicates. Statistical analysis compared the indicated data point to the control (a) or the previous data point (b) using a two-tailed Student's t test for equal or unequal variance depending on F test for variance.
DISCUSSION
Flavonoids Move to Sites Distal of Aglycone Application in Tissues That Transport Auxin
These experiments used two approaches to show that flavonoids move from their site of synthesis or local application to distant tissues. First, flavonoids are not made in roots of etiolated wild-type seedlings (Buer and Muday, 2004), but flavonoids are present in the roots of wild-type seedlings when the shoots are grown in the light and the roots are grown in the dark. Treatment of shoots with N further enhanced flavonoid accumulation in these dark-grown roots. This result was consistent with flavonoid movement in planta. Secondly, tt4 mutants cannot make flavonoids and show no DPBA staining, but treatment of these mutants with locally applied aglycones led to the restoration of flavonoid synthesis. The application of N, DHK, DHQ, K, or Q to tt4 mutants was used to observe flavonoid movement, as no background flavonoids interfere with the visualization of movement.
The addition of N, DHK, or DHQ resulted in the uptake, rapid conversion to downstream products, and the long-range movement of downstream products, irrespective of application site. The local application of N, DHK, or DHQ to various tissue types bypassed the chalcone synthase lesion in tt4, resulting in the rapid production (<5 min) of intracellular gold fluorescence likely resulting from the Q-DPBA complex. Additionally, the rapid movement of either flavonoid precursors or downstream products to distal locations in the plant was detected using DPBA. No differences in the flavonoid movement were noted whether DPBA was added via the agar cylinder (Fig. 3) or used after flavonoid application as in conventional staining procedures (Supplemental Fig. S2). HPLC analysis confirmed the formation of downstream flavonoid compounds in distal tissues after exogenous application of N, DHK, or DHQ to root tips or cotyledons of tt4-1. K and Q application to root tips resulted in limited basipetal movement to approximately 10 mm after 24-h incubation. When K and Q were applied either midroot or to the cotyledons, fluorescence was never observed at sites distant from these application sites. The more limited mobility of K and Q in all tissues suggests that N, DHK, and DHQ are more likely the mobile flavonoids that are then detected at distant sites after conversion to K or Q, which generate the most intense fluorescence products when bound to DPBA. Additionally, plasmolysis experiments combined with confocal microscopy showed that the flavonoid products accumulated inside cells and were not detected in regions between cells, suggesting that the long-distance movement of these molecules was symplastic.
Flavonoid movement approximated the rates and direction of auxin (indole-3-butyric acid and indole-3-acetic acid [IAA]) transport (8–10 mm h−1; Rashotte et al., 2003). Flavonoid movement also occurred in the cell layers proposed for auxin movement (for review, see Blancaflor and Masson, 2003), wherein basipetal transport in roots was in the epidermal and cortical cell layers and acropetal transport was in the vascular tissue (Tsurumi and Ohwaki, 1978; Rashotte et al., 2003). The intracellular localization of flavonoids in root tissues supported the hypothesis that these compounds may regulate auxin transport, as flavonoids were found in the same tissues that transport auxin and in an appropriate cellular compartment to regulate transport across the plasma membrane.
The movement of flavonoids should be considered in the context of physiological processes that are modulated by these molecules. Consistent with elevated IAA transport, the flavonoid-deficient tt4 mutants have enhanced lateral root formation (Brown et al., 2001), delayed gravity responses (Buer and Muday, 2004), and reduced inhibitory effects of ethylene on root gravitropism (Buer et al., 2006). These mutant phenotypes are evident in tt4 roots grown on agar and exposed to light, whereas in nature roots are underground and presumably unable to synthesize flavonoids. The results presented here, which indicate that flavonoids can move long distances in plants, suggest that flavonoid synthesis in the light-grown shoot tissues and subsequent transport of flavonoids to the roots are adequate to modulate auxin transport and dependent physiological processes in this organ.
The Sensitivity and Specificity of DPBA Staining
Two issues required consideration relating to the sensitivity and specificity of DPBA staining. The limit of detection for DPBA-enhanced flavonoid fluorescence in vivo is not known. However, our maximum dose of aglycone was in the order of 50 ng per plant, assuming total uptake from the flavonoid supplied in the agar cylinder at the point of application. This result implied high sensitivity of fluorescence microscopy at detecting the DPBA-flavonoid complex in situ. In addition, the application to root tips of agar cylinders with lower flavonoid concentrations (as low as 1 × 10−9 m for 1 h) was also able to cause gold fluorescence to form in the root tips, consistent with the Q-DPBA complex (not shown). Several lines of evidence suggested that DPBA is specific for intermediates of the flavonoid biosynthetic pathway. Mutants that make no flavonoids, such as tt4, exhibit no DPBA fluorescence (Peer et al., 2001; Buer and Muday, 2004). Spectrofluorometric analyses showed that equimolar concentrations of K and Q resulted in 200- to 400-fold greater fluorescence than the precursor N, and, therefore, would dominate fluorescence detection even if present in relatively low concentrations compared to other intermediates. Furthermore, the tt7 mutant, which lacks the F3′H enzyme, had green fluorescence, except when treated with DHQ, which confirmed that golden fluorescence required DHQ and Q synthesis. In contrast, golden fluorescence was enhanced in the tt3 mutant, which lacks the dihydroflavonol reductase enzyme that blocks DHQ and DHK conversion into anthocyanins and has elevated K and Q levels (Peer et al., 2001).
The 24-h incubation of tt4 mutants with N resulted in golden fluorescence indicative of Q formation; however, HPLC analysis showed the relative concentration of Q was only 2 to 60 ng mg−1 fresh weight after hydrolysis. The small quantities measured indicate the sensitivity of the DPBA fluorescence techniques for visualizing the presence of flavonoid compounds, in particular K and Q. The observation that adding N at 1 nm resulted in detectable fluorescence at the root tip after 1-h incubations (not shown) supported the sensitivity of fluorescence detection, as this concentration is well below the detection limits of Q and K by HPLC (100 nm; not shown). Our measured quantities compared favorably with a previous report of K and Q concentrations in Arabidopsis (Peer et al., 2001).
Is Flavonoid Movement Bulk Flow or Active Transport?
The ability of glybenclamide, GSH, and GSSG to inhibit the movement of flavonoids represents a step toward determining the mechanism by which flavonoids are transported. These results suggest that flavonoid movement may be mediated by ABCC proteins, which are ABC transporters, likely to mediate active transport. Active movement is also supported by the tissue-specific localization of flavonoids, their cell-to-cell movement, and their unidirectional movement when applied midroot. If the movement is diffusion mediated, the flavonoid distribution should be less localized and movement bidirectional.
Interestingly, ABC transporters are also implicated in auxin transport (Geisler et al., 2005; Terasaka et al., 2005) and p-glycoproteins are inhibited by some flavonoids (Zhang and Morris, 2003). Geisler et al. (2005) show that specific MDR/PGP proteins may be the target of flavonoids, as PGP1-mediated IAA efflux is sensitive to inhibition by Q. Additionally, the mdr4 mutation enhances root gravitropism, while tt4 roots have delayed gravity response (Lewis et al., 2007). A tt4/mdr4 double mutant shows enhanced gravity response consistent with flavonoids targeting MDR4 (Lewis et al., 2007).
The interaction of GSH and GSSG may reflect issues of redox involving F3′H (TT7; Schoenbohm et al., 2000), a cytochrome P450-dependent monooxygenase requiring NADPH and O2 to function, interactions with glutathione S-transferases through competitive interactions, or sulfur residues attaching to protein cysteinyl residues resulting in compromised protein interactions (May et al., 1998). Smith et al. (2003) showed that an Arabidopsis glutathione S-transferase has a flavonoid interaction, but the function of the gene remains unknown. Imin et al. (2006) showed that flavonoids inhibited rooting in Medicago truncatula callus cells and that GSH and GSSG reverses this inhibition. There are many ABC proteins in Arabidopsis and more work is required to determine how and which are involved with the processes described here.
Conversion of K to Q in Arabidopsis
The interconversion of flavonoid intermediates as examined in vivo followed the predicted biosynthetic pathway of flavonoid compounds with one exception. The unexpected result was that after treatment with K, the formation of Q was obvious (Fig. 2; Supplemental Fig. S2K; Supplemental Movie S4). According to the standard pathway (Schoenbohm et al., 2000; Fig. 1), flavonol synthase is indicated to be a unidirectional enzyme that does not convert K back to DHK so that F3′H can subsequently form DHQ and then flavonol synthase catalyze the further reaction to Q. An enzyme previously thought unidirectional was recently shown reversible (Zhang et al., 2006). However, we cannot discount the possibility that we are forcing a reverse reaction by adding excess substrate. This appeared a less favored pathway, as the Q fluorescence was consistently less intense during K applications (Fig. 2; Supplemental Fig. S2, E and K; Supplemental Movie S4) compared to the addition of the other compounds. Alternatively, this could be due to an unknown enzymatic activity. For example, there is the possibility that F3′H could catalyze the direct conversion of K to Q (Graham, 1998), but this has not been demonstrated in enzyme assays.
In conclusion, these results showed that flavonoids are capable of long-distance movement throughout Arabidopsis plants. As flavonoids are important for lateral root development (Brown et al., 2001), maximal gravitropic response (Buer and Muday, 2004), and regulation of this response to ethylene (Buer et al., 2006), this movement of flavonoids provides a method for these processes to proceed while roots are growing normally in a dark environment. Further experiments to determine the mechanisms of how flavonoids move are under way. Rigorous phenotype comparisons and changes occurring in flavonoid and transport mutants after exogenously adding flavonoid compounds should further unravel the physiological roles for flavonoids in plants and the mechanisms by which their localization is specified.
MATERIALS AND METHODS
Chemicals
Chemicals were obtained from Sigma or TransMIT.
Seedling Growth
The Arabidopsis flavonoid mutants tt3-1 and tt7-1 were obtained from the Arabidopsis Biological Resource Centre (The Ohio State University, Columbus, Ohio). These mutants plus tt4(W85) (now known as tt4-1), tt4(2YY6), tt4-2 [generated by backcrossing tt4(2YY6) to remove a second unlinked mutation, max4; Bennett et al., 2006], and the respective wild-type Landsberg erecta (Ler) or Columbia were used in this work. Some experiments were conducted with tt4(2YY6), but with identical results to tt4-2. Seeds were sterilized and seedlings grown as described previously (Buer and Muday, 2004). Temperatures were maintained at 21°C to 23°C in growth chambers with 100 μmol m−1 s−1 continuous light regimes. Seedlings were grown to 5 to 6 d and then transplanted to new control medium plates or media containing the various indicated drugs, and flavonoid compounds were applied as detailed below. For HPLC analyses, seedlings were sown in 15-cm petri plates in two rows (approximately 1,000 seedlings per plate) to facilitate gathering of large amounts of tissue. For light-grown seedlings with light-shielded roots, seeds were germinated in a charcoal layer in G-7 magenta boxes (Sigma) covered with aluminum foil. The roots penetrated the charcoal layer and grew into the lower agar stratum providing a dark-growing environment.
Supplying Flavonoid Compounds
The various aglycones were locally applied at the root tip, midroot, or on the cotyledons using agar cylinders as described previously for tritiated auxin applications (Rashotte et al., 2000). The flavonoid compounds were supplied at 100 μm concentrations unless described otherwise (total amount in the agar was in the nanogram range [see below]). The aglycones were made up freshly from 1 or 10 mm stocks dissolved into dimethyl sulfoxide (DMSO) in 1% agar (A6686; Sigma) cooled to 40°C plus MES (5 mm):Suc (30 mm), pH 5.5, in 3-mL scintillation vials, allowed to harden, and transferred to the desired tissues via 1-mm diameter agar cylinders with a thin-stem transfer pipette. In the case of N, this delivered approximately 200 fg μm−1 agar. The volume of the agar cylinders was approximately 30 μL, thus containing approximately 80 ng of N. This cylinder was used to supply a minimum of 15 seedling roots. Therefore, assuming total uptake of flavonoid from the cylinder, the total maximum dose of flavonoid per plant was approximately 50 ng. Similarly, for cotyledon applications the maximum dose per plant was calculated to be approximately 200 ng per plant. An agar cylinder was usable for several treatments; however, new cylinders were always used. The stocks were stored at −20°C. Although the compounds were freshly made each time, analyses of week-old, dark-stored agar formulations showed they produced comparable fluorescence in all tt4 seedlings.
Two methods of local flavonoid application were used. For generating movies and testing inhibitors, DPBA was added to the agar cylinder with the various aglycones and seedlings were visualized horizontally. To compensate for this addition, extra agar was added to the MES:Suc portion to make a 1% final agar concentration. A slide was coated with molten control media or inhibitors by pipetting the agar directly onto it. Once the agar cooled, a seedling was moved to the agar and placed on the microscope. To visualize flavonoid fluorescence generation and movement, movies were created from individual micrographs taken at specific intervals following flavonoid application at the root tip. A bright-field image was captured and then an image under 488-nm excitation. The agar cylinder with the desired compound was carefully moved near the root tip and individual images were captured over time. Generally there was a slight gap between the root tip and the agar cylinder. To normalize the time for compounds to travel over this gap, a regression line was generated to determine the average time required (not shown). This time was subtracted from the overall time to obtain the true zero. To minimize photobleaching, the excitation shutter was closed between micrograph captures. Due to the nature of the microscopy, the incubation of seedlings with flavonoid compounds required the seedling to lie horizontally versus the vertical orientation as for the other experiments. Images were obtained every 15 s (except for K, every minute). A final bright-field image was captured, and a montage of the entire root tip region was assembled to allow visualization of the overall fluorescence. After adding the time to each image in PhotoShop, the images were joined into QuickTime format (**.mov) using Apple's iMovie3 software. As the epifluorescent microscope is an inverted style, imaging was performed through the slide and agar.
For all other experiments, the DPBA staining was done following the incubation of the various aglycones. The agar cylinders were applied at the extreme tip of the root and the seedlings were then oriented vertically in holders for the duration of the incubation. For midroot and cotyledon application, the seedlings were rotated approximately 120° in the vertical plane to minimize possible capillarity where the compounds could flow down the sides of tissues.
Inhibition Experiments
Seedlings were transplanted to petri dishes containing normal Murashige and Skoog media plus the inhibitor of choice and incubated vertically in the light for 24 h. The seedlings were then moved to slides coated with the same concentration of inhibitor and viewed under the microscope as for generating movies. N plus DPBA was supplied at the root tip and the movement of flavonoids was followed over 10 min. The results were compared to the control using equal or unequal variance two-tailed Student's t tests determined from F-test analysis of variance in Excel. At least six replicates were in each category.
Fluorescence Spectroscopy
Fluorescence intensities of N, DHK, DHQ, K, and Q were tested with a spectrofluorometer (FluorMax-3; Jobin Yvon Horiba) with excitation and emission slit widths at 5 nm. Individual compounds at 10 μm were conjugated with DPBA (0.25% plus 0.0005% Triton X-100) and excited at 488 nm, and the emission spectra were collected from 490 to 800 nm. The results were plotted in Origin 7.0, and the calculus integration option was used to integrate the areas under the curves using zero as the baseline.
Microscopy and Kinetics Measurements
Epifluorescence microscopy was performed on an inverted Zeiss Axiovert 200M microscope equipped with P.A.L.M. robotics (Millennium Science). Fluorescence excitation and emission wavelengths were with standard FITC filters with excitation from 450 to 490 nm and an emission long-pass filter from 510 nm. A Hitachi HV-D30 digital camera was used to capture epifluorescent micrographs. Measurements were made using the inbuilt measuring capabilities of the P.A.L.M. system. The aglycones were applied at the root tip or 5 mm from the root tip (termed midroot) and incubated in the light for the required time, and the distance of fluorescence was measured from the local application site after DPBA staining.
Confocal microscopy was performed on a Leica TCS SP2 laser scanning microscope (Leica Microsystems). Fluorescence was excited by an argon laser (Enterprise II-651; Coherent) with the 488-nm line and with an emission filter from 510 to 580 nm. The aglycones were applied locally at the desired location, incubated for approximately 1 h, and DPBA stained, and optical sections were collected from half the root diameter. Photobleaching becomes a problem when trying to section through entire roots and the distal half of the root becomes increasingly nonfluorescent.
HPLC Analysis
N (100 μm) was applied to Arabidopsis seedlings on day 5 following seeding in 15-cm petri dishes. For the data in Figure 5, the compounds were applied locally at the root tip and incubated vertically for 24 h, and then the seedlings were excised at the root/shoot junction. The aerial tissue was immediately frozen with liquid nitrogen and pulverized with a mortar and pestle. For the samples in Table III, N was either applied at the root tip or on the leaves. After 24-h treatment, the roots and aerial tissues were harvested separately and immediately frozen with liquid nitrogen. For each 100 mg of fresh tissue, 300 μL of cold acetone was added, and the mixture was vortexed, sonicated for 5 min, and then centrifuged at 16,100g to separate the crude extract from the tissue. The supernatant was used for analysis and the hydrolysis of aglycones from the glycosylated flavonoid compounds. The glycosylated flavonoids from crude extracts were hydrolyzed following the protocol of Burbulis et al. (1996) by heating equal volumes of crude extract and 2 n HCl at 70°C for 40 min. The flavonoids were separated from the aqueous volume with an equal volume of ethyl acetate by vortexing and centrifuging at 16,100g for 10 min. The upper organic layer was removed with a needle and syringe, and placed in new Eppendorf tubes, dried under N2 gas, and resuspended in acetone.
HPLC analysis was performed on crude extracts or the hydrolyzed samples from wild-type and tt4-1 seedlings on a Shimadzu LC-10 VP Series HPLC with a C18 column (Alltima; 250 × 4.6 mm i.d.). The column was injected with 40 μL from the various treatments and eluted with an acetonitrile plus 1% acetic acid gradient from 10% to 40% (35 min) and then from 40% to 88% (10 min). The column eluent was monitored for absorbance by diode array (190–800 nm). The phenolic standards used were N, DHK, DHQ, K, and Q.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. K and Q fluorescence overwhelms other fluorescing compounds.
Supplemental Figure S2. Comparison of 5-min and 1-h incubations of aglycones at the root tip of tt4-1 seedlings.
Supplemental Figure S3. The aglycone standards have unique absorption spectra.
Supplemental Table S1. Control experiments for eluent and Suc indicate no interference with flavonoid movement.
Supplemental Table S2. Comparison of the absorption maxima of the exogenously applied aglycones from HPLC.
Supplemental Table S3. Summary of flavonoid fluorescence localization by CLSM.
Supplemental Movies S1 to S3 and S5. Adding N, DHK, DHQ, or Q near the root tip of a tt4 seedling resulted in the nearly immediate formation of Q fluorescence in specific cells in the distal elongation zone.
Supplemental Movie S4. Adding K reveals a possible reverse reaction through flavonol synthase.
Supplementary Material
Acknowledgments
We thank Daryl Webb, Australian National University Electron Microscopy Unit, for help with CLSM. Julie Christie and Riccardo Natoli are thanked for help with the P.A.L.M. system. Paul Ahn from Shimadzu and Charles Horcart, Research School of Biological Sciences (RSBS) Mass Spec Facility, are thanked for assistance with the HPLC. Jeremy Weinman, Genomic Interactions Group, RSBS, is thanked for help with the video clips. Warwick Hillier from the Biomolecular Spectroscopy Facility, RSBS, is thanked for his help with the spectrofluorometer. The valuable comments of Barry Rolfe are appreciated.
This work was supported by the Australian Research Council Centre of Excellence for Integrative Legume Research (project no. CEO348212) and the Biotechnology Resource Centre (Australian National University; to M.A.D.), and by the U.S. Department of Agriculture (grant no. 2006–03406 to G.K.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Charles S. Buer (charles.buer@anu.edu.au).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bais HP, Park S-W, Weir TL, Callaway RM, Vivanco JM (2004) How plants communicate using the underground information superhighway. Trends Plant Sci 9 26–32 [DOI] [PubMed] [Google Scholar]
- Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O (2006) The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol 16 553–563 [DOI] [PubMed] [Google Scholar]
- Blancaflor EB, Masson PH (2003) Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiol 133 1677–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Muday GK (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16 1191–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Sukumar P, Muday GK (2006) Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol 140 1384–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbulis IE, Iacobucci M, Shirley BW (1996) A null mutation in the first enzyme of flavonoid biosynthesis does not affect male fertility in Arabidopsis. Plant Cell 8 1013–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolo V, Braidot E, Chiandussi E, Vianello A, MacrÌ F (2003) KATP+ channel opening prevents succinate-dependent H2O2 generation by plant mitochondria. Physiol Plant 118 313–318 [Google Scholar]
- Coe EH, McCormick SM, Modena SA (1981) White pollen in maize. J Hered 72 318–320 [Google Scholar]
- Debeaujon I, Léon-Kloosterziel KM, Koornneef M (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic MA, Mathesius U, Arioli T, Weinman JJ, Gärtner E (1997) Chalcone synthase gene expression in transgenic subterranean clover correlates with localised accumulation of flavonoids. Aust J Plant Physiol 24 119–132 [Google Scholar]
- Djordjevic MA, Redmond JW, Batley M, Rolfe BG (1987) Clovers secrete specific phenolic compounds which either stimulate or repress nod gene expression in Rhizobium trifolii. EMBO J 6 1173–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espunya MC, Díaz M, Moreno-Romero J, Martínez MC (2006) Modification of intercellular levels of glutathione-dependent formaldehyde dehydrogenase alters glutathione homeostasis and root development. Plant Cell Environ 29 1002–1011 [DOI] [PubMed] [Google Scholar]
- Forestier C, Frangne N, Eggmann T, Klein M (2003) Differential sensitivity of plant and yeast MRP (ABCC)-mediated organic anion transport processes towards sulfonylureas. FEBS Lett 554 23–29 [DOI] [PubMed] [Google Scholar]
- Geisler M, Blakeslee JJ, Bouchard R, Lee OR, Vincenzetti V, Bandyopadhyay A, Titapiwatanakun B, Peer WA, Bailly A, Richards EL, et al (2005) Cellular efflux of auxin catalyzed by the Arabidopsis MDR/PGP transporter AtPGP1. Plant J 44 179–194 [DOI] [PubMed] [Google Scholar]
- Graham TL (1998) Flavonoid and flavonol glycoside metabolism in Arabidopsis. Plant Physiol Biochem 36 135–144 [Google Scholar]
- Grotewold E (2004) The challenges of moving chemicals within and out of cells: insights into the transport of plant natural products. Planta 219 906–909 [DOI] [PubMed] [Google Scholar]
- Harrison MJ (2005) Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 59 19–42 [DOI] [PubMed] [Google Scholar]
- Imin N, Nizamidin M, Wu T, Rolfe BG (2006) Factors involved in root formation in Medicago truncatula. J Exp Bot 58 439–451 [DOI] [PubMed] [Google Scholar]
- Jacobs M, Rubery PH (1988) Naturally occurring auxin transport regulators. Science 241 346–349 [DOI] [PubMed] [Google Scholar]
- Jenkins GI, Long JC, Wade HK, Shenton MR, Bibikova TN (2001) UV and blue light signalling: pathways regulating chalcone synthase gene expression in Arabidopsis. New Phytol 151 121–131 [DOI] [PubMed] [Google Scholar]
- Klein M, Martinoia E, Hoffmann-Thoma G, Weissenböck G (2000) A membrane-potential dependent ABC-like transporter mediates the vacuolar uptake of rye flavone glucuronides: regulation of glucuronide uptake by glutathione and its conjugates. Plant J 21 289–304 [DOI] [PubMed] [Google Scholar]
- Kubasek WL, Ausubel FM, Shirley BW (1998) A light-independent developmental mechanism potentiates flavonoid gene expression in Arabidopsis seedlings. Plant Mol Biol 37 217–223 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Miller ND, Splitt BL, Wu G, Spalding EP (2007) Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis Multidrug Resistance-Like ABC transporter genes. Plant Cell 19 1838–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ou-Lee T-M, Raba R, Amundson RG, Last RL (1993) Arabidopsis flavonoid mutants are hypersensitive to UV-B irradiation. Plant Cell 5 171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyola-Vargas VM, Broeckling CD, Badri D, Vivanco JM (2007) Effect of transporters on the secretion of phytochemicals by the roots of Arabidopsis thaliana. Planta 225 301–310 [DOI] [PubMed] [Google Scholar]
- Mathesius U, Schlaman HRM, Spaink HP, Sautter C, Rolfe BG, Djordjevic MA (1998) Auxin transport inhibition precedes root nodule formation in white clover roots and is regulated by flavonoids and derivatives of chitin oligosaccharides. Plant J 14 23–34 [DOI] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D (1998) Glutathione homeostasis in plants: implications for environmental sensing and plant development. J Exp Bot 49 649–667 [Google Scholar]
- Mo Y, Nagel C, Taylor LP (1992) Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc Natl Acad Sci USA 89 7213–7217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol J, Grotewold E, Koes R (1998) How genes paint flowers and seeds. Trends Plant Sci 3 212–217 [Google Scholar]
- Morris AC, Djordjevic MA (2006) The Rhizobium leguminosarum biovar trifolii ANU794 induces novel developmental responses on the subterranean clover cultivar Woogenellup. Mol Plant Microbe Interact 19 471–479 [DOI] [PubMed] [Google Scholar]
- Murphy A, Peer WA, Taiz L (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211 315–324 [DOI] [PubMed] [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16 1898–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Brown DE, Tague BW, Muday GK, Taiz L, Murphy AS (2001) Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiol 126 536–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier MK, Burbulis IE, Winkel-Shirley B (1999) Disruption of specific flavonoid genes enhances the accumulation of flavonoid enzymes and end-products in Arabidopsis seedlings. Plant Mol Biol 40 45–54 [DOI] [PubMed] [Google Scholar]
- Pelletier MK, Shirley BW (1996) Analysis of flavanone 3-hydroxylase in Arabidopsis seedlings. Plant Physiol 111 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Poupart J, Waddell CS, Muday GK (2003) Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiol 133 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea PA (1999) MRP subfamily ABC transporters from plants and yeast. J Exp Bot 50 895–913 [Google Scholar]
- Redmond JW, Batley M, Djordjevic MA, Innes RW, Kuempel PL, Rolfe BG (1986) Flavones induce expression of nodulation genes in Rhizobium. Nature 323 632–635 [Google Scholar]
- Saslowsky DE, Dana CD, Winkel-Shirley B (2000) An allelic series for the chalcone synthase locus in Arabidopsis. Gene 255 127–138 [DOI] [PubMed] [Google Scholar]
- Saslowsky D, Winkel-Shirley B (2001) Localization of flavonoid enzymes in Arabidopsis roots. Plant J 27 37–48 [DOI] [PubMed] [Google Scholar]
- Schoenbohm C, Martens S, Eder C, Forkmann G, Weisshaar B (2000) Identification of the Arabidopsis thaliana flavonoid 3′-hydroxylase gene and functional expression of the encoded P450 enzyme. Biol Chem 381 749–753 [DOI] [PubMed] [Google Scholar]
- Scott AC, Allen NS (1999) Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol 121 1291–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan JJ, Cheong H, Rechnitz GA (1998) The colorless flavonoids of Arabidopsis thaliana (Brassicaceae). I. A model system to study the orthodihydroxy structure. Am J Bot 85 467–475 [PubMed] [Google Scholar]
- Sheahan JJ, Rechnitz GA (1993) Differential visualization of transparent testa mutants in Arabidopsis thaliana. Anal Chem 65 961–963 [Google Scholar]
- Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM (1995) Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J 8 659–671 [DOI] [PubMed] [Google Scholar]
- Smith AP, Nourizadeh SD, Peer WA, Xu J, Bandyopadhyay A, Murphy AS, Goldsbrough PB (2003) Arabidopsis AtGSTF2 is regulated by ethylene and auxin, and encodes a glutathione S-transferase that interacts with flavonoids. Plant J 36 433–442 [DOI] [PubMed] [Google Scholar]
- Sorefan K, Booker J, Haurogné K, Goussot M, Bainbridge K, Foo E, Chatfield S, Ward S, Beveridge C, Rameau C, et al (2003) MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev 17 1469–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama A, Shitan N, Yazaki K (2007) Involvement of a soybean ATP-binding cassette-type transporter in the secretion of genistein, a signal flavonoid in legume-Rhizobium symbiosis. Plant Physiol 144 2000–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LP, Grotewold E (2005) Flavonoids as developmental regulators. Curr Opin Plant Biol 8 317–323 [DOI] [PubMed] [Google Scholar]
- Taylor LP, Jorgensen R (1992) Conditional male fertility in chalcone synthase-deficient petunia. J Hered 83 11–17 [Google Scholar]
- Terasaka K, Blakeslee JJ, Titapiwatanakun B, Peer WA, Bandyopadhyay A, Makam SN, Lee OR, Richards EL, Murphy AS, Sato F, et al (2005) PGP4, an ATP binding cassette p-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell 17 2922–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutter D (2005) Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol 7 581–591 [DOI] [PubMed] [Google Scholar]
- Tsurumi S, Ohwaki Y (1978) Transport of 14C-labeled indoleacetic acid in Vicia root segments. Plant Cell Physiol 19 1195–1206 [Google Scholar]
- Van Norman JM, Frederick RL, Sieburth LE (2004) BYPASS1 negatively regulates a root-derived signal that controls plant architecture. Curr Biol 14 1739–1746 [DOI] [PubMed] [Google Scholar]
- Waller GR, Nowacki EK (1978) Alkaloid Biology and Metabolism in Plants. Plenum Press, New York, pp 121–141
- Wasson AP, Pellerone FI, Mathesius U (2006) Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 18 1617–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5 218–223 [DOI] [PubMed] [Google Scholar]
- Wuyts N, Lognay G, Swennen R, De Waele D (2006) Nematode infection and reproduction in transgenic and mutant Arabidopsis and tobacco with an altered phenylpropanoid metabolism. J Exp Bot 57 2825–2835 [DOI] [PubMed] [Google Scholar]
- Yazaki K (2006) ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett 580 1183–1191 [DOI] [PubMed] [Google Scholar]
- Zhang C, Griffith BR, Fu Q, Albermann C, Fu X, Lee I-K, Li L, Thorson JS (2006) Exploiting the reversibility of natural product glycosyltransferase-catalyzed reactions. Science 313 1291–1294 [DOI] [PubMed] [Google Scholar]
- Zhang SZ, Morris ME (2003) Effects of the flavonoids biochanin A, morin, phloretin, and silymarin on P-glycoprotein-mediated transport. J Pharmacol Exp Ther 304 1258–1267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.