Abstract
Na+ uptake by plant roots has largely been explored using species that accumulate little Na+ into their shoots. By way of contrast, the halophyte Suaeda maritima accumulates, without injury, concentrations of the order of 400 mm NaCl in its leaves. Here we report that cAMP and Ca2+ (blockers of nonselective cation channels) and Li+ (a competitive inhibitor of Na+ uptake) did not have any significant effect on the uptake of Na+ by the halophyte S. maritima when plants were in 25 or 150 mm NaCl (150 mm NaCl is near optimal for growth). However, the inhibitors of K+ channels, TEA+ (10 mm), Cs+ (3 mm), and Ba2+ (5 mm), significantly reduced the net uptake of Na+ from 150 mm NaCl over 48 h, by 54%, 24%, and 29%, respectively. TEA+ (10 mm), Cs+ (3 mm), and Ba2+ (1 mm) also significantly reduced 22Na+ influx (measured over 2 min in 150 mm external NaCl) by 47%, 30%, and 31%, respectively. In contrast to the situation in 150 mm NaCl, neither TEA+ (1–10 mm) nor Cs+ (0.5–10 mm) significantly reduced net Na+ uptake or 22Na+ influx in 25 mm NaCl. Ba2+ (at 5 mm) did significantly decrease net Na+ uptake (by 47%) and 22Na+ influx (by 36% with 1 mm Ba2+) in 25 mm NaCl. K+ (10 or 50 mm) had no effect on 22Na+ influx at concentrations below 75 mm NaCl, but the influx of 22Na+ was inhibited by 50 mm K+ when the external concentration of NaCl was above 75 mm. The data suggest that neither nonselective cation channels nor a low-affinity cation transporter are major pathways for Na+ entry into root cells. We propose that two distinct low-affinity Na+ uptake pathways exist in S. maritima: Pathway 1 is insensitive to TEA+ or Cs+, but sensitive to Ba2+ and mediates Na+ uptake under low salinities (25 mm NaCl); pathway 2 is sensitive to TEA+, Cs+, and Ba2+ and mediates Na+ uptake under higher external salt concentrations (150 mm NaCl). Pathway 1 might be mediated by a high-affinity K transporter-type transporter and pathway 2 by an AKT1-type channel.
Various mechanisms have evolved in plants to allow them to cope with growing in saline soils; all are based on limiting the concentration of sodium and chloride ions accumulating in the cytosol, since cytosolic enzymes are sensitive to these ions in both glycophytes and halophytes (Flowers et al., 1977, 1986; Greenway and Munns, 1980). The cytosolic concentrations of sodium and chloride are determined by the net fluxes across the plasma membrane and the tonoplast. Of the tonoplast proteins, those involved in the transport of Na+ are a Na+/H+ antiporter (Apse et al., 1999; Fukuda et al., 1999; Hamada et al., 2001; Ma et al., 2004), which is energized by the proton gradient generated by the vacuolar H+-ATPase and the H+-PPiase (Gaxiola et al., 1999, 2002; Sze et al., 1999, 2002). Compartmentalization of Na+ in vacuoles is a particularly important aspect of the way plant cells deal with external salt, because the vacuolar ions contribute to osmotic adjustment. The extrusion of cytoplasmic Na+ out of the cell, effected by SALT OVERLY SENSITIVE1 (SOS1; Shi et al., 2002, 2003; Martínez-Atienza et al., 2007), a H+ exchanger present in the plasma membrane and a member of the NhaP group of proteins (Pardo et al., 2006), is another putative route to avoid Na+ accumulation in the cytosol (Blumwald et al., 2000) and to transfer Na+ to xylem vessels (Shi et al., 2002; see Davenport et al., 2007). Reducing Na+ influx must, though, be the key step for controlling Na+ accumulation compared with vacuolar Na+ compartmentalization and Na+ extrusion by SOS1, neither of which would be sufficient alone. However, the pathways by which plants take up Na+ are uncertain.
Electrophysiological studies suggest that Na+ influx across the plasma membrane occurs via voltage-independent channels or nonselective cation channels (NSCCs), but their precise molecular identities are not known (Tyerman and Skerrett, 1999; Demidchik et al., 2002). A further candidate for mediating sodium transport across the plasma membrane is a low-affinity cation transporter (LCT) from wheat (Triticum aestivum), although its physiological role remains to be established in planta (Schachtman et al., 1997; Amtmann et al., 2001). In the plasma membrane, the HKT proteins (high affinity K transporters), which have also been shown to transport Na+ (see Rodriguez-Navarro and Rubio, 2006), fall into two subfamilies, one of which (subfamily 2) has only been found in the grasses (Platten et al., 2006). Wheat TaHKT2;1 functions as a K+-Na+ symporter (Schachtman and Schroeder, 1994; Rubio et al., 1995; Gassmann et al., 1996) and homologs have since been cloned from many species of plants. The HKT proteins can be divided into two distinct types according to their properties of Na+ and K+ transport in heterologous expression systems: The Arabidopsis (Arabidopsis thaliana) AtHKT1;1 transported only Na+ (Uozumi et al., 2000); rice (Oryza sativa) OsHKT2;1 showed an AtHKT1;1-like Na+-specific transport activity and mediates Na+ uptake in the absence of external K+ (Horie et al., 2007), whereas OsHKT2;2 showed a TaHKT2;1-like K+-Na+ transport activity (Horie et al., 2001); both eucalyptus EcHKT1;1 and EcHKT1;2 mediated Na+ and K+ uptake (Fairbairn et al., 2000). Based on the capacity of Arabidopsis hkt1 mutations to suppress Na+ accumulation and hypersensitivity of the sos3-1 mutant to Na+, Rus et al. (2001) proposed that AtHKT1;1 is a determinant of Na+ entry into plant roots. The evidence from wheat also supports this viewpoint: Transgenic wheat plants in which native TaHK2;1 expression is significantly reduced through the introduction of antisense showed decreased Na+ uptake into roots (Laurie et al., 2002). Recent evidence shows, however, that AtHKT1;1 is a determinant of accumulation of Na+ in the root and retrieval of Na+ from the xylem (Davenport et al., 2007). The analysis by Rodriguez-Navarro and Rubio (2006) suggests that HKT transporters mediate high-affinity Na+ uptake but also function in low-affinity Na+ transport.
At present, two obvious approaches to the functional identification of the genes encoding K+ and Na+ transporters, gene knock out and expression in heterologous systems, present problems that do not generally occur with other genes (Rodriguez-Navarro and Rubio, 2006). Rodriguez-Navarro and Rubio (2006) note “The problem of gene knock out is the pleiotropic effects caused by mutations that affect K+ transporters. In fungi it is known that the disruption of the TRK (Madrid et al., 1998) but not HAK genes (Bañuelos et al., 2000) produces hyperpolarization and a consequent enhancement of K+ uptake through non-K+ transporters (Madrid et al., 1998)….these problems have not been reported in plants but possibly only because they have not been investigated” (p. 1156). Even if a gene of a putative transporter were cloned, Na+ uptake tests cannot be carried out in yeast trk1 trk2 mutants at high external Na+ concentrations since they have intrinsic low-affinity transporters (Santa-María et al., 1997). Moreover, heterologous expression system may not reproduce kinetic characteristics of transporters in planta (Garciadeblas et al., 2003; Haro et al., 2005). There is no perfect system currently available to investigate K+ and Na+ uptake in plants.
To date, Na+ uptake by plant roots has been explored using glycophytes (e.g. Arabidopsis, rice, or wheat) and a few halophytes (e.g. Thellungiella halophila, Puccinellia tenuiflora, and Mesembryanthemum crystallinum). Most glycophytes (Läuchli, 1984; Huang et al., 2006; James et al., 2006) and some halophytes, such as T. halophila (Volkov et al., 2003; Inan et al., 2004; Taji et al., 2004) and P. tenuiflora (Wang et al., 2002, 2004) are relatively salt-excluding plants and have a strong selectivity for K+ over Na+ that limits the uptake of Na+ while maintaining the uptake of K+. M. crystallinum is a salt-secreting plant and possesses epidermal bladder cells in its aerial parts, which store Na+ (Adams et al., 1998; Su et al., 2002): Understanding ion transport in such plants may be confounded with the complex processes of salt secretion and the development of salt bladders.
Although high-affinity Na+ uptake in plant roots can be tested by applying the depletion method (Garciadeblas et al., 2003; Haro et al., 2005), the lack of a suitable system has restricted the identification of low-affinity Na+ uptake pathways, such as operate in plants growing under salinity. Species from within the Chenopodiaceae, the family with the highest proportion of halophytes, offer potential physiological models. Species in the genus of Suaeda are salt-accumulating plants (Yeo and Flowers, 1980; Wang et al., 2002), which accumulate substantial amounts of Na+ in their shoots with apparent weak selectivity for K+ over Na+ (Reimann, 1992; Reimann and Breckle, 1993; Wang et al., 2002); the selectivity of roots of Suaeda salsas for K+ over Na+ (SK:Na) was between 2% and 14% of that of Hordeum vulgare, Echinochloa frumentacea, Calamagrostis pseudophragmites, Sophora alopecuroides, and P. tenuiflora and the selectivity of transport from root to stem between 5% and 33% of that in the same species (Wang et al., 2002). We have used S. maritima, a species where net uptake of Na+ in 50 mm NaCl is an order of magnitude greater than that of durum wheat (Triticum durum) growing in the same salinity (0.13 μmol/g fresh weight root/min, interpolated from the data in Yeo and Flowers, 1986; net uptake of Na+ by durum wheat in 50 mm NaCl is 0.01–0.02 μmol/g fresh weight root/min; Davenport et al., 2005) to examine Na+ influx and found two distinct low-affinity Na+ uptake pathways: Pathway 1 is insensitive to TEA+ or Cs+, but sensitive to Ba2+ and mediates Na+ uptake under low salinities such as 25 mm NaCl; pathway 2 is sensitive to TEA+, Cs+, or Ba2+ and mediates Na+ uptake under higher external salt concentrations, such as 150 mm NaCl. We also present physiological evidence that NSCCs and LCT are not the major pathways for Na+ entry into root cells of S. maritima.
RESULTS
S. maritima grows optimally in salt concentrations of about 150 mm sodium chloride (Yeo and Flowers, 1980) and in 200 mm NaCl accumulates sodium ions in its leaves to concentrations of around 5.5 mmol per g dry weight or about 430 mm based on the tissue water content (plants grown for 35 d: Clipson, 1984; see also Flowers et al., 1986). To throw light on the way in which sodium ions enter the root of S. maritima, we have examined the effects of a range of inhibitors on growth and ion uptake.
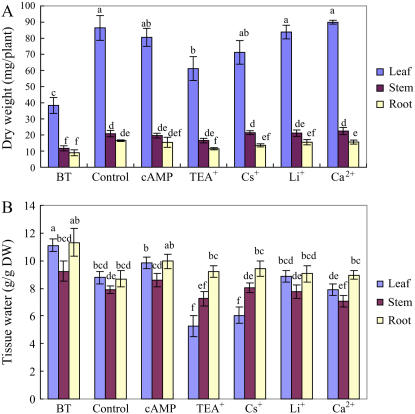
Effects of cAMP, Ca2+, and Li+ on Growth and Ion Accumulation of S. maritima Growing at Near-Optimal Salinity (150 mm NaCl)
We evaluated the effects of cAMP and Ca2+, which inhibit Na+ influx through NSCCs (Maathuis and Sanders, 2001; Demidchik and Tester, 2002), and Li+, which is considered an analog of Na+ and is a competitive inhibitor for Na+ uptake and transport (Serrano et al., 1999; Rubio et al., 2004) on growth and ion accumulation of S. maritima. Compared to control plants (plus NaCl, but no inhibitor), cAMP (500 μm), Li+(10 mm), and Ca2+(10 mm) did not have any significant effect on dry weight or tissue water content of leaf, stem, or root (Fig. 1); neither did these compounds have any significant effect on Na+ or K+ concentrations in different plant parts (Fig. 2). Prolonging the treatment time from 48 to 144 h did not induce any significant effect of cAMP, Li+, or Ca2+ on whole plant Na+ content of S. maritima (data not presented). Since these data suggest that NSCCs do not mediate Na+ uptake in S. maritima, we investigated the potential roles of other ion channels.
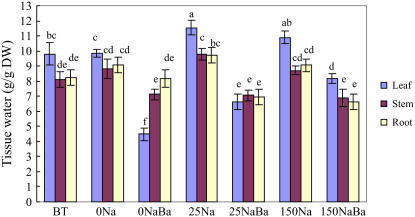
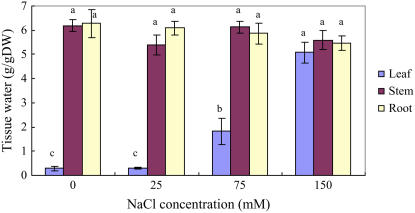
Figure 1.
Growth and tissue water content of S. maritima seedlings in response to treatment with NaCl (150 mm) together with cAMP (500 μm), TEA+ (10 mm), Cs+ (3 mm), Li+ (10 mm), or Ca2+ (10 mm). Three-week-old seedlings were transferred to Hoagland solution supplemented with 75 mm NaCl and 24 h later the concentration was increased to 150 mm NaCl for another 24 h (Control); for the inhibitor treatments, the plants were subjects to the same salinization procedure but in the presence of the inhibitor (i.e. from the time of salinization with 75 mm NaCl). Plants were grown in 100% relative humidity. BT represents before treatment with NaCl and inhibitors. Values are means ± sd (n = 24) and bars indicate sd. Columns with different letters indicate significant difference at P < 0.05 (Duncan test). [See online article for color version of this figure.]
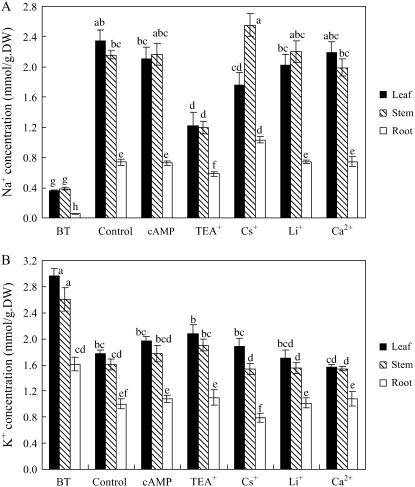
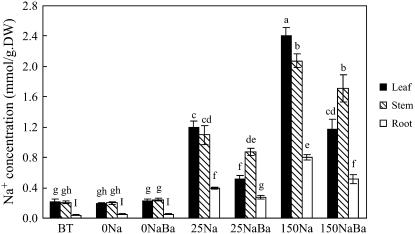
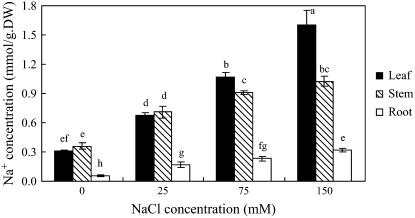
Figure 2.
Na+ and K+ concentrations in S. maritima seedlings treated with NaCl (150 mm) together with cAMP (500 μm), TEA+ (10 mm), Cs+ (3 mm), Li+ (10 mm), or Ca2+ (10 mm). Three-week-old seedlings were transferred to Hoagland solution supplemented with 75 mm NaCl and 24 h later the concentration was increased to 150 mm NaCl for another 24 h (Control); for the inhibitor treatments, the plants were subjects to the same salinization procedure but in the presence of the inhibitor (i.e. from the time of salinization with 75 mm NaCl). Plants were grown in 100% relative humidity. BT represents before treatment with NaCl and inhibitors. Values are means ± sd (n = 8) and bars indicate sd. Columns with different letters indicate significant difference at P < 0.05 (Duncan test).
Effects of Various K+ Channel Blockers on Growth and Ion Accumulation of S. maritima Growing at Near-Optimal Salinity (150 mm NaCl)
TEA+ and Cs+ inhibit K+ uptake through most K+ channels and some other transporters (Hedrich and Schroeder, 1989; Tester, 1990). In preliminary experiments (data not presented), we found that plants whose roots were treated with 10 mm TEA+ and 3 mm Cs+ wilted, an effect not seen with other inhibitors that we used. So, in the experiments that we report involving TEA+ and Cs+ treatments, we reduced transpiration by keeping the plants in a chamber with 100% relative humidity (compare with Nublat et al., 2001; Shi et al., 2002). In these experiments, we added the inhibitors at the same time as the salt; S. maritima is a salt-accumulating plant and has a strong capacity for Na+ accumulation, so it adjusts very rapidly to increase in external Na+ concentration (Clipson, 1987). Using non-Na+-adapted plants allowed us to investigate the properties of those proteins involved in initial Na+ uptake and avoid any possible consequences of a high internal Na+ concentration interfering with the uptake of the inhibitors. Under these conditions, TEA+ and Cs+ significantly decreased leaf fresh weight (by 46% and 42% compared with the control, respectively; data not presented) of plants growing in 150 mm NaCl. TEA+ also decreased leaf dry weight (by 29%; Fig. 1); the effect of Cs+ was not statistically significant. Leaf tissue water content was significantly reduced by both TEA+ (by 40%) and Cs+ (by 31%) treatments compared with the control (Fig. 1).
The exposure of plants to external Na+ invariably leads to an increase in the concentration of that ion in the plant. TEA+, however, significantly decreased Na+ concentrations in leaf, stem, and root by 48%, 45%, and 21%, respectively, compared with corresponding organs of control plants, which were treated with NaCl (150 mm) but not the inhibitor (Fig. 2A). Although Cs+ treatment also reduced Na+ concentrations in leaves, the concentrations in stems and roots increased (Fig. 2A). Further analysis of the data showed that while TEA+ altered the quantity of Na+ in different plant parts, it had little effect on its distribution within the plant (Table I). However, Cs+ reduced the quantity of Na+ in leaf tissue and increased the proportion in the stem (Table I).
Table I.
Effect of TEA+ and Cs+ on Na+ relative distribution in different plant parts
The quantity of sodium in each plant part was calculated from the data in Figures 1 and 2 and used to calculate the proportions of the total quantity in each part. Different letters within the second column indicate significant differences at P < 0.05 (Duncan test).
| Treatments | Total Quantity | Na+ Proportion
|
||
|---|---|---|---|---|
| Leaf | Stem | Root | ||
| μmol/plant | % | |||
| Control | 259 ± 23 a | 78 | 17 | 5 |
| 10 mm TEA+ | 101 ± 11 c | 74 | 20 | 6 |
| 3 mm Cs+ | 194 ± 17 b | 65 | 28 | 7 |
K+ concentrations in salinized plants fell when compared with the situation before salinization (Fig. 2B). However, neither TEA+ nor Cs+ (nor any of the other inhibitors used) had any effect on the K+ concentration in leaf, stem, or root (Fig. 2B). After a NaCl treatment of 48 h, TEA+ and Cs+ did not significantly reduce K+ content of the whole plant compared with Na+ content (data not presented). These plants had been grown in 6 mm K+ external solution and we did not add Na+ to the solution until the treatment, so the K+ content was high (159 ± 21.2 μmol/plant) and Na+ content was low (18.9 ± 2.51 μmol/plant). The high K+ background is the most likely reason why TEA+ and Cs+, the inhibitors of K+ channels and transporters, did not significantly affect whole plant K+ content in our experiments.
Effects of Inhibitors on Growth, Tissue Water, and Ion Accumulation of S. maritima Growing in 25 mm NaCl
Although the optimal salinity for the growth of S. maritima is around 150 mm NaCl, about 85% of growth measured as organic dry weight is achieved in 25 mm NaCl (Yeo and Flowers, 1980), allowing separate assessment of the response that stimulates growth from that which requires osmotic adjustment for survival. Treating plants with 25 mm NaCl and Li+ or cAMP or Ca2+ for 144 h did not have any significant effect on whole plant Na+ and K+ contents (Supplemental Fig. S1), even though cAMP and Ca2+ improved water status and Na+ or K+ concentrations in some parts of S. maritima (Supplemental Fig. S2).
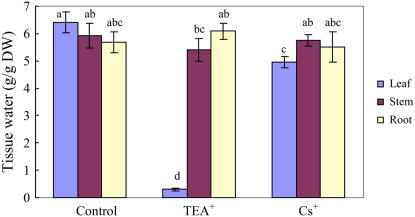
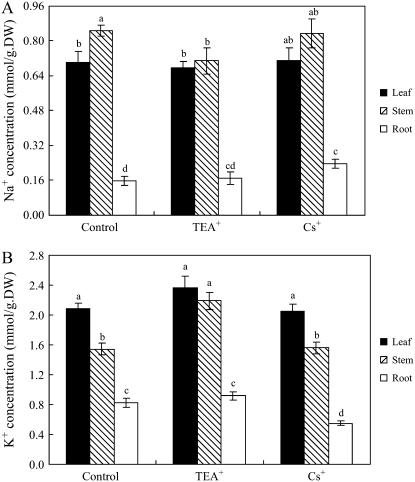
We also examined the effects of TEA+ (10 mm) and Cs+ (3 mm) on S. maritima growing at the lower concentration of 25 mm NaCl in a chamber with 100% relative humidity. TEA+ severely reduced leaf fresh weight and tissue water (Fig. 3); Cs+ significantly decreased leaf fresh weight and tissue water by 26% and 23% compared with the control, respectively. Neither TEA+ nor Cs+ had any effect on the dry weight of leaves or roots: TEA+ reduced the dry weight of stem by 32%; Cs+ did not significantly affect stem dry weight (data not presented). Although TEA+ and Cs+ significantly affected Na+ or K+ concentrations in some parts of S. maritima (Fig. 4), they did not significantly affect whole plant Na+ and K+ contents (data not presented).
Figure 3.
Tissue water content of S. maritima seedlings in response to treatment with NaCl (25 mm), TEA+ (10 mm), or Cs+ (3 mm). Three-week-old seedlings were transferred to Hoagland solution supplemented with 25 mm NaCl (Control), or 25 mm NaCl with different inhibitors for 48 h (to change each 24 h) in 100% relative humidity. Values are means ± sd (n = 24) and bars indicate sd. Columns with different letters indicate significant difference at P < 0.05 (Duncan test). [See online article for color version of this figure.]
Figure 4.
Na+ and K+ concentrations in S. maritima seedlings treated with NaCl (25 mm), TEA+ (10 mm), or Cs+ (3 mm). Three-week-old seedlings were transferred to Hoagland solution supplemented with 25 mm NaCl (Control), or 25 mm NaCl with different inhibitors for 48 h (to change each 24 h) in 100% relative humidity. Values are means ± sd (n = 8) and bars indicate sd. Columns with different letters indicate significant difference at P < 0.05 (Duncan test).
Effects of TEA+ and Cs+ on Net Uptake of Na+ in 25 mm and 150 mm NaCl
TEA+ significantly reduced the net uptake of Na+, measured over a period of 48 h, from 0.56 ± 0.04 μmol g−1 fresh weight min−1 in the untreated plants growing in 150 mm NaCl to 0.26 ± 0.03 μmol g−1 fresh weight min−1 (Table II). Net uptake of Na+ in the presence of Cs+ (0.43 ± 0.03 μmol g fresh weight−1 min−1) was also significantly less than that of the control plants (plus 150 mm NaCl, but without inhibitor; Table II). These results suggest that TEA+ strongly inhibits Na+ absorption by the root, but did not affect Na+ transport within the plant; Cs+ not only inhibited Na+ uptake, but also blocked Na+ transport to the leaves, causing a greater portion of Na+ to be retained in the stem and root in comparison with control (Table I).
Table II.
Effect of TEA+ and Cs+ on net Na+ flux (μmol g−1 fresh weight root min−1) of S. maritima growing in different salt concentrations for 48 h
Na+ fluxes for S. maritima treated with 150 mm NaCl were calculated from the data of Figures 1 and 2; Na+ fluxes of S. maritima treated with 25 mm NaCl were calculated from the data of Figures 3 and 4. Net uptake was calculated, using the control data as an example, as (Na+ content in whole plant of control − Na+ content in whole plant before NaCl and inhibitor treatments)/(fresh weight of control root × 48 × 60). Different letters within the same row indicate significant differences at P < 0.05 (Duncan test).
| NaCl Concentration | Inhibitor
|
||
|---|---|---|---|
| None (Control) | TEA+ | Cs+ | |
| mm | 10 mm | 3 mm | |
| 150 | 0.56 ± 0.04 a | 0.26 ± 0.03 c | 0.43 ± 0.03 b |
| 25 | 0.20 ± 0.02 a | 0.15 ± 0.01 a | 0.18 ± 0.03 a |
In contrast to the situation in 150 mm NaCl, 10 mm TEA+ or 3 mm Cs+ did not significantly reduce net Na+ flux (the net quantity of Na+ absorbed by the plant per unit of root and per unit of time) of S. maritima growing in 25 mm NaCl (Table II).
Ba2+ Inhibits Na+ Uptake of S. maritima Both in 25 mm and 150 mm External NaCl
Ba2+ is known to be another K+ channel blocker in plants (Tester, 1988; Kelly et al., 1995), dramatically reducing K+ uptake mediated by K+ transporters AtKUP1, EcHKT1;1, or EcHKT1;2 (Fu and Luan, 1998; Fairbairn et al., 2000). Ba2+ also inhibited Na+ uptake mediated by OsHKT2;1 or OsHKT1;1 (formerly OsHKT4) and completely inhibited Na+ uptake in rice roots (Garciadeblas et al., 2003). Thus, we tested the effects of Ba2+ on Na+ and K+ uptake in S. maritima under different external NaCl concentrations.
Plant dry weight was not affected significantly over the period of treatment (48 h) by the different concentrations of NaCl with or without 5 mm Ba2+ (data not presented). However, Ba2+ had a remarkable effect on water content. In the absence of added NaCl to the culture solution, Ba2+ severely decreased tissue water both in leaf and stem of S. maritima (Fig. 5). For plants growing in 25 mm NaCl, although Ba2+ still significantly reduced fresh weight (data not presented) and tissue water, leaf water status was much better in the presence (25NaBa treatment) than in the absence of salt (0NaBa treatment). In 150 mm NaCl, Ba2+ again significantly reduced tissue water in leaf, stem, and root, but again the water content was higher than in the absence of salt or in 25 mm NaCl (Fig. 5).
Figure 5.
The effect of different external concentrations of NaCl, with or without 5 mm Ba2+, on the water content of S. maritima seedlings. Three-week-old seedlings were transferred to Hoagland solution supplemented with different concentrations of NaCl with or without 5 mm Ba2+ for 48 h (150 mm NaCl was added at 75 mm NaCl/24 h) in 100% relative humidity. BT represents before treatments. The figure preceding Na is its concentration in the culture solution; Ba signifies the presence of 5 mm Ba2+. Values are means ± sd (n = 24) and bars indicate sd. Columns with different letters indicate significant difference at P < 0.05 (Duncan test). [See online article for color version of this figure.]
In the absence of added NaCl (the culture solution contains a trace quantity of NaCl), Ba2+ did not significantly affect Na+ concentrations in different plant parts of S. maritima although it decreased leaf and root K+ concentrations by 12% and 18%, respectively, compared to control plants. In 25 mm NaCl, Ba2+ significantly decreased leaf and root Na+ concentrations by 58% and 30%, respectively; Ba2+ also significantly decreased stem and root K+ concentrations by 19% and 26%, respectively. In 150 mm NaCl, Ba2+ again significantly decreased leaf and root Na+ concentrations (by 51% and 36%, respectively) and root K+ concentrations (by 29%; Fig. 6). However, Ba2+ did not significantly affect whole plant K+ contents under either 25 mm NaCl or 150 mm NaCl (data not presented).
Figure 6.
Na+ concentrations in S. maritima seedlings treated under different concentrations of NaCl with or without 5 mm Ba2+. Three-week-old seedlings were transferred to Hoagland solution supplemented with different concentrations of NaCl with or without 5 mm Ba2+ for 48 h (150 mm NaCl was added at 75 mm NaCl/24 h) in 100% relative humidity chamber. BT represents before treatments. The figure preceding Na is its concentration in the culture solution; Ba signifies the presence of 5 mm Ba2+. Values are means ± sd (n = 8) and bars indicate sd. Columns with different letters indicate significant difference at P < 0.05 (Duncan test).
These changes in Na+ contents and concentrations resulted from the effects of Ba2+ in significantly lowering net Na+ fluxes (calculated from uptake data obtained over 48 h) by 47% and 29%, in 25 mm and 150 mm NaCl, respectively (Table III). Ba2+ decreased whole plant Na+ content by 56% in 25 mm NaCl and by 49% in 150 mm NaCl. In contrast, Na+ uptake was not inhibited under low 25 mm external NaCl by TEA+ and Cs+ (Table II). Since net fluxes confound influx and retranslocation, we investigated the effects of various inhibitors on 22Na+ influx into the roots.
Table III.
Effect of Ba2+ (5 mm) on whole plant Na+ content (μmol/plant) and root net Na+ flux (μmol g−1 fresh weight root min−1) of S. maritima
The data were calculated from those of Figures 5 and 6. Net uptake was calculated, using the 25Na data as an example, as (Na+ content in whole plant of 25Na − Na+ content in whole plant before NaCl and inhibitor treatments)/(fresh weight of 25Na root × 48 × 60). Different letters within the same row indicate significant differences at P < 0.05 (Duncan test); BT, before NaCl and inhibitor treatment.
| BT | 25Na | 25NaBa | 150Na | 150NaBa | |
|---|---|---|---|---|---|
| Na+ content | 7.0 ± 0.56 d | 50 ± 5.61 b | 22 ± 1.4 c | 100 ± 7.7 a | 51 ± 4.4 b |
| Na+ net flux | 0.19 ± 0.02 c | 0.10 ± 0.01 d | 0.43 ± 0.02 a | 0.31 ± 0.03 b |
Effects of K+ and Inhibitors on 22Na+ Influx
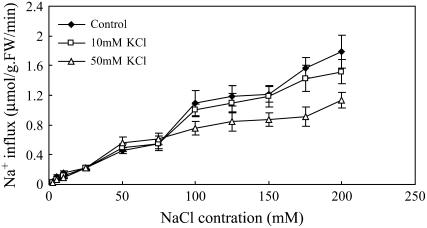
To investigate the effects of inhibitors on 22Na+ influx, rather than net accumulation, we used whole root systems of S. maritima attached to about 4 cm of stem. We confirmed that influx of 22Na+ to the roots was linear over the first 2 min of exposure to 22Na+, as for Arabidopsis (Essah et al., 2003); after that, root 22Na+ content increased gradually and over the same time 22Na+ began to accumulate in the remnant of the stem. The influx of 22Na+ into the roots showed evidence of a plateau (of some 0.5 μmol g−1 fresh weight root min−1) as the external salt concentration increased between 50 and 75 mm NaCl; above this concentration, the influx more than doubled as the external concentration doubled (Fig. 7). Double reciprocal plots (Lineweaver-Burk) were linear over the concentration ranges 2.5 to 75 mm Na+ (1/influx = 58.6[1/{NaCl}] + 0.73; R2 = 0.97) and 100 to 200 mm Na+ (1/influx = 68.1[1/{NaCl}] + 0.27; R2 = 0.98), allowing approximate estimates to be made of the Km and Vmax values, which were 80 mm and 1.4 μmol g−1 fresh weight root min−1 and 243 mm and 3.6 μmol g−1 fresh weight root min−1 over the low and high concentration ranges, respectively.
Figure 7.
Effects of NaCl and KCl concentration on root 22Na+ influx of S. maritima seedlings. Seventeen-day-old seedlings were transferred to non-KNO3 Hoagland solution supplemented with three concentrations (0, 10, and 50 mm) of KCl and various concentrations (2.5 to 200 mm) of NaCl for 3 d. For measurements of influx, the seedlings were transferred to equivalent solutions labeled with 22Na+. Values are means ± sd (n = 6) and bars indicate sd.
K+ (10 or 50 mm) had no effect on 22Na+ influx at concentrations below 75 mm NaCl, but the influx of 22Na+ was inhibited by 50 mm K+ when the external concentration of NaCl was above 75 mm (Fig. 7). However, the double reciprocal plots were not characteristic of K+ being either a competitive or noncompetitive inhibitor (Supplemental Fig. S3).
Li+ (1 and 10 mm) and Ca2+ (5 and 10 mm) did not have any significant effect on 22Na+ influx of S. maritima growing in either 25 mm or 150 mm external NaCl (Supplemental Fig. S4) in agreement with their lack of effect on growth and ion accumulation.
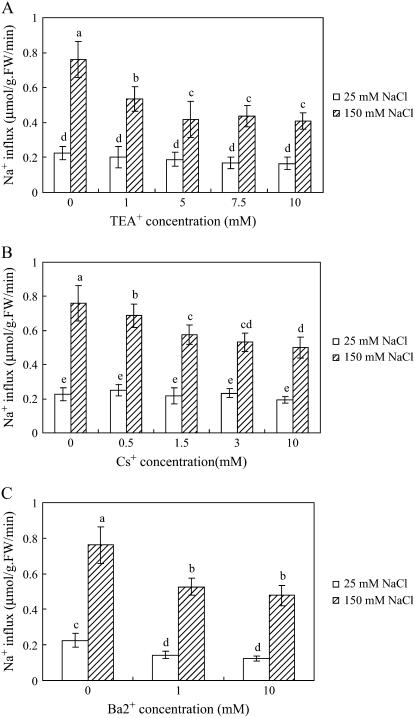
We found similarities between the effects of TEA+, Cs+, and Ba2+ on growth and net ion accumulation and on 22Na+ influx. Neither TEA+ (1, 5, 7.5, or 10 mm) nor Cs+ (0.5, 1.5, 3, or 10 mm) had any significant effects on 22Na+ influx of S. maritima growing in 25 mm external NaCl (Fig. 8, A and B). However, in 150 mm external NaCl, influx of 22Na+ into the roots decreased significantly with the increase of TEA+ and Cs+ concentration (Fig. 8, A and B). 22Na+ influx into the roots of S. maritima was also significantly reduced by Ba2+, but in this case in both 25 mm and 150 mm external NaCl (Fig. 8C). Even 1 mm Ba2+ decreased 22Na+ influx, by 36% and 31% in 25 mm and 150 mm external NaCl, respectively, compared to a control without Ba2+.
Figure 8.
Root 22Na+ influx of S. maritima seedlings treated with TEA+ (A), Cs+ (B), and Ba2+(C). Seventeen-day-old seedlings were transferred to Hoagland solution supplemented with 25 mm NaCl and 150 mm NaCl for 4 d, respectively. Then seedlings were transferred to Hoagland solution supplemented with corresponding concentrations of NaCl and TEA+, Cs+, or Ba2+ for 10 min before they were transferred into the above corresponding solution labeled with 22Na+. Values are means ± sd (n = 6) and bars indicate sd. Columns with different letters indicate significant difference at P < 0.05 (Duncan test).
So, Li+ and Ca2+ have no inhibitory effects on 22Na+ influx of S. maritima in either 25 mm or 150 mm external NaCl; K+, Cs+, and TEA+ have no inhibitory effects on 22Na+ influx in 25 mm NaCl, but inhibit 22Na+ influx in 150 mm NaCl. Ba2+ inhibits 22Na+ influx both in 25 mm and 150 mm external NaCl. The effects of Cs+ and TEA+ on 22Na+influx measured over 2 min matches closely the effects on net uptake estimated over 48 h.
Interestingly, in 25 mm NaCl, the 22Na+ influx of 0.23 ± 0.04 μmol g−1 fresh weight root min−1 (Fig. 8) measured over 2 min was virtually the same as net flux (0.20 ± 0.02 μmol g−1 fresh weight root min−1) determined over 48 h (Table II). 22Na+ influx of 0.76 ± 0.10 μmol g−1 fresh weight root min−1 in 150 mm NaCl (Fig. 8) exceeds net flux of 0.56 ± 0.04 μmol g−1 fresh weight root min−1, but this net flux is the average of 24 h in 75 mm NaCl and 24 h in 150 mm NaCl (Table II).
Na+ Alleviation of Wilting of S. maritima Induced by Ba2+ or TEA+
We observed that plants of S. maritima wilted severely after adding Ba2+ (5 mm) for 48 h in the absence of NaCl, even at 100% relative humidity; in the presence of 25 mm NaCl, Ba2+ treatment brought about only slight wilting and in 150 mm NaCl the plants did not wilt at all when treated with Ba2+. This observation was in accordance with leaf water status shown in Figure 5, where for plants grown in the presence of Ba2+, leaf water content was higher, the higher external Na+. Na+ concentrations in leaf, stem, and root of 25NaBa treatment increased 2.3-, 3.6-, and 5.5-fold in comparison with the corresponding parts of 0NaBa treatment, and for 150NaBa treatment, Na+ concentrations increased by 5.2-, 7.1-, and 10.3-fold, respectively (Fig. 6). However, K+ concentrations in leaf and root of 25NaBa or 150NaBa treatment were not significantly different from the corresponding parts of plants in the 0NaBa treatment; only stem K+ concentrations were lowered by 15% for the 25NaBa treatment and by 17% for the 150NaBa treatment (data not shown). It appears that Na+ alleviates the wilt symptom of S. maritima caused by Ba2+.
A similar phenomenon was also observed in the presence of TEA+ (10 mm). With decreasing external NaCl concentrations from 150 to 0 mm wilting increased: Na+ alleviated TEA+-induced wilting of S. maritima. These visible symptoms again reflected changes in leaf water content (Fig. 9): Leaf water content increased 7-fold in 75 mm NaCl and 18-fold in 150 mm NaCl in comparison with the plants growing in the presence of TEA+ but the absence of external NaCl. These changes in wilting and water content were correlated with changes in the Na+ concentration in the leaves (the Na+ concentration of roots and stems also increased with increasing external Na+; Fig. 10; there was, however, no effect of increasing external Na+ on the concentration of K+; Supplemental Fig. S5).
Figure 9.
Tissue water content in S. maritima seedlings treated with 10 mm TEA+ and different concentrations of NaCl. Three-week-old seedlings were transferred to Hoagland solution supplemented with 10 mm TEA+ and different concentrations of NaCl for 48 h (150 mm NaCl was added at 75 mm NaCl/24 h) in 100% relative humidity chamber. Values are means ± sd (n = 24) and bars indicate sd. Columns with different letters indicate significant difference at P < 0.05 (Duncan test). [See online article for color version of this figure.]
Figure 10.
Na+ concentrations in S. maritima seedlings treated with 10 mm TEA+ and different concentrations of NaCl. Three-week-old seedlings were transferred to Hoagland solution supplemented with 10 mm TEA+ and different concentrations of NaCl for 48 h (150 mm NaCl was added at 75 mm NaCl/24 h) in 100% relative humidity chamber. Values are means ± sd (n = 8) and bars indicate sd. Columns with different letters indicate significant difference at P < 0.05 (Duncan test).
DISCUSSION
S. maritima Is Valuable for Characterizing Na+ Uptake and Transport
Net accumulation of Na+ in a plant is the end result of the balance of influx and efflux, moderated by the capacity for storage synonymous with the vacuolar volume in leaf cells. Among 15 species of the Chenopodiaceae tested for Na+ and K+ accumulation under similar conditions, S. maritima had among the highest accumulation of Na+ and the lowest K+/Na+ ratio (Reimann and Breckle, 1993). In this strongly Na+-accumulating species growing in low external NaCl (25 mm), 22Na+ influx (0.23 ± 0.04 μmol g−1 fresh weight root min−1; Fig. 8) measured over 2 min was virtually the same as net flux (0.20 ± 0.02 μmol g−1 fresh weight root min−1; Table II) determined over 48 h. At higher salinity (150 mm external NaCl), close to the optimal concentration for growth (approximately 200 mm NaCl; Flowers, 1972; Yeo and Flowers, 1980; Khan et al., 2000; Lu et al., 2003), 22Na+ influx (0.76 ± 0.10 μmol g−1 fresh weight root min−1; Fig. 8) exceeded net flux (0.56 ± 0.04 μmol g−1 fresh weight root min−1; Table II; determined in 75 mm NaCl for 24 h + 150 mm NaCl for 24 h). However, the difference between influx and net accumulation is relatively small, especially in comparison with the situation in Arabidopsis, where influx (1.88 μmol g−1 fresh weight root min−1) exceeded uptake over 3 weeks (0.03 μmol g−1 fresh weight root min−1; both in 50 mm NaCl and 0.2 mm Ca2+) by more than 60-fold (Essah et al., 2003).
Since for S. maritima growing in 150 mm NaCl, 95% of the Na+ that is accumulated is in the shoot (78% in the leaves and 17% in the stems; Table I) and since there is evidence that little or no Na+ is retranslocated from the shoots (Yeo, 1981), then this suggests that virtually all the sodium that enters roots is transported to the shoots where it is accumulated. S. maritima has succulent leaves with enlarged cells in which the vacuoles, where Na+ is accumulated, occupy most of the volume (Hajibagheri et al., 1984). In contrast to S. maritima, glycophytes (Läuchli, 1984; Huang et al., 2006; James et al., 2006) and some halophytes, such as T. halophila (Volkov et al., 2003; Inan et al., 2004; Taji et al., 2004) and P. tenuiflora (Wang et al., 2002, 2004) do not accumulate such high ion concentrations in the leaves, except when those leaves are dying or dead. To date, several mechanisms of limiting Na+ accumulation in leaves of salt-excluding plants have been proposed. It has been suggested that SOS1 mediates not only Na+ extrusion to the soil solution but also Na+ retrieval from the xylem into the surrounding parenchyma cells and so affects long-distance Na+ transport (Shi et al., 2002, 2003). Arabidopsis AtHKT1;1 and rice OsHKT1;5 (OsHKT8) are also proposed to function in Na+ unloading from xylem vessels to xylem parenchyma cells (Berthomieu et al., 2003; Ren et al., 2005; Sunarpi et al., 2005) and AtHKT1;1 in retrieval of Na+ from the root xylem (Rus et al., 2001; Davenport et al., 2007). Durum wheat TmHKT7-A2 and TmHKT1;5-A withdraw Na+ from the xylem in the roots, and also TmHKT7-A2 withdraws Na+ from the xylem in the leaf (Huang et al., 2006; James et al., 2006; Byrt et al., 2007). Whatever the mechanism, limiting Na+ accumulation in leaves of salt-excluding plants is central to tolerance (Munns, 2002; Wang et al., 2002). However, in S. maritima because the bulk of Na+ (95%) entering the plant is transported to shoot this is valuable material for characterizing the pathway of Na+ transport from root to shoot.
The Evidence in Planta Did Not Support NSCCs and LCT as the Major Pathways for Na+ Entry into Root Cells
It has been suggested that voltage-independent channels or NSCCs and a LCT are the major pathways for Na+ uptake (Schachtman et al., 1997; Amtmann and Sanders, 1999; Tyerman and Skerrett, 1999; Amtmann et al., 2001; Demidchik et al., 2002). NSCCs and the LCT are characterized by Ca2+, cAMP, and cGMP inhibition of ion conductance and Na+ influx (Clemens et al., 1998; Maathuis and Sanders, 2001; Demidchik and Tester, 2002; Tyerman, 2002; Essah et al., 2003; Antosiewicz and Hennig, 2004). However, our results indicate that 10 mm Ca2+ and 500 μm cAMP did not significantly affect whole plant Na+ contents of S. maritima under either 25 mm or 150 mm NaCl treatment for 48 or 144 h (Supplemental Fig. S1A). Furthermore, neither 5 mm nor 10 mm Ca2+ have any inhibitory effects on Na+ influx of S. maritima in either 25 mm or150 mm external NaCl (Supplemental Fig. S4B). The absence of any effects of 5 mm or 10 mm Ca2+ suggests that LCT is not involved in the uptake of sodium by S. maritima. Even though S. maritima was grown in a medium with 0.5 mm Ca2+ in our whole plant experiments (except for the special cases of the 10 mm Ca2+ treatments), LCT is unlikely to be the major pathway of Na+ influx in Suaeda, since in most soils calcium levels are high enough to inhibit Na+ transport through LCT substantially (Schachtman and Liu, 1999; Hirschi, 2004). Ca2+ is more common than Na+, being the fifth most abundant element in the earth's crust and true Ca2+ deficiencies in soils are rather rare; seawater calcium concentrations are about 10 mm (Harvey, 1966). Furthermore, adding 5 mm or 10 mm Ca2+ still did not have any significant effect on Na+ influx of S. maritima cultured in a medium with 0.1 mm Ca2+ (Supplemental Fig. S4B). As far as NSCCs are concerned, these are also unlikely conduits for Na+ in S. maritima as they are not inhibited by Cs+ and TEA+ (Demidchik and Tester, 2002; Table I shows that both Cs+ and TEA+ permeate the channel, so they do not block it and in T. halophila, neither TEA+ nor Cs+ block the voltage-independent current; V. Volkov and A. Amtmann, personal communication).
Pathway 1 of Low-Affinity Na+ Uptake
Over 48 h in 25 mm NaCl, the K+ channel blocker Ba2+ (5 mm) significantly decreased (by 47%; Table III) the net flux of Na+ by the roots of S. maritima, but two other K+ channel blockers, TEA+ (10 mm) and Cs+(3 mm), were without effect (Table II). 22Na+ influx measurements made over 2 min exhibited exactly the same characteristics: Ba2+ (1 mm) significantly reduced 22Na+ influx (by 36%; Fig. 8C), but TEA+ (1–10 mm) and Cs+ (0.5–10 mm) had no effect (Fig. 8, A and B). This finding is in agreement with previous reports about the influx of 22Na+ into the roots of Arabidopsis where neither TEA+ nor Cs+ had any inhibitory effect, but Ba2+ significantly reduced uptake in 50 mm NaCl (Essah et al., 2003). In the yeast (Saccharomyces cerevisiae) heterologous expression system and in Na+-depletion experiments, Garciadeblas et al. (2003) found that Ba2+ inhibited Na+ uptake mediated by OsHKT2;1 or OsHKT1;1 and completely inhibited Na+ uptake in rice roots, but TEA+ showed no effect under low external Na+ concentrations. EcHKT1;1 and EcHKT1;2 mediating K+ uptake were also strongly blocked by Ba2+ but less so by Cs+; TEA+ was ineffective (Fairbairn et al., 2000; Liu et al., 2001). Influx was also inhibited by K+ although the nature of the inhibition has yet to be fully resolved. The uptake of 22Na+ from a low external concentration (5 mm) measured over a period of 24 h had previously been shown to be inhibited by K+ (by 5%, 9%, and 44% in 1, 10, and 100 mm K+; Yeo, 1974 [The uptake of 42K from a 5 mm solution was also inhibited by the presence of Na, by 37%, 47%, and 67% in 1, 10, and 100 mm Na+.]). These results demonstrate that HKT possesses the pharmacological property of insensitivity to TEA+ and Cs+ and great sensitivity to Ba2+. This makes HKT a prime candidate for mediating the uptake of Na+ through pathway 1.
Pathway 2 of Low-Affinity Na+ Uptake
At a higher concentration of NaCl (150 mm), TEA+ (10 mm), Cs+ (3 mm), and Ba2+ (5 mm) significantly reduced net Na+ fluxes in S. maritima by 54%, 24%, and 29% (Tables II and III); after 48 h treatment, whole plant Na+ content was decreased by 61%, 25%, and 49%, respectively (Tables I and III); TEA+ (10 mm), Cs+ (3 mm), and Ba2+ (1 mm) significantly reduced Na+ influx by 47%, 30%, and 31% (Fig. 8). The Km for Na+ influx of 243 mm was some three times higher than that for pathway 1—80 mm estimated from influx rates between 2.5 and 75 mm NaCl. This result is in contrast to the situation in T. halophila, where TEA+ (20 mm) and Cs+ (5 mm) both increased rather than decreased the influx of Na+ from a solution of 100 mm NaCl (Wang et al., 2006). As mentioned above, HKT is insensitive to TEA+ and Cs+, so it could not be involved in Na+ absorption in S. maritima in conditions of high salinity. The inward-rectifying K+ channel AKT1 is sensitive to TEA+, Cs+, and Ba2+, and is involved in plant root K+ uptake from the soil solution (Maathuis et al., 1997; Hirsch et al., 1998). It also has been proposed that AKT1 could mediate a significant Na+ uptake with an increase in external Na+ concentrations (Amtmann and Sanders, 1999; Blumwald et al., 2000). This viewpoint was supported by the coincidence of OsAKT1 expression with Na+ accumulation in rice: In the relatively salt-tolerant rice varieties Pokkali and BK, OsAKT1 transcripts disappeared from the exodermis and endodermis in plants treated with 150 mm NaCl for 48 h but OsAKT1 transcription was not changed in these cells in the salt-sensitive variety IR29; at this time the plants of IR29 accumulated Na+ in leaf tissue to concentrations of 1,400 μmol per gram fresh weight; in contrast, Pokkali had accumulated 200 μmol/g Na+ in the leaves, and BK about 100 μmol/g Na+ (Golldack et al., 2003). However, cell specificity of the expression pattern of the OsAKT1 was identical in all tissues for the three rice lines irrespective of whether the plants were grown in 100 μm or 4 mm K+ (Golldack et al., 2003). Therefore, Golldack et al. (2003) concluded that OsAKT1 expression did not respond to external K+ concentrations and that Na+ accumulation or exclusion in the whole plant depended on the OsAKT1 expression in specific cells of plants exposed to 150 mm NaCl. Furthermore, plant AKT1-type K+ channels show homology to animal Shaker-type K+ channels, both at the sequence and structure levels (Very and Sentenac, 2003). Several studies have shown that substantial Na+ can permeate animal Shaker K+ channels at very positive potentials; this Na+ permeation is often most prominent in the C-type inactivated state of the channels (Lopez-Barneo et al., 1993; Callahan and Korn, 1994; Starkus et al., 1997, 1998, 2000; Kiss et al., 1998, 1999; Ogielska and Aldrich, 1998, 1999; Yellen, 1998; Wang et al., 2000). Taken together, the available data, including our study showing classical K+ channel blockers TEA +, Cs+, or Ba2+ strongly inhibit root Na+ uptake, indicate involvement of AKT1-type channel in the absorption of Na+ at high external salt concentration.
Alleviation by Na+ of the Effects of K+ Channel Blockers on Tissue Water Relations
Our data show that TEA+, Cs+, and Ba2+ significantly reduced leaf water content (TEA+ by 95%, Cs+ by 23%, and Ba2+ by 42% in 25 mm NaCl; and by 40%, 31%, and 25%, respectively, in 150 mm NaCl for 48 h; Figs. 1, 3, and 5). This suggests that the three typical K+ channels blockers either reduced the hydraulic conductivity of S. maritima and cause wilting of the leaves or caused an increase in the ion concentrations in the leaf cell walls, a process that reduces leaf turgor (Clipson et al., 1985). Our results show that under TEA+ and Ba2+ treatments, with increasing external NaCl concentrations, the leaf Na+ concentrations in S. maritima increased (Figs. 6 and 10), improving leaf water status (Figs. 5 and 9) and alleviating wilting. Perhaps at the low external Na+ concentration, Na+ and/or K+ uptake is blocked at the leaf plasma membrane, leaving these ions in the cell walls and reducing turgor. At higher external sodium concentrations, the higher vacuolar ion concentrations alter the balance between cell walls and the protoplast: Such changes are not reflected in tissue ion concentrations owing to the small capacity of the cell wall fraction. However, it has been demonstrated that TEA+ blocks the water permeability through some aquaporin channels expressed in Xenopus oocytes (Brooks et al., 2000; Detmers et al., 2006), so this effect may also influence the movement of water into the leaf cells.
CONCLUSION
Our results provide clear evidence for differences in the characteristics of Na+ uptake with increasing external concentration. It is too early to speculate why different pathways might have evolved, as little is known of how Na+ uptake is effected in other halophytes. S. maritima represents an extreme in terms of Na/K selectivity, where the supply of Na+ ions appears obligately coupled to growth rate (Yeo and Flowers, 1986), where the ability to maintain sodium uptake for growth and osmotic adjustment at high external salt concentrations is essential for survival in seawater and on drying salt marshes. A comparison with other members of the Chenopodiaceae and the Poaceae should be informative.
MATERIALS AND METHODS
Plant Materials and Treatments
Seeds of Suaeda maritima originating from Cuckmere Haven in East Sussex, UK were germinated at 25°C on filter paper wetted with sterile water. After about 6 d, seedlings were transplanted to sand irrigated with modified Hoagland nutrient solution containing 6 mm KNO3, 1 mm NH4H2PO4, 0.5 mm MgSO4, 0.5 mm Ca(NO3)2, 60 μm Fe citrate, 92 μm H3BO3, 18 μm MnCl2, 1.6 μm ZnSO4, 0.6 μm CuSO4, and 0.7 μm (NH4)6Mo7O24. Solutions were changed every 3 d. After 18 d in sand culture, the plants were transferred to beakers containing 70 mL of the same modified Hoagland solution and left for 3 d to acclimatize before being used in experiments: Each beaker contained three plants. The solution was changed everyday, but was not aerated; separate measurements showed that the oxygen concentrations in these beakers was approximately 4.4 mg/L and changed little in 2 d. The plants were grown in a room where the temperature ranged from 23°C to 28°C and the relative humidity averaged 65%/75% (day/night); the daily photoperiod was 16/8 h (light/dark and the light flux density during the light period was between 220 and 240 μmol m−2 s−1).
Seedlings, which were 21 to 23 d old (with shoots that were about 6 cm in height with young lateral branches) were used to evaluate the effect of inhibitors of ion transport on growth and ion accumulation. The choice of concentrations of inhibitors was based on previous studies: 500 μm 8-bromo-cAMP (Maathuis and Sanders, 2001), 10 mm CaCl2 (Demidchik and Tester, 2002), 10 mm LiCl (Rubio et al., 2004), 10 mm TEA-Cl (Essah et al., 2003), 3 mm CsCl (Hampton et al., 2004), and 5 mm BaCl2 (Garciadeblas et al., 2003). Each treatment, which lasted between 48 and 144 h (see figure and table legends or the text for details) under the conditions described above, had eight replicates with three plants per beaker. Where TEA-Cl, CsCl, or BaCl2 was included in the treatment, the relative humidity of the growth chamber (Sanyo MLR-350HT growth cabinet; Sanyo Electrical and Biomedical; photosynthetically active radiation of 200 μmol m−2 s−1) was maintained at 100% to minimize the effect of transpiration on Na+ accumulation (compare with Nublat et al., 2001; Shi et al., 2002), since these three inhibitors caused plants to wilt. For treatments with BaCl2, MgCl2 (0.5 mm) replaced MgSO4 (0.5 mm) in the Hoagland nutrient solution.
Growth Measurements, and Na+ and K+ Concentration Determination
At the end of the treatments, plant roots were washed twice for 8 min in ice-cold 20 mm CaCl2 to exchange cell wall-bound Na+ and the shoots rinsed in deionized water to remove surface salts. Root, stem, and leaf were separated and blotted; fresh weights were determined immediately and samples oven dried at 70°C for 3 d to obtain dry weights. Tissue water content was calculated from the difference between fresh and dry weights. Na+ and K+ were extracted from dried plant tissue in 100 mm acetic acid at 90°C for 2 h and ion analysis was performed using an atomic absorption spectrophotometer (Unicam SP919).
22Na+ Influx Experiments
S. maritima seedlings were grown in Hoagland nutrient solution [except for the experiment using Ca2+ as an inhibitor where the external Ca(NO3)2 concentration was reduced to 0.1 mm] in sand for 14 d and in beakers (without sand) for 3 d. They were subsequently transferred to Hoagland solution supplemented with 25 mm NaCl or 150 mm NaCl for 4 d. The seedlings were then used to evaluate 22Na+ influx according to the method described by Essah et al. (2003). Briefly, roots with 4 cm of attached main stem were excised and pretreated for 10 min in nutrient solution (except for the experiment of Ba2+ inhibitor with 0.5 mm MgSO4 replaced by 0.5 mm MgCl2) containing inhibitors and 25 mm or 150 mm unlabeled NaCl, with a change of solution after 5 min, and then transferred to above corresponding solution (10 mL) labeled with 185 to 370 kBq L−1 of 22Na+ as uptake solution. After 2 min, roots were removed from the uptake solution, blotted, and transferred to 200 mL of ice-cold NaCl (25 or 150 mm plus 20 mm CaCl2) for two successive rinses of 2 min and then a further rinse of 3 min. Finally, the roots were blotted gently, weighed, and transferred to glass vials containing 2.5 mL of Optiphase Hisafe (Fisher Chemicals) and Na uptake was determined using a scintillation counter (Wallac Rackbeta 1217, LKB Wallac).
Statistical Analysis
Results of growth, ion concentration, water status, and 22Na+ influx rate of plants are presented as means with sds. Statistical analyses, one-way ANOVA, and Duncan's multiple range test were performed using software (SPSS).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Whole plant Na+ and K+ contents in S. maritima seedlings treated with NaCl, cAMP, Li+, or Ca2+.
Supplemental Figure S2. Na+ and K+ concentrations in S. maritima seedlings treated with NaCl, cAMP, Li+, or Ca2+.
Supplemental Figure S3. Double reciprocal plots showing the effects of KCl concentration on root Na+ influx of S. maritima seedlings at different NaCl concentrations.
Supplemental Figure S4. Root Na+ influx of S. maritima seedlings treated with Li+ and Ca2+.
Supplemental Figure S5. K+ concentrations in S. maritima seedlings treated with 10 mm TEA+ and different concentrations of NaCl.
Supplementary Material
Acknowledgments
The authors thank Dr. M. Tester, Dr. R.J. Davenport, Dr. P.J. White, and Dr. F.J.M. Maathuis for helpful discussion, especially Dr. R.J. Davenport for technical guidance on the tracer flux experiments and Dr. Xing-You Gu for support of our work. The authors are grateful to Professor A. Rodriguez-Navarro, Dr. A. Amtmann, and Dr. R.J. Davenport for critically reading the manuscript. S.-M. Wang and J.-L. Zhang acknowledge the support of the Royal Society of London; J.-L. Zhang would like to thank Anne Wetson and Zhan Li for their help with the 22Na+ influx experiments. We also thank anonymous reviewers for their constructive suggestions on the manuscript
This work was supported by the National Natural Science Foundation of China (grant nos. 30671488 and J0630962), the National High Tech Project of China (grant no. 2006AA10Z126), a Sino-British Fellowship Trust Award, and an International Joint Project from the Royal Society of the United Kingdom.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Timothy J. Flowers (t.j.flowers@sussex.ac.uk).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Adams P, Nelson DE, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H (1998) Growth and development of Mesembryanthemum crystallinum (Aizoaceae). New Phytol 138 171–190 [DOI] [PubMed] [Google Scholar]
- Amtmann A, Fischer M, Marsh EL, Stefanovic A, Sanders D, Schachtman DP (2001) The wheat cDNA LCT1 generates hypersensitivity to sodium in a salt-sensitive yeast strain. Plant Physiol 126 1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann A, Sanders D (1999) Mechanisms of Na+ uptake by plant cells. In J Callow, ed, Advances in Botanical Research, Vol 29. Academic Press, San Diego, pp 75–112
- Antosiewicz DM, Hennig J (2004) Overexpression of LCT1 in tobacco enhances the protective action of calcium against cadmium toxicity. Environ Pollut 129 237–245 [DOI] [PubMed] [Google Scholar]
- Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285 1256–1258 [DOI] [PubMed] [Google Scholar]
- Bañuelos MA, Madrid R, Rodríguez-Navarro A (2000) Individual functions of the HAK and TRK potassium transporters of Schwanniomyces occidentalis. Mol Microbiol 37 671–679 [DOI] [PubMed] [Google Scholar]
- Berthomieu P, Conejero G, Nublat A, Brackenbury WJ, Lambert C, Savio C, Uozumi N, Oiki S, Yamada K, Cellier F, et al (2003) Functional analysis of AtHKT1 in Arabidopsis shows that Na+ recirculation by the phloem is crucial for salt tolerance. EMBO J 22 2004–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E, Aharon GS, Apse MP (2000) Sodium transport in plant cells. Biochim Biophys Acta 1465 140–151 [DOI] [PubMed] [Google Scholar]
- Brooks HL, Regan JW, Yool AJ (2000) Inhibition of aquaporin-1 water permeability by tetraethylammonium: involvement of the loop E pore region. Mol Pharmacol 57 1021–1026 [PubMed] [Google Scholar]
- Byrt CS, Platten JD, Spielmeyer W, James RA, Lagudah ES, Dennis ES, Tester M, Munns R (2007) HKT1;5-like cation transporters linked to Na+ exclusion loci in wheat, Nax2 and Kna1. Plant Physiol 143 1918–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MJ, Korn SJ (1994) Permeation of Na+ through a delayed rectifier K+ channel in chick dorsal root ganglion neurons. J Gen Physiol 104 747–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI (1998) The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc Natl Acad Sci USA 95 12043–12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipson NJW (1984) Salt tolerance in Suaeda maritima L. Dum. PhD thesis. University of Sussex, Falmer, Brighton, UK
- Clipson NJW (1987) Salt tolerance in the halophyte Suaeda maritima L. Dum. growth, ion and water relations and gas exchange in response to altered salinity. J Exp Bot 38 1996–2004 [Google Scholar]
- Clipson NJW, Tomos AP, Flowers TJ, Wyn Jones RG (1985) Salt tolerance in the halophyte Suaeda maritima (L.) Dum.: the maintenance of turgor pressure and water potential gradients in plants growing at different salinities. Planta 165 392–396 [DOI] [PubMed] [Google Scholar]
- Davenport R, James RA, Zakrisson-Plogander A, Tester M, Munns R (2005) Control of sodium transport in durum wheat. Plant Physiol 137 807–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport RJ, Munoz-Mayor A, Jha D, Essah PA, Rus A, Tester M (2007) The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ 30 497–507 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Davenport RJ, Tester M (2002) Nonselective cation channels in plants. Annu Rev Plant Biol 53 67–107 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Tester M (2002) Sodium fluxes through nonselective cation channels in the plasma membrane of protoplasts from Arabidopsis roots. Plant Physiol 128 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmers FJM, De Groot BL, Muller EM, Hinton A, Konings IBM, Sze M, Flitsch SL, Grubmuller H, Deen PMT (2006) Quaternary ammonium compounds as water channel blockers—specificity, potency, and site of action. J Biol Chem 281 14207–14214 [DOI] [PubMed] [Google Scholar]
- Essah PA, Davenport R, Tester M (2003) Sodium influx and accumulation in Arabidopsis. Plant Physiol 133 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbairn DJ, Liu WH, Schachtman DP, Gomez-Gallego S, Day SR, Teasdale RD (2000) Characterisation of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis. Plant Mol Biol 43 515–525 [DOI] [PubMed] [Google Scholar]
- Flowers TJ (1972) Salt tolerance in the halophyte Suaeda maritima L. Dum: the effect of sodium chloride on growth, respiration, and soluble enzymes in a comparative study with Pisum sativum L. J Exp Bot 23 310–321 [Google Scholar]
- Flowers TJ, Hajibagheri MA, Clipson NJW (1986) Halophytes. Q Rev Biol 61 313–337 [Google Scholar]
- Flowers TJ, Troke PF, Yeo AR (1977) The mechanism of salt tolerance in halophytes. Annu Rev Plant Physiol 28 89–121 [Google Scholar]
- Fu H-H, Luan S (1998) AtKUP1: a dual affinity K+ transporter from Arabidopsis. Plant Cell 10 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda A, Nakamura A, Tanaka Y (1999) Molecular cloning and expression of the Na+/H+ exchanger gene in Oryza sativa. Biochim Biophys Acta 1446 149–155 [DOI] [PubMed] [Google Scholar]
- Garciadeblas B, Senn ME, Banuelos MA, Rodriguez-Navarro A (2003) Sodium transport and HKT transporters: the rice model. Plant J 34 788–801 [DOI] [PubMed] [Google Scholar]
- Gassmann W, Rubio F, Schroeder JI (1996) Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J 10 869–882 [DOI] [PubMed] [Google Scholar]
- Gaxiola RA, Fink GR, Hirschi KD (2002) Genetic manipulation of vacuolar proton pumps and transporters. Plant Physiol 129 967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaxiola RA, Rao R, Sherman A, Grisafi P, Alper SL, Fink GR (1999) The Arabidopsis thaliana proton transporters, AtNHX1 and AVP1, can function in cation detoxification in yeast. Proc Natl Acad Sci USA 96 1480–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golldack D, Quigley F, Michalowski CB, Kamasani UR, Bohnert HJ (2003) Salinity stress-tolerant and -sensitive rice (Oryza sativa L.) regulate AKT1-type potassium channel transcripts differently. Plant Mol Biol 51 71–81 [DOI] [PubMed] [Google Scholar]
- Greenway H, Munns R (1980) Mechanisms of salt tolerance in nonhalophytes. Annu Rev Plant Physiol 31 149–190 [Google Scholar]
- Hajibagheri MA, Hall JL, Flowers TJ (1984) Stereological analysis of leaf cells of the halophyte Suaeda maritima (L.) Dum. J Exp Bot 35 1547–1557 [Google Scholar]
- Hamada A, Shono M, Xia T, Ohta M, Hayashi Y, Tanaka A, Hayakawa T (2001) Isolation and characterization of a Na+/H+ antiporter gene from the halophyte Atriplex gmelini. Plant Mol Biol 46 35–42 [DOI] [PubMed] [Google Scholar]
- Hampton CR, Bowen HC, Broadley MR, Hammond JP, Mead A, Payne KA, Pritchard J, White PJ (2004) Cesium toxicity in Arabidopsis. Plant Physiol 136 3824–3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro R, Bañuelos MA, Senn ME, Barrero-Gil J, Rodríguez-Navarro A (2005) HKT1 mediates sodium uniport in roots: pitfalls in the expression of HKT1 in yeast. Plant Physiol 139 1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey HW (1966) The Chemistry and Fertility of Sea Waters. The University Press, Cambridge, UK, p 240
- Hedrich R, Schroeder JI (1989) The physiology of ion channels and electrogenic pumps in higher plants. Annu Rev Plant Physiol Plant Mol Biol 40 539–569 [Google Scholar]
- Hirsch H, Lewis B, Spalding E, Sussman M (1998) A role for the AKT1 potassium channel in plant nutrition. Science 280 918–921 [DOI] [PubMed] [Google Scholar]
- Hirschi KD (2004) The calcium conundrum: both versatile nutrient and specific signal. Plant Physiol 136 2438–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Costa A, Kim TH, Han MJ, Horie R, Leung H-Y, Miyai A, Hirochika H, An G, Schroedr JI (2007) Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J 26 3003–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie T, Yoshida K, Nakayama H, Yamada K, Oiki S, Shinmyo A (2001) Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J 27 129–138 [DOI] [PubMed] [Google Scholar]
- Huang S, Spielmeyer W, Lagudah ES, James RA, Platten JD, Dennis ES, Munns R (2006) A sodium transporter (HKT7) is a candidate for Nax1, a gene for salt tolerance in durum wheat. Plant Physiol 142 1718–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inan G, Zhang Q, Li PH, Wang ZL, Cao ZY, Zhang H, Zhang CQ, Quist TM, Goodwin SM, Zhu JH, et al (2004) Salt cress. A halophyte and cryophyte Arabidopsis relative model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol 135 1718–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RA, Davenport RJ, Munns R (2006) Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiol 142 1537–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly WB, Esser JE, Schroeder JI (1995) Effects of cytosolic calcium and limited, possible dual, effects of G-protein modulators on guard-cell inward potassium channels. Plant J 8 479–489 [Google Scholar]
- Khan MA, Ungar IA, Showalter AM (2000) The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. J Arid Environ 45 73–84 [Google Scholar]
- Kiss L, Immke D, LoTurco J, Korn S (1998) The interaction of Na+ and K+ in voltage-gated potassium channels: evidence for cation binding sites of different affinity. J Gen Physiol 111 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss L, LoTurco J, Korn S (1999) Contribution of the selectivity filter to inactivation in potassium channels. Biophys J 76 253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuchli A (1984) Salt exclusion: an adaptation of legumes for crops and pastures under saline conditions. In RC Staples, ed, Salinity Tolerance in Plants: Strategies for Crop Improvement. Wiley, New York, pp 171–187
- Laurie S, Feeney KA, Maathuis FJM, Heard PJ, Brown SJ, Leigh RA (2002) A role for HKT1 in sodium uptake by wheat roots. Plant J 32 139–149 [DOI] [PubMed] [Google Scholar]
- Liu WH, Fairbairn DJ, Reid RJ, Schachtman DP (2001) Characterization of two HKT1 homologues from Eucalyptus camaldulensis that display intrinsic osmosensing capability. Plant Physiol 127 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Hoshi T, Heinemann S, Aldrich R (1993) Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels 1 61–71 [PubMed] [Google Scholar]
- Lu C, Qiu N, Wang B, Zhang J (2003) Salinity treatment shows no effects on photosystem II photochemistry, but increases the resistance of photosystem II to heat stress in halophyte Suaeda salsa. J Exp Bot 54 851–860 [DOI] [PubMed] [Google Scholar]
- Ma XL, Zhang Q, Shi HZ, Zhu JK, Zhao YX, Ma CL, Zhang H (2004) Molecular cloning and different expression of a vacuolar Na+/H+ antiporter gene in Suaeda salsa under salt stress. Biol Plant 48 219–225 [Google Scholar]
- Maathuis FJM, Ichida AM, Sanders D, Schroeder JI (1997) Roles of higher plant K+ channels. Plant Physiol 114 1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D (2001) Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol 127 1617–1625 [PMC free article] [PubMed] [Google Scholar]
- Madrid R, Gómez MJ, Ramos J, Rodríguez-Navarro A (1998) Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J Biol Chem 273 14838–14844 [DOI] [PubMed] [Google Scholar]
- Martínez-Atienza J, Jiang X, Garciadeblas B, Mendoza I, Zhu JK, Pardo JM, Quintero FJ (2007) Conservation of the SOS salt tolerance pathway in rice. Plant Physiol 143 1001–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25 239–250 [DOI] [PubMed] [Google Scholar]
- Nublat A, Desplans J, Casse F, Berthomieu P (2001) sas1, an Arabidopsis mutant overaccumulating sodium in the shoot, shows deficiency in the control of the root radial transport of sodium. Plant Cell 13 125–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogielska E, Aldrich R (1998) A mutation in S6 of Shaker potassium channels decreases the K+ affinity of an ion binding site revealing ion-ion interactions in the pore. J Gen Physiol 112 243–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogielska E, Aldrich R (1999) Functional consequences of a decreased potassium affinity in a potassium channel pore ion interactions and C-type inactivation. J Gen Physiol 113 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo JM, Cubero B, Leidi EO, Quintero FJ (2006) Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot 57 1181–1199 [DOI] [PubMed] [Google Scholar]
- Platten JD, Cotsaftis O, Berthomieu P, Bohnert H, Davenport RJ, Fairbairn DJ, Horie T, Leigh RA, Lin HX, Luan S, et al (2006) Nomenclature for HKT transporters, key determinants of plant salinity tolerance. Trends Plant Sci 11 372–374 [DOI] [PubMed] [Google Scholar]
- Reimann C (1992) Sodium exclusion by Chenopodium species. J Exp Bot 43 503–510 [Google Scholar]
- Reimann C, Breckle SW (1993) Sodium relations in Chenopodiaceae—a comparative approach. Plant Cell Environ 16 323–328 [Google Scholar]
- Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HX (2005) A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet 37 1141–1146 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro A, Rubio F (2006) High-affinity potassium and sodium transport systems in plants. J Exp Bot 57 1149–1160 [DOI] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI (1995) Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270 1660–1663 [DOI] [PubMed] [Google Scholar]
- Rubio L, Rosado A, Linares-Rueda A, Borsani O, Garcia-Sanchez MJ, Valpuesta V, Fernandez JA, Botella MA (2004) Regulation of K+ transport in tomato roots by the TSS1 locus: implications in salt tolerance. Plant Physiol 134 452–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rus A, Yokoi S, Sharkhuu A, Reddy M, Lee BH, Matsumoto TK, Koiwa H, Zhu JK, Bressan RA, Hasegawa PM (2001) AtHKT1 is a salt tolerance determinant that controls Na+ entry into plant roots. Proc Natl Acad Sci USA 98 14150–14155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santa-María GE, Rubio F, Dubcovsky J, Rodríguez-Navarro A (1997) The HAK1 gene of barley is a member of a large gene family and encodes a high affinity potassium transporter. Plant Cell 9 2281–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Kumar R, Schroeder JI, Marsh EL (1997) Molecular and functional characterization of a novel low-affinity cation transporter (LCT1) in higher plants. Proc Natl Acad Sci USA 94 11079–11084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Liu W (1999) Molecular pieces to the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci 4 281–287 [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI (1994) Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature 370 655–658 [DOI] [PubMed] [Google Scholar]
- Serrano R, Mulet JM, Rois G, Marquez JA, de Larrinoa IF, Leube MP, Mendizabal I, Pascual-Ahuir A, Proft M, Ros R, et al (1999) A glimpse of the mechanisms of ion homeostasis during salt stress. J Exp Bot 50 1023–1036 [Google Scholar]
- Shi H, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi HZ, Lee BH, Wu SJ, Zhu JK (2003) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21 81–85 [DOI] [PubMed] [Google Scholar]
- Starkus J, Heinemann S, Rayner M (2000) Voltage dependence of slow inactivation in Shaker potassium channels results from changes in relative K+ and Na+ permeabilities. J Gen Physiol 115 107–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J, Kuschel L, Rayner M, Heinemann S (1997) Ion conduction through C-type inactivated Shaker channels. J Gen Physiol 110 539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J, Kuschel L, Rayner M, Heinemann S (1998) Macroscopic Na+ currents in the “Nonconducting” Shaker potassium channel mutant W434F. J Gen Physiol 112 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H, Golldack D, Zhao CS, Bohnert HJ (2002) The expression of HAK-type K+ transporters is regulated in response to salinity stress in common ice plant. Plant Physiol 129 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunarpi, Horie T, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan WY, Leung HY, Hattori K, et al (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J 44 928–938 [DOI] [PubMed] [Google Scholar]
- Sze H, Li X, Palmgren MG (1999) Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 11 677–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H, Schumacher K, Muller ML, Padmanaban S, Taiz L (2002) A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+-ATPase. Trends Plant Sci 7 157–161 [DOI] [PubMed] [Google Scholar]
- Taji T, Seki M, Satou M, Sakurai T, Kobayashi M, Ishiyama K, Narusaka Y, Narusaka M, Zhu JK, Shinozaki K (2004) Comparative genomics in salt tolerance between Arabidopsis and Arabidopsis-related halophyte salt cress using Arabidopsis microarray. Plant Physiol 135 1697–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tester M (1988) Blockade of potassium channels in the plasmalemma of Chara corallina by tetraethylammonium Ba2+, Na+ and Cs+. J Membr Biol 105 77–85 [Google Scholar]
- Tester M (1990) Plant ion channels: whole-cell and single-channel studies. New Phytol 114 305–340 [DOI] [PubMed] [Google Scholar]
- Tyerman SD (2002) Nonselective cation channels: multiple functions and commonalities. Plant Physiol 128 327–328 [Google Scholar]
- Tyerman SD, Skerrett IM (1999) Root ion channels and salinity. Sci Hortic (Amsterdam) 78 175–235 [Google Scholar]
- Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder JI (2000) The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol 122 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Very AA, Sentenac H (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54 575–603 [DOI] [PubMed] [Google Scholar]
- Volkov V, Wang B, Dominy PJ, Fricke W, Amtmann A (2003) Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana, possesses effective mechanisms to discriminate between potassium and sodium. Plant Cell Environ 27 1–14 [Google Scholar]
- Wang B, Davenport RJ, Volkov V, Amtmann A (2006) Low unidirectional sodium influx into root cells restricts net sodium accumulation in Thellungiella halophila, a salt-tolerant relative of Arabidopsis thaliana. J Exp Bot 57 1161–1170 [DOI] [PubMed] [Google Scholar]
- Wang SM, Zhao GQ, Gao YS, Tang ZC, Zhang CL (2004) Puccinellia tenuiflora exhibits stronger selectivity for K+ over Na+ than wheat. J Plant Nutr 27 1841–1857 [Google Scholar]
- Wang SM, Zheng WJ, Ren JZ, Zhang CL (2002) Selectivity of various types of salt-resistant plants for K+ over Na+. J Arid Environ 52 457–472 [Google Scholar]
- Wang Z, Hesketh J, Fedida D (2000) A high-Na+ conduction state during recovery from inactivation in the K+ channel Kv1.5. Biophys Chem 79 2416–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G (1998) The moving parts of voltage-gated ion channels. Q Rev Biophys 31 239–295 [DOI] [PubMed] [Google Scholar]
- Yeo AR (1974) Salt tolerance in the halophyte Suaeda maritima (L.) Dum. PhD thesis. University of Sussex, Falmer, Brighton, UK
- Yeo AR (1981) Salt tolerance in the halophyte Suaeda maritima (L.) Dum.: intracellular compartmentation of ions. J Exp Bot 32 487–497 [Google Scholar]
- Yeo AR, Flowers TJ (1980) Salt tolerance in the halophyte Suaeda maritima (L.) Dum.: evaluation of the effect of salinity upon growth. J Exp Bot 31 1171–1183 [Google Scholar]
- Yeo AR, Flowers TJ (1986) Ion transport in Suaeda maritima: its relation to growth and implications for the pathway of radial transport of ions across the root. J Exp Bot 37 143–159 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.