Abstract
Nitrogen fixation (NF) in soybean (Glycine max L. Merr.) is highly sensitive to soil drying. This sensitivity has been related to an accumulation of nitrogen compounds, either in shoots or in nodules, and a nodular carbon flux shortage under drought. To assess the relative importance of carbon and nitrogen status on NF regulation, the responses to the early stages of drought were monitored with two soybean cultivars with known contrasting tolerance to drought. In the sensitive cultivar (‘Biloxi’), NF inhibition occurred earlier and was more dramatic than in the tolerant cultivar (‘Jackson’). The carbon flux to bacteroids was also more affected in ‘Biloxi’ than in ‘Jackson’, due to an earlier inhibition of sucrose synthase activity and a larger decrease of malate concentration in the former. Drought provoked ureide accumulation in nodules of both cultivars, but this accumulation was higher and occurred earlier in ‘Biloxi’. However, at this early stage of drought, there was no accumulation of ureides in the leaves of either cultivar. These results indicate that a combination of both reduced carbon flux and nitrogen accumulation in nodules, but not in shoots, is involved in the inhibition of NF in soybean under early drought.
Drought-related inhibition of nitrogen fixation (NF) seriously limits legume yield in many arid and semiarid regions of the world. Three major factors have been proposed to be involved in drought effects on NF: oxygen limitation, carbon shortage, and regulation by nitrogen metabolism. The role of oxygen limitation in the response of nitrogenase activity to drought has been discussed extensively (Díaz del Castillo and Layzell, 1995; Serraj and Sinclair, 1996a; Minchin, 1997). However, while it is clear that drought causes an increase in nodular oxygen diffusion resistance (Durand et al., 1987), it is unlikely that such changes are the only cause of the decline in NF since the inhibition of NF by drought cannot be reverted by simply increasing the O2 concentration around the rhizosphere (Díaz del Castillo et al., 1994).
An alternative explanation for the decrease in NF under drought is a reduced carbon supply to bacteroids (Arrese-Igor et al., 1999). The main carbon source transported from shoots into nodules is Suc, which may be hydrolyzed by either Suc synthase (SS) or alkaline invertase (AI), and studies with rug4 mutants of pea (Pisum sativum) have shown that SS activity is essential for nodule functioning (Gordon et al., 1999). SS has been shown to be the first nodule enzyme activity that declines under drought in both tropical legumes, such as soybean (Glycine max L. Merr.; González et al., 1995), and temperate legumes, such as pea (González et al., 1998; Gálvez et al., 2005). The decrease in SS activity results in an accumulation of Suc and a reduced concentration of organic acids, mainly malate, in pea nodules (Gálvez et al., 2005), causing a shortage of substrates for bacteroid respiration. As a consequence, a transient accumulation of oxygen in the infected region would take place, leading to an increase in the resistance of the oxygen diffusion barrier in order to avoid nitrogenase damage. Both the depletion of respiratory substrates and consequent closure of the oxygen diffusion barrier would cause the observed decline in NF (González et al., 2001). However, this major role of SS for nodule functioning and the response to drought has been recently challenged for the pasture legumes Lotus japonicus (Horst et al., 2007) and alfalfa (Medicago sativa; Naya et al., 2007).
Nitrogen metabolism has also been proposed to play a role in the regulation of NF under drought conditions by a nitrogen feedback mechanism involving shoot nitrogen status, with several molecules suggested to be involved in such a mechanism (see King and Purcell, 2005, and refs. therein). Moreover, it has been reported that NF in ureide-exporter legumes is a more drought-sensitive process than in amide-exporter ones (Sinclair and Serraj, 1995). The ureides allantoin and allantoate are the main nitrogenous compounds exported from soybean nodules to shoots (Herridge, 1982), where they are metabolized. An accumulation of ureides under drought in both shoots (Serraj and Sinclair, 1996b; Serraj et al., 1999b) and nodules (Serraj et al., 1999b; Vadez et al., 2000) has been reported. Serraj et al. (2001) suggested that ureide inhibition of nodular activity could occur as either a direct feedback within the nodule or an indirect feedback originated from shoots. The latter has been suggested to depend on ureide catabolism pathways (Sinclair et al., 2003). Allantoin is first metabolized into allantoate by allantoinase, and the subsequent step of allantoate degradation in soybean has been attributed to two enzymatic pathways: allantoate amidinohydrolase (Shelp and Ireland, 1985) and allantoate amidohydrolase (Winkler et al., 1985), which requires manganese as a cofactor (Lukaszewski et al., 1992). Moreover, supply of external ureides increased ureide concentration in leaves and inhibited nitrogenase activity (Serraj et al., 1999b; Vadez et al., 2000). Also, Vadez and Sinclair (2001) reported an inverse relationship between shoot ureide concentrations in well-watered plants and the drought sensitivity of NF among nine soybean cultivars, supporting the role of elevated shoot ureides in NF inhibition, although there was no strict correlation between drought sensitivity itself and shoot ureide concentration under drought conditions.
In this study two soybean genotypes, which show different drought sensitivity, have been analyzed. ‘Jackson’ was identified as having substantial drought tolerance (Sall and Sinclair, 1991), and, subsequently, its NF performance under drought was confirmed under both controlled (Serraj and Sinclair, 1996b; Purcell et al., 1997) and field conditions (Serraj et al., 1997). This drought tolerance was associated with low concentrations of ureides in shoots under drought (Serraj and Sinclair, 1997; Serraj et al., 1997). ‘Biloxi’ is sensitive to soil drying (Serraj and Sinclair, 1996b) and under drought there is a higher accumulation of ureides in shoots compared to ‘Jackson’ (Serraj and Sinclair, 1996b; Purcell et al., 1998). This differential accumulation of ureides has been associated with distinct ureide catabolism pathways in leaves (Vadez and Sinclair, 2002), supporting the idea of a systemic regulation of NF. However, Todd and Polacco (2004) have shown that soybean cultivars with contrasting tolerance to drought, such as ‘Maple Arrow’ and ‘Williams’, use the same enzymatic pathway for allantoate degradation in shoots. Moreover, King and Purcell (2005) recently showed that ureide catabolism in ‘Jackson’ is not strictly manganese independent, as earlier proposed. Furthermore, so far there is no available information about nodule carbon fluxes in soybean cultivars showing contrasting tolerance to drought and how these carbon fluxes are correlated with the nitrogen metabolism response at the nodule level. The aim of this study is to characterize the carbon and nitrogen nodule metabolism of these two soybean cultivars at early drought stages to ascertain the relevance of nodule carbon metabolism in NF tolerance to drought.
RESULTS
Drought Effects on Nodule Water Status, Plant Biomass, and NF
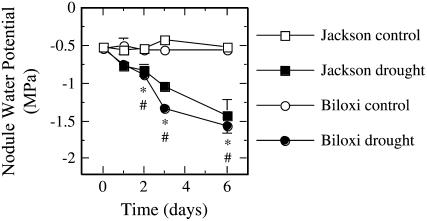
Plants of both cultivars irrigated at field capacity maintained a similar nodule water potential of −0.50 to −0.60 MPa throughout the study period. However, drought provoked a gradual and progressive decline in the nodule water potential of both cultivars (Fig. 1), with stressed plants showing significant differences from their respective controls 2 d after starting the stress treatment. At the end of the study period, stressed plants of both cultivars reached nodule water potential values of approximately −1.5 MPa (Fig. 1). Plant biomass was similar for both cultivars, with values around 8 g dry weight (DW) plant−1 at the beginning of the experimental period. The mild drought conditions reached in this study did not provoke significant biomass differences between control and stressed plants in either of the cultivars (data not shown).
Figure 1.
Effects of drought on nodule water potential of ‘Jackson’ and ‘Biloxi’ soybean plants. For ‘Jackson’ an asterisk (*) and for ‘Biloxi’ a hash (#) represent significant differences with the corresponding control values at P ≤ 0.05. Values represent mean ± se (n = 4).
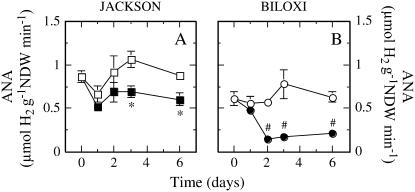
Specific NF rates, measured as the apparent release of H2 (apparent nitrogenase activity [ANA]) on a nodule biomass basis, were slightly lower in ‘Biloxi’ than in ‘Jackson’ under unrestricted water availability. In ‘Jackson’, drought provoked a 30% decrease of ANA 3 d after the onset of drought, maintaining this level of activity throughout the study period (Fig. 2A). In contrast, ‘Biloxi’ showed a 75% ANA decrease within 2 d of the onset of drought (Fig. 2B). The protein content of the nodule plant fraction showed similar values for both cultivars, with no differences between well-watered and stressed plants in either of them (data not shown). This suggests that drought level was not sufficient to trigger major, irreversible changes in nodule status.
Figure 2.
Effects of drought on ANA of ‘Jackson’ (A) and ‘Biloxi’ (B). Legends and statistical analysis are as described in the Figure 1 legend. Values represent mean ± se (n = 4).
Drought Effects on Ureide Content of Leaves and Nodules
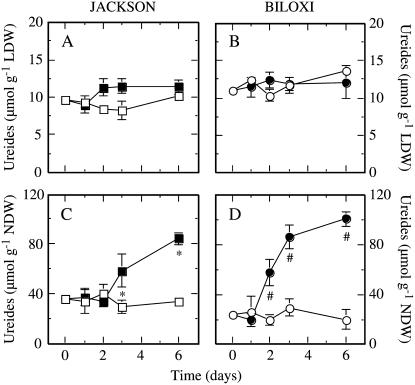
In ‘Jackson’ the ureide content in leaves was around 9 μmol g−1 DW in control plants (Fig. 3A), which was lower than the level in ‘Biloxi’ (12 μmol g−1 DW; Fig. 3B). Drought did not significantly modify shoot ureide levels in either cultivar throughout the study period (Fig. 3, A and B). The nodular ureide content in well-watered ‘Jackson’ plants was around 35 μmol g−1 nodule DW (NDW) and was higher than that in ‘Biloxi’ nodules (24 μmol g−1 NDW). Drought caused a 2-fold accumulation of ureides in ‘Jackson’ nodules after 3 d of treatment that reached a 2.5-fold accumulation at the end of the study (Fig. 3C). In contrast, ‘Biloxi’ nodules showed a 2-fold ureide accumulation 2 d after the onset of drought that increased to a 5-fold accumulation at the end of the drought period (Fig. 3D).
Figure 3.
Effects of drought on ureides levels (allantoin and allantoic acid) of ‘Jackson’ (A) and ‘Biloxi’ (B) leaves and ‘Jackson’ (C) and ‘Biloxi’ (D) nodules. Legends and statistical analysis are as described in the Figure 1 legend. LDW, Leaf DW. Values represent mean ± se (n = 4).
Drought Effects on Nodular Enzyme Activities and Organic Acid Content
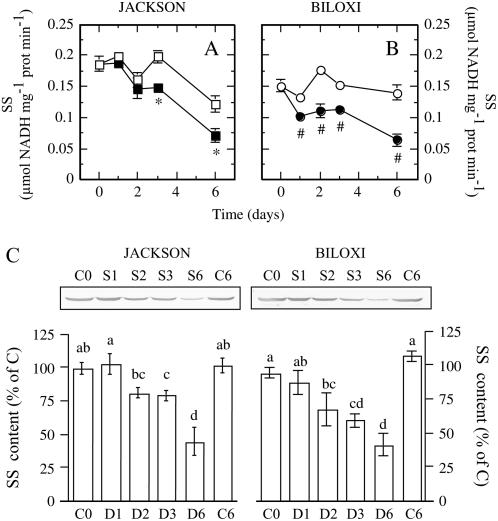
Regarding the enzyme activities monitored, the most immediate and marked response to drought was that observed for SS (Fig. 4, A and B). Under unlimited water supply, nodule SS activity was slightly, although significantly, higher in ‘Jackson’ than in ‘Biloxi’ (0.174 ± 0.009 versus 0.151 ± 0.005 μmol NADH mg−1 protein min−1). In the sensitive cultivar ‘Biloxi’, SS activity showed a 25% decrease within 1 d of water deprivation, declining to 55% at the end of the study (Fig. 4B). However, in ‘Jackson’, a significant decrease of this activity was not observed until 3 d after the onset of drought and was only reduced by 40% at the end of the study period (Fig. 4A). To test whether this decline of activity was due to a reduction of protein content, an immunodetection assay was carried out (Fig. 4C). SS immunodetection showed a reduction in the protein amount similar for both cultivars.
Figure 4.
Effects of drought on SS activity of ‘Jackson’ (A) and ‘Biloxi’ (B) nodules. Legends and statistical analysis are as described in the Figure 1 legend. Values represent mean ± se (n = 4). C shows western immunoblots of host plant SS levels from control (C) and stressed (D) plants, at days 0, 1, 2, 3, and 6 after the onset of drought. Equal amounts of protein were loaded on each lane. Densitometry analysis displayed, expressed as a percentage of the control values, corresponds to four independent biological experiments.
Both cultivars showed similar values for AI, malate dehydrogenase, NADP+-dependent isocitrate dehydrogenase (ICDH), Asp aminotransferase (AAT), and Glu synthase (GOGAT) activities (average values for control nodules of both cultivars were 0.18, 8.5, 0.08, 0.5, and 0.026 μmol mg−1 protein min−1, respectively). Only the activity of phosphoenolpyruvate carboxylase (PEPC) was markedly higher in ‘Jackson’ (0.14 ± 0.004 μmol mg−1 protein min−1) than in ‘Biloxi’ (0.06 ± 0.004 μmol mg−1 protein min−1). In ‘Jackson’ none of these activities was significantly affected by drought, whereas in ‘Biloxi’ AI, AAT, and GOGAT activities decreased by 25%, at the end of the treatment period (data not shown).
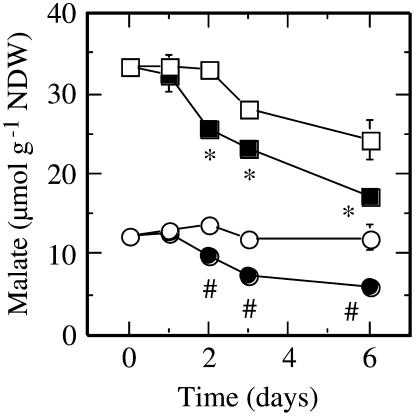
Nodular malate content in ‘Jackson’ control plants was 30.2 ± 1.2 μmol g−1 NDW and was 2.5-fold higher than in control plant nodules of ‘Biloxi’ (Fig. 5). Succinate concentrations in ‘Jackson’ nodules were also significantly higher than those of ‘Biloxi’, although succinate concentrations were much lower than those of malate (1.25 ± 0.08 and 0.31 ± 0.03 μmol g−1 NDW in ‘Jackson’ and ‘Biloxi’, respectively). Citrate and α-ketoglutarate contents did not show significant differences between cultivars (data not shown). Malate decreased significantly in both cultivars 2 d after the onset of drought. However, the decline of malate content was greater in ‘Biloxi’ as nodule water potential became more negative (Fig. 5). At the end of the study period, ‘Jackson’ nodules exhibited malate levels approximately 70% of those of control plants, whereas in ‘Biloxi’ nodules the levels were reduced to 50% of control values (Fig. 5).
Figure 5.
Effects of drought on malate levels of ‘Jackson’ and ‘Biloxi’ nodules. Legends and statistical analysis are as described in the Figure 1 legend. Values represent mean ± se (n = 4).
Table I shows the linear regressions between ANA and ureide concentration, malate concentration, and the ratio of malate to ureide concentrations, respectively, in nodules of stressed plants. Regressions were obtained using values previously normalized against their respective controls for each cultivar. For ureides, the correlation coefficients (r2 values) for both cultivars are very similar, at around 0.5. However, the correlation of ANA with malate increased to a range of 0.7 to 0.77 and was even higher with the malate to ureide ratio, with values above 0.9 for both cultivars. Indeed, when regression analysis was performed including both cultivars, the correlation between normalized ANA and normalized malate to ureide ratio remained 0.76, despite the relative different response of each cultivar to drought.
Table I.
Regression line equations between normalized ANA (y) and normalized ureide content, malate content, and the ratio between malate and ureide content of ‘Jackson’ and ‘Biloxi’ nodules (x in each column, respectively)
Each equation is followed by its correlation coefficient (r2; in bold). Values were obtained from those given in Figures 2 to 5, normalized against their respective daily control values.
| Cultivar | Ureides | Malate | Malate/Ureides |
|---|---|---|---|
| ‘Jackson’ | y = − 0.132 × + 0.895 | y = 0.591 × + 0.258 | y = 0.261 × + 0.570 |
| r2 = 0.50 | r2 = 0.77 | r2 = 0.94 | |
| ‘Biloxi’ | y = − 0.129 × + 0.781 | y = 1.179 × − 0.384 | y = 0.639 × + 0.155 |
| r2 = 0.52 | r2 = 0.70 | r2 = 0.90 |
DISCUSSION
Nodule Water Status and NF
There is a high diversity among the response of different legume species to water stress (Sinclair and Serraj, 1995). Indeed, such diversity can be found within different lines of the same species, i.e. different soybean cultivars showing distinct tolerances or sensitivities to drought (Sall and Sinclair, 1991; Serraj and Sinclair, 1997). In this study drought provoked a similar nodule water potential decline in both ‘Jackson’ and ‘Biloxi’ soybeans (Fig. 1). Most published papers linking drought and ureide effects on NF express water stress as either fractions of transpirable soil water, a parameter of relevant agronomical significance, or leaf water potential, which is the usual way to express plant water status. Less attention has been given to nodule water potential' which, although closely related to leaf water potential (González et al., 1995), has been recently shown to be more relevant to NF than plant (leaf) water status (Marino et al., 2007a).
Drought inhibited NF in both ‘Jackson’ and ‘Biloxi’, but this inhibition occurred earlier and more severely in ‘Biloxi’ (Fig. 2). These results, measured by a flow-through gas system detecting H2 evolution, confirm that NF in ‘Jackson’ is more tolerant to drought, as already reported in field and greenhouse experiments, using acetylene reduction techniques (Serraj and Sinclair, 1996b; Serraj et al., 1997).
Ureides Accumulate in Nodules, But Not in Leaves, in Early Drought
Studies of Sinclair's group established a relationship between the greater tolerance to drought of ‘Jackson’ and a smaller ureide accumulation in leaves (Serraj and Sinclair, 1997; Serraj et al., 1997; Vadez and Sinclair, 2002). Indeed, Sinclair et al. (2003) suggested that the differences in leaf ureide metabolism were the main cause of the distinct sensitivity of the cultivars to drought. In general, these studies were carried out over medium-term periods of drought or following the supply of external ureides. It can be argued that experiments using an external supply of ureides, in order to mimic the physiological response to drought, do not allow these ureides to reach the nodules (the site of their physiological synthesis) via the xylem. Instead, they are directly targeted to leaves. This would then preclude the possibility of a buildup of these compounds in nodules and, therefore, would not reflect the natural physiological consequences of a drought-induced impairment of long-distance transport. Furthermore, King and Purcell (2005) suggested that shoot ureide accumulation in soybean is not responsible for NF inhibition. In this study, no accumulation of ureides in leaves of ‘Jackson’ or ‘Biloxi’ has been detected (Fig. 3, A and B) despite the fact that NF was already inhibited (Fig. 2, A and B). Taken together, these results indicate that leaf ureides are not involved at the early stages of NF inhibition under drought, although a role in later stages of a more severe drought (Serraj et al., 1999b) cannot be discarded.
A possible role of nodule ureide content in soybean NF has received much less attention, despite the fact that more than 30 years ago Minchin and Pate (1974) recorded an increase in the soluble amino acid content of pea nodules under low transpiration conditions. There is only one very recent comparative study on nodule ureide content profiles between drought-tolerant and -sensitive cultivars (King and Purcell, 2005), which showed that nodular ureide accumulation mirrored the decline in NF. Therefore, these authors concluded that ureides, along with other nitrogen compounds, are potential candidates for inducing a feedback inhibition of NF. In this work, drought provoked ureide accumulation in nodules of both cultivars. As this accumulation was greater and occurred earlier in ‘Biloxi’ (Fig. 3, C and D), it appears to be correlated with the NF sensitivity to drought of this cultivar. It is noteworthy that ureide accumulation occurs in nodules despite a decreased NF. Although some other causative factors may also lead to this effect, such as an increase in the activity of ureide biosynthetic enzymes, this seems to be unlikely since, based on our previous experience, nodular metabolic activities of primary metabolism tend to be stable or to decline under mild or moderate drought (Larrainzar et al., 2007). The only exception to this trend is ICDH, whose activity has been shown to slightly increase in pea nodules both under drought (Gálvez et al., 2005) and under oxidative stress (Marino et al., 2007b). Thus, it can be concluded that export is impaired, resulting in the accumulation of certain metabolites. The circumstances leading to this impaired export have been discussed elsewhere (Streeter, 1993; Walsh, 1995; Serraj et al., 1999a). Our results do not provide evidence for nodule ureides being the actual compounds that induce the decrease in NF, but it is likely that an accumulation of fixation products is involved. Indeed, Lodwig et al. (2003) have shown that a complex amino acid cycling occurs in nodules, with the plant providing amino acids to the bacteroids, enabling them to shut down their ammonium assimilation, and bacteroids acting like plant organelles to cycle amino acids back to the plant for Asn synthesis. However, it is not yet known if this exchange could be directly disrupted by a drought-induced accumulation of nitrogen compounds.
Furthermore, the present results are also in agreement with the hypothesis that the cause of NF inhibition under drought is of a local origin, rather than relying on a systemic signal (Marino et al., 2007a).
Carbon Flux in Nodules Is More Affected in the Sensitive Cultivar
SS has already been described as a key enzyme that regulates NF under drought conditions in soybean (González et al., 1995) and pea (González et al., 1998; Gálvez et al., 2005). The activity of this enzyme has also been observed to decline under other abiotic stresses, such as salinity, defoliation, nitrate, and oxidative stress (Fernández-Pascual et al., 1996; Gordon et al., 1997; Marino et al., 2006). Moreover, there is a high correlation between SS inhibition and the NF decline under abiotic stresses (Arrese-Igor et al., 1999). However, it has yet to be proven that diversity in SS activity can be associated with a differential tolerance to drought. In this work, SS was the first nodule enzyme to show reduced activity under drought in both cultivars (Fig. 4, A and B), with the inhibition occurring at the first day of reduced watering in ‘Biloxi’ (Fig. 4B), and before any observed effect on NF (Fig. 2B). In contrast, ‘Jackson’ maintained SS activity rates at control values until the third day of drought (Fig. 4A), and the decline was concomitant with that of NF (Fig. 2A). The SS activity decline was also related to a reduction in SS content (Fig. 4C). However, in ‘Biloxi’ the activity decline was observed prior to any effect on SS protein content, suggesting that SS activity could be modulated through posttranscriptional modifications (Komina et al., 2002; Geigenberger, 2003).
In pea nodules, decreased SS activity leads to a decline in malate content (Gálvez et al., 2005; Marino et al., 2007a). It had been earlier suggested that a likely decrease in nodule malate content under certain environmental constraints may lead to the inhibition of NF (Arrese-Igor et al., 1999). In this work, malate content in ‘Jackson’ nodules ranged between 2- and 3-fold the measured values in ‘Biloxi’ nodules (Fig. 5), a situation that is consistent with the higher NF rates of the former and seems to be related to the higher SS and, particularly, PEPC activity rates. A decline in nodular malate content was observed after 2 d of drought treatment in both cultivars, but it was more pronounced in ‘Biloxi’ (Fig. 5), a fact that is not related to PEPC, whose activity was maintained throughout the study period in both cultivars at control levels, but instead to the decline of SS activity. The higher constitutive content of malate may also be an important component of the drought tolerance of ‘Jackson’ nodules since a greater malate availability would reduce the effect of a carbon-skeleton shortage due to SS down-regulation.
Carbon/Nitrogen Interactions in the Inhibition of NF under Drought in Soybean
To determine the relative importance of carbon or nitrogen in the inhibition of NF under drought, correlation analyses were performed between normalized ANA and normalized ureide content, malate content, and the malate to ureide ratio (Table I) in nodules of stressed plants. The correlation between malate and ANA was higher than that of ureides and ANA, reflecting the involvement of carbon shortage in the inhibition of NF during early drought conditions. However, when both factors were combined, NF dependence on the malate to ureide ratio showed a higher correlation than that of ureides or malate alone. A strong relation between carbon and nitrogen metabolism is widely accepted as a crucial interplay in the regulation of plant performance, and it has also been shown to occur in water-stressed pea nodules (Gálvez et al., 2005). Taken together, these results indicate that there are at least two major mechanisms involved in the drought-induced inhibition of NF in soybean nodules: (1) an impairment of long-distance transport, leading to an accumulation of ureides in nodules, which is likely to provoke a nitrogen feedback regulation of NF; and (2) an impairment of metabolic carbon flux in nodules, resulting in a shortage of carbon substrate for bacteroid NF. Both factors seem to be crucial for the regulation of NF under drought, although the results obtained so far do not allow for a definitive statement, such as whether the accumulation of nitrogen compounds has a direct effect on NF or an indirect one through an induced decline in SS activity. Furthermore, both nitrogen accumulation and SS decline could be independently induced by oxidative signaling. This has been recently suggested for pea (Marino et al., 2006) and alfalfa (Naya et al., 2007) and is consistent with the regulation of NF under drought occurring at the local/nodule level (Marino et al., 2007a), rather than systemically.
MATERIALS AND METHODS
Growth Conditions
‘Jackson’ and ‘Biloxi’ soybean plants (Glycine max L. Merr.) were inoculated with the hup− Bradyrhizobium japonicum strain UPM792, to allow for the detection of H2 evolution. Plants were grown in 1-L pots with 2:1 (v/v) vermiculite:perlite as rooting substrate in a controlled environmental chamber (24°C/18°C day/night temperature, 60%/70% day/night relative humidity, and 16-h photoperiod). They were watered three times a week with nutrient solution lacking nitrogen (Rigaud and Puppo, 1975).
Experimental Procedures, NF, and Water Potential
To obtain plants with similar biomass and developmental stage, experiments were carried out when plants were 5 and 6 weeks old for ‘Biloxi’ and ‘Jackson’, respectively. Previous experiments using 6-week-old ‘Biloxi’ plants gave an identical metabolic profiling, but, due to their bigger size, transpiration rates were higher and the water content of pots was more rapidly depleted. Therefore, when analyzed under these conditions, drought effects were more dramatic and less comparable between cultivars. Plants were separated randomly into two sets: control and drought. During the study period, control plants were supplied daily with nutrient solution to field capacity, whereas stressed plants were supplied daily with one-quarter of the measured evapotranspirational water loss volume. Four plants per treatment were harvested at days 1, 2, 3, and 6 after the onset of drought in order to obtain data at different levels of stress.
For ANA determinations, H2 evolution of intact plants, whose root systems were sealed into the growth pots, was measured in an open flow-through system under N2/O2 (79%/21%) according to Witty and Minchin (1998) using an electrochemical H2 sensor (Qubit Systems). The H2 sensor was calibrated using high-purity gases (Praxair) employing a gas mixer (Air Liquide) flowing at the same rate as the sampling system (500 mL min−1).
Nodule water potential was determined by a Wescor HR-33T psychrometer. Nodules were harvested, frozen in liquid N2, and stored at −80°C for further analysis. Roots and shoots were separated and dried for 48 h at 70°C for DW determinations.
Extraction and Assay of Enzymes
All enzymes were extracted from nodules at 4°C with mortar and pestle in an optimized medium consisting of 50 mm MOPS, pH 7, 20% polyvinylpolypyrrolidone, 10 mm dithiothreitol, 10 mm 2-mercaptoethanol, 1 mm EDTA, 20 mm KCl, and 5 mm MgCl2 (Marino et al., 2006). The homogenates were centrifuged for 30 min at 20,000g, 4°C. Aliquots of the supernatants were retained for determination of plant fraction protein (Bradford, 1976) and for PEPC (EC 4.1.1.31) activity. The rest of the supernatant was desalted by low-speed centrifugation (180g, 2 min) through 5-mL columns of Bio Gel P6DG (Bio-Rad) previously equilibrated with 50 mm MOPS, pH 7, 20 mm KCl, 5 mm MgCl2. Desalted extracts were used to assay SS (EC 2.4.1.13), AI (EC 3.2.1.26), malate dehydrogenase (EC 1.1.1.37), GOGAT (EC 1.4.1.14), AAT (EC 2.6.1.1), and ICDH (EC 1.1.1.42). All activities were measured within the linear range at 30°C, as described elsewhere (Marino et al., 2006).
Malate and Ureide Determination
To analyze malate and ureide content of nodules, frozen nodules were homogenized to a fine powder in liquid N2 with mortar and pestle. Then 1.5 mL of 10% (w/v) TCA in water was added and the homogenate was centrifuged for 10 min at 1,750g, 4°C. The aqueous phase was washed six times with diethyl ether saturated with water. The diethyl ether was discarded and the aqueous phase was purged with helium for 2 min and then filtered through a 0.45-μm syringe filter (Wilson and Harris, 1966).
Malate levels were determined by ion chromatography in a DX-500 system (Dionex) by gradient separation with a Dionex IonPac AS11 column according to the manufacturer's instructions (2.5 mm NaOH/18% methanol to 45 mm NaOH/18% methanol in 13 min; Gálvez et al., 2005).
Nodule ureides (allantoin and allantoate) were determined by capillary electrophoresis. The length of the capillary tube was 60 cm and 0.1 m Na2B4O7·10H2O, pH 9.2, 25 mL L−1 OFM-Anion BT (Waters) solution was used as electrolyte. Samples were injected for 5 s by the hydrostatic method and electrophoresed under 10 kV for 30 min. Allantoin and allantoate were detected by optical density at 190 nm (Sato et al., 1998).
Ureide content of leaves was analyzed following extraction in 1 mL of 0.2 n NaOH to 10 mg of dry tissue, boiling of extracts for 30 min, and centrifugation at 12,000g for 10 min. Ureides were quantified using a colorimetric detection method (Trijbels and Vogel, 1966).
Immunoblotting
SDS-PAGE was performed according to Laemmli (1970) with a 1-mm-thick 10% (w/v) polyacrylamide resolving gel and a 4.6% (w/v) stacking gel in a vertical electrophoresis cell (MiniProtean III; Bio-Rad) at 150 V for 60 min. Gels were electroblotted onto PVDF membranes for 75 min at 100 V in a Mini Trans-Blot electrophoretic transfer Cell (Bio-Rad). Blots were blocked in 5% (w/v) skim milk in 20 mm Tris-buffered saline at 4°C overnight. As primary antibody anti-SS (1:5,000, v/v) was used. As the secondary antibody, goat anti-rabbit IgG conjugated to alkaline phosphatase (1:10,000, v/v; Sigma-Aldrich) was employed. Immunoreactive bands were visualized with a bCIP/NBT liquid substrate system (Sigma-Aldrich).
Statistical Analysis
Results were examined by two-way ANOVA. All effects discussed in this study were significant at P ≤ 0.05 in Fisher's lsd among means.
Acknowledgments
Antibodies against SS, Bradyrhizobium japonicum strain, and soybean seeds were kindly provided by Dr. Anthony J. Gordon, Prof. Tomás Ruiz-Argüeso, and Dr. Thomas R. Sinclair, respectively. The authors would also like to thank Dr. Frank R. Minchin and Dr. Thomas R. Sinclair for fruitful discussions; Elena Denia and Gustavo Garijo for technical assistance; and Itziar Tirapu for preliminary work. C.A.-I. wishes to acknowledge the support provided by the Mobility Programme of the Spanish Ministry of Education and Science.
This work was supported by the Dirección General de Investigación, Ministry of Education and Science (Spain; grant no. AGL2005–0274/AGR), and its associated Fondo Europeo de Desarrollo Regional funding. R.L., E.L., and D.M. are holders of predoctoral fellowships of the “Formación de Personal Investigador” and “Formación de Profesorado Universtario” programs of the Spanish Ministry of Education and Science and of the Basque Government, respectively.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Cesar Arrese-Igor (cesarai@unavarra.es).
Open Access articles can be viewed online without a subscription.
References
- Arrese-Igor C, González EM, Gordon AJ, Minchin FR, Gálvez L, Royuela M, Cabrerizo PM, Aparicio-Tejo PM (1999) Sucrose synthase and nodule nitrogen fixation under drought and other environmental stresses. Symbiosis 27 189–212 [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Díaz del Castillo L, Hunt S, Layzell DB (1994) The role of oxygen in the regulation of nitrogenase activity in drought-stressed soybean nodules. Plant Physiol 106 949–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz del Castillo L, Layzell DB (1995) Drought stress, permeability to O2 diffusion and the respiratory kinetics of soybean roots nodules. Plant Physiol 107 1187–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand JL, Sheehy JE, Minchin FR (1987) Nitrogenase activity, photosynthesis and nodule water potential in soybean plants experiencing water-deprivation. J Exp Bot 38 311–321 [Google Scholar]
- Fernández-Pascual M, de Lorenzo CA, de Felipe MR, Rajalakshmi S, Gordon AJ, Thomas BJ, Minchin FR (1996) Possible reasons for relative salt stress tolerance in nodules of white lupin cv. Multolupa. J Exp Bot 47 1709–1716 [Google Scholar]
- Gálvez L, González EM, Arrese-Igor C (2005) Evidence for carbon flux shortage and strong carbon/nitrogen interactions in pea nodules at early stages of water stress. J Exp Bot 56 2551–2561 [DOI] [PubMed] [Google Scholar]
- Geigenberger P (2003) Regulation of sucrose to starch conversion in growing potato tubers. J Exp Bot 54 457–465 [DOI] [PubMed] [Google Scholar]
- González EM, Aparicio-Tejo PM, Gordon AJ, Minchin FR, Royuela M, Arrese-Igor C (1998) Water-deficit effects on carbon and nitrogen metabolism of pea nodules. J Exp Bot 49 1705–1714 [Google Scholar]
- González EM, Gálvez L, Royuela M, Aparicio-Tejo PM, Arrese-Igor C (2001) Insights into the regulation of nitrogen fixation in pea nodules: lessons from drought, abscisic acid and increased photoassimilate availability. Agronomie 21 607–613 [Google Scholar]
- González EM, Gordon AJ, James CL, Arrese-Igor C (1995) The role of sucrose synthase in the response of soybean nodules to drought. J Exp Bot 46 1515–1523 [Google Scholar]
- Gordon AJ, Minchin FR, James CL, Komina O (1999) Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol 120 867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Minchin FR, Skøt L, James CL (1997) Stress-induced declines in soybean N2 fixation are related to nodule sucrose synthase activity. Plant Physiol 114 937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge DF (1982) Relative abundance of ureides and nitrate in plant tissues of soybean as a quantitative assay of nitrogen fixation. Plant Physiol 70 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst I, Welham T, Kelly S, Kaneko T, Sato S, Tabata S, Parniske M, Wang TL (2007) TILLING mutants of Lotus japonicus reveal that nitrogen assimilation and fixation can occur in the absence of nodule-enhanced sucrose synthase. Plant Physiol 144 806–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CA, Purcell LC (2005) Inhibition of N2 fixation in soybean is associated with elevated ureides and amino acids. Plant Physiol 137 1389–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komina O, Zhou Y, Sarath G, Chollet R (2002) In vivo and in vitro phosphorylation of membrane and soluble forms of soybean nodule sucrose synthase. Plant Physiol 129 1664–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- Larrainzar E, Wienkoop S, Ladrera R, Weckwerth W, Arrese-Igor C, Gonzalez EM (2007) Medicago truncatula root nodule proteome analysis reveals differential plant and bacteroid responses to drought stress. Plant Physiol 144 1495–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodwig EM, Hosie AHF, Bordes A, Findlay K, Allaway D, Karunakaran R, Downie JA, Poole PS (2003) Amino-acid cycling drives nitrogen fixation in the legume-Rhizobium symbiosis. Nature 422 722–726 [DOI] [PubMed] [Google Scholar]
- Lukaszewski KM, Blevins DG, Randall DD (1992) Asparagine and boric acid cause allantoate accumulation in soybean leaves by inhibiting manganese-dependent allantoate amidohydrolase. Plant Physiol 99 1670–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D, Frendo P, Ladrera R, Zabalza A, Puppo A, Arrese-Igor C, González EM (2007. a) Nitrogen fixation control under drought stress: localized or systemic? Plant Physiol 143 1968–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino D, González EM, Arrese-Igor C (2006) Drought effects on carbon and nitrogen metabolism of pea nodules can be mimicked by paraquat: evidence for the occurrence of two regulation pathways under oxidative stresses. J Exp Bot 57 665–673 [DOI] [PubMed] [Google Scholar]
- Marino D, González EM, Frendo P, Puppo A, Arrese-Igor C (2007. b) NADPH recycling systems in oxidative stressed pea nodules: a key role for the NADP+-dependent isocitrate dehydrogenase. Planta 225 413–421 [DOI] [PubMed] [Google Scholar]
- Minchin FR (1997) Regulation of oxygen diffusion in legume nodules. Soil Biol Biochem 29 881–888 [Google Scholar]
- Minchin FR, Pate JS (1974) Diurnal functioning of the legume root nodule. J Exp Bot 25 295–308 [Google Scholar]
- Naya L, Ladrera R, Ramos J, González EM, Arrese-Igor C, Minchin FR, Becana M (2007) The response of carbon metabolism and antioxidant defenses of alfalfa nodules to drought stress and to the subsequent recovery of plants. Plant Physiol 144 1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell LC, de Silva M, King CA, Kim WH (1997) Biomass accumulation and allocation in soybean associated with genotypic differences in tolerance of nitrogen fixation to water deficits. Plant Soil 196 101–113 [Google Scholar]
- Purcell LC, Serraj R, de Silva M, Sinclair TR, Bona S (1998) Ureide concentration of field-grown soybean in response to drought and the relationship to nitrogen fixation. J Plant Nutr 21 949–966 [Google Scholar]
- Rigaud J, Puppo A (1975) Indole-3-acetic-acid catabolism by soybean bacteroids. J Gen Microbiol 88 223–228 [Google Scholar]
- Sall K, Sinclair TR (1991) Soybean genotypic differences in sensitivity of symbiotic nitrogen-fixation to soil dehydration. Plant Soil 133 31–37 [Google Scholar]
- Sato T, Yashima H, Ohtake N, Sueyoshi K, Akao S, Harper JE, Ohyama T (1998) Determination of leghemoglobin components and xylem sap composition by capillary electrophoresis in hypernodulation soybean mutants cultivated in the field. Soil Sci Plant Nutr 44 635–645 [Google Scholar]
- Serraj R, Sinclair TR (1996. a) Inhibition of nitrogenase activity and nodule oxygen permeability by water deficit. J Exp Bot 47 1067–1073 [Google Scholar]
- Serraj R, Sinclair TR (1996. b) Processes contributing to N2-fixation insensitivity to drought in the soybean cultivar Jackson. Crop Sci 36 961–968 [Google Scholar]
- Serraj R, Sinclair TR (1997) Variation among soybean cultivars in dinitrogen fixation response to drought. Agron J 89 963–969 [Google Scholar]
- Serraj R, Purcell LC, Bona S, Sinclair TR (1997) Nitrogen accumulation and nodule activity of field grown Jackson soybean in response to water deficits. Field Crops Res 52 109–116 [Google Scholar]
- Serraj R, Sinclair TR, Purcell LC (1999. a) Symbiotic N2 fixation response to drought. J Exp Bot 50 143–155 [Google Scholar]
- Serraj R, Vadez V, Denison RF, Sinclair TR (1999. b) Involvement of ureides in nitrogen fixation inhibition in soybean. Plant Physiol 119 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R, Vadez V, Sinclair TR (2001) Feedback regulation of symbiotic N2 fixation under drought stress. Agronomie 21 621–626 [Google Scholar]
- Shelp BJ, Ireland RJ (1985) Ureide metabolism in leaves of nitrogen-fixing inhibition in soybean. Plant Physiol 77 779–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair TR, Serraj R (1995) Legume nitrogen fixation and drought. Nature 378 344 [Google Scholar]
- Sinclair TR, Vadez V, Chenu K (2003) Ureide accumulation in response to Mn nutrition by eight soybean genotypes with N-2 fixation tolerance to soil drying. Crop Sci 43 592–597 [Google Scholar]
- Streeter JG (1993) Translocation—a key factor limiting the efficiency of nitrogen-fixation in legume nodules. Physiol Plant 87 616–623 [Google Scholar]
- Todd CD, Polacco JC (2004) Soybean cultivars ‘Williams 82’ and ‘Maple Arrow’ produce both urea and ammonia during ureide degradation. J Exp Bot 55 867–877 [DOI] [PubMed] [Google Scholar]
- Trijbels F, Vogel GD (1966) Degradation of allantoine by Pseudomonas acidovirans. Biochim Biophys Acta 113 292–301 [DOI] [PubMed] [Google Scholar]
- Vadez V, Sinclair TR (2001) Leaf ureide degradation and N2 fixation tolerance to water deficit in soybean. J Exp Bot 52 153–159 [PubMed] [Google Scholar]
- Vadez V, Sinclair TR (2002) Sensitivity of N2 fixation traits in soybean cultivar Jackson to manganese. Crop Sci 42 791–796 [Google Scholar]
- Vadez V, Sinclair TR, Serraj R (2000) Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiol Plant 110 215–223 [Google Scholar]
- Walsh KB (1995) Physiology of the legume nodule and its response to stress. Soil Biol Biochem 27 637–655 [Google Scholar]
- Wilson AM, Harris GA (1966) Hexose-, inositol-, and nucleoside phosphate esters in germinating seeds of crested wheatgrass. Plant Physiol 41 1416–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler RG, Polacco JC, Blevins DG, Randall DD (1985) Enzymic degradation of allantoate in developing soybeans. Plant Physiol 79 787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JF, Minchin FR (1998) Methods for the continuous measurement of O2 consumption and H2 production by nodulated legume root systems. J Exp Bot 49 1041–1047 [Google Scholar]