Abstract
A review of current biopsy techniques that are used in obtaining specimens from which to make a diagnosis of primary intraocular lymphoma (PIOL) is presented. Methods for obtaining and subsequently testing vitrectomy specimens are discussed. In addition, the yields of external and internal approaches for obtaining chorioretinal tissue, and diagnostic vitrectomies, are reviewed.
Keywords: Primary intraocular lymphoma, Vitrectomy, Chorioretinal biopsy, Transvitreal retinal biopsy
Introduction
Primary intraocular lymphoma (PIOL) is a subset of primary central nervous system lymphoma (PCNSL) with predilection for intraocular regions that sit behind the blood-retina barrier [1-4]. PIOL is a unique malignant lymphoproliferation because of its affectation of an immune privileged site, involving the subretinal pigment epithelium (RPE), retina, vitreous, and optic nerve. Frequently masquerading as a uveitis, PIOL is often misdiagnosed for intraocular inflammation and is treated with corticosteroids [5] or, infrequently, PIOL can masquerade as a viral retinitis and is treated with antiviral medication [6]. It is not until the presumed uveitis fails to respond to corticosteroid therapy that another cause is sought. Prompt diagnosis of PIOL is imperative because consultation with a neurooncologist and initiation of chemotherapy and/or radiation therapy can extend the patient’s life. Furthermore, most cases of PIOL will eventually involve the brain (PCNSL) [1, 7], which has a poor prognosis. Thus, if disease can be halted within the eye, there is a possibility that morbidity and mortality may be improved in patients with PIOL. Diagnosis of PIOL requires histopathologic evidence of the malignant lymphoma cells. A tissue biopsy must be obtained from which to make a pathologic diagnosis and perform further testing including molecular analyses. When undertaking a biopsy it is important to have a diagnostic plan laid out. Because an ocular pathologist will ultimately receive the biopsy specimen and will be making the diagnosis in a suspected case of PIOL, it is important to involve this member of the patient’s care team prior to bringing the patient to the operating room. The ocular pathologist can help determine which affected intraocular structure may provide the best opportunity to result in the highest yield of lymphoma cells.
In this article, we review the biopsy techniques and yields that may be employed in order to provide tissue from which to make a diagnosis of PIOL in suspect cases. It is important to realize that, if a patient is suspected of having PIOL, a lumbar puncture with cytologic analysis of the cerebrospinal fluid (CSF) should be performed because of the high possibility of brain involvement in PIOL [1, 3, 7-9]. In addition, patients should also receive a brain magnetic resonance imaging (MRI) scan to determine whether lesions are present in the absence of obvious lymphoma cells in the CSF. When brain involvement has been ruled out by the above procedures, we focus on the involved eye from which to make a diagnosis.
Historical background
Prior to the advent of pars plana vitrectomy in the 1970s pioneered by Machemer [10-15], enucleation [16, 17] was the first surgical procedure employed by ophthalmic surgeons to make a diagnosis of the so called reticulum cell sarcoma (as PIOL was known prior to the 1970s) [8, 17, 18]. Enucleation could be performed in patients’ eyes in which there was no possibility of restoring vision (vision worse than count fingers) due to massive involvement or in eyes that had become intractably painful. In addition, in patients succumbing to their disease, enucleation could be performed to confirm a clinical suspicion of reticulum cell sarcoma. Enucleation, however, is not an ideal diagnostic procedure to undertake when a patient still has potential functional vision and an otherwise normal eye.
Vitreous biopsy
The vitreous remains the preferred tissue to sample when an eye exhibits chronic uveitis of unknown cause or intraocular malignancy or infection is suspected. Vitrectomy is also indicated when there is potential for treatment to be initiated or changed upon the diagnostic procedures used on the vitrectomy specimen. Indeed, often masquerading as a vitritis, PIOL cells are commonly found in the vitreous and this structure is most frequently biopsied to make a pathologic diagnosis of PIOL.
Cytologic examination of vitreous biopsy specimens has been employed to make a diagnosis of PIOL since the mid-1970s in cases that were initially diagnosed as uveitis [19, 20]. However, lack of improvement with typical corticosteroid treatment prompted vitreous biopsy with cytology, revealing the atypical lymphocytes associated with PIOL [19, 20]. Importantly, a final diagnosis of PIOL allows the appropriate treatment to commence (in the mid-1970s this was primarily radiation treatment [19, 20]). Vitrectomy can also yield a diagnosis of PIOL when lumbar puncture and cytologic analysis of CSF fail to reveal PCNSL cells [21].
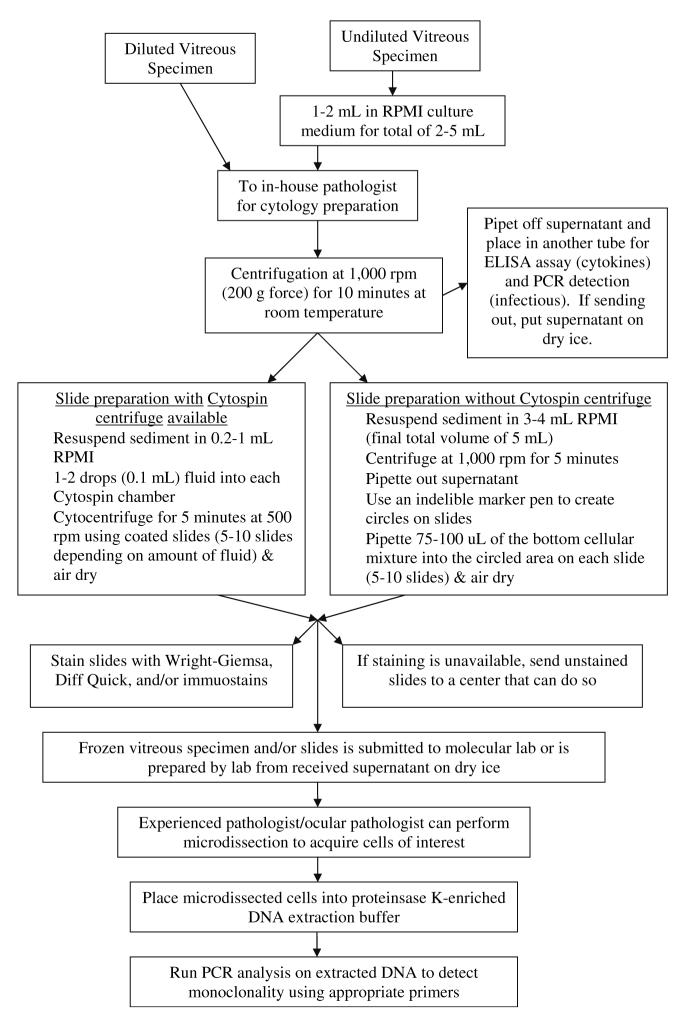
The technique for performing a complete pars plana vitrectomy in a suspected case of PIOL follows typical protocol. A standard three-port pars plana approach is employed. Because cytology [22] is standard and we frequently use molecular analysis with polymerase chain reaction amplification (PCR) [23-25] and cytokine-level analysis [26, 27], a complete core vitrectomy is recommended. Undiluted vitreous is first obtained for cytological analysis. A dilute specimen (in which cytokine levels could be measured) is then obtained by using the infuser and cutting the remaining vitreous. Vitreous wash fluid that has been collected in the vitrector cassette should also be used for microbiologic cultures. In order to prevent cell death, 1-2 ml of vitreous specimen may be expediently placed into a tube containing Roswell Park Memorial Institute (RPMI) culture medium for a total volume of 2-5 ml of fluid. This fluid should then be sent immediately to an awaiting cytologist or ocular pathologist who will perform the appropriate steps to obtain the best yield [28]. It must be stressed that time is of the essence because lymphoma cells rapidly begin to degenerate. Centrifugation of the submitted sample at 1,000 rpm and pipeting the supernatant into another tube for enzyme-linked immunosorbent assay (ELISA) assay and molecular investigation (PCR for infective virus) are recommended [23-25, 29-31]. The sediment in the original tube is then resuspended in 0.2-1 ml of RPMI and cytocentrifuged again. They are used for cytology and molecular analyses (PCR for monoclonality of malignant B, or rarely T, cells). Figure 1 demonstrates the protocol one may follow after collecting the vitreous specimen.
Fig. 1.
Diagram of handling and processing vitreous specimen
Vitreous specimens may not always contain neo-plastic cells and, thus, be negative for the diagnosis of PIOL. This might especially be the case if there is minimal vitreal involvement by the PIOL cells [32] or the cells have degenerated. Sometimes the quality of the cytology is poor, therefore making it unfeasible to make the diagnosis. In such events, it may be necessary to perform another vitrectomy and send to a well-qualified cytological laboratory [3].
Davis and colleagues submitted vitrectomy specimens from 27 patients suspected of having an intraocular malignancy to analysis by cytology [33]. A final diagnosis of lymphoma was achieved in 13 patients with cytology yielding four true positive lymphoma cases (a sensitivity of 31%) and no false positive cases. The positive predictive value (PPV) and negative predictive value (NPV) of cytologic evaluation, then, was 100 and 60.9%, respectively. Thus, while a positive cytologic evaluation was certain for lymphoma, a negative evaluation certainly does not completely rule out the possibility of intraocular lymphoma. Earlier, in a series of 87 patients who, by clinical examination, were suspicious for PIOL it was found by cytologic analysis of the vitreous specimen that 42 were positive for PIOL, another three were suspicious for PIOL, and 42 were negative [34].
A negative cytology, then, is not always reassuring. When suspicion for PIOL still runs high, it is important to consider other adjunctive testing to which the vitrectomy specimen might be submitted. In addition, while the ability to acquire vitreous specimens exists at many surgical ophthalmology centers, the appropriate analyses and experienced pathologists to evaluate such specimens reliably may not. In these instances, it is important to consider sending the vitreous specimen to a center that has the capacity to evaluate it. However, even though the vitreous specimen may be sent to a major hospital with an experienced histopathologist, the very real possibility that any lymphoma cells within the vitreous biopsy could degenerate exists. It is imperative that the appropriate handing of the vitreous specimen occur and to be aware of adjunctive tests that may be performed at a nearby laboratory by those experienced with the basic techniques of molecular biology [2].
Diagnostic testing of vitrous biopsy specimen (sample)
Cytology
Cytology is the cornerstone by which a diagnosis of PIOL is achieved. The malignant B-cells of PIOL exhibit characteristic features that can be revealed using either Giemsa or Diff Quick staining [2, 23]. Large round or oval nuclei (frequently segmented and often containing prominent nucleoli) surrounded by scant basophilic cytoplasm are the defining features of this intraocular lymphoproliferation (Fig. 2) [1, 2,20, 22, 23, 35].
Fig. 2.
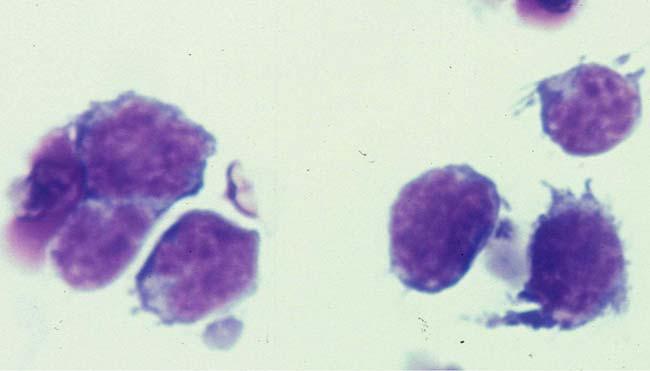
Cytology showing classical PIOL cells with large nuclear, prominent nucleoli, and scant basophilic cytoplasm (Giemsa, original magnification, 640×)
Cytologic examination of the vitreous specimen can be difficult because there may be a relative lack of lymphoma cells compared to reactive inflammatory cells [4]. In addition, atypical lymphoma cells may be in a state of necrosis such that necrotic debris does not allow for a diagnosis of PIOL to be secured (Fig. 3) [2, 36].
Fig. 3.
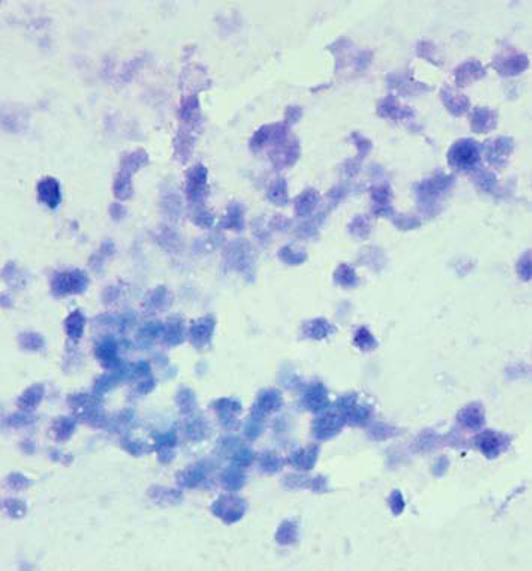
Cytology showing degenerative cells with poor morphology (Giemsa, original magnification, 400×)
Cytokine measurements
B-cell malignancies can secrete high levels of IL-10 [37], an immunosupressive cytokine, while inflammatory conditions (such as uveitis [38]) are associated with high levels of IL-6 [39-41], a pro-inflammatory cytokine [42, 43]. We [24, 26, 44, 45] and others [27, 46] have shown that PIOL can exhibit high IL-10 levels with IL-10:IL-6 ratios greater than 1.0 being suggestive of PIOL [47]. Cytokine levels and IL-10:IL-6 ratios are by no means diagnostic of PIOL, but they can be useful adjunctive tests in corroborating suspicion of PIOL and determining whether there is a durable response to treatment. For example, one of our patients had IL-10 levels that seemed to correlate with the amount of cells in the vitreous and degree of vision [48]. The patient received systemic and intrathecal methotrexate (receiving six cycles). Over six years, however, vitreous biopsy showed that there continued to be intraocular disease. When IL-10 levels were high and IL-10:IL-6 ratios were much greater than 1.0, there were noted to be many more vitreous cells and relatively poor vision. After intravitreal methotrexate administration, the patient would respond by having low to undetectable levels of IL-10 and IL-10:IL-6 ratios less than 1.0, correlating with a relative lack of vitreous cells and improvement in vision. This patient, however, developed new cerebral lesions (PCNSL) and eventually expired. We have shown that cytokine levels can be significantly different between intraocular lymphoma and uveitis [26]. By determining a IL-10:IL-6 ratio greater than 1.0 in suspected cases of PIOL, we correctly classified such cases as PIOL 74.7% of the time (with a sensitivity and specificity for the cutoff of 74.3 and 75.0%, respectively) [26]. Figure 1 shows an appropriate step in which to submit part of the vitreous specimen to cytokine analysis.
Molecular analysis
Perhaps one of the most promising methods for demonstrating malignancy outside the realm of cytology is the use of PCR to show a monoclonal proliferation of malignant B-cells (or, in very rare instances, T-cells) [49]. Unfixed and unstained cytospin slides should be sent to a molecular lab, such as at the National Eye Institute (NEI), where microdissection of a minimal of 15 atypical cells may then be subjected to molecular analysis (Fig. 4) [24, 25, 50]. When using PCR to detect monoclonality [specifically, rearrangements of the immunoglobulin heavy chain (IgH) gene] of the malignant B-cell, the malignant cells most frequently involved in PIOL, knowledge of the typical primers used is essential. At the NEI we use primers including those for the second framework region (FR2A 5′- TGGRTCCGMCAGSCVYCNGG-3′), the third frame work region (FR3A 5 ′- A C A - CGGCYSTGTATTACTGT-3′), and CDR3 (5′- CCGGRAARRGTCTGGAGTGG-3′) to detect monoclonality within the variable region of the third complementary determining region (CDR3) in the IgH gene (Fig. 4B) [24, 25, 29]. Very rarely are PIOLs of T-cell lineage and if there is strong clinical evidence for suggesting such an entity (as in the setting of cutaneous T-cell lymphoma/mycosis fungoides) then we identify gene rearrangements in the T-cell receptor (TCR) gene by using primers for CDR3 and the variable region in the gamma chain (γ) gene [51]. All primers and necessary reagents are purchased from commercial sources.
Fig. 4.

(A) Photomicrograph showing the microdissected atypical cells to be submitted to molecular analysis (Giemsa, original magnification, 400×); (B) PCR amplification showing positive IgH gene rearrangement in these cells (first lane) as compared to the positive control (last lane) and negative control (middle lane)
It remains to be seen, however, what the PPV and NPV of PCR testing might be. Currently, cytologic evaluation is still the gold standard by which diagnoses of PIOL are made. If PCR, in fact, yields sensitivity far superior to that of cytology (while maintaining the same specificity, i.e., 100%) perhaps PCR analysis of vitrectomy specimens may supplant cytology for making a diagnosis of PIOL. Vitreous samples that contain very few lymphoma cells that are outnumbered by inflammatory cells may result in the polyclonal inflammatory cells overshadowing the monoclonality of the PIOL cells. Histo- and cytopathologic evaluations will never truly be supplanted because identification of malignant cells to describe immunohistological subtyping and to identify atypical cells for microdissection (which can improve the sensitivity of PCR analysis) will always be necessary. Indeed, at the NEI we are familiar with cytologic evaluation being noncontributory in making a diagnosis of PIOL. While we frequently employ molecular pathologic analysis (microdissection and PCR) to determine monoclonal rearrangement of the IgH gene [24, 25, 29] this test is still considered to be adjunctive to cytology. Appropriate time points to perform PCR are shown in Fig. 1.
External chorioretinal biopsy
While vitrectomy with the aforementioned evaluations may provide the ophthalmic surgeon with a diagnosis, this technique requires that there be malignant cells within the vitreous. However, failure to identify malignant cells in the vitreous can occur and may be due to degeneration of the malignant lymphoma cells, paucity of cells in the vitreous, or lack of involvement altogether of the vitreous. Lymphomatous involvement may be confined solely to the sub-RPE and chorioretinal biopsy (pioneered by Peyman and colleagues [52-55]) may yield tissue with which to make a diagnosis of PIOL. Interestingly, Peyman and colleagues were the first to report making a diagnosis of PIOL from tissue obtained via external chorioretinal biopsy [55].
The surgical technique for performing an external chorioretinal biopsy is fairly straightforward and is discussed with references to its essential points [56, 59]. First, provided that the fundus is clearly visible, laser photocoagulation is applied 1-3 days before surgery in a zone of the area to be biopsied. If the vitreous is too hazy, endolaser is placed immediately following vitrectomy. After a 360° conjunctival incision and isolation and tying of the rectus muscles a three-port pars plana vitrectomy is performed (in addition to endolaser application in the area to be biopsied if it was not placed prior to the surgery). The vitreous specimen (dilute and undilute) is sent for cytologic and microbiologic analyses as well as cytokine (IL-10 and IL-6) levels [1, 2, 24, 25, 30, 31, 57]. A nearly full-thickness scleral flap is made, leaving one side attached to act as a hinge. When the flap of sclera is retracted, the surgeon is able to visualize the choroids, which is practically bare. Next, a penetrating diathermy is placed through the choroid and retina along the outer margin of the inner choroidal bed. Two incisions parallel to the limbus are made. Next, by inserting one blade of a 0.12 forceps through the incision, the full thickness of the choroid and retina may be grasped at one edge. Then, two more incisions, perpendicular to the limbus, are made with Vannas scissors, thereby yielding a block of chorioretinal tissue. Extreme care should be taken to grasp the full-thickness tissue only once with the forceps so that the architecture of the tissue remains intact [56, 59]. The scleral flap is then closed over the wound and is sutured closed followed by fluid-gas exchange.
Chorioretinal biopsy, then, can be an important surgical procedure when other diagnostic tests have not resulted in identification of an infectious, malignant, or inflammatory etiology and when instituted empirical therapy has not changed the disease course [58-60]. Chorioretinal biopsy can provide excellent anatomy for the diagnosis of PIOL (Fig. 5).
Fig. 5.
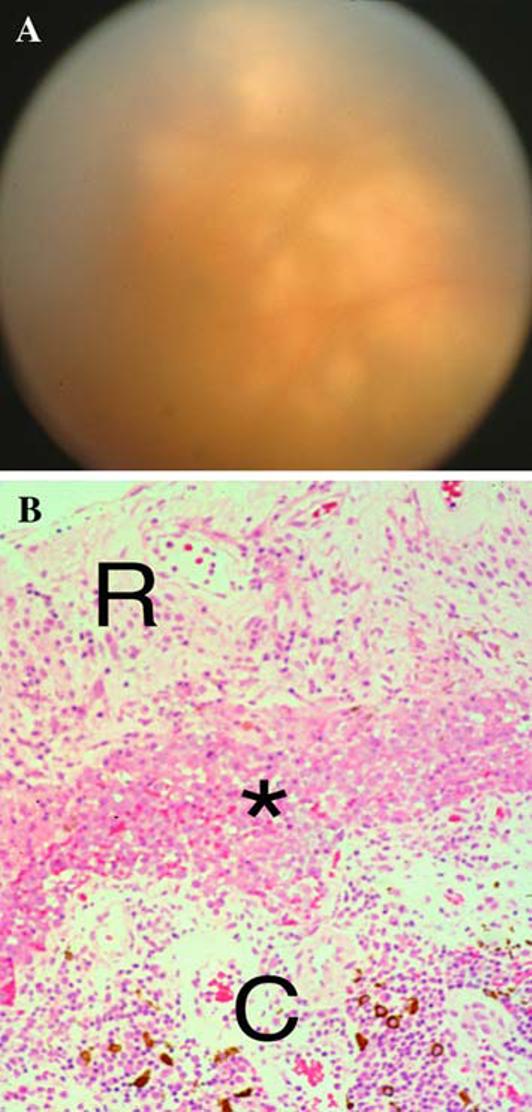
(A) Ophthalmoscopic examination showing haze vitreous and multiple yellowish subretinal lesions; (B) Chorioretinal biopsy of a methacrylate section showing PIOL cells (asterisk) located between the retina (R) and choroids (C), where inflammatory cells infiltrate (hematoxylin & eosin, original magnification, 100×)
Transvitreal retinochoroidal biopsy
Transvitreal retinochoroidal (endoretinal) biopsy is another approach by which chorioretinal tissue is aquired [56]. Briefly, in this technique a standard three-port vitrectomy is performed (sending undiluted and diluted vitrectomy to the pathology lab for analysis) with endodiathermy used to outline an area of retina that is of interest. The demarcated retinal tissue is then removed, intraocular scissors are introduced into the vitreous space and a small hole is created in the retina such that the area of interest is excised. With intraocular forceps, the retinal tissue is brought to the opening for the vitrector and is gently drained out of the eye using intraocular irrigation [56]. This technique was used in the past to obtain retinal tissue in difficult cases of PIOL. For example, one 67-year-old woman presented with lesions reminiscent of viral retinitis (Fig. 6A), but was recalcitrant to corticosteroid and anti-viral therapy (Desai, Sundar, Chan, unpublished data). Due to the patient’s rapidly deteriorating vision as well as bilaterally ominous lesions (with those in the right eye encroaching on the fovea), transvitreal retinochoroidal biopsy was performed (Fig. 6B). Cytology of the vitreous fluid obtained from vitrectomy (and cerebrospinal fluid from lumbar puncture) failed to reveal lymphoma cells. However, assay for IL-10 in the vitreous and CSF were 5,456 pg/ml (normal = 0 pg/ml) and 25 pg/ml (normal = 0 pg/ml), respectively and IL-6 levels were 193 pg/ml (normal = 0 pg/ml) and undetectable in the vitreous and CSF, respectively. Immunopathology of the retinal specimen (snap frozen) showed a predominance of B-lymphocytes (positive for CD19, CD20, and CD22) with monoclonal restriction (κ light chain positive). Thus, PIOL was highly suggested by these findings and a MRI with gadolinium of the brain was performed showing a 1 × 1 cm mass lesion in the right frontal lobe that enhanced with contrast. Biopsy of this mass revealed a diffuse large B-cell lymphoma (PCNSL). Whole-brain radiation therapy was then instituted.
Fig. 6.
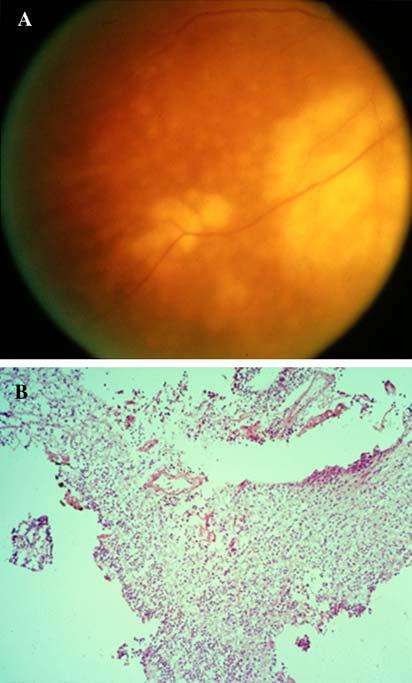
(A) Ophthalmoscopic examination showing multiple large and small orange-yellowish subretinal lesions; (B) Endoretinal biopsy of a frozen section showing disorganized retinal tissues and poor morphology (hematoxylin & eosin, original magnification, 200×)
Cassoux and colleagues have also used endoretinal biopsy to diagnose PIOL when vitrectomy was nondiagnostic [61]. The patient had undergone vitrectomy twice that failed to reveal any malignant lymphocytes, but a high IL-10 level was detected [61]. Cassoux et al. noted that their technique for performing an endoretinal biopsy involved the induction of a localized retinal detachment (if one was not already present) by injecting sodium hyaluronate into the subretina. The resultant bulging retina was subsequently excised by cutting around the perimeter with scissors and extricating the biopsy with forceps. Endolaser was then applied around the biopsy site [61].
Diagnostic testing of chorioretinal and endoretinal biopsy tissue
The biopsy tissue is immediately processed by an ophthalmic pathologist in the operating room. It is generally divided into three portions [62]. One third of the tissue is fixated for routine histopathologic studies, including light and electron microscopic examinations. The second portion is snap frozen in optimal cutting temperature (OCT) embedding compound and is used for immunopathologic and molecular characterization. The third portion is sent for culture with the preference for viral and other microorganisms cultures and/or tissue culture [62].
Again, external chorioretinal and transvitreal retinochoroidal biopsies are typically performed when diagnostic vitrectomy in inconclusive but there remains a very high suspicion for PIOL. Risks of biopsying, which include hypotony, hemorrhages, endophthalmitis, retinal detachment, and cataract formation should be outweighed by the risk of allowing lesions that threaten the macula, or are infectious or malignant in etiology to progress. In addition, a plan should be designed so that proper testing of the biopsy tissue is carried out. This requires coordinating care amongst the surgeons, pathologist, microbiologist, and molecular biologists. The information obtained from the biopsy can often lead to a change in clinical treatment (such as initiating chemotherapy) with benefits not only seen in visual acuity improvement and tumor regression, but in potentially extending the life of the patient [59].
Endoretinal biopsy tissues are often small and delicate and require handling with extra care [63]. The biopsy specimen may not be able to be divided into three portions, but, rather, only enough to be processed for frozen sections. These sections can then undergo routine histology, immunohistochemistry and molecular analysis.
In summary, vitreal, chroioretinal and/or endoretinal biopsy is needed to provide the diagnosis of PIOL if lymphoma cells are not found in the CSF. Prior to the biopsy procedure, it is critical to have a thorough discussion among the surgeons (vitreoretinal surgeon and uveitis specialist), pathologists and molecular biologists. The discussion and plan will help to minimize surgical risks and to carry out the biopsy tissue properly. The information obtained from the biopsy can often lead to a correct diagnosis, appropriate clinical treatment, and prolonging the PIOL patient’s life [64].
Acknowledgement
Support: The NEI intramural research program
Abbreviations
- CSF
cerebrospinal fluid
- PCNSL
primary central nervous system lymphoma
- PIOL
primary intraocular lymphoma
- PCR
polymerase chain reaction amplification
- RPE
retinal pigment epithelium
Contributor Information
John A. Gonzales, Immunopathology Section, Laboratory of Immunology, National Eye Institute, NationalInstitutes of Health Building 10, Room 10N103, MSC 1857, 10 Center Drive, Bethesda, MD 20892-1857, USA; Howard Hughes Medical Institute, Chevy Chase, MD, USA & Keck School of Medicine, University of Southern California, Los Angeles, CA, USA
Chi-Chao Chan, Immunopathology Section, Laboratory of Immunology, National Eye Institute, National Institutes of Health Building 10, Room 10N103, MSC 1857, 10 Center Drive, Bethesda, MD 20892-1857, USA.
References
- 1.Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol. 2002;13(6):411–418. doi: 10.1097/00055735-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Chan CC, Wallace DJ. Intraocular lymphoma: up-date on diagnosis and management. Cancer Control. 2004;11(5):285–295. doi: 10.1177/107327480401100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Char DH, Ljung BM, Miller T, Phillips T. Primary intraocular lymphoma (ocular reticulum cell sarcoma) diagnosis and management. Ophthalmology. 1988;95:625–630. doi: 10.1016/s0161-6420(88)33145-3. [DOI] [PubMed] [Google Scholar]
- 4.Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol. 2004;242(11):901–913. doi: 10.1007/s00417-004-0973-0. [DOI] [PubMed] [Google Scholar]
- 5.Hormigo A, Abrey L, Heinemann MH, DeAngelis LM. Ocular presentation of primary central nervous system lymphoma: diagnosis and treatment. Br J Haematol. 2004;126(2):202–208. doi: 10.1111/j.1365-2141.2004.05028.x. [DOI] [PubMed] [Google Scholar]
- 6.de Smet MD, Nussenblatt RB, Davis JL, Palestine AG. Large cell lymphoma masquerading as a viral retinitis. Int Ophthalmol. 1990;14(56):413–417. doi: 10.1007/BF00163568. [DOI] [PubMed] [Google Scholar]
- 7.Rockwood eJ, Zakov ZN, Bay JW. Combined malignant lymphoma of the eye and CNS (reticulum-cell sarcoma) J Neurosurg. 1984;61:369–374. doi: 10.3171/jns.1984.61.2.0369. [DOI] [PubMed] [Google Scholar]
- 8.Char DH, Margolis L, Newman AB. Ocular reticulum cell sarcoma. Am J Ophthalmol. 1981;91(4):480–483. doi: 10.1016/0002-9394(81)90236-1. [DOI] [PubMed] [Google Scholar]
- 9.Baehring JM, Androudi S, Longtine JJ, et al. Analysis of clonal immunoglobulin heavy chain rearrangements in ocular lymphoma. Cancer. 2005;104(3):591–597. doi: 10.1002/cncr.21191. [DOI] [PubMed] [Google Scholar]
- 10.Machemer R. Advances in vitrectomy through the pars-plana (author’s transl) Klin Monatsbl Augenheilkd. 1974;164(4):572–579. [PubMed] [Google Scholar]
- 11.Machemer R. Subtotal vitrectomy through the pars plana. Trans Am Acad Ophthalmol Otolaryngol. 1973;77(2):OP198–OP201. [PubMed] [Google Scholar]
- 12.Machemer R, Buettner H, Norton EW, Parel JM. Vitrectomy: a pars plana approach. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(4):813–820. [PubMed] [Google Scholar]
- 13.Machemer R, Buettner H, Parel JM. Vitrectomy, a pars plana approach. Instrumentation. Mod Probl Ophthalmol. 1972;10:172–177. [PubMed] [Google Scholar]
- 14.Machemer R, Norton EW. Vitrectomy, a pars plana approach. II. Clinical experience. Mod Probl Ophthalmol. 1972;10:178–185. [PubMed] [Google Scholar]
- 15.Machemer R, Parel JM, Norton EW. Vitrectomy: a pars plana approach. Technical improvements and further results. Trans Am Acad Ophthalmol Otolaryngol. 1972;76(2):462–466. [PubMed] [Google Scholar]
- 16.Currey TA, Deutsch AR. Reticulum cell sarcoma of the uvea. South Med J. 1965;58:919. [Google Scholar]
- 17.Vogel MH, Font RL, Zimmerman LE, Levine RA. Reticulum cell sarcoma of the retina and uvea. Report of six cases and review of the literature. Am J Ophthalmol. 1968;66(2):205–215. doi: 10.1016/0002-9394(68)92065-5. [DOI] [PubMed] [Google Scholar]
- 18.Nevins RC, Jr, Frey WW, Elliott JH. Primary, solitary, intraocular reticulum cell sarcoma (microgliomatosis). (A clinicopathologic case report) Trans Am Acad Ophthalmol Otolaryngol. 1968;72(6):867–876. [PubMed] [Google Scholar]
- 19.Klingele TG, Hogan MJ. Ocular reticulum cell sarcoma. Am J Ophthalmol. 1975;179(1):39–47. doi: 10.1016/0002-9394(75)90453-5. [DOI] [PubMed] [Google Scholar]
- 20.Michels RG, Knox DL, Erozan YS, Green WR. Intraocular reticulum cell sarcoma. Diagnosis by pars plana vitrectomy. Arch Ophthalmol. 1975;93(12):1331–1335. doi: 10.1001/archopht.1975.01010020961005. [DOI] [PubMed] [Google Scholar]
- 21.Parver LM, Font RL. Malignant lymphoma of the retina and brain. Initial diagnosis by cytologic examination of vitreous aspirate. Arch Ophthalmol. 1979;97(8):1505–1507. doi: 10.1001/archopht.1979.01020020167016. [DOI] [PubMed] [Google Scholar]
- 22.Whitcup SM, de Smet MD, Rubin BI, et al. Intraocular lymphoma. Clinical and histopathologic diagnosis. Ophthalmology. 1993;100:1399–1406. doi: 10.1016/s0161-6420(93)31469-7. [DOI] [PubMed] [Google Scholar]
- 23.Tuaillon N, Chan CC. Molecular analysis of primary central nervous system and primary intraocular lymphoma. Curr Mol Med. 2001;1(2):259–272. doi: 10.2174/1566524013363915. [DOI] [PubMed] [Google Scholar]
- 24.Shen DF, Zhuang Z, LeHoang P, et al. Utility of microdissection and polymerase chain reaction for the detection of immunoglobulin gene rearrangement and translocation in primary intraocular lymphoma. Ophthalmology. 1998;105(9):1664–1669. doi: 10.1016/S0161-6420(98)99036-4. [DOI] [PubMed] [Google Scholar]
- 25.Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc. 2003;101:275–292. [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf LA, Reed GF, Buggage RR, et al. Vitreous cytokine levels. Ophthalmology. 2003;110(8):1671–1672. doi: 10.1016/S0161-6420(03)00811-X. [DOI] [PubMed] [Google Scholar]
- 27.Cassoux N, Giron A, Bodaghi B, et al. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma (PIOL) Invest Ophthalmol Vis Sci. 2007 doi: 10.1167/iovs.06-0031. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitcup SM, Chan CC, Buggage RR, et al. Improving the diagnostic yield of vitrectomy for intraocular lymphoma. Arch Ophthalmol. 2000;118(3):446. [PubMed] [Google Scholar]
- 29.Chan CC, Shen D, Nussenblatt RB, et al. Detection of molecular changes in primary intraocular lymphoma by microdissection and polymerase chain reaction. Diagn Mol Pathol. 1998;7(1):63–64. doi: 10.1097/00019606-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Chan CC, Shen DF, Whitcup SM, et al. Detection of human herpesvirus-8 and Epstein-Barr virus DNA in primary intraocular lymphoma. Blood. 1999;93(8):2749–2751. [PubMed] [Google Scholar]
- 31.Shen DF, Herbort CP, Tuaillon N, et al. Detection of toxoplasma gondii DNA in primary intraocular B-cell lymphoma. Mod Pathol. 2001;14(10):995–999. doi: 10.1038/modpathol.3880424. [DOI] [PubMed] [Google Scholar]
- 32.Blumenkranz MS, Ward T, Murphy S, et al. Applications and limitations of vitreoretinal biopsy techniques in intraocular large cell lymphoma. Retina. 1992;12(3):S64–S70. doi: 10.1097/00006982-199212031-00014. [DOI] [PubMed] [Google Scholar]
- 33.Davis JL, Miller DM, Ruiz P. Diagnostic testing of vitrectomy specimens. Am J Ophthalmol. 2005;140(5):822–829. doi: 10.1016/j.ajo.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 34.Palexas GN. Diagnostic pars plana vitrectomy report of a 21-year retrospective study. Trans Am Ophthalmol Soc. 1995;93:281–308. [PMC free article] [PubMed] [Google Scholar]
- 35.Char DH, Ljung BM, Deschenes J, Miller TR. Intraocular lymphoma: immunological and cytological analysis. Br J Ophthalmol. 1988;72(12):905–911. doi: 10.1136/bjo.72.12.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaldivar RA, Martin DF, Holden JT, Grossniklaus HE. Primary intraocular lymphoma: clinical, cytologic, and flow cytometric analysis. Ophthalmology. 2004;111(9):1762–1767. doi: 10.1016/j.ophtha.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Blay JY, Burdin N, Rousset F, et al. Serum interleukin-10 in non-Hodgkin’s lymphoma: a prognostic factor. Blood. 1993;82(7):2169–2174. [PubMed] [Google Scholar]
- 38.Murray PI, Hoekzema R, van Haren MA, et al. Aqueous humor interleukin-6 levels in uveitis. Invest Ophthalmol Vis Sci. 1990;31(5):917–920. [PubMed] [Google Scholar]
- 39.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D’Andrea A, Aste-Amezaga M, Valiante NM, et al. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178(3):1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Waal Malefyt R, Haanen J, Spits H, et al. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horn F, Henze C, Heidrich K. Interleukin-6 signal transduction and lymphocyte function. Immunobiology. 2000;202(2):151–167. doi: 10.1016/S0171-2985(00)80061-3. [DOI] [PubMed] [Google Scholar]
- 43.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Chan CC, Whitcup SM, Solomon D, Nussenblatt RB. Interleukin-10 in the vitreous of primary intraocular lymphoma. Am J Ophthalmol. 1995;120(5):671–673. doi: 10.1016/s0002-9394(14)72217-2. [DOI] [PubMed] [Google Scholar]
- 45.Whitcup SM, Stark-Vancs V, Wittes RE, et al. Association of interleukin-10 in the vitreous and cerebral spinal fluid and primary central nervous system lymphoma. Arch Ophthalmol. 1997;115:1157–1160. doi: 10.1001/archopht.1997.01100160327010. [DOI] [PubMed] [Google Scholar]
- 46.Merle-Beral H, Davi F, Cassoux N, et al. Biological diagnosis of primary intraocular lymphoma. Br J Haematol. 2004;124(4):469–473. doi: 10.1046/j.1365-2141.2003.04800.x. [DOI] [PubMed] [Google Scholar]
- 47.Yokota M, Takase H, Imai Y, et al. A case of intraocular malignant lymphoma diagnosed by immunoglobulin gene rearrangement and translocation, and IL-10/ IL-6 ratio in the vitreous fluid. Nippon Ganka Gakkai Zasshi. 2003;107(5):287–291. [PubMed] [Google Scholar]
- 48.Velez G, de Smet MD, Whitcup SM, et al. Iris involvement in primary intraocular lymphoma: report of two cases and review of the literature. Surv Ophthalmol. 2000;44(6):518–526. doi: 10.1016/s0039-6257(00)00118-1. [DOI] [PubMed] [Google Scholar]
- 49.Coupland SE, Hummel M, Muller HH, Stein H. Molecular analysis of immunoglobulin genes in primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2005;46(10):3507–3514. doi: 10.1167/iovs.05-0401. [DOI] [PubMed] [Google Scholar]
- 50.Wallace DJ, Shen DF, Reed GF, et al. Detection of the bcl-2 t(14;18) translocation and proto-oncogene expression in primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2006;47(7):2750–2756. doi: 10.1167/iovs.05-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coupland SE, Anastassiou G, Bornfeld N, et al. Primary intraocular lymphoma of T-cell type: report of a case and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2005;243(3):189–197. doi: 10.1007/s00417-004-0890-2. [DOI] [PubMed] [Google Scholar]
- 52.Peyman GA, Dodich NA. Full-thickness eye wall resection: an experimental approach for treatment of choroidal melanoma. I. Dacron-graft. Invest Ophthalmol. 1972;11(2):115–121. [PubMed] [Google Scholar]
- 53.Peyman GA, Homer P, Kasbeer R, Vlchek J. Full-thickness eye wall biopsy. II. In primates. Invest Ophthalmol. 1975;14(7):565–567. [PubMed] [Google Scholar]
- 54.Peyman GA, Meisels HI, Batko KA, Vlchek JK. Full-thickness eye wall biopsy. I. An experimental approach to the tissue diagnosis and study of choroidal and retinal lesions. Invest Ophthalmol. 1975;14(6):484–487. [PubMed] [Google Scholar]
- 55.Peyman GA, Juarez CP, Raichand M. Full-thickness eye-wall biopsy: long-term results in 9 patients. Br J Ophthalmol. 1981;65(10):723–726. doi: 10.1136/bjo.65.10.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nussenblatt RB, Davis JL, Palestine AG. Chorioretinal biopsy for diagnostic purposes in cases of intraocular inflammatory disease. Dev Ophthalmol. 1992;23:133–138. doi: 10.1159/000429641. [DOI] [PubMed] [Google Scholar]
- 57.Chan CC. Primary intraocular lymphoma: clinical features, diagnosis, and treatment. Clin Lymphoma. 2003;4(1):30–31. doi: 10.1016/s1526-9655(11)70005-7. [DOI] [PubMed] [Google Scholar]
- 58.Johnston RL, Tufail A, Lightman S, et al. Retinal and choroidal biopsies are helpful in unclear uveitis of suspected infectious or malignant origin. Ophthalmology. 2004;111(3):522–528. doi: 10.1016/j.ophtha.2002.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Martin DF, Chan CC, de Smet MD, et al. The role of chorioretinal biopsy in the management of posterior uveitis. Ophthalmology. 1993;100(5):705–714. doi: 10.1016/s0161-6420(93)31585-x. [DOI] [PubMed] [Google Scholar]
- 60.Ridley ME, McDonald HR, Sternberg P, Jr, et al. Retinal manifestations of ocular lymphoma (reticulum cell sarcoma) Ophthalmology. 1992;99(7):1153–1160. doi: 10.1016/s0161-6420(92)31834-2. discussion 1160-1161. [DOI] [PubMed] [Google Scholar]
- 61.Cassoux N, Charlotte F, Rao NA, et al. Endoretinal biopsy in establishing the diagnosis of uveitis: a clinicopathologic report of three cases. Ocul Immunol Inflamm. 2005;13(1):79–83. doi: 10.1080/09273940590909149. [DOI] [PubMed] [Google Scholar]
- 62.Nussenblatt RB, Whitcup SM. Uveitis: Fundamental and Clinical Practice. 3rd edn. Mosby; 2004. [Google Scholar]
- 63.Liang X, Shen D, Huang Y, et al. Molecular pathology and CXCR4 expression in surgically excised retinal. Ophthalmology. 2007;114(1):147–156. doi: 10.1016/j.ophtha.2006.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan CC, Gonzales JA. Primary Intraocular Lymphoma: World Scientific. Singapore, London, Hackensack NJ: 2007. (in press) [Google Scholar]