Abstract
Objective
To investigate whether the sheep xenograft model of human hematopoiesis can be used to mimic mobilization of human hematopoietic stem cells in vivo.
Material and Methods
Sheep transplanted with 3.6×106 CD34+ from human adult bone marrow were mobilized 1.5 years post-transplant with human G-CSF for 5 days. At day 3 and 4 of mobilization, human cells were harvested from PB and BM and were injected into secondary sheep recipients (n=6) and these animals were analyzed for the presence of human cells in their BM and PB starting at 3.5 months post-transplantation.
Results
Maximum mobilization of human cells in PB occurred at day 3, with a 21-fold increase in total numbers of human cells, and a recovery of 5.5×104/ml of CD34+. In the BM, maximal numbers of human cells were achieved at day 4, with a 6.3-fold increase and a recovery of 1.5×104/ml CD34+ cells. PB and BM mobilized human cells were then transplanted into new sheep recipients, and analysis at 3.5 months post-transplant demonstrated that levels of human cell engraftment in BM of the group transplanted with mobilized PB were significantly lower than those transplanted with BM cells (0.6±0.1 versus 8.0±1.8 %). Furthermore, in sheep transplanted with mobilized PB, the levels of human cells in circulation remained 2.5-fold higher than the levels of human cells found in their BM.
Conclusion
Mobilization of human cells in the sheep model parallels human PB and BM HSC mobilization in healthy human donors in their ability to engraft, differentiate and repopulate secondary hosts. Thus, this model can become a useful tool to study mobilization regimens, mechanisms and quality of products obtained.
INTRODUCTION
Mobilized peripheral blood stem cells (PBSC) are increasingly used as an alternative to bone marrow derived stem cells (BMHSC) for both allogeneic and autologous transplantation. [1-9] The faster hematopoietic and immune reconstitution which is obtained when PBSC are used as a hematopoietic graft, and the simplicity and safety of collection methods have allowed PBSC to quickly replace BMHSC as the cells of choice for HSC transplantation [1,2, 5-6] In addition, it has also been suggested that there is a lower incidence of tumor contamination of PBSC preparations compared with BM [6,9]. The large amount of accessory cells present in peripheral blood could potentially also allow for immunomodulatory approaches to enhance graft versus tumor response following transplantation [1,2]. While several authors have reported differences in the phenotype, cell cycle status, and amount of stem cells present in PBSC versus BMHSC [10-21], other research groups have devoted their attention to the mechanisms by which HSC are mobilized [22-27]. Still, a further understanding of the mechanisms of mobilization and the quality and biological characteristics of stem cell subsets in peripheral blood with respect to both the level and durability of engraftment they provide would likely contribute to the development of safer, more predictable and efficacious mobilization protocols with higher safety. In addition, a better understanding of the mobilization process would allow the study of new mobilization regimens and the use of new molecules for mobilization, potentially leading to the development of protocols that enable differential mobilization of normal versus tumor cells.
Although in vitro studies have proven invaluable in the analysis of HSC, it has proven very difficult to establish a clear-cut relationship between LTC-IC and in vivo repopulation and durability of engraftment in vivo. Several non-human primate and murine models have been developed to study the engraftment and differentiation of HSC [24,27], and Van der Loo et al. have described a model in which mobilization of human cells was possible following transplantation of mice with human PBSC [28].
The human–to-sheep model of in utero transplantation has proven to be an invaluable tool in the identification, characterization and quantification of human hematopoietic stem cells from several sources [29-32]. In this model, we are able to study the behavior of the engrafted human HSC within a physiologically relevant animal that is large enough to allow for multiple samplings over long periods of time, and in which human cells expand sufficiently to allow us to sort engrafted human cells back out of peripheral blood or marrow following in vivo treatments and evaluate their engraftment/differentiative potential in secondary recipients [33-35]. In the present studies, we demonstrate that the human-to-sheep xenograft model of human hematopoiesis can serve as an accurate model to study in vivo mobilization of human hematopoietic stem cells, and that the human cells mobilized from sheep PB and BM parallel human PB and BM HSC mobilized in healthy human donors in their ability to engraft, differentiate and repopulate secondary hosts.
MATERIAL AND METHODS
Human donor cell preparation
Heparinized human bone marrow (HBM) was obtained from healthy donors after informed consent. Low density bone marrow mononuclear cells (BMNC) were separated by a Ficoll density gradient (1.077g/ml) (Sigma, St Louis, MO) and washed twice in Iscove’s modified Dulbecco’s media (IMDM), (Gibco Laboratories, Grand Island, NY). BMNCs were then enriched for the CD34+ fraction using magnetic cell sorting (Miltenyi Biotec, Inc., Auburn, CA).
Creation of the human sheep chimeras and assessment of engraftment
Fetal sheep (n=3) at 55-60 days of gestation were transplanted in utero with 3.6×106 human CD34+ cells following the procedure that has been described in detail previously.29 Adult sheep bone marrow (SBM) was obtained from the posterior iliac crest of normal adult sheep following standard procedures that had been approved by the University of Nevada Institutional Animal Care and Use Committee (IACUC). The transplanted sheep were analyzed for donor (human) cell engraftment in bone marrow and peripheral blood at 3.5 5.5, 6.5 and 11.5 months after transplantation.
Assessment of human donor cell engraftment and selection of human cells by cell sorting
The presence of donor cells in hematopoietic tissues of the recipients was determined at intervals post-transplantation using flow cytometric analysis and hematopoietic progenitor assays. Flow cytometric analysis of the cell populations was performed in a FACScan (Becton Dickinson Immunosystems [BDIS], San Jose, CA). Monoclonal antibodies to various cluster designations (CDs) directly conjugated with FITC or PE were used according to the manufacturer’s recommendation. These included: CD45, CD34, CD20, CD3, CD7, HLA-DR (BDIS), and Glycophorin A (Immunotech, Miami, FL).
Sorting of the CD45+ fraction of human cells from the BM and PB of chimeric sheep was performed at days on which the highest levels of human cells were seen, corresponding to days 2 and 3 of mobilization, on a FACS Vantage after labeling with CD45 and CD34 (BDIS).
Assays for human clonogenic progenitors were performed in triplicate in MethoCult GF H4434 (StemCell Technologies Inc, Vancouver, Canada) from either BM or PB cells that were collected at day 0, 1, 2 and 3 of culture. Assays for sheep clonogenic progenitors were performed as previously reported29-32. In brief 5×105 BM or PB mononuclear cells were plated in 2mls of methylcellulose in the presence of erythropoietin (0.4 IU/mL) and a preparation of PHA-LCM (5% vol/vol) derived from sheep leukocytes. The cultures were then incubated in a humidified incubator at 37°C in 5% CO2 in air. After 5-10 days for sheep and 9-14 days for human, colonies were counted and categorized according to standard criteria.
Mobilization of chimeric and control sheep with G-CSF
Human recombinant G-CSF, Neupogen, (Amgen, Inc., Thousand Oaks, CA) was administereed for 5 days once a day subcutaneously in the morning. Animals were individually weighed before injection and a dose of 4.8-5.7μg/Kg was used.
Transplant of mobilized human cells into secondary sheep recipients
After sorting of human CD45+cells from either BM or PB, these cells were transplanted into 6 new 55-60 days old fetal sheep recipients. The lambs were allowed to complete gestation and undergo normal birth, and human cell engraftment and/or differentiation was then evaluated in these lambs.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). Comparisons between experimental results were determined by two-sided non-paired Student’s t-test analysis. A p value < 0.05 was considered statistically significant.
RESULTS
Recombinant human G-CSF is able to mobilize sheep stem/progenitor cells
The first question that we wanted to address was whether human G-CSF would be able to mobilize sheep stem/progenitor cells, or it would be able to exclusively mobilize human cells in a chimeric animal. To this end, 5 non-transplanted sheep between 1-3 years of age were mobilized with human recombinant G-CSF for 5 days. Animals were sampled every day for evaluation of their total white blood cell count in the PB and progenitor cell content in PB and BM. In 4 out of 5 animals, G-CSF caused an efficient increase in total numbers of white blood cells in PB when compared to day 0 of mobilization and/or control un-mobilized sheep (n=3) (p<0.01) (Figure 1). Furthermore, short-term clonogenic assays, at day 0, gave rise to CFU-GM 170±19.2; BFU-E 35.8±6.2 and GEMM 4.4±1.2 in the BM while in the PB, the number of CFU-GM was 15±2.2; BFU-E 2±0.8 and GEMM 1±0.1. At day 3 of mobilization, there was a 2-fold increase in total colony forming units in the BM, and a 10 fold increase in the PB with numbers of colonies increasing to CFU-GM 161±4.2; BFU-E 15±0.8 and GEMM 7±2.1, demonstrating the ability of human G-CSF to mobilize sheep stem/progenitor cells.
Figure 1. Human r-G-CSF is able to mobilize sheep white blood cells in control non-transplanted sheep.
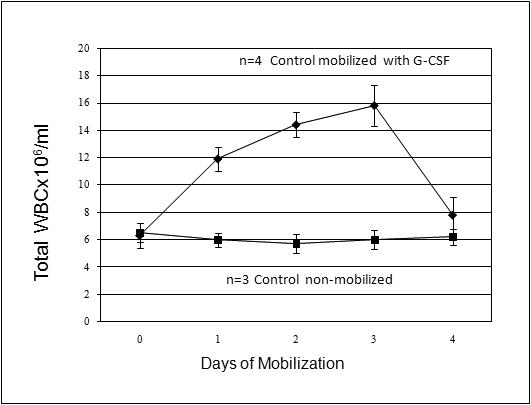
The results show that an efficient increase in total numbers of white blood cells in PB in all of the mobilized sheep (◆) (n=4) occurred when compared to day 0 of mobilization and a control un-mobilized sheep (■) (n=3) (p<0.01).
Selection of human chimeric animals for G-CSF mobilization studies
Sheep that had been made chimeric by in utero transplantation of 3.6×106 adult human BM CD34+cells at 55-60 gestational days were evaluated for the presence of human cells in their BM and PB starting shortly after birth (3.5 months post-transplant) and at regular intervals thereafter. 3 animals that exhibited stable chimerism since birth were selected to participate in this study. The overall levels of human cell engraftment as determined by flow cytometric analyses of the peripheral blood and bone marrow at the different time points post-transplant are shown in Figure 2. As can be seen at all time points post-transplant, significant levels of human cell engraftment were found in these chimeric sheep in both BM and PB.
Figure 2. Selection of human chimeric animals for mobilization.
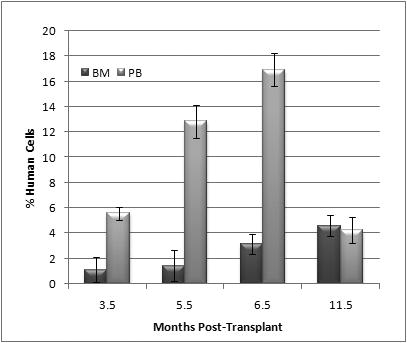
3 animals that had been made chimeric by in utero HSC transplantation and displayed stable chimerism were selected to participate in this study. Flow cytometric analyses of the peripheral blood and bone marrow at the different time points post-transplant show consistent levels of human cell engraftment
Mobilization of human-sheep chimeras with recombinant human G-CSF
In order to evaluate the efficacy of mobilization of human progenitor cells in chimeric animals, we used evaluation of total white blood cell counts pre-, during and post-mobilization, combined with flow cytometric analysis using human-specific antibodies against human cell progenitors and differentiated cells. Figure 3 displays data from PB of 2 mobilized chimeric sheep that responded to G-CSF. The 3rd chimeric animal that received a dose of 4.8μg/Kg yielded only a slight, non-significant increase in WBC count in PB, and was thus considered a “poor mobilizer” and was not included in the study. Of note is that in this animal the percentage of human cells in the PB did not increase with G-CSF administration. In the other 2 chimeric animals that received G-CSF, maximal mobilization of total WBC (sheep + human) into the PB occurred at days 2 and 3 of mobilization. Within the same animal, the number of human versus sheep WBC was calculated at the different time points by multiplying the total number of WBC by the percentage of respectively human or sheep CD45+ cells present in the sample that day. The total number of human leucocytes ,CD45+ cells, was significantly increased at days 2 and 3 of mobilization as compared with levels of human cells at day 0 (p<0.01)
Figure 3. Mobilization of human and sheep white blood cells in chimeric sheep.
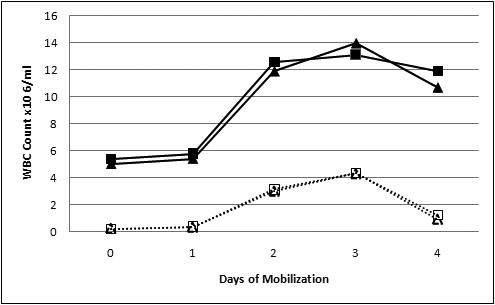
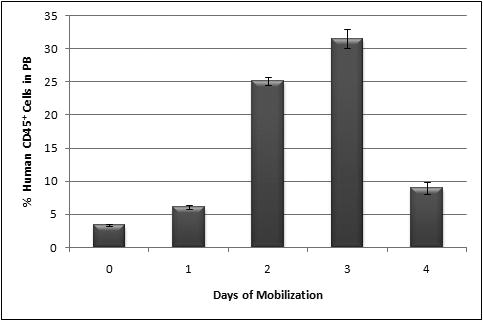
(A) Both human and sheep WBC were mobilized to the PB in the chimeric animals. Solid lines depict total number of WBC in the 2 animals that responded to G-CSF. One animal is represented by ■ while the other is represented by ▲. Doted lines represent the total number of human cells mobilized to the PB at each time point. In order to calculate the specific numbers of human cells mobilized, the total number of WBC was multiplied by the percentage of human CD45+ cells. Maximal mobilization of human cells to the PB occurred at days 2 and 3 of mobilization. The average of human cells mobilized at day 3 was significantly increased as compared with levels of human cells at day 0 (p<0.01). (B) Percentage of human CD45+ cells mobilized to the PB at each time point.
In order to evaluate whether G-CSF was more efficient at mobilizing human cells than sheep cells within the same animal, we determined the fold increase of human WBC cell counts at day 0 and day 3, and compared this to the fold increase of sheep WBC at the same time points. G-CSF was much more efficient in mobilizing human cells, since the total human WBC counts went from 0.2±0.02×106 at day 0 to 4.3±0.04×106 at day 3 corresponding to a 21.5-fold increase of total human white blood cells. By contrast, in these same chimeric animals, the total number of WBC only increased 3 fold, since it was 4.45±1.3×106 at day 0 and increased to 13.5±10.6 2.2 ×106 at day 3.
The percentage of human CD45+ cells in PB of mobilized sheep can be seen in Figure 3b. At day 0, the levels of CD45+ in PB were 3.4±0.14 %, increased to 6.12±0.3 at day 1, 25.11±0.7 at day 2, and achieved a maximum at day 3 of mobilization with 31.55±1.5% of CD45+ cells. The human cells mobilized into circulation were not only of myeloid origin, but were in fact multilineage, with the presence of T cells, B cells, dendritic cells and red blood cells (Day3: CD7 14.8-15.8%; CD20 3.84-4.9%; CD1a 27.9-28.5%; CD86 0-1.7%; GlyA 7.1-7,7%). In order to evaluate the number of human progenitor cells mobilized into the peripheral blood, the percentage of human CD34+ cells at each day of mobilization was determined. While no human CD34+ cells were found in the PB on days 0, 1 and 2 of mobilization, at day 3 and 4 we found respectively 5.5±2.2×104/ml and 0.7±0.4 ×104/ml CD34+cells, corresponding to the percentages of 0.4±0.1 and 0.07±0.01 of CD34+ cells in PB. The number of CD34+ cells in PB correlated well with human colony formation in short-term colony assays. The total clonogenic potential of PB at day 0 was 35±9.8, and increased at day 3 to 240.6±52.4 CFU/ml, demonstrating that human progenitor cells were indeed in circulation.
Expansion of human cells in the bone marrow of the mobilized sheep
We also wished to determine the effect of human recombinant G-CSF in the human-cell content of the BM of the chimeric sheep. Figure 4 shows the percentage of human CD45+ cells in the BM of mobilized sheep versus days of mobilization. There was a progressive and significant expansion of the levels of human cells with the mobilization time, reaching the highest level at day 4 of mobilization (8.4±0.7 % of CD45+ cells). Also, at day 4 of mobilization, BM reached the highest percentage of CD34+ cells (0.1-0.2%), CD7 cells (1.5-1.6%) and CD1a+ dendritic cells (4.3-10.4%). Of note is that the total numbers of human cells in the marrow of G-CSF mobilized sheep reached their highest levels at a later time point than what was seen in the PB.
Figure 4. Percentage of human CD45+ cells in BM of mobilized sheep.
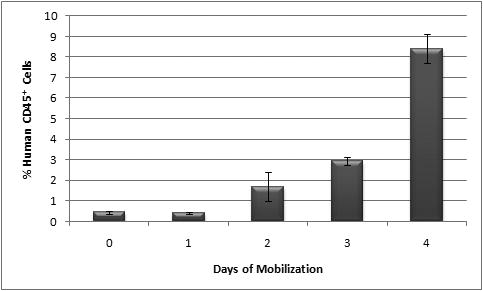
Percentage of human CD45+ increased in BM of mobilized sheep to reach a maximum at day 4 of mobilization.
Transplant of Mobilized PB and BM into secondary sheep recipients
We had previously demonstrated that differences existed in the short- and long-term repopulating ability between human mobilized BM and PB when these cells were transplanted directly into our model. Thus, we wanted to investigate whether human stem cells from mobilized chimeric sheep peripheral blood (PB) differed from mobilized chimeric sheep bone marrow (BM) in their potential to engraft following transplantation. To this end, CD45+ cells from either mobilized PB or BM were sorted from the mobilized chimeric sheep in a FACS vantage as described in the materials and methods section. 4.5 ×105 CD45+ cells from BM and 2×106 CD45+ cells from PB were obtained from one animal and 3.2×105 and 1.8 ×106, respectively from the other one. For the first animal, the CD34 content of these preparations was respectively for the BM and PB, 6.1×103 and 1.9×104. For the other animal, the CD34 content was 5.8×103 for the BM and 1.7×104 for the PB.
The number of CD45+ injected into secondary recipients from either BM (n=3) or PB (n=3) was adjusted for the CD34+ content of the graft with each animal receiving 4×103CD34+ cells. At 3.5 months post-transplant, we analyzed these secondary recipients for the presence of human cells in their BM and PB, and the results are displayed in Figure 5. The levels of human cell engraftment in BM were significantly higher in sheep transplanted with CD45+ cells from BM than the ones transplanted with CD45+ cells from mobilized peripheral blood (8.0±1.8 versus 0.6±0.1 %) (p<0.01). Furthermore, the presence of human cells in PB was also consistently higher in animals transplanted with BM-derived cells than with their PB counterpart (5±0.98 versus 1.5±0.15 %) Of note is that while in the sheep that were transplanted with HSC from BM the levels of human cells were higher in BM, in the ones transplanted with PB, the levels of human cells were higher in their PB.
Figure 5. Percentage of human cells at 3.5 months post-transplant in secondary sheep recipients transplanted with either mobilized BM or PB.
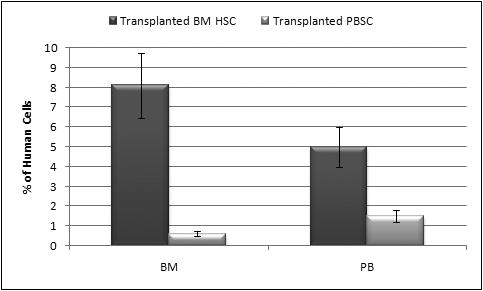
The levels of human cells present in BM and in PB were significantly higher in sheep transplanted with CD45+ cells from BM than the ones transplanted with CD45+ cells from mobilized peripheral blood (p<0.01).
We followed sheep transplanted with the mobilized PB for an additional 3 months, and at 6 months post-transplant, the total levels of human cells persisted at higher levels in their PB (3.3%) than their BM (1.3%) (Figure 6). We also looked at the distribution of CD34+ cells in the animals transplanted with PBSC, and, as can be seen in Figure 6, levels of CD34+ cells in circulation were higher than the levels of CD34+ cells in their BM.
Figure 6. Levels of human cells in BM and PB of sheep transplanted with mobilized PB at 6 months post-transplant.
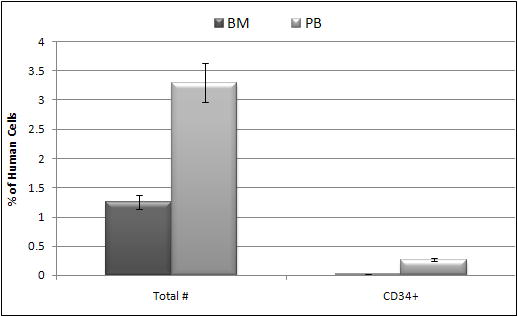
In these animals the total levels of human cells persisted in higher levels in the PB in the BM. Also CD34+ cells in the animals transplanted with PBSC remained higher in circulation.
DISCUSSION
Mobilized peripheral blood HSC (PBSC) are rapidly replacing marrow HSC as a clinical source of HSC for hematopoietic reconstitution, due largely to the ease and safety of collection. Although recent studies have shed light on the possible mechanism(s) of mobilization [12, 22-27], few studies have been performed that compare the quality of the HSC from peripheral blood versus marrow, with respect to the levels and durability of engraftment [16-17,33]. This is mainly due to the lack of a suitable model system in which to study mobilization and in vivo engraftment behavior of mobilized cells. In the present studies, we assessed the feasibility of using sheep made chimeric with human cells by in utero transplantation to serve as a large animal model of human HSC mobilization in vivo.
To this end, we first examined whether the administration of human G-CSF to normal control sheep (non-transplanted) would affect their steady-state hematopoiesis. We observed a significant increase in the peripheral blood white count starting at day 1, and demonstrated that hematopoietic progenitors capable of forming colonies in short-term methylcellulose assays were efficiently mobilized into the PB of the sheep receiving human G-CSF. Having demonstrated that human G-CSF could be administered to sheep without toxicity and could in fact exert clinically relevant effects on the hematopoietic system of control sheep, we next looked at the suitability of the human-sheep xenograft to the study of human HSC mobilization in vivo. At one year post-transplant, sheep that had been made chimeric by in utero transplantation of adult human bone marrow CD34+ cells were mobilized for 4 days with human G-CSF. Each day of the mobilization protocol, peripheral blood and bone marrow were harvested and evaluated by both flow cytometry and hematopoietic colony assays to assess the in vivo mobilization of human progenitors. Our results show that human progenitor cells are mobilized more efficiently by human G-CSF than are the endogenous sheep progenitors, with levels of human cells in the circulation of the mobilized sheep as high as 32% at day 3 of mobilization. This increased efficiency in mobilizing human stem /progenitor cells versus self HSC can likely be attributed to the partial homology (82%) found between human and sheep G-CSF protein [36]. The absolute number of human CD34+ cells within the circulation increased during mobilization to levels as high as 5.5±2.2×104/ml CD34+ cells/ml of blood, and human-specific colony assays confirmed that these were indeed multipotent human hematopoietic progenitors that were being mobilized. As reported by others [37-39], we saw an increase in numbers of dendritic cells in both bone marrow and PB of mobilized sheep.
We also examined whether the administration of human G-CSF to the chimeric sheep would affect the human progenitor content of the chimeric bone marrow. Our data demonstrate that significant increases in the levels of human cells occurred in the marrow. However, the mobilization of human cells in the marrow was delayed in comparison to the effects in the peripheral blood, with the maximal levels of human cells in the marrow being achieved after the peak levels of human progenitors in the blood had already been reached and was returning to baseline.
In order to investigate the quality of the human cells mobilized in the sheep model, and assess how they compared to human stem cells mobilized from healthy human donors, we transplanted mobilized PB and BM into new sheep recipients in order to compare the quality of the HSC obtained from mobilized PB and BM with respect to their ability to provide high level durable engraftment following transplantation. In agreement with a previous study, sheep transplanted with both mobilized PBSC and marrow HSC exhibited durable engraftment [33]. However, animals receiving PBSC exhibited lower levels of engraftment of human cells in both BM and PB than other animals transplanted with cells from the mobilized BM. Furthermore, animals receiving human cells from mobilized peripheral blood persistently exhibited higher levels of human cell activity within their peripheral blood than in their BM. This finding correlates well with existing clinical data showing that patients who have received mobilized PB grafts rather than marrow HSC exhibit more rapid hematologic recovery in their periphery. In addition, although CD34+ cells were present in the secondary recipients transplanted with PBSC, they located preferentially in their PB rather than the BM, suggesting that important differences may exist between HSC from mobilized peripheral blood when compared to those from marrow. Of note is that on a cell-per-cell basis, engraftment of human cells in secondary recipients seems to be more efficient than in primary recipients. This is noticeable by the fact that 4×103 CD34+ cells transplanted into secondary recipients generated levels of engraftment similar to those in primary recipients that had received a much higher dose of CD34+cells. Although we see this effect in most of our studies, we have thus far been unable to determine whether the first transplant is selecting cells with higher engraftment capabilities or is favoring cells more apt to survive and differentiate within the sheep BM’s microenvironment.
In conclusion, we demonstrate that human hematopoietic progenitors capable of long-term hematopoietic engraftment in sheep recipients can be efficiently mobilized into the peripheral blood of human-sheep hematopoietic chimeras using a standard clinical mobilization protocol with G-CSF. As in humans, our results demonstrate that important qualitative differences may exist between PBSC and marrow-derived HSC with respect to the speed of hematologic recovery and the level of engraftment that they provide following transplantation. These studies suggest that the human-sheep xenograft model can be used to study human HSC mobilization in vivo, and potentially contribute to the elucidation of the mechanisms responsible for mobilization, thus allowing the development of novel, safer and more predictable mobilization strategies for clinical use.
Acknowledgments
Supported by: Grants HL 70566, HL73737, HL52955, NAG9-1340 from National Institutes of Health, and NASA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arai S, Klingemann HG. Hematopoietic stem cell transplantation: bone marrow vs. mobilized peripheral blood. Arch Med Res. 2003 Nov-Dec;34(6):545–53. doi: 10.1016/j.arcmed.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Russell NH, Byrne JL. Allogeneic transplantation using peripheral blood stem cells. Best Pract Res Clin Haematol. 2001 Dec;14(4):685–700. doi: 10.1053/beha.2001.0167. [DOI] [PubMed] [Google Scholar]
- 3.Theilgaard-Mönch K, Raaschou-Jensen K, Andersen H, Russell CA, Vindeløv L, Jacobsen N, Dickmeiss E. Single leukapheresis products collected from healthy donors after the administration of granulocyte colony-stimulating factor contain ten-fold higher numbers of long-term reconstituting hematopoietic progenitor cells than conventional bone marrow allografts. BMT. 1999;23:243. doi: 10.1038/sj.bmt.1701579. [DOI] [PubMed] [Google Scholar]
- 4.Jansen J, Hanks S, Thompson JM, Dugan MJ, Akard LP. Transplantation of hematopoietic stem cells from the peripheral blood. J Cell Mol Med. 2005 Jan-Mar;9(1):37–50. doi: 10.1111/j.1582-4934.2005.tb00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S, Robison LL, Francisco L, Carter A, Liu Y, Grant M, Baker KS, Fung H, Gurney JG, McGlave PB, Nademanee A, Ramsay NK, Stein A, Weisdorf DJ, Forman SJ. Late mortality in survivors of autologous hematopoietic cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005 Feb 8; doi: 10.1182/blood-2005-01-0035. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderlini P, Korbling M. The use of mobilized peripheral blood stem cells from normal donors for allografting. Stem Cells. 1997;15:9. doi: 10.1002/stem.150009. [DOI] [PubMed] [Google Scholar]
- 7.To LB, Haylock DN, Simmons PJ, Juttner CA. The Biology and Clinical Uses of Blood Stem Cells. Blood. 1997;89:2233. [PubMed] [Google Scholar]
- 8.Sohn SK, Kim JG, Kim DH, Baek JH, Lee KB. Diverse clinical applications using advantages of allogeneic peripheral blood stem cell transplantation. Int J Hematol. 2004 Jun;79(5):457–61. doi: 10.1532/ijh97.a10313. [DOI] [PubMed] [Google Scholar]
- 9.Platzbecker U, Ehninger G, Bornhauser M. Allogeneic transplantation of CD34+ selected hematopoietic cells--clinical problems and current challenges. Leuk Lymphoma. 2004 Mar;45(3):447–53. doi: 10.1080/10428190310001615684. [DOI] [PubMed] [Google Scholar]
- 10.Prosper F, Stroncek D, Verfaillie CM. Phenotypic and functional characterization of long-term culture-initiating cells present in peripheral blood progenitor collections of normal donor treated with granulocyte colony-stimulating factor. Blood. 1996;88:2033. [PubMed] [Google Scholar]
- 11.To LB, Haylock DN, Dowse T, Simmons PJ, Trimboli S, Ashman LK, Juttner CA. A Comparative Study of the Phenotype and Proliferative Capacity of Peripheral Blood (PB) CD34+ Cells Mobilized by Four Different Protocols and Those of Steady-Phase PB and Bone Marrow CD34+ Cells. Blood. 1994;84:2930. [PubMed] [Google Scholar]
- 12.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002 Sep;30(9):973–81. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 13.Leung W, Ramirez M, Civin CI. Quantity and quality of engrafting cells in cord blood and autologous mobilized peripheral blood. BMT. 1999;5:69. doi: 10.1053/bbmt.1999.v5.pm10371358. [DOI] [PubMed] [Google Scholar]
- 14.Leung W, Ramirez M, Novelli EM, Civin CI. In vivo engraftment potential of clinical hematopoietic grafts. J Investig Med. 1998;46:303. [PubMed] [Google Scholar]
- 15.Yan XQ, Chen Y, Hartley C, McElroy P, Fletcher F, McNiece IK. Marrow repopulating cells in mobilized PBPC can be serially transplanted for up to five generations or be remobilized in PBPC reconstituted mice. BMT. 1998;21:975. doi: 10.1038/sj.bmt.1701219. [DOI] [PubMed] [Google Scholar]
- 16.Benboubker L, Domenech J, Linassier C, Desbois I, Delain M, Lamagnere JP, Colombat P, Binet C. Long-term cultures to evaluate engraftment potential of CD34+ cells from peripheral blood after mobilization by chemotherapy with and without GM-CSF. Exp Hematol. 1995;23:1568. [PubMed] [Google Scholar]
- 17.Turner CW, Yeager AM, Waller EK, l, Wingard JR, Fleming WH. Engraftment potential of different sources of human hematopoietic progenitor cells in BNX mice. Blood. 1996;87:3237. [PubMed] [Google Scholar]
- 18.Steidl U, Kronenwett R, Rohr UP, Fenk R, Kliszewski S, Maercker C, Neubert P, Aivado M, Koch J, Modlich O, Bojar H, Gattermann N, Haas R. Gene expression profiling identifies significant differences between the molecular phenotypes of bone marrow-derived and circulating human CD34+ hematopoietic stem cells. Blood. 2002 Mar 15;99(6):2037–44. doi: 10.1182/blood.v99.6.2037. [DOI] [PubMed] [Google Scholar]
- 19.Roberts AW, Metcalf D. Noncycling state of peripheral blood progenitor cells mobilized by granulocyte colony-stimulating factor and other cytokines. Blood. 1995 Aug 15;86(4):1600–5. [PubMed] [Google Scholar]
- 20.Uchida N, He D, Friera AM, Reitsma M, Sasaki D, Chen B, Tsukamoto A. The unexpected G0/G1 cell cycle status of mobilized hematopoietic stem cells from peripheral blood. Blood. 1997 Jan 15;89(2):465–72. [PubMed] [Google Scholar]
- 21.Prosper F, Vanoverbake K, Stroncek D, Verfaillie CM. Primitive Long-Term Culture Initiating Cells (LTC-ICs) in Granulocyte Colony-Stimulating Factor Mobilized Peripheral Blood Progenitor Cells Have Similar Potential for Ex Vivo Expansion as Primitive LTC-ICs in Steady State Bone Marrow. Blood. 1997;89:3991. [PubMed] [Google Scholar]
- 22.Ulyanova T, Scott LM, Priestley GV, Jiang Y, Nakamoto B, Koni PA, Papayannopoulou T. VCAM-1 expression in adult hemopoietic and non-hemopoietic cells is controlled by tissue inductive signals and reflects their developmental origin. Blood. 2005 Mar 15; doi: 10.1182/blood-2004-09-3417. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papayannopoulou T, Priestley GV, Nakamoto B, Zafiropoulos V, Scott LM. Molecular pathways in bone marrow homing: dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood. 2001 Oct 15;98(8):2403–11. doi: 10.1182/blood.v98.8.2403. [DOI] [PubMed] [Google Scholar]
- 24.Mahmud N, Patel H, Hoffman R. Growth factors mobilize CXCR4 low/negative primitive hematopoietic stem/progenitor cells from the bone marrow of nonhuman primates. Biol Blood Marrow Transplant. 2004 Oct;10(10):681–90. doi: 10.1016/j.bbmt.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004 Jul 1;104(1):65–72. doi: 10.1182/blood-2003-05-1589. Epub 2004 Mar 9. [DOI] [PubMed] [Google Scholar]
- 26.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001 Sep 1;98(5):1289–97. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 27.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha-4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol. 2003 Dec;23(24):9349–60. doi: 10.1128/MCB.23.24.9349-9360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Loo JCM, Hanenberg H, Cooper RJ, Luo FY, Lazaridis EN, Williams DA. Nonobese Diabetic/Severe Combined Immunodeficiency (NOD/SCID) Mouse as a Model System to Study the Engraftment and Mobilization of Human Peripheral Blood Stem Cells. Blood. 1998;92:2556. [PubMed] [Google Scholar]
- 29.Kawashima I, Zanjani ED, Almeida-Porada G, Flake AW, Zeng H, Ogawa M. CD34+ human marrow cells that express low levels of Kit protein are enriched for long-term marrow-engrafting cells. Blood. 1996 May 15;87(10):4136–42. [PubMed] [Google Scholar]
- 30.Civin CI, Almeida-Porada G, Lee MJ, Olweus J, Terstappen LW, Zanjani ED. Sustained, retransplantable, multilineage engraftment of highly purified adult human bone marrow stem cells in vivo. Blood. 1996 Dec 1;88(11):4102–9. [PubMed] [Google Scholar]
- 31.Ziegler BL, Valtieri M, Porada GA, De Maria R, Muller R, Masella B, Gabbianelli M, Casella I, Pelosi E, Bock T, Zanjani ED, Peschle C. KDR receptor: a key marker defining hematopoietic stem cells. Science. 1999 Sep 3;285(5433):1553–8. doi: 10.1126/science.285.5433.1553. [DOI] [PubMed] [Google Scholar]
- 32.Almeida-Porada G, El Shabrawy D, Porada C, Zanjani ED. Differentiative potential of human metanephric mesenchymal cells. Exp Hematol. 2002 Dec;30(12):1454–62. doi: 10.1016/s0301-472x(02)00967-0. [DOI] [PubMed] [Google Scholar]
- 33.Verfaillie CM, Almeida-Porada G, Wissink S, Zanjani ED. Kinetics of engraftment of CD34(-) and CD34(+) cells from mobilized blood differs from that of CD34(-) and CD34(+) cells from bone marrow. Exp Hematol. 2000 Sep;28(9):1071–9. doi: 10.1016/s0301-472x(00)00506-3. [DOI] [PubMed] [Google Scholar]
- 34.Almeida-Porada G, Porada CD, Chamberlain J, Torabi A, Zanjani ED. Formation of human hepatocytes by human hematopoietic stem cells in sheep. Blood. 2004 Oct 15;104(8):2582–90. doi: 10.1182/blood-2004-01-0259. Epub 2004 Jul 1. [DOI] [PubMed] [Google Scholar]
- 35.Zanjani ED, Flake AW, Rice H, Hedrick M, Tavassoli M. Long-term repopulating ability of xenogeneic transplanted human fetal liver hematopoietic stem cells in sheep. J Clin Invest. 1994 Mar;93(3):1051–5. doi: 10.1172/JCI117054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Brien PM, Seow HF, Rothel JS, Wood PR. Cloning and sequencing of an ovine granulocyte colony-stimulating factor cDNA. DNA Seq. 1994;4(5):339–42. doi: 10.3109/10425179409020862. [DOI] [PubMed] [Google Scholar]
- 37.Gazitt Yair. Immunologic Profiles of Effector Cells and Peripheral Blood Stem Cells Mobilized with Different Hematopoietic Growth Factors. Stem Cells. 2000 November;18(No 6):390–398. doi: 10.1634/stemcells.18-6-390. [DOI] [PubMed] [Google Scholar]
- 38.Shier LR, Schultz KR, Imren S, Regan J, Issekutz A, Sadek I, Gilman A, Luo Z, Panzarella T, Eaves CJ, Couban S. Differential effects of granulocyte colony-stimulating factor on marrow- and blood-derived hematopoietic and immune cell populations in healthy human donors. Biol Blood Marrow Transplant. 2004 Sep;10(9):624–34. doi: 10.1016/j.bbmt.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Ings SJ, Balsa C, Leverett D, Mackinnon S, Linch DC, Watts MJ. Peripheral blood stem cell yield in 400 normal donors mobilised with granulocyte colony-stimulating factor (G-CSF): impact of age, sex, donor weight and type of G-CSF used. British Journal of Haematology. 2007;134(5):517–525. doi: 10.1111/j.1365-2141.2006.06223.x. [DOI] [PubMed] [Google Scholar]


