Abstract
Heterochromatin normally has prescribed chromosomal positions and must not encroach on adjacent regions. We demonstrate that the fission yeast protein Epe1 stabilises silent chromatin, preventing the oscillation of heterochromatin domains. Epe1 loss leads to two contrasting phenotypes: alleviation of silencing within heterochromatin and expansion of silent chromatin into neighbouring euchromatin. Thus, we propose that Epe1 regulates heterochromatin assembly and disassembly, thereby affecting heterochromatin integrity, centromere function and chromosome segregation fidelity. Epe1 regulates the extent of heterochromatin domains at the level of chromatin, not via the RNAi pathway. Analysis of an ectopically silenced site suggests that heterochromatin oscillation occurs in the absence of heterochromatin boundaries. Epe1 requires predicted iron- and 2-oxyglutarate (2-OG)-binding residues for in vivo function, indicating that it is probably a 2-OG/Fe(II)-dependent dioxygenase. We suggest that, rather than being a histone demethylase, Epe1 may be a protein hydroxylase that affects the stability of a heterochromatin protein, or protein–protein interaction, to regulate the extent of heterochromatin domains. Thus, Epe1 ensures that heterochromatin is restricted to the domains to which it is targeted by RNAi.
Keywords: centromere, Epe1, fission yeast, heterochromatin, JmjC domain
Introduction
Heterochromatin is a conserved feature of eukaryotic chromosomes and plays an important role in chromosome segregation, genomic stability and gene regulation. In the fisson yeast Schizosaccharomyces pombe, heterochromatin is formed at centromeres, telomeres and the mating-type (mat) locus (Verdel and Moazed, 2005). Centromeres are composed of a central domain (cnt), which has a specialised chromatin structure associated with the histone H3 variant Cnp1/CENP-A, flanked by heterochromatic outer repeats (otr) (Pidoux and Allshire, 2004). At centromeres, tRNA genes (Scott et al, 2006) and the IRCs (Cam et al, 2005) have been implicated in confining heterochromatin. At the mating-type locus, the mat2 and mat3 silent donor loci and the K region are packaged into heterochromatin constrained by the IR-R and IR-L barrier elements which recruit TFIIIC (Noma et al, 2001, 2006; Thon et al, 2002).
In regions of silent chromatin, histones are generally underacetylated (Ekwall et al, 1997; Mellone et al, 2003) and are methylated at lysine 9 of histone H3 (H3K9me) by the histone methyltransferase (HMTase) Clr4, a member of the highly conserved Suv39 family (Rea et al, 2000). The H3K9 methylation is a binding site for the chromodomain proteins: Swi6, Chp1 and Chp2 (Ekwall et al, 1995; Bannister et al, 2001; Nakayama et al, 2001; Sadaie et al, 2004).
Transcription of the outer repeats by RNA polymerase II (RNAPII) generates noncoding RNA transcripts that are processed into small interfering RNAs (siRNAs) by the RNaseIII-like ribonuclease Dicer (Dcr1). siRNAs associate with the RNA-induced Initiation of Transcriptional Silencing (RITS) complex, which consists of Chp1, Argonaute (Ago1) and Tas3. The RITS complex uses the siRNAs to target it to homologous chromatin for silencing (Motamedi et al, 2004; Noma et al, 2004; Verdel et al, 2004). Mutants in RNAi pathway proteins such as dcr1Δ, ago1Δ and rpb2, the second largest subunit of RNA polymerase II, lose centromeric silencing (reviewed by Verdel and Moazed, 2005). However, RNAi is dispensable for the maintenance of heterochromatin at the mat locus (Jia et al, 2004; Kim et al, 2004).
Previously, we proposed that S. pombe protein Epe1 and other members of the JmjC domain family are 2-OG/Fe(II)-dependent dioxygenases that may act as histone demethylases (Trewick et al, 2005). Recently, several JmjC domain proteins have been demonstrated to have this activity (reviewed by Klose et al, 2006). Epe1 is distributed across all the major heterochromatic domains and certain meiotic genes (Zofall and Grewal, 2006). The observation that Epe1 blocks heterochromatin from forming beyond the IR-L barrier at the mat locus lead to the proposal that Epe1 is a negative regulator of heterochromatin (Ayoub et al, 2003). Loss of Epe1 leads to the downregulation of genes that are known to be upregulated in cells with defective silent chromatin, suggesting that Epe1 counteracts silencing of repressed genes (Isaac et al, 2007). It has also been suggested that Epe1 directly facilitates the access of RNAPII to centromeric repeats and that Epe1 has a role at heterochromatin boundaries by facilitating transcription of the IRC boundary elements (Zofall and Grewal, 2006).
Here we show that contrary to previous reports, predicted Fe(II)- and 2-OG-binding residues are required for Epe1 function, suggesting that Epe1 is a 2-OG/Fe(II)-dependent dioxygenase. We also demonstrate that Epe1 acts at the chromatin level to prevent heterochromatin domains from both expanding and contracting.
Results
Epe1 restrains heterochromatin to its normal domain
We initially identified Epe1 as an Swi6 interacting protein in a yeast two-hybrid screen. The Epe1 cDNA obtained corresponded to the region spanning from amino acid 652 to the C-terminus, indicating that the region containing the JmjC domain of Epe1 is not required for the interaction with Swi6 (Supplementary Figure 1A). Consistent with this and the observations of others (Zofall and Grewal, 2006; Isaac et al, 2007), GFP-tagged Epe1 was found to colocalise with Swi6 at heterochromatin. This localisation is dependent on Swi6, Clr4 and Rik1 (Supplementary Figure 1B).
As Epe1 is localised to heterochromatin, we investigated its role in heterochromatin stability using marker genes inserted within and outside centromeric heterochromatin at centromere 1 (cen1). Genes placed within the centromeres are transcriptionally silenced due to the formation of H3K9 methylation/Swi6-dependent heterochromatin (Allshire et al, 1995; Grewal and Klar, 1996). In the case of the ura4+ marker gene, this silencing results in restricted growth on selective plates that lack uracil (−URA) and good growth on counter-selective plates that contain 5-fluoroorotic acid (FOA). Genes inserted in the distal extremity of cen1 are less silent (sites 3 and 4: Figure 1A; Allshire et al, 1995) and genes inserted in the euchromatin immediately adjacent to cen1 are expressed well (sites 1 and 2: Figure 1A). Deletion of the gene encoding Epe1 (epe1Δ) results in enhanced silencing of markers inserted at the extremities of the cen1 outer repeat (sites 3 and 4), indicated by increased growth on FOA. In addition, loss of Epe1 causes significant silencing of the normally fully expressed marker genes in adjacent euchromatin (sites 1 and 2; Figure 1B). Chromatin immunoprecipitation (ChIP) analysis was performed to examine the level of H3K9me2, a well-characterised histone modification associated with silent chromatin. In epe1Δ cells, high levels of H3K9me2 are found at the normally euchromatic region outside of the centromere (Figure 1C). This agrees with previous observations showing that in epe1Δ cells, silent chromatin extends into nearby euchromatic regions and results in gene silencing (Ayoub et al, 2003; Zofall and Grewal, 2006).
Figure 1.
Epe1 restrains heterochromatin to its normal domain. (A) Location of ura4+ markers at the extremities of cen1. Outside of cen1 markers were inserted at the HpaI (site 1) and XhoI (site 2) sites. At the extremities of the otr, markers were inserted in opposite orientations at the BglII site (sites 3 and 4). (B) epe1Δ causes expansion of centromeric heterochromatin. epe1Δ cells with the ura4+ marker inserted at the indicated site were spotted onto the indicated media. (C) H3K9me2 ChIP analysis of wild-type (WT) and epe1Δ strains containing the indicated ura4+ insertion compared with the uraDS/E mini-gene.
Epe1 is required for centromeric heterochromatin integrity
Loss of Epe1 promotes spreading of silent chromatin from heterochromatin domains into euchromatin (Figure 1; Ayoub et al, 2003; Zofall and Grewal, 2006). However, the effect of epe1Δ on heterochromatin integrity has not been tested. To address this, we examined the effect of the epe1Δ mutation on silencing of an ade6+ gene inserted within the centromeric outer repeats (Figure 2A). In wild-type cells, this ade6+ gene (cen1-otr1R(SphI):ade6+) is strongly repressed, resulting in the formation of red colonies on plates with limiting adenine, due to the accumulation of a red adenine precursor. Conversely, clr4Δ cells that lack the histone H3K9 methyltransferase are unable to assemble heterochromatin, resulting in full expression of the ade6+ marker and the formation of white colonies. Unusually, epe1Δ colonies exhibit variegation, resulting in white, pink and red colonies (Figure 2B). The state of silencing of cen1-otr1:ade6+ switches frequently in an epe1Δ population, so that when white colonies are replated they often give rise to red and pink colonies and vice versa (Supplementary Figure 2). Thus, although these epe1Δ isolates are genetically identical, they display distinct metastable silent and expressed states, which must reflect epigenetic differences in centromeric heterochromatin integrity.
Figure 2.
Epe1 is required for normal centromeric heterochromatin integrity. (A) A ura4+ marker was inserted at the NcoI site of the imr and an ade6+ marker was inserted in the SphI site in the otr. (B) Loss of Epe1 causes variegation of silencing at the centromere otr. Single colonies of epe1Δ with ade6+ at the SphI site in the otr were spotted onto media containing a low level of adenine. (C) Loss of Epe1 causes loss of silencing at the centromere imr. Cells containing ura4+ marker inserted in the NcoI site of the imr were preselected on either media lacking uracil or containing FOA. Colonies were then spotted onto the indicated plates. (D) Loss of Epe1 causes chromosome segregation defects. Single colonies of epe1Δ mutants with ade6+ at the otr were spotted onto media containing low levels of adenine and media containing 15 μg/ml TBZ. (E) epe1Δ cells exhibit lagging chromosomes (indicated by arrow). The number of anaphase cells with lagging chromosomes was assessed in white and red/pink otr1:ade6+ epe1Δ cells as well as wild-type (WT) and swi6Δ.
We also tested whether loss of Epe1 affects silencing of a ura4+ gene located within centromeric heterochromatin (cen1-imr1L(NcoI):ura4+; Figure 2A). In wild-type strains, this ura4+ gene is strongly silenced, allowing good growth on FOA and low levels of growth on media lacking uracil. In contrast, the silencing in epe1Δ cells variegates (data not shown). Colonies with the ura4+ gene in the transcriptionally silent or active states were picked from FOA or −URA plates and their ability to grow was assessed when challenged with selective (−URA) or counter selective (FOA) medium. epe1Δ colonies expressing the ura4+ gene (from −URA plates) were consistently able to sustain this active state, allowing better growth on −URA plates than similarly preselected wild-type colonies (Figure 2C). In reciprocal experiments, FOA-resistant epe1Δ cells selected for silencing of imr1L(NcoI):ura4+ gene (from FOA plates) were less capable than wild type in sustaining this repressed state resulting in more growth on −URA (Figure 2C). Together, this indicates that silent chromatin at centromeres is less stable in the absence of Epe1.
We show that loss of Epe1 causes not only spreading of heterochromatin into euchromatic regions, but also the destabilisation of heterochromatin within the centromere. The destabilisation of silencing observed in the absence of Epe1 is inconsistent with the previously proposed role for Epe1 as a factor that acts to prevent heterochromatin spreading past specific boundary elements (Ayoub et al, 2003; Zofall and Grewal, 2006).
Epe1 is required for normal centromere function
It is well established that mutants with defective centromeric heterochromatin, such as swi6Δ, clr4Δ and rik1Δ, have chromosome segregation defects; they display lagging chromosomes on late anaphase spindles and are sensitive to microtubule-destabilising drug thiabendazole (TBZ) (Ekwall et al, 1995; Ekwall et al, 1996). If loss of Epe1 leads to disruption of heterochromatin, then epe1Δ cells would be expected to display similar chromosome segregation defects. epe1Δ colonies in which cen1-otr1(SphI):ade6+ was silent (red) or expressed (white) were replated in a serial dilution assay on plates containing 15 μg/ml TBZ. Cells derived from white epe1Δ colonies consistently displayed greater TBZ sensitivity than wild type; however, genetically identical red epe1Δ colonies were not very TBZ sensitive compared to wild type (Figure 2D). This indicates that TBZ sensitivity covariegates with the silent/expressed state, implying that cells with less intact silent chromatin are more prone to chromosome mis-segregation events.
epe1Δ cells exhibit lagging chromosomes at an elevated frequency compared to wild-type cells. A higher incidence of lagging chromosomes was observed in cultures derived from white otr1(SphI):ade6+ colonies than their genetically identical red/pink relatives (Figure 2E). This indicates that the white-expressed state caused by loss of Epe1 is incompatible with normal chromosome segregation and is consistent with Epe1 being required for centromeric heterochromatin integrity and centromere function. Therefore, white epe1Δ colonies, in a manner similar to swi6Δ cells, exhibit defective centromeric heterochromatin, which results in loss of silencing, lagging chromosomes and TBZ sensitivity.
Loss of Epe1 causes heterochromatin to oscillate
Epe1 is required to restrict domains of heterochromatin, and in its absence heterochromatin spreads into surrounding euchromatin (Figure 1; Ayoub et al, 2003; Zofall and Grewal, 2006). However, contrary to this, our analysis demonstrates that Epe1 is required for normal heterochromatin integrity since loss of Epe1 destabilises silencing at centromeres and causes chromosome segregation defects. It is surprising that these seemingly opposing effects could be caused by absence of the same protein. A possible explanation for this phenotype is that expansion of a silent domain disrupts silencing at more internal sites, perhaps by titrating away essential components of heterochromatin. An alternative explanation is that in the absence of Epe1, the silent chromatin domains oscillate, either expanding into euchromatin or retreating to allow alleviation of silencing.
To address these possibilities, a strain was constructed with a ura4+ in a normally nonsilent euchromatic site (otr1R(XhoI):ura4+) and, on the same side of the centromere, an ade6+ gene within the silent otr region (otr1R(SphI):ade6+; Figure 3A). Wild-type cells silence the centromeric ade6+ gene, forming red colonies and express the euchromatic ura4+ gene. Some epe1Δ colonies form an extended heterochromatin domain, silencing the euchromatic ura4+ gene (FOAR colonies). The majority of these epe1Δ colonies are red or pink (Figure 3A), indicating that in the absence of Epe1 centromeric heterochromatin is not disrupted when silent chromatin extends into neighbouring euchromatin.
Figure 3.
Loss of Epe1 causes heterochromatin to oscillate. Strains were constructed with an ade6+ marker inserted at the SphI site on the right otr of cen1 and a ura4+ marker at the XhoI site on the same side of the centromere (A) or on the opposite side of the centromere (B). Single colonies were picked from wild-type (WT) and epe1Δ strains and spotted onto the indicated plates. Single red or white colonies were replated onto the indicated media, 500 of the resulting colonies were classified according to their colour. The numbers indicated are representative of several experiments.
Also, when centromeric heterochromatin is disrupted in epe1Δ cells, silencing does not spread into the euchromatin. This is demonstrated by white (cen1-otr1:ade6+ expressing) epe1Δ cells which when replated showed good expression of the euchromatic ura4+ gene (poor growth on FOA). However, occasionally, white epe1Δ colonies with disrupted centromeric silencing gave rise to a few colonies on FOA plates, however, these were red/pink rather than white. This again indicates that in epe1Δ cells, repression of ura4+ outside the normal silent domain requires silencing to be intact in the adjacent outer repeats (Figure 3A).
A similar experiment was performed with a strain in which the ura4+ gene was inserted at the equivalent position in the euchromatin on the opposite side of the centromere to the ade6+ marker (Figure 3B). Again, when epe1Δ cells were plated on FOA to select colonies in which heterochromatin has spread over the ura4+ gene, the colonies formed were red or pink, indicating that ade6+ is repressed on the other side of the centromere (Figure 3B). Therefore, silencing must be maintained across the outer repeats on the left-hand side of the centromere to allow the expansion of the heterochromatin domain on the right-hand side.
Together, these data indicate that in epe1Δ cells the spreading of heterochromatin beyond the normal centromeric domain does not destabilise silent chromatin within the centromere. It also indicates that epe1Δ mutants require intact heterochromatin on both left and right centromeric otr repeats in order to form an extended heterochromatin domain. Therefore, loss of Epe1 leads to a more erratic form of silent chromatin, allowing heterochromatin to oscillate, retreating or extending over greater distances than observed in the wild-type cells.
Heterochromatin expansion occurs independently of boundaries in epe1Δ cells
Epe1 has been proposed to act at boundaries because peaks of Epe1 localisation have been found to coincide with, and promote the transcription of IRC elements (Zofall and Grewal, 2006). Moreover, IRC elements have been demonstrated to act as boundary elements (Noma et al, 2006). However, if Epe1 functions only at boundary elements, loss of Epe1 would be expected to have no effect on an ectopically silenced locus where no known boundary elements are present. The ectopic silencer strain contains a 1.6 kb fragment from the outer repeat of centromere 3 (L5) inserted at the ade6 locus (Figure 4A) and has been shown to efficiently silence an adjacent ura4+ marker gene but not the ade6+ gene 1.3 kb downstream of the ura4+ ORF. H3K9me2, Swi6 and Chp1 are associated with this ectopically silenced ura4+ and this silencing is dependent on RNAi and heterochromatin components. Thus, the silent chromatin formed at this ectopic site (ade6:L5-ura4+) is indistinguishable from that found at the centromeric repeats themselves (Partridge et al, 2002; Volpe et al, 2003). In wild-type strains, silencing of this ade6:L5-ura4+ reporter allows good growth on FOA relative to −URA plates (Figure 4A). However, some epe1Δ cells display increased growth on −URA relative to wild type (Figure 4A). This suggests that loss of Epe1 destabilises heterochromatin at the ectopic silencer. ChIP analysis shows that in epe1Δ cells with disrupted silencing, H3K9me2 decreases and H3K9 acetylation increases on the ura4+ marker (Supplementary Figure 4).
Figure 4.
Heterochromatin oscillates independently of boundaries and the RNAi pathway. (A) Loss of Epe1 causes disruption of silencing at the ade6:L5-ura4 ectopic silencer; a fragment of the otr of cen3 (L5) is inserted at the euchromatic ade6+ locus adjacent to a ura4+ gene. Unselected colonies were plated onto indicated media. (B) Loss of Epe1 allows spreading of heterochromatin in the absence of boundary elements. White colonies containing the ade6:L5-ura4 ectopic silencer were replated and the colour of the resulting colonies was assessed. The numbers indicated are representative of several experiments. (C) Loss of Epe1 alleviates silencing of a ura4+ marker genes inserted 150 bp distal to mat3 (mat3-M(EcoRV):ura4+). Unselected single colonies were plated onto indicated media.
Conversely, we examined whether deletion of epe1 allows spreading of heterochromatin at this ectopic site to silence the ade6+ gene that resides 1.3 kb downstream of ura4+ (Figure 4A). In wild-type cells, this ade6+ gene remains expressed, resulting in white colonies. In contrast, epe1Δ cells containing the same ade6:L5-ura4+ form a significant number of red and pink colonies on nonselective plates. The frequency of these red/pink (ade6 repressed) colonies increases when cells with a silent ura4+ gene are selected on FOA plates (Figure 4B). Thus, in the absence of Epe1, silent chromatin can extend further from the L5/centromeric repeat fragment and silence both ura4+ and ade6+ genes. When epe1Δ mutants are grown on −URA media to select for cells that are expressing the ura4+ gene, the frequency of colonies in which heterochromatin has spread from the L5 to silence the downstream ade6+ is very low. This suggests that in epe1Δ mutants, heterochromatin spreads in a contiguous and directional fashion and is consistent with previous data (Ayoub et al, 2003).
This analysis indicates that at an ectopic silencer, where there is no boundary between adjacent ura4+ and ade6+ genes, Epe1 is required both for robust silencing and to counteract heterochromatin spreading. As at cen1, in the absence of Epe1, silencing variegates and heterochromatin domains fluctuate. Therefore, although Epe1 may indeed have a role at boundaries, Epe1 does not act solely at boundaries. Epe1 clearly acts both to prevent spreading at sites that lack known boundary elements and to prevent disruption of heterochromatin, suggesting that Epe1 has a direct role in regulating the extent and integrity of heterochromatin domains.
Epe1 does not act via the RNAi pathway
It has been proposed that Epe1 acts in the RNAi pathway to recruit RNAPII to centromeric repeats, and thereby promoting the production of noncoding transcripts (Zofall and Grewal, 2006). An alternative possibility is that Epe1 acts independently of RNAi and functions at the chromatin level, for example by directly modifying a heterochromatin factor. To determine if Epe1 resides in the RNAi pathway, we examined hallmark criteria that distinguish between RNAi factors and heterochromatin factors. RNAi components are required both for the establishment and maintenance of silencing at centromeres. Thus, reintroduction of the histone H3 lysine 9 methyltransferase Clr4 into chp1Δclr4Δ cells does not allow the reestablishment of silent chromatin (Sadaie et al, 2004). However, when Clr4 is reintroduced into a clr4Δepe1Δ double mutant, heterochromatin is formed (Supplementary Figure 3). Thus, functional Epe1 is not an absolute requirement for the establishment of the silent state at centromeres.
The RNAi machinery has been shown to be dispensable for maintenance of silencing at the mating-type locus (Jia et al, 2004; Kim et al, 2004). Silencing at the mating-type locus was examined using a ura4+ marker gene inserted 150 bp distal to mat3 (mat3-M(EcoRV):ura4+; Figure 4C). In wild-type cells and dcr1Δ mutants, the marker at this site is strongly silenced, resulting in poor growth on −URA plates. However, we find that in some epe1Δ and epe1Δdcr1Δ colonies, silencing is alleviated giving better growth than wild type on −URA plates (Figure 4C). This indicates that loss of Epe1 causes variable silencing at the mat locus with some colonies exhibiting disrupted silencing. epe1Δ cells have also been shown to form extended domains of heterochromatin at the mat locus (Ayoub et al, 2003). Therefore, it is possible that at the mat locus, silencing oscillates in a similar fashion to that observed at centromeres. Defective silencing of mat3(EcoRV):ura4+ in epe1Δ cells is consistent with Epe1 not acting in the RNAi pathway, this suggests that Epe1 acts to regulate silent chromatin and/or is a component of silent chromatin itself.
Loss of Epe1 allows heterochromatin to expand without active RNAi
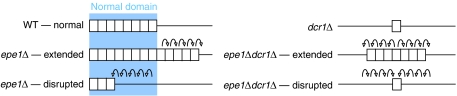
To determine if in the absence of the RNAi pathway, loss of Epe1 can still result in the formation of extended heterochromatin domains, we examined silencing in dcr1Δ mutants and cells bearing a mutation in Rpb2. rpb2-m203 mutants lack centromeric siRNA and loses centromeric silencing due to the inability of the mutant RNAPII to recruit RNAi components (Kato et al, 2005). We examined rpb2-m203 and dcr1Δ strains with ura4+ markers inserted in the centromeric heterochromatin of cen1 (otr1R(SphI):ura4+ and imr1L(NcoI):ura4+ respectively). Silencing in the epe1Δrpb2-m203 double mutant variegates, so colonies were preselected on −URA and FOA. Analysis demonstrates that an epe1Δ mutant can suppress the silencing defect of an rpb2-m203 mutant (Figure 5A). Stronger silencing (FOAR) is observed in the epe1Δrpb2-m203 double mutant compared to the rpb2-m203 mutant alone. Mutations in epe1 are also able to suppress the loss of centromeric silencing observed in the RNAi-deficient dcr1Δ mutant, the enzyme responsible for siRNA generation (Figure 5B). Therefore, in the absence of Epe1, heterochromatin spreads along the centromeric repeats even without intact RNAi. Previous analyses have demonstrated that a moderate level of H3K9me2 methylation persists in an RNAi-deficient background (Sadaie et al, 2004). The most plausible explanation for our observations is that in the absence of Epe1, silent chromatin can extend outwards using this residual H3K9 methylation as a nucleation point for the expansion of silent chromatin along the chromatin fibre without RNAi components or siRNAs.
Figure 5.
Loss of Epe1 allows heterochromatin to expand without active RNAi. epe1Δ can partially rescue the phenotypes of rpb2-m203 (A) and dcr1Δ (B) mutants, which have defective RNAi. (C) ChIP analysis of levels of H3K9me2 on the centromeric outer repeats (dg region) was compared to that of the euchromatic fbp1+ gene. (D) epe1Δ causes expansion of telomeric heterochromatin. Strains were used which contain a minichromosome that has an ade6+ gene next to a telomere. Red wild-type (WT) and epe1Δ colonies were picked and spotted or plated onto media containing limited adenine, the colour of the resulting colonies was assessed. The numbers presented are representative of several experiments.
We next examined centromeric heterochromatin formed in epe1Δ, dcr1Δ and an epe1Δdcr1Δ double mutant. ChIP analysis demonstrates that in dcr1Δ cells, the level of H3K9me2 is reduced below wild-type levels. Compared to dcr1Δ, H3K9me2 is significantly increased in the epe1Δdcr1Δ double mutant (Figure 5C). This indicates that epe1Δ cells can maintain H3K9 methylation in the absence of RNAi.
Furthermore, to confirm that in the absence of the RNAi pathway epe1Δ mutants can form extended heterochromatin domains, silent chromatin was examined at a synthetic telomere. At this synthetic telomere, heterochromatin is established independently of the RNAi pathway as it is composed of terminal TTACAG1−6 repeats but lacks the proximal telomere-associated repeats through which RNAi mediates silencing (Kanoh et al, 2005). The synthetic telomere was created adjacent to the ade6+ gene on the minichromosome Ch16. In wild-type cells, the ade6+ gene juxtaposed to the synthetic telomere exhibits variegated expression resulting in red, pink, white and sectored colonies (Nimmo et al, 1994). To assess silencing of the ade6+ gene, red (repressed) colonies were replated. The red epe1Δ colonies maintain the red silent state more effectively than red wild-type cells (Figure 5D). This suggests that loss of Epe1 allows more robust heterochromatin to form at the synthetic telomere, consistent with an extended silent domain. Again, no known boundary exists between telomere repeats and the ade6+ gene.
Together, these data indicate that in the absence of Epe1, heterochromatin can expand (Figure 5) and be disrupted (Figure 4C) in the absence of functional RNAi.
epe1Δ cells display low levels of siRNAs derived from centromeric transcripts
Noncoding RNAPII transcripts derived from the centromeric outer repeats are processed by the RNAi pathway to produce siRNAs. To determine if Epe1 affects or is required for the production of these noncoding centromeric RNAs, transcript levels were assessed by Northern blot and RT–PCR. As expected, the level of centromeric transcript in the wild type is low, but transcripts accumulate in dcr1Δ cells. We observe that in epe1Δdcr1Δ double mutants, the levels of transcript observed are significantly reduced compared to that of the dcr1Δ background (Figure 6A). This is consistent with previous observations (Zofall and Grewal, 2006). However, in epe1Δdcr1Δ cells (and also in epe1Δ), the levels of centromeric transcript detected are inversely correlated with the level of phenotypic silencing. Strains containing a ura4+ marker within the centromeric heterochromatin (cen1-imr1L(NcoI):ura4+) were grown in media containing FOA to select for the repressed state and media lacking uracil to enrich for cells in which heterochromatin is disrupted. In epe1Δdcr1Δ cells, more transcripts accumulate when silencing is disrupted than when silencing is intact (Figure 6B). This suggests that in epe1Δ cells, the stochastic loss and formation of heterochromatin over the centromeric repeats (and the transcript promoters) regulates the amount of centromeric transcription. Therefore, the overall reduction in centromeric transcript detected in epe1Δdcr1Δ cells is caused by loss of regulation of heterochromatin. This suggests that the effect Epe1 has on centromeric transcription is indirect and provides an alternative explanation for the reduced levels of RNAPII associated with the heterochromatic repeats and IRC elements observed in epe1Δ cells (Zofall and Grewal, 2006).
Figure 6.
epe1Δ mutants display low levels of siRNAs derived from centromeric transcripts. (A) Centromeric transcripts levels are reduced in an epe1Δdcr1Δ compared with a dcr1Δ mutant. The transcripts were detected using a centromere-specific probe. As a loading control, levels of larger ribosomal RNAs were visualised by EtBr staining. (B) RT–PCR to analyse levels of centromeric transcript in cells containing a ura4+ marker in the centromere (cen1-imr1L(NcoI):ura4+) grown in the absence of uracil and in media containing FOA. (C) Levels of siRNAs are reduced in an epe1Δ mutant. siRNAs were detected using a probe specific for the dh repeats. As a loading control, the blot was also hybridised with a probe specific for a snoRNA.
Consistent with a misregulation of heterochromatin in epe1 mutants causing reduced centromere repeat transcription, Northern analyses of siRNAs homologous to centromeric dh repeats revealed that siRNAs levels are variable but lower in epe1Δ cells compared to the wild type (Figure 6C). siRNA levels do not cause the variegation in silencing as siRNA levels are not higher in cells with extended heterochromatin domains, than in cells with disrupted heterochromatin (Supplementary Figure 5). Also, the low level of siRNAs is not the cause of the disruption of silencing, because epe1Δ cells have defective silencing at the mat locus where siRNAs are not required to maintain silencing (Figure 4C). Therefore, since we have demonstrated that in epe1Δ cells the expansion and disruption of heterochromatin is independent of the RNAi pathway, the erratic behaviour of heterochromatin observed must be due to defective regulation of heterochromatin rather than reduced siRNA levels.
Epe1 requires iron- and 2-OG-binding residues for activity
Previously, we demonstrated that the JmjC domain of Epe1 can be modelled on the structure of FIH (Factor inhibiting HIFα) (Trewick et al, 2005). FIH is a member of the 2-OG/Fe(II)-dependent dioxygenase superfamily, which bind Fe(II) using the consensus amino-acid residues HXD/EXnH (Schofield and Ratcliffe, 2005). Epe1 contains a variant of this motif in which the second histidine is replaced with tyrosine (HXEX70Y), therefore, we predicted that Epe1 coordinates Fe(II) with the residues H297, E299 and Y370. The structural model also suggested that Epe1 would interact with its co-substrate 2-OG via K314 along with two additional amino acids (Trewick et al, 2005).
More recently it has been suggested that Epe1 is not an active dioxygenase enzyme. This was proposed due to the lack of in vitro histone demethylase activity (data not shown; Tsukada et al, 2006; Zofall and Grewal, 2006) and Epe1 overexpression studies. Overexpression of either wild-type protein or Epe1 mutated in a predicted Fe(II)-binding residue causes the disruption of centromeric heterochromatin (Zofall and Grewal, 2006). This suggested that the critical H297 residue, predicted to bind Fe(II), is dispensable for Epe1 function. However, this interpretation neglects the possibility that the defective silencing observed is due to Epe1 overexpression rather than Epe1 activity.
To investigate further, wild-type Epe1 and the mutant proteins Epe1-H297A and Epe1-K314A (Figure 7A), with defective Fe(II)-binding and 2-OG-interacting residues, respectively, were overexpressed from the nmt41 promoter on a high copy plasmid. The Epe1-H297A and Epe1-K314A proteins are stable and expressed at a similar level to the wild-type Epe1 protein (Figure 7E). Overexpression of the wild-type Epe1, Epe1-H297A or Epe1-K314A in a strain containing the normally strongly repressed cen1-otr1R(SphI):ade6+ marker, alleviated silencing, so that mainly white colonies were formed compared with the red colonies formed with the empty plasmid control (Figure 7C). Similarly, in a strain bearing otr1R(SphI):ura4+, overexpression of wild-type or the Epe1 point mutants resulted in the majority of colonies exhibiting increased growth on −URA plates, consistent with defective centromeric heterochromatin formation (Figure 7D). Therefore, the H297 and K314 residues are not required for the disruption of silencing observed when Epe1 is overexpressed.
Figure 7.
Epe1 requires iron- and 2-OG-binding residues for activity. (A) Multiple alignments of JmjC domain proteins. The Saccharomyces cerevisiae (sc) JHD1, Homo sapiens (hs) JHDM1A and S. pombe (sp) Epe1 proteins are shown. Predicted Fe(II)-binding residues are highlighted in red and the predicted 2-OG-binding residues are highlighted in green. The conserved Fe(II)- and 2-OG-binding residues, H297 and K314, of Epe1 are indicated by ♦. (B) Strains containing the ura4+ or ade6+ marker inserted at the SphI site of the otr. (C, D) Plasmids containing Epe1 or Epe1 point mutants were overexpressed from an nmt41 promoter in strains with ade6+ (C) or ura4+ (D) at the SphI site of otr1. Overexpression of Epe1 and epe1H297A and K314A point mutants cause disruption of centromeric silencing. (E) Western blot of extracts from cells overexpressing Epe1 or Epe1 point mutants from an nmt41 promoter in a wild-type background. (F) Genomic epe1H297A and K314A mutants exhibit variegation of centromeric silencing. epe1H297A and K314A point mutants with otr1R(SphI):ade6+ were spotted onto media containing low adenine. (G) Genomic epe1H297A and K314A mutants of Epe1 cause spreading of centromeric heterochromatin. epe1H297A and K314A point mutants with ura4+ at the XhoI site adjacent to cen1 were spotted onto the indicated media.
However, to determine if these critical residues are really required for Epe1 function, the same H297A and K314A alterations were made in the open reading frame of endogenous epe1+ gene expressed from its native promoter. Interestingly, the epe1-H297A and epe1-K314A mutants have phenotypes that are indistinguishable from epe1Δ. Like epe1Δ cells, both epe1-H297A and epe1-K314A cells exhibit variegated expression of cen1-otr1R(SphI):ade6+, resulting in red, pink and white colonies (Figure 7F). Moreover, epe1-H297A and epe1-K314A cells have extended centromeric chromatin domains, H3K9me2 can spread from the centromere (Supplementary Figure 6) resulting in silencing of a ura4+ marker gene located in a normally expressed euchromatic site as indicated by increased growth on FOA (Figure 7G). These analyses clearly demonstrate that the in vivo activity of the Epe1 protein is abolished by the H297A and K314A mutations. This indicates that the predicted Fe(II)-binding and 2-OG-interacting residues are essential for Epe1 function. Furthermore, this is consistent with Epe1 being an active enzyme of the 2-OG/Fe(II)-dependent dioxygenase superfamily. The disruption of silencing observed when Epe1 is overexpressed is therefore not due to the enzymatic activity of Epe1. These analyses contradict previous reports that suggested that these residues are not important for Epe1 function and that Epe1 is not a 2-OG/Fe(II)-dependent dioxygenase.
Discussion
Here we have demonstrated that Epe1 regulates the stability of heterochromatin domains. Our analyses have show that loss of Epe1 causes silencing to variegate with some epe1Δ cells forming extended silent chromatin domains (Figures 1 and 3) while other genetically identical epe1Δ cells have destabilised heterochromatin (Figures 2, 3 and 4). These silent and expressed states are metastable (Supplementary Figure 2). We propose that in epe1Δ cells, silent chromatin domains oscillate, expanding into the surrounding euchromatin or contracting to cause alleviation of silencing (Figure 3).
We suggest that Epe1 acts directly to prevent the oscillation of heterochromatin domains rather than via boundary elements. Although Epe1 may have a specific role at heterochromatin boundaries, our data plainly demonstrates that Epe1 stabilises heterochromatin in the absence of known boundary elements (Figures 4 and 5D).
Epe1 could either act in the RNAi pathway or at the chromatin level to regulate the stability of heterochromatin domains. Our analyses show that Epe1 is not a component of the RNAi pathway and therefore must function at the chromatin level. Unlike RNAi factors, Epe1 is not required for the establishment of centromeric heterochromatin (Supplementary Figure 3). Also, loss of Epe1 causes variegated silencing at the mat locus (Figure 4C), where RNAi is dispensable for maintenance of heterochromatin (Hall et al, 2002). In fact, in epe1Δ cells, heterochromatin can spread independently of RNAi (Figure 5). We postulate that residual pockets of H3K9me2 that remain in RNAi mutants (Sadaie et al, 2004) may act as nucleation sites from which, in the absence of Epe1, heterochromatin can spread. Therefore, the erratic behaviour of heterochromatin observed is due to aberrant regulation of heterochromatin rather than by an RNAi defect. Our data suggest that Epe1 does not regulate RNAPII (Zofall and Grewal, 2006), but that Epe1 regulates the integrity of heterochromatin and therefore indirectly effects access of RNAPII to centromeric chromatin.
Heterochromatin may spread along fibres in a transcription/RNAi-coupled manner. Or, in an alternative model, spreading might be caused by the polymerisation of chromatin factors in a step-wise fashion, for example, a nucleation site of Swi6, bound to H3K9me2, could recruit a histone deacetylase and Clr4, allowing H3K9 methylation of adjacent nucleosomes and binding of additional Swi6 (reviewed by Talbert and Henikoff, 2006). Our results demonstrate that in the absence of Epe1, heterochromatin can spread or collapse without active RNAi, therefore, suggesting that Epe1 may prevent heterochromatin from spreading and collapsing via the step-wise assembly mechanism. We propose that Epe1 dampens the natural tendency of silent chromatin to assemble or disassemble. Thus, in the absence of Epe1, minor fluctuations in the extent of silent chromatin domains remain unchecked and the process is unregulated, resulting in frequent expansion–contraction of the silent domain (Figure 8).
Figure 8.
Model for the function of Epe1. Epe1 prevents the oscillation of silent chromatin. In dcr1Δ cells, residual pockets of H3K9me2 may act as nucleation sites from which, in the absence of Epe1, heterochromatin can spread.
Epe1 is likely to be an active 2-OG/Fe(II)-dependent dioxygenase. Contrary to other reports, we have demonstrated that a predicted iron-binding residue (H297) and 2-OG-binding residue (K314) are essential for the in vivo activity of Epe1 (Figure 7). However, no histone demethylase activity can be detected in vitro for Epe1 (unpublished observation; Tsukada et al, 2006; Zofall and Grewal, 2006). Although Epe1 could be a histone demethylase, the lack of in vitro activity leads us to propose an alternative mechanism for Epe1. It is possible that Epe1 acts analogously to another JmjC domain protein, FIH, to which Epe1 has strong structural homology (Trewick et al, 2005). FIH is a protein hydroxylase that hydroxylates Asn803 of HIF and prevents it binding to the histone acetylase p300 (Schofield and Ratcliffe, 2005). We propose that Epe1 could be a protein hydroxylase that affects the stability of a heterochromatin protein, or protein–protein interaction, to regulate the extent and stability of heterochromatin domains. Hydroxylation of a heterochromatin factor could regulate the stability of silent chromatin, effectively buffering the extent of heterochromatin formed adjacent to a nucleation site. There are many potential substrates for Epe1. As Epe1 interacts with Swi6 (Supplementary Figure 1; Zofall and Grewal, 2006; Isaac et al, 2007), it is possible that Epe1 hydroxylates Swi6. However, it is equally possible that Epe1 prevents silent chromatin from oscillating by hydroxylating other components of silent chromatin. For example, Epe1 could directly regulate the activity or stability of the Clr4 methyltransferase, histone deacetylases or another heterochromatin protein.
Materials and methods
Fission yeast strains and genetic methods
The media and standard genetic procedures used were described previously (Moreno et al, 1991; Allshire et al, 1994). Epe1 was deleted by homologous integration of ura4+ to replace the ORF. The epe1∷KanMX4 mutant was derived from a diploid strain obtained from Bioneer (Korea). The otr1R(SphI):ade6+ otr1R(XhoI):ura4+ strain was constructed by transformation of the BamHI fragment of the pPhe-otr1(XhoI)-ura4 plasmid (Allshire et al, 1995) into cells containing otr1R(SphI):ade6+. The successful integration of ura4+ at the XhoI site was determined by PCR and Southern blot.
Serial dilution assays
Strains or single colonies were spotted in either a 10- or five-fold dilution onto the appropriate plates and incubated for 4 days at 32°C. To assess the sensitivity to TBZ, serial dilutions were spotted onto YES+15 μg/ml TBZ. For the analysis at the mating-type locus, h90 colonies were identified for analysis by their brown colour when stained with iodine.
Immunostaining and lagging chromosome analysis
Cells were fixed and stained as previously described (Ekwall et al, 1996). For lagging chromosome analysis, 100 late anaphases (spindle >5 μm) for each strain were analysed. Details of microscopy were described previously (Pidoux et al, 2000, 2003).
Western blot
Whole-cell extracts were prepared from logarithmically growing cells. Cells were harvested, resuspended in trichloroacetic acid and vortexed with beads. The acid-soluble proteins were boiled in SDS–PAGE loading buffer and used for immunoblotting. Blots were probed with an anti-Epe1 antibody and anti-BIP antibody as a loading control.
ChIP
ChIP was performed as described (Pidoux et al, 2004) except for the following modifications. For H3K9me2 ChIP, cells were fixed with 1% PFA for 15 min at room temperature. Cells were lysed using a bead beater (Biospec products) and sonicated using a Bioruptor (Diagenode) sonicator for a total of 15 min (30 s ON and OFF cycle). Monoclonal H3K9me2 antibody (1 μl) was used per ChIP. Multiplex PCR products were separated on 1.7% agarose gels and post-stained with ethidium bromide. Quantitation of bands was performed using the Kodak EDAS 290 system and 1D Image Analysis Software (Eastman Kodak).
siRNA and centromeric transcript preparation
For RT–PCR, cells were resuspended in 10 mM Tris–HCl, pH 7.5, 10 mM EDTA, pH 8, 0.5% SDS and lysed with the addition of phenol:chloroform 5:1, acid washed beads and vortexed for 30 min at 65°C. The aqueous phase was chloroform extracted and the RNA ethanol precipitated. The RT–PCR was performed using SuperScript III Reverse Transcriptase (Invitrogen). For northern blots, RNA was extracted by resuspending cells in 50 mM Tris–HCl pH 7.5, 10 mM EDTA pH 8, 100 mM NaCl, 1% SDS, lysing by the addition of phenol:chloroform 5:1, acid washed beads and vortexing for 30 min at 4°C. The soluble fraction was extracted with phenol/chloroform and ethanol precipitated. Centromeric transcripts were precipitated with 10% polyethylene glycol 8000 and 0.5 M NaCl on ice for 30 min followed by centrifugation. siRNAs were precipitated by addition of ethanol and sodium acetate and incubation at −20°C for 3 h. Transcripts were run on a 1% agarose 6% formaldehyde gel. siRNA samples were run on an 8% polyacrylamide gel. To check for loading, siRNA gels were cut above the xylene cyanol band and stained with ethidium bromide. siRNA and transcript gels were blotted by capillary transfer onto Hybond-NX (Amersham) and UV crosslinked. Transcript gels were probed with a 32P-labelled PCR product homologous to the dg centromeric repeats. siRNA gels were probed with a PCR product homologous to the dh repeats, and as a loading control an oligonucleotide homologous to a snoRNA.
Construction of plasmid mutants
Epe1 was cloned into the pDONR201 entry plasmid (Invitrogen). Point mutations were introduced into the Epe1 entry plasmid using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). The wild-type and mutated Epe1 ORFs were LR recombined into a S. pombe expression plasmid under control of the nmt41 promoter. The wild-type and point mutated Epe1 ORFs were checked by sequencing.
Construction of genomic mutants
A PCR product containing the epe1H297A point mutation was obtained by amplification of the mutated epe1 gateway entry plasmid. Primers were used with 80 base pair of homology to the region surrounding Epe1. The epe1K314A mutant DNA fragment was obtained using a two-step PCR protocol, creating a product with 200 base pair of homology either side of the ORF. PCR products were transformed into epe1∷ura4+ or epe1∷KanMX4 strains. Colonies were screened for replacement of the marker and point mutants were checked by sequencing.
Supplementary Material
Supplementary Figures
Supplementary Figure Legends
Acknowledgments
We thank A Pidoux for helpful discussions, A Buscaino and A Pidoux for critical reading of the manuscript and members of the EU funded Epigenome Network of Excellence for discussion. Research in the Allshire lab was supported by the Wellcome Trust (065061/Z). RCA is a Wellcome Trust Principal Research Fellow and; SCT is a Dorothy Hodgkin Fellow of the Royal Society.
References
- Allshire RC, Javerzat JP, Redhead NJ, Cranston G (1994) Position effect variegation at fission yeast centromeres. Cell 76: 157–169 [DOI] [PubMed] [Google Scholar]
- Allshire RC, Nimmo ER, Ekwall K, Javerzat JP, Cranston G (1995) Mutations derepressing silent centromeric domains in fission yeast disrupt chromosome segregation. Genes Dev 9: 218–233 [DOI] [PubMed] [Google Scholar]
- Ayoub N, Noma K, Isaac S, Kahan T, Grewal S, Cohen A (2003) A novel jmjC domain protein modulates heterochromatization in fission. Mol Cell Biol 23: 4356–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister AJ, Zegerman P, Partridge JF, Miska EA, Thomas JO, Allshire RC, Kouzarides T (2001) Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120–124 [DOI] [PubMed] [Google Scholar]
- Cam HP, Sugiyama T, Chen ES, Chen X, FitzGerald PC, Grewal SI (2005) Comprehensive analysis of heterochromatin- and RNAi-mediated epigenetic control of the fission yeast genome. Nat Genet 37: 809–819 [DOI] [PubMed] [Google Scholar]
- Ekwall K, Javerzat JP, Lorentz A, Schmidt H, Cranston G, Allshire R (1995) The chromodomain protein Swi6: a key component at fission yeast centromeres. Science 269: 1429–1431 [DOI] [PubMed] [Google Scholar]
- Ekwall K, Nimmo ER, Javerzat JP, Borgstrom B, Egel R, Cranston G, Allshire R (1996) Mutations in the fission yeast silencing factors clr4+ and rik1+ disrupt the localisation of the chromo domain protein Swi6p and impair centromere function. J Cell Sci 109 (Part 11): 2637–2648 [DOI] [PubMed] [Google Scholar]
- Ekwall K, Olsson T, Turner BM, Cranston G, Allshire RC (1997) Transient inhibition of histone deacetylation alters the structural and functional imprint at fission yeast centromeres. Cell 91: 1021–1032 [DOI] [PubMed] [Google Scholar]
- Grewal SI, Klar AJ (1996) Chromosomal inheritance of epigenetic states in fission yeast during mitosis and meiosis. Cell 86: 95–101 [DOI] [PubMed] [Google Scholar]
- Hall IM, Shankaranarayana GD, Noma K, Ayoub N, Cohen A, Grewal SI (2002) Establishment and maintenance of a heterochromatin domain. Science 297: 2232–2237 [DOI] [PubMed] [Google Scholar]
- Isaac S, Walfridsson J, Zohar T, Lazar D, Kahan T, Ekwall K, Cohen A (2007) Interaction of Epe1 with the heterochromatin assembly pathway in Schizosaccharomyces pombe. Genetics 175: 1549–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Noma K, Grewal SI (2004) RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science 304: 1971–1976 [DOI] [PubMed] [Google Scholar]
- Kanoh J, Sadaie M, Urano T, Ishikawa F (2005) Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr Biol 15: 1808–1819 [DOI] [PubMed] [Google Scholar]
- Kato H, Goto DB, Martienssen RA, Urano T, Furukawa K, Murakami Y (2005) RNA polymerase II is required for RNAi-dependent heterochromatin assembly. Science 309: 467–469 [DOI] [PubMed] [Google Scholar]
- Kim HS, Choi ES, Shin JA, Jang YK, Park SD (2004) Regulation of Swi6/HP1-dependent heterochromatin assembly by cooperation of components of the mitogen-activated protein kinase pathway and a histone deacetylase Clr6. J Biol Chem 279: 42850–42859 [DOI] [PubMed] [Google Scholar]
- Klose RJ, Kallin EM, Zhang Y (2006) JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet 7: 715–727 [DOI] [PubMed] [Google Scholar]
- Mellone BG, Ball L, Suka N, Grunstein MR, Partridge JF, Allshire RC (2003) Centromere silencing and function in fission yeast is governed by the amino terminus of histone H3. Curr Biol 13: 1748–1757 [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Motamedi MR, Verdel A, Colmenares SU, Gerber SA, Gygi SP, Moazed D (2004) Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell 119: 789–802 [DOI] [PubMed] [Google Scholar]
- Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI (2001) Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292: 110–113 [DOI] [PubMed] [Google Scholar]
- Nimmo ER, Cranston G, Allshire RC (1994) Telomere-associated chromosome breakage in fission yeast results in variegated expression of adjacent genes. EMBO J 13: 3801–3811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K, Allis CD, Grewal SI (2001) Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science 293: 1150–1155 [DOI] [PubMed] [Google Scholar]
- Noma K, Cam HP, Maraia RJ, Grewal SI (2006) A role for TFIIIC transcription factor complex in genome organization. Cell 125: 859–872 [DOI] [PubMed] [Google Scholar]
- Noma K, Sugiyama T, Cam H, Verdel A, Zofall M, Jia S, Moazed D, Grewal SI (2004) RITS acts in cis to promote RNA interference-mediated transcriptional and post-transcriptional silencing. Nat Genet 36: 1174–1180 [DOI] [PubMed] [Google Scholar]
- Partridge JF, Scott KS, Bannister AJ, Kouzarides T, Allshire RC (2002) Cis-acting DNA from fission yeast centromeres mediates histone H3 methylation and recruitment of silencing factors and cohesin to an ectopic site. Curr Biol 12: 1652–1660 [DOI] [PubMed] [Google Scholar]
- Pidoux A, Mellone B, Allshire R (2004) Analysis of chromatin in fission yeast. Methods 33: 252–259 [DOI] [PubMed] [Google Scholar]
- Pidoux AL, Allshire RC (2004) Kinetochore and heterochromatin domains of the fission yeast centromere. Chromosome Res 12: 521–534 [DOI] [PubMed] [Google Scholar]
- Pidoux AL, Richardson W, Allshire RC (2003) Sim4: a novel fission yeast kinetochore protein required for centromeric silencing and chromosome segregation. J Cell Biol 161: 295–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, Uzawa S, Perry PE, Cande WZ, Allshire RC (2000) Live analysis of lagging chromosomes during anaphase and their effect on spindle elongation rate in fission yeast. J Cell Sci 113 (Part 23): 4177–4191 [DOI] [PubMed] [Google Scholar]
- Rea S, Eisenhaber F, O'Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T (2000) Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406: 593–599 [DOI] [PubMed] [Google Scholar]
- Sadaie M, Iida T, Urano T, Nakayama J (2004) A chromodomain protein, Chp1, is required for the establishment of heterochromatin in fission yeast. EMBO J 23: 3825–3835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield CJ, Ratcliffe PJ (2005) Signalling hypoxia by HIF hydroxylases. Biochem Biophys Res Commun 338: 617–626 [DOI] [PubMed] [Google Scholar]
- Scott KC, Merrett SL, Willard HF (2006) A heterochromatin barrier partitions the fission yeast centromere into discrete chromatin domains. Curr Biol 16: 119–129 [DOI] [PubMed] [Google Scholar]
- Talbert PB, Henikoff S (2006) Spreading of silent chromatin: inaction at a distance. Nat Rev Genet 7: 793–803 [DOI] [PubMed] [Google Scholar]
- Thon G, Bjerling P, Bunner CM, Verhein-Hansen J (2002) Expression-state boundaries in the mating-type region of fission yeast. Genetics 161: 611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trewick SC, McLaughlin PJ, Allshire RC (2005) Methylation: lost in hydroxylation? EMBO Rep 6: 315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y (2006) Histone demethylation by a family of JmjC domain-containing proteins. Nature 439: 811–816 [DOI] [PubMed] [Google Scholar]
- Verdel A, Jia S, Gerber S, Sugiyama T, Gygi S, Grewal SI, Moazed D (2004) RNAi-mediated targeting of heterochromatin by the RITS complex. Science 303: 672–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdel A, Moazed D (2005) RNAi-directed assembly of heterochromatin in fission yeast. FEBS Lett 579: 5872–5878 [DOI] [PubMed] [Google Scholar]
- Volpe T, Schramke V, Hamilton GL, White SA, Teng G, Martienssen RA, Allshire RC (2003) RNA interference is required for normal centromere function in fission yeast. Chromosome Res 11: 137–146 [DOI] [PubMed] [Google Scholar]
- Zofall M, Grewal SI (2006) Swi6/HP1 recruits a JmjC domain protein to facilitate transcription of heterochromatic repeats. Mol Cell 22: 681–692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures
Supplementary Figure Legends