Abstract
During postnatal development, ascending and descending auditory inputs converge to form fibrodendritic layers within the central nucleus of the inferior colliculus (IC). Before the onset of hearing, specific combinations of inputs segregate into bands separated by interband spaces. These bands may define functional zones within the IC. Previous studies in our laboratory have shown that unilateral or bilateral cochlear ablation at postnatal day 2 (P2) disrupts the development of afferent bands from the dorsal nucleus of the lateral lemniscus (DNLL) to the IC. These results suggest that spontaneous activity propagated from the cochlea is required for the segregation of afferent bands within the developing IC. To test if spontaneous activity from the cochlea also may be required to maintain segregated bands of DNLL input, we performed cochlear ablations in rat pups at P9, after DNLL bands already are established. All animals were killed at P12 and glass pins coated with carbocyanine dye, DiI (1,1′-dioctodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate), subsequently were placed in the commissure of Probst to label the crossed projections from both DNLLs. When compared with surgical controls, experimental results showed a similar pattern of DNLL bands in the IC contralateral to the ablated cochlea, but a disruption of DNLL bands in the IC ipsilateral to the cochlear ablation. The present results suggest that cochlear ablation after DNLL bands have formed may affect the maintenance of banded DNLL projections within the central nucleus of the IC.
Keywords: DNLL, DiI, development, afferent bands, GABA, deafferentation
The central nucleus of the inferior colliculus (IC) is composed of a series of cochleotopically ordered layers that preserve frequency-specific processing of various auditory features. Multiple auditory nuclei target the IC and terminate in afferent compartments along these cochleotopic layers (Oliver and Shneiderman, 1989b). It is likely that these afferent compartments within the IC create an intrinsic organization capable of integrating inputs from different auditory nuclei (Oliver and Shneiderman, 1989a; Cant and Benson, 2006).
A banded organization of afferent projections to the IC develops quite early, in fact, well before auditory experience could shape functioning synaptic circuits. For example, crossed projections from the dorsal nucleus of the lateral lemniscus (DNLL) to the central nucleus of the IC have organized into bands about one week before hearing onset in rat (Gabriele et al., 2000b). Similarly, banded projections from LSO to the IC have been observed in kittens at birth (Brunso-Bechtold and Henkel, 2005; Henkel et al., 2005), several days before acoustically driven activity is present in the auditory midbrain (Moore and Irvine, 1979). Consequently, the timing of these events suggests that patterns of spontaneous activity or spatially distributed molecular guidance cues may well be vital for initial assembly and maintenance of integrative circuits in the IC.
As for the retina, cochlear activity begins quite early in development (Jhaveri et al., 1991; Lippe, 1994, 1995; Lin and Chen, 2000; Jones et al., 2001). The intrinsic bursting characterizing that activity may well have important implications for regulation of developing afferent projections in IC. Maturation along cochleotopic gradients has been observed for a variety of morphological features of neural circuitry in lower auditory nuclei (Cant, 1998). Furthermore, when spontaneous cochlear activity was reduced or eliminated in neonatal animals, neuron survival and the development of neural circuits in the cochlear and superior olivary nuclei were altered (Moore and Kitzes, 1985; Rubel and Hyson, 1992; Kitzes et al., 1995). Of particular importance in the present study, the critical period in the rodent (rat or gerbil) cochlear nucleus (CN) in which cells are dependent on cochlear input appears to end quite abruptly around postnatal day 8 or 9 (P8 or P9) (Tierney et al., 1997; Moore et al., 1998).
A previous study from our laboratory showed that unilateral cochlear ablation at birth in rat pups altered the symmetric distribution of bands from crossed DNLL nuclei to IC (Gabriele et al., 2000a). The changes in DNLL projections may have resulted from spontaneous activity-dependent mechanisms, from reactive synaptogenesis after cell death related to deafferentation of the IC, or from a combination of both effects. In other studies, neonatal cochlear ablation altered both the strength and timing of inhibitory and excitatory responses to lemniscal stimulation recorded from the postnatal IC in slice preparations (Vale and Sanes, 2000, 2002; Vale et al., 2004). Thus, the consequences of neonatal cochlear ablation are complex.
In the present study, we continued our investigation of the role of cochlear activity in the development of crossed DNLL projections in the IC. By delaying unilateral cochlear ablation until P9 in rat pups, and labeling DNLL projections to IC on P12, we were able to study the maintenance of DNLL bands in the IC after their initial formation. In addition, because P9 is after the critical period of dependence of auditory neurons on cochlear input for survival, we were able to evaluate the effects of cochlear ablation in the absence of significant loss of other IC inputs that could result in reactive synaptogenesis. Crossed DNLL projections to IC were isolated from other auditory fibers in the midline at the tegmental commissure (of Probst) and labeled with carbocyanine dye (DiI, 1,1′-dioctodecyl-3,3, 3′,3′-tetramethylindocarbocyanine perchlorate). The results show that unilateral cochlear ablation after DNLL bands have formed, at the end of the critical period for cell death in lower auditory nuclei, alters the pattern of DNLL afferent bands in IC.
EXPERIMENTAL PROCEDURES
Axonal projections from the DNLL to the IC were studied in two groups of postnatal rat pups near the time of hearing onset at P12. The experimental group had been operated 3–4 days earlier to ablate the right cochlea and the control group underwent anesthesia and skin incision but the cochlea was not removed. A carbocyanine dye, DiI, was used to label DNLL axons as they crossed the midline. Carbocyanine dyes diffuse retrogradely back to the cell bodies as well as anterogradely through the axolemma to label the axons, their branches, and endings. All animal care and experimental procedures were in compliance with the Guide for the Care and Use of Laboratory Animals in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care (AAALAC). Prior to the experiments, all protocols were approved by the Institutional Animal Care and Use Committee at Wake Forest University School of Medicine. Every effort was made to minimize the number of animals used in this study and their suffering.
Cochlear ablation
As previously described (Gabriele et al., 2000a) the cochlea was ablated by aspiration. Briefly, P8–P9 rat pups in the experimental group (n=8) were anesthetized by hypothermia and a skin incision was made ventral to the right pinna. The external auditory canal was exposed by blunt dissection and followed to the tympanic membrane, taking care to avoid injury to the root of the facial motor nerve. The tympanic membrane then was pierced and the middle ear cavity cleared of mesenchyme. After removing the ossicles, a syringe needle was inserted through the round window and sterile water was flushed through the perilymphatic spaces of the cochlea to destroy hair cells. Next the bone between the oval and round windows was pinched and the cochlear contents were aspirated. Half of each litter was used as surgical controls to minimize developmental variation. In the control animals (n=6), the skin was incised but the middle and inner ears were not opened. The wound was closed with cyanoacrylate glue (VetBond Tissue Adhesive, 3M, St. Paul, MN, or equivalent) and the rat placed on a warming pad to recover. After recovery, ablated and control pups were returned to the litter and reared to P12.
Labeling DNLL commissural axons
On P12, rat pups were given an overdose of ketamine and xylazine and perfused through the heart with 4% paraformaldehyde. The brains were removed and postfixed in 4% paraformaldehyde overnight. Brains were blocked in the coronal plane, embedded in a 2% gelatin-egg yolk mixture, and immersed overnight in 4% paraformaldehyde. Using a Vibratome (St. Louis, MO, USA), sections were cut from the rostral face of the embedded block until commissural fibers ventral to the periaqueductal gray matter were visualized at the superior colliculus–IC junction. At this site on midline, the block was impaled with a glass pin coated with DiI (Invitrogen-Molecular Probes, Eugene, OR, USA, #22885) and any stray flecks of dye were rinsed off the block. The tissue block was then returned to 4% paraformaldehyde and incubated in the dark at 37 °C for 6 weeks to allow diffusion of the dye to branches and endings in the IC.
Histology
After 6 weeks’ incubation, the brains were returned to the refrigerator for 24 h before vibratome sectioning in the coronal plane at 75 μm. Serial sections through the IC and CN were collected in 0.1 M phosphate buffer, mounted onto±charged slides, and coverslipped immediately using a permanent aqueous mounting media (Gel/Mount, Biomeda, Foster City, CA, USA, Catalogue #M01). Sections were viewed with an Olympus BX2 epifluorescent photomicroscope (Melville, NY, USA) equipped with a Cy5 filter set (Chroma Technologies, Brattleboro, VT, USA) for DiI visualization. Images of all sections containing the IC and DNLL were collected within 24 h using a Spot RT Slider digital camera (1600×1200 pixel resolution) and software (Diagnostic Instruments, Sterling Heights, MI, USA).
It has been well documented in the literature that cochlear ablation in neonatal animals results in a marked reduction in CN volume on the side of the ablation (Trune, 1982; Nordeen et al., 1983; Moore and Kowalchuk, 1988; Hashisaki and Rubel, 1989; Hardie and Shepherd, 1999; Gabriele et al., 2000a). During a critical period that ends rather abruptly around P9 in rats (Moore et al., 1998) and between P7 and P9 in gerbils (Tierney et al., 1997), significant shrinkage of the CN is in large part due to deafferentation-induced neuron loss. After the critical period, shrinkage still occurs due to presynaptic and postsynaptic changes in the CN although significant neuronal loss does not occur (Moore et al., 1998). In the present study, changes in CN volume ipsilateral and contralateral to the ablation were assessed as a relative index of the extent of cochlear ablation. Neurolucida software (Microbrightfield, Colchester, VT, USA) was used to trace the CN in a series of every other Nissl-stained section and the total volume of each CN was derived from the compiled data. Volumetric comparisons were calculated for individual cases (percentage reduction; right CN relative to left CN; positive percentages reflect a right CN volume reduction), and means for the experimental group were compared with those of the control group using a paired T test (Prism, GraphPad Software, Inc., San Diego, CA, USA).
Analysis of DiI labeling
Images of DiI-labeled DNLL projections to the central nucleus of the IC were processed and analyzed in a similar manner to that described previously for analysis of DNLL bands (Gabriele et al., 2000a; Henkel et al., 2005). Briefly, to assess the relative density of labeled fibers and the pattern of distribution in the IC on each side in ablated and control groups, brightness profiles of the IC were collected from 4× images (1600×1200 pixels, 0.86 pixels/micron) and compared using ImageJ software (NIH, Bethesda, MD, USA). The high frequency noise in the density plot profiles was smoothed digitally with a Gaussian 15×15 filter before analysis. IC sections containing the heaviest area of label were consistently located in the middle third of the CNIC in the rostral caudal axis, usually corresponding to the middle three 75 μm sections. From these three sections, in control and ablated cases, the section containing the most clearly defined banding pattern was selected for analysis. Images were acquired and cropped in order to center the IC within the frame. Using Image J software a macro was programmed to create a line perpendicular to the banded projection and/or cell layers if there were no apparent bands. Line density plot profiles were acquired from this line. Slight variations in the mounting of the tissue block, the angle of pin placement, or the presence of artifacts in the section often dictated the exact position of the line drawn for analysis.
As a first step in the analysis of the distribution of DNLL axons, variation was examined in a brightness profile acquired along a line drawn from ventromedial to dorsolateral orthogonal to the axonal layers. The mean gray level and standard deviation for the data points along this line were determined. Nonlinear regression was used to fit the spatial-brightness function of the labeled profile to a sine curve modeling regular density changes across a series of layers (Prism, GraphPad Software, Inc.). The best fit for a sine curve was calculated by setting the starting point for baseline as the mean, amplitude as standard deviation, and period of equally spaced sublayers as 160 μm based on previous measurements of band thickness (Gabriele et al., 2000a). The baseline, standard deviation, period and R2 value of the nonlinear regression analysis for the right IC and the left IC in the control group were compared and statistical differences (paired t- and Wilcoxon tests) were not significant (P>0.05). In the unilateral cochlear ablation group, density profiles of labeled areas in the ipsilateral and contralateral IC relative to the ablation were compared with the control density profiles using an ANOVA design (Statistica, StatSoft, Inc., Tulsa, OK, USA).
Sections through the ventral nucleus of the lateral lemniscus, superior olivary complex (SOC) and CN were examined for retrogradely labeled cells to confirm that DiI-pins had isolated commissural fibers and had not labeled axons in other ascending auditory tracts. Cases with retrograde labeling in these nuclei were omitted from the study. Also, as a further analysis of deafferentation-induced changes in the auditory brainstem, retrograde labeling was compared in the DNLL ipsilateral and contralateral to the cochlear ablation.
RESULTS
DiI pins labeled axonal projections of DNLL that cross at the dorsal tegmental commissure and innervate the IC of P12 rat pups (Fig. 1). In both the unilateral cochlear ablation and surgical control groups, DiI-labeled fibers coursed in fascicles from the pin site through the tegmentum ventral to the periaqueductal gray and cuneiform area and entered the ventral part of IC on each side.
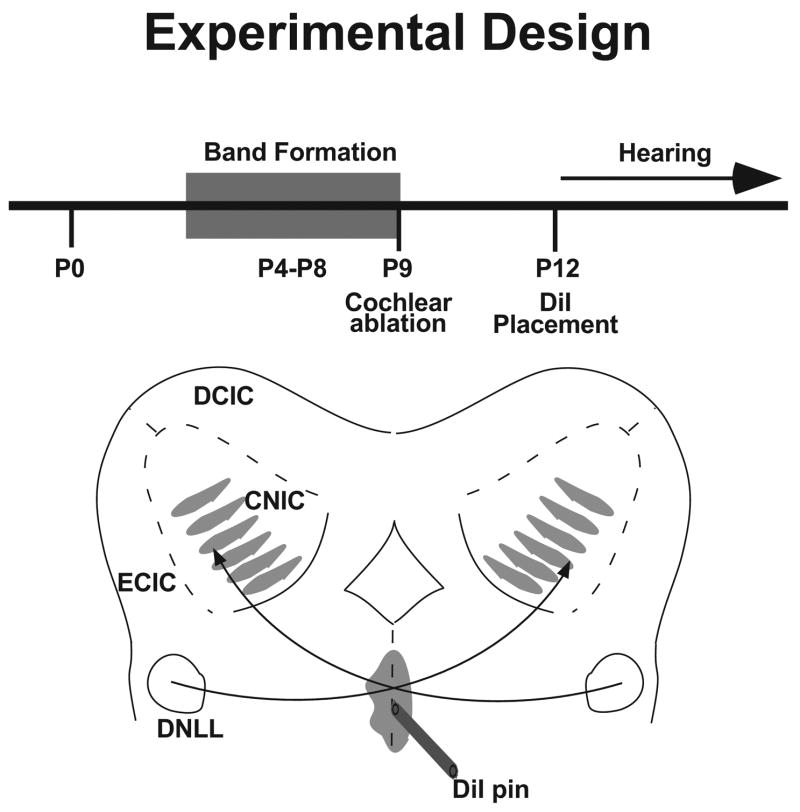
Fig. 1.
Schematic of the experimental design. Unilateral cochlear ablation was performed at P9 in rat pups after banded projections from the dorsal nucleus of the lateral lemniscus (DNLL) already have formed in the central nucleus of the inferior colliculus (CNIC). Three days later, at the time of hearing onset, rat pups were killed, the brains harvested, and DNLL axons labeled with the carbocyanine dye DiI where they crossed the midline. Other abbreviations: DCIC, dorsal cortex of the inferior colliculus; ECIC, external cortex of the inferior colliculus.
DNLL fibers in the IC of untreated rat pups
In the control group (n=6), DiI-labeled fibers entered the central nucleus of the IC and were distributed parallel to the orientation of fibrodendritic layers (Fig. 2A and B). It is likely that some of these labeled fibers extended into the deepest layers of the dorsal cortex of the rat IC. The relation of the labeled fibers to the boundaries of the IC was confirmed by comparing DiI labeling with changes in cell packing and size at the borders of the central nucleus and the external and dorsal cortex of the IC in adjacent Nissl-stained sections.
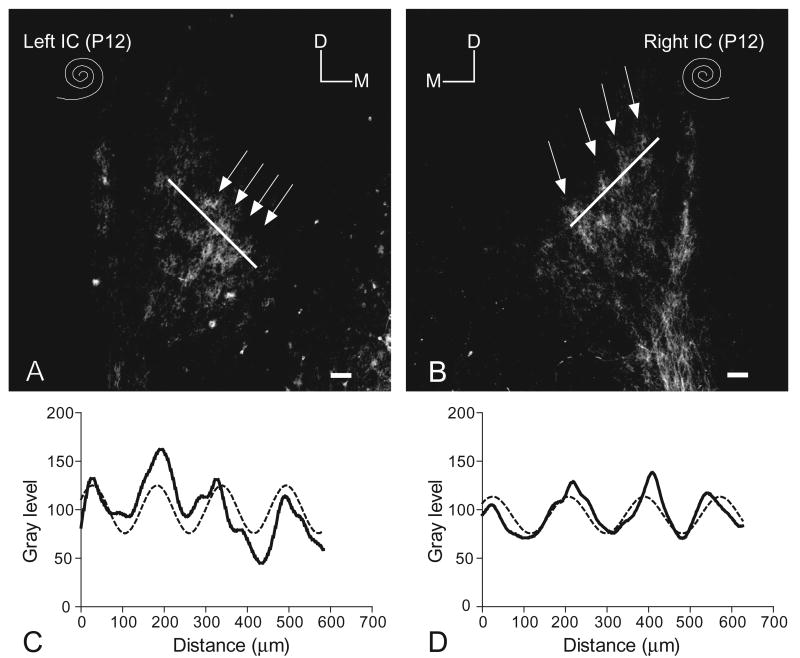
Fig. 2.
(A, B) Paired images illustrating symmetrical DiI labeling of the banded pattern (arrows) of crossed DNLL fibers in the central nucleus of the IC on each side in a 12-day-old (P12), sham-operated (intact condition of cochleas indicated by spiral symbols) control rat pup. For orientation, dorsal (D) and medial (M) are indicated in each image. Scale bars=100 μm. (C, D) Line plots of DiI labeling sampled along a line across axon layers in the IC (see corresponding lines in A and B, respectively) demonstrating periodic changes in gray level (solid lines in graphs) corresponding to the bands of label in A and B, respectively. A sine function that best fit the gray level profile is shown (hatched line).
Labeled DNLL axons typically distributed throughout the rostral to caudal (longitudinal) axis of the central nucleus of the IC as well as across its tonotopic axis. In the middle third, DiI-labeled fibers formed a pattern of alternating densely labeled and sparsely labeled bands in the central nucleus of IC (Fig. 2A and B, arrows). Bands of labeled fibers and endings were most distinct in the ventral half of the central nucleus, although in some cases the pattern extended to the dorsolateral region as well. Parallel bands of labeled DNLL fibers in the IC appeared to be of similar intensity and distributed in a symmetrical banded pattern on each side (Fig. 2A and B).
DNLL fibers in the IC of unilateral cochlear ablation rat pups
After unilateral cochlear ablation, the distribution of labeled DNLL fibers in the IC ipsilateral to the ablation was compared with that in the contralateral IC. In addition, the distribution of labeled fibers in both inferior colliculi in ablated animals (n=8) was compared with the distribution in controls (Fig. 3A and B). Parallel dense bands of labeling separated by regions of sparse labeling (interband space) were readily apparent in the IC contralateral to the ablation, but less obvious in the IC ipsilateral to the ablation (Fig. 3B). Qualitatively, the overall density of the labeled fibers in the IC ipsilateral to the ablation consistently appeared greater than in the IC contralateral to the ablation. In addition, a patterned distribution of labeling in the IC ipsilateral to the ablation was obscured somewhat by increased label in the interband spaces. Whether the disruption of bands in the IC ipsilateral to the ablation was due to differences in branching and/or distribution of labeled endings will require analysis of fiber morphology in future studies.
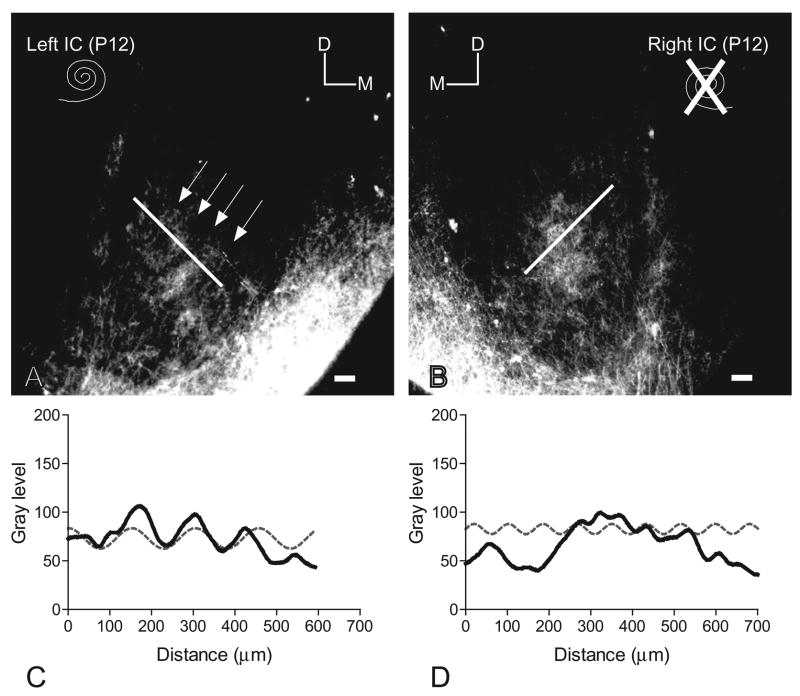
Fig. 3.
(A, B) Paired images illustrating the patterns of DiI labeling of crossed DNLL fibers in the central nucleus of the IC on each side relative to unilateral cochlear ablation (condition of cochleas indicated by spiral symbols) in a P12 rat pup. For orientation, dorsal (D) and medial (M) are indicated in each image. Note that a banded pattern (arrows) is maintained in the IC contralateral to the ablation, but not in the IC ipsilateral to the ablation. Scale bars=100 μm. (C, D) Line plots illustrating gray level sampled along a line (see corresponding lines in A and B, respectively) across DiI labeled axons in the IC. Note the relative goodness of fit of the gray level plot (solid line in graph) for labeling contralateral to the cochlear ablation (C) with the sine function (hatched line in graph) and the poor fit ipsilateral to the ablation (D).
Semiquantitative analysis of DNLL fibers in the IC
To compare systematically the changes in the banded pattern of labeled DNLL fibers in the central nucleus of IC as a result of cochlear ablation, image density was plotted along lines (Fig. 2A and B, white lines) placed orthogonal to bands of labeled DNLL axons in the IC layers. Peaks in these line density profiles (Fig. 2C and D) represented the most densely labeled areas in the IC of DNLL projections (arrows in Fig. 2A and B) and valleys in the line density profiles represented regions of sparse or absent labeling. The periodicity of the line density profiles as well as the variance in density between bands and the interband spaces was analyzed in the control cases and in unilateral cochlear ablation cases.
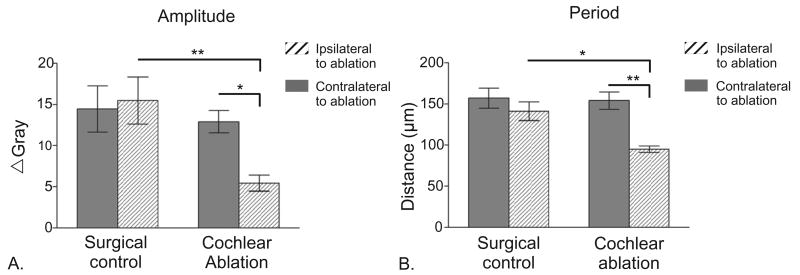
Using nonlinear regression, a sine curve modeling density across regularly spaced bands of labeled DNLL fibers (Fig. 2C and D; dashed lines) was fit to the line density profiles for labeled areas in IC (Fig. 2C and D; solid lines). Best-fit sine curves in the control group had a mean R2>0.30±0.07 and a mean period of 142 μm, representing the center-to-center distance between banded densities in the line density profiles. There was no significant difference in the center-to-center distance by this measure between the left and right IC in the control cases (see Table 1, Student’s paired t-test; P>0.1, n=6). The best-fit sine curves in controls had a mean amplitude of 15.46±2.86, representing the difference between the image density within and between labeled afferent bands in IC. Analysis revealed no significant difference in amplitude for the left and right IC in the control cases (see Table 1, Student’s paired t-test; P>0.1, n=6).
Table I.
Comparison of best-fit sine curve parameters from line density analysis of DNLL labeling in the left and right IC of control cases and in the ICs following cochlear ablation
| Left surgical control | Right surgical control | Contralateral ablation | Ipsilateral ablation | |
|---|---|---|---|---|
| Baseline | 64.87±6.56 | 76.96±6.73 | 73.45±9.97 | 81.84±9.96 |
| Amplitude | 14.46±2.82 | 15.46±2.86 | 12.91±1.35 | 5.44±0.98†,** |
| Period (μm) | 157.51±12.22 | 142±11.29 | 154.94±10.62 | 95.71±3.80††,* |
| R2 | 0.4896±0.05 | 0.4941±0.07 | 0.5102±0.02 | 0.1854±0.03 |
Ipsilateral ablation compared to control group ANOVA P<0.005.
Ipsilateral ablation compared to control group ANOVA P<0.005.
To analyze the effect of unilateral cochlear ablation (n=8) on the spatial distribution of DNLL fibers in the central nucleus of the IC, best-fit sine curves were derived for the spatial distribution of labeling in IC contralateral and ipsilateral to the ablated side (Fig. 3C and D). As shown in Fig. 4, best-fit curves for labeling in the IC contralateral to the ablated side were not different from control cases (R2=0.5102±0.02, P=0.99; period= 154±10.62, P=0.99; amplitude=12.91±1.35, P=0.94, multivariate ANOVA). However the best-fit sine curves in the IC ipsilateral to the ablation were significantly different from both the contralateral IC and the IC in control cases (Table 1). For line density profiles in the ipsilateral IC, the best-fit sine curves had poorer fits to the data (R2<0.20) and the mean period of the best-fit curves was significantly less than in controls, indicating that the labeling pattern no longer had the same regular spacing (Fig. 4B). Finally, the results show that the amplitude of the best-fit curves in the ablated group was reduced compared with the controls (Fig. 4A). Our results suggest that the disruption of DNLL bands in the IC is due to expansion of labeled projections in the IC.
Fig. 4.
(A) Bar graph comparing the mean amplitude of fluctuation of gray level for patterns of DiI labeling of DNLL fibers in the left (solid bar) and right IC (hatched bar) for the control group and in the ipsilateral (solid bar) and contralateral IC (hatched bar) for the unilateral cochlear ablation group. * P<0.05; ** P<0.005 ANOVA. Whiskers on bars are standard errors of the mean (n=6). (B) Bar graph comparing the mean period of fluctuation of gray level for patterns of DiI labeling of DNLL fibers in the left (solid bar) and right IC (hatched bar) for the control group and in the ipsilateral (solid bar) and contralateral IC (hatched bar) for the unilateral cochlear ablation group. * P<0.05; ** P<0.005 ANOVA. Whiskers on bars are standard errors of the mean (n=6).
Retrogradely labeled cells in both control and ablation groups were present and distributed symmetrically in the left and right DNLL, but were absent from other hindbrain auditory nuclei that are known to project to the IC (Fig. 5). The DNLL also receives inputs from the contralateral DNLL via the commissure of Probst (the site of DiI injection). These fibers also may contribute to retrograde labeling in the DNLL, making it difficult to assess possible changes in labeling of the contralateral and ipsilateral DNLL after unilateral cochlear ablation. Nevertheless, this result is in marked contrast to the asymmetry of label observed between the DNLL following unilateral ablation on P2 (Gabriele et al., 2000a).
Fig. 5.
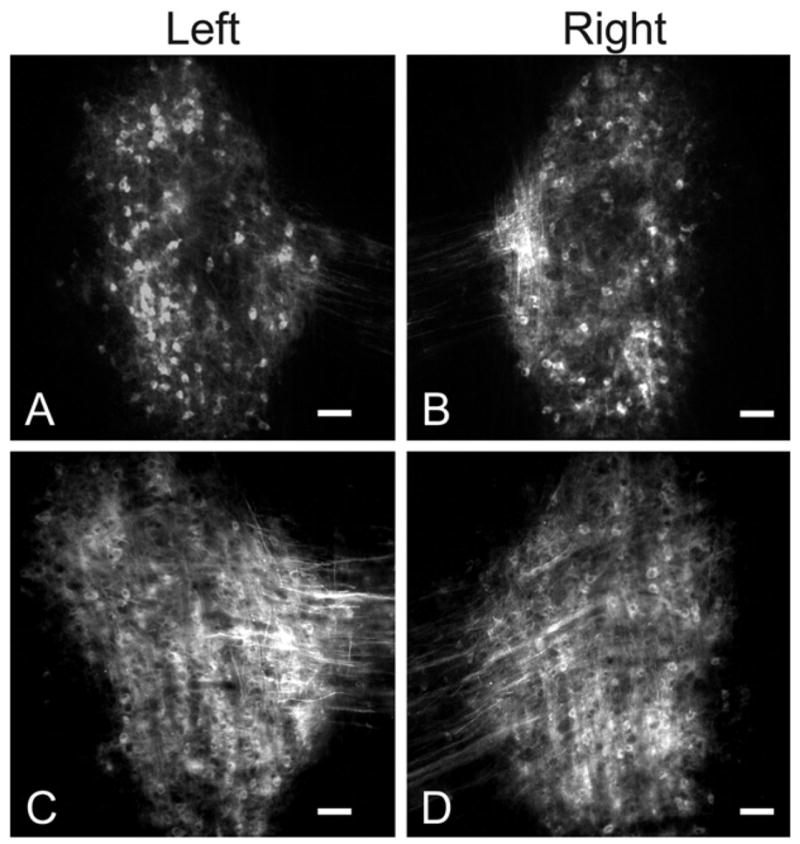
(A, B) Paired images of retrogradely labeled neurons in the left (A) and right (B) DNLL of a control animal after DiI placement in the dorsal tegmental commissure (of Probst) (see experimental design in Fig. 1). Note the symmetric labeling on the left and right side. Scale bars=100 μm. (C, D) Paired images illustrating retrogradely labeled neurons in the contralateral (C) and ipsilateral (D) DNLL after DiI placement in the dorsal tegmental commissure (of Probst) of an ablated animal (see experimental design in Fig. 1). Note the labeling is symmetric on each side although it is more difficult to resolve individual cells than in A and B. Scale bars=50 μm.
Volumetric analysis of cochlear nuclei
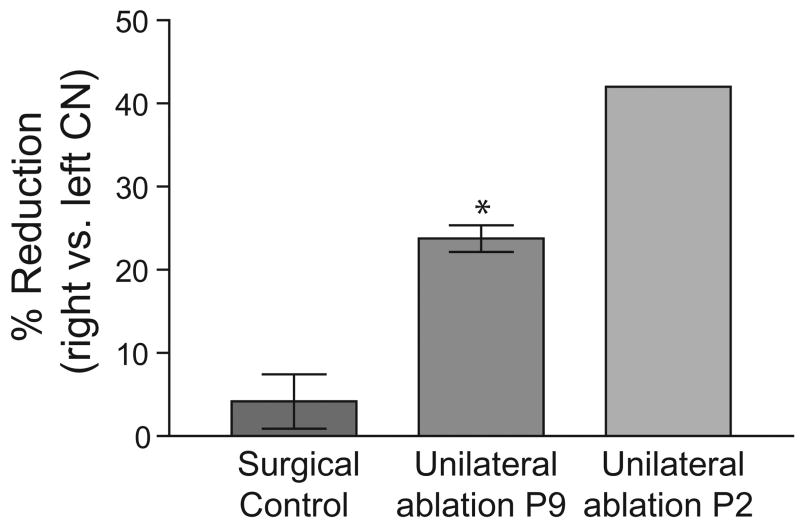
In the present study unilateral cochlear ablations and sham surgeries were performed on P9 rat pups, a time at which the CN is no longer sensitive to deafferentation-induced cell loss. Nevertheless, CN volume is known to be affected by removal of the cochlea and has been used in previous studies as an index of atrophy resulting from ablation (Gabriele et al., 2000a). In the present study volumes of the left and right CN in control cases were not significantly different (Student’s paired t-test P>0.1, n=6). After unilateral cochlear ablation, the CN volume contralateral to the ablation was not significantly different than the CN volume in control cases. However, CN volume ipsilateral to the cochlear ablation was reduced by 25–35% compared with the contralateral CN and was significantly smaller than controls (Fig. 6).
Fig. 6.
Bar graph illustrating the mean percent difference in volume of the CN on each side for the control group (left bar) and a P9 cochlear ablation group (center bar). For comparison, data from the P2 ablation group in Gabriele et al., 2000b are shown in the right bar. Note that the 25% reduction in CN volume on the side of the cochlear ablation is significantly different than variation in the control group (* P<0.05 t-test). However, the reduction is considerably less than for the P9 ablated group (about 50% reduction). All ablations are considered right ablations. Whiskers indicate standard error of the mean for present data, n=8.
DISCUSSION
The organization of crossed projections from DNLL to the IC was examined three days after unilateral cochlear ablation in nine-day-old rat pups. By P9, DNLL projections have segregated into a series of bands in the central nucleus of the IC (Gabriele et al., 2000b). On P12, after cochlear ablation on P9, crossed DNLL axons no longer are segregated into regularly spaced bands in the IC ipsilateral to the ablation. In contrast, there is no change in the banded distribution of DNLL axons in the IC contralateral to the ablation. The results show that DNLL fibers remain plastic and anatomical changes in DNLL fiber distribution in the IC occur rapidly (within three days) after cochlear ablation. Thus, the territory of DNLL projections in IC ipsilateral to the ablation has expanded or reorganized so that labeled fibers intermingle across boundaries of the banded compartments.
Analysis of IC banded architecture
In order to analyze changes in the distribution pattern of projections from the crossed DNLL to the IC, a model based on an orderly series of evenly spaced afferent bands was compared with the actual pattern of axonal labeling. The validity of correlation of image density with distribution of axon endings was shown previously for DNLL projections to IC in ferret (Henkel et al., 2003). Thickness and spacing of the afferent bands was comparable whether measured as peaks in image density or as spread of synaptic swellings across layers. Accordingly, the distance between labeled afferent bands for DNLL projections in P12 rat in the present study was approximately 142 μm based on peak to peak measures from line density profiles. Analysis of the left and right control IC in P12 rat pups showed symmetric banded patterns of labeled DNLL projections that were consistent with the model. Based on these findings, the banded pattern in the IC of control cases was compared with the pattern in the IC after cochlear ablation.
Activity-dependent changes in developing circuits
It is important to distinguish developmental mechanisms that construct neural circuits prior to sensory experience from those that shape neural circuits through experience or evoked activity. It is also well established that activity-dependent processes work in concert with activity-independent processes to create the topographical organization of afferents within target sensory structures (Katz and Shatz, 1996; Friauf and Lohmann, 1999; Crowley and Katz, 2002; Rubel and Cramer, 2002). Ablation of peripheral sensory structures and pharmaceutical blockade of activity in developing primary afferent fibers have been shown to affect activity-dependent processes during critical periods of circuit development (Shatz and Stryker, 1988; Shatz, 1990; Sanes and Takacs, 1993; Tucci et al., 2002). When spontaneous waves of retinal wave activity were silenced prior to eye opening, the refinement of afferent projections within the visual thalamus and primary visual cortex was disrupted (Shatz and Stryker, 1988; Meister et al., 1991; Penn et al., 1998) Consequently, in the developing visual system, there is strong evidence that spontaneous activity of retinal ganglion cells guides the initial development of visual projection patterns.
Before the onset of hearing, spontaneous activity in higher order auditory neurons may be driven in some measure by peripheral bursting from the cochlea reminiscent of the waves of activity that occur in the developing retina (Walsh and McGee, 1987; Kotak and Sanes, 1995; Lippe, 1995). By carefully measuring labeled primary afferent fibers in the cochlear nuclei of postnatal kittens, Leake and Snyder (Snyder and Leake, 1997; Leake et al., 2002; Leake et al., 2006) showed that the topography of spiral ganglion inputs in the CN develops immediately prior to the onset of hearing and is dependent on spontaneous bursting activity in the auditory nerve. Similar early development of afferent circuits in IC has been reported. For instance, LSO projections have formed a banded pattern in IC before hearing onset in both kittens and ferrets (Brunso-Bechtold and Henkel, 2005; Henkel et al., 2005) and DNLL projections are banded before hearing onset in rat as well (Gabriele et al., 2000b). In the present study, the disruption of a normal banded pattern of crossed DNLL axons in the IC of P12 rat following unilateral cochlear ablation on P9 is consistent with spontaneous activity in ascending auditory pathways being a critical factor in shaping the spatial arrangement of DNLL projections in IC.
It is clear that the central nucleus of the IC is the site of convergence for inputs from multiple lower auditory nuclei. For example, the LSO projects to both the ipsilateral and contralateral IC. Injection of anterograde tracer into the LSO on one side revealed a pattern of reciprocal alternating bands of labeled LSO axons with intervening unlabeled spaces in both the left and right IC (Shneiderman and Henkel, 1987). The DNLL also projects bilaterally to the IC (Kudo, 1981; Zook and Casseday, 1987; Shneiderman et al., 1988). In the present paradigm, only the contralateral projections from both DNLL nuclei were labeled and a symmetrical pattern of labeled bands separated by unlabeled spaces can be observed in the left and right IC. Together these observations suggest that a labeled band of DNLL input together with the adjacent unlabeled space is likely to form a layer composed of ipsilateral and contralateral projections.
DNLL neurons utilize the neurotransmitter GABA and make symmetric synaptic contacts with the soma and proximal dendrites of IC neurons (Brunso-Bechtold et al., 1981; Adams and Mugnaini, 1984; Shneiderman et al., 1988, 1993; Bajo et al., 1993; Merchan et al., 1994; Gonzalez-Hernandez et al., 1996; Kelly and Li, 1997; Chen et al., 1999; van Adel et al., 1999). Although much more is known about the developmental mechanisms of glutamatergic circuit formation, important progress has been made in understanding the development of inhibitory circuits in the auditory brainstem such as those in the SOC (Kandler, 2004; Kandler and Gillespie, 2005). In the mature SOC, inhibitory projections from the medial nucleus of the trapezoid body (MNTB) are distributed systematically along narrow strips in LSO corresponding to precise tonotopic organization (Ryan et al., 1982; Friauf, 1992). Before hearing onset in the gerbil and the rat, the initial distribution of MNTB axonal arbors and synapses in the LSO is much broader than that present in the mature animal. Subsequently, aberrant branches and synapses are eliminated and MNTB projections become more precisely aligned along the tonotopic axis (Sanes and Siverls, 1991; Kim and Kandler, 2003).
We have hypothesized that DNLL axons undergo a refinement of distribution similar to that of MNTB axons and, furthermore, that the development and maintenance of the banded pattern of DNLL inputs in the IC is influenced by Hebbian mechanisms. Importantly, there is clear evidence that during embryonic and early postnatal periods lower auditory inputs as well as those from DNLL initially arborize profusely in the IC. Specifically (Gabriele et al., 2000b) have shown that, beginning on P4 and continuing through P8, the diffuse arrangement of DNLL projections in the IC becomes refined into the banded pattern that resembles the adult distribution of DNLL inputs by P12. In our model, we propose that ipsilateral and contralateral inputs from the DNLL first project diffusely in the IC, then compete for a target zone and become segregated into bands of ipsilateral and contralateral input.
Following cochlear ablation in postnatal and adult animals, the IC ipsilateral to the intact ear is more responsive than the contralateral IC to acoustically evoked activity (Kitzes, 1984; McAlpine et al., 1997). Down-regulation of inhibition in the ipsilateral IC has been suggested as one mechanism for this imbalance of evoked activity. The DNLL predominantly receives excitation from ascending pathways stimulated by the contralateral ear and inhibition from pathways stimulated by the ipsilateral ear. Indeed, decreases in GABA receptor expression, in glutamate decarboxylase (GAD) expression (Mossop et al., 2000), and in inhibitory neurotransmitter release have been shown following ablation (Bledsoe et al., 1995; Suneja et al., 1998; Vale and Sanes, 2002; Vale et al., 2004). Consequently, it seems likely that following unilateral cochlear ablation, the DNLL contralateral to the ablation would be deactivated while the DNLL ipsilateral to the ablation would be activated (Fig. 7). Thus, we predicted that following unilateral cochlear ablation, inactivated DNLL inputs in the IC ipsilateral to the ablation would be at a competitive disadvantage for space and that those inputs would retract as the activated DNLL inputs expanded their target zone.
Fig. 7.
(A) Simplified diagram illustrating some of the major excitatory inputs to the DNLL (LSO, lateral superior olive) and organization of DNLL projections to the IC at P12. (B) Schematic diagram summarizing plastic changes in the DNLL projections to IC after cochlear ablation. Alternating light and dark gray bars represent the pattern of afferent bands that are characteristic in the central nucleus of the IC. The condition of the cochleas is indicated by the spiral symbol. Altered influence of the cochlea on DNLL is indicated by the arrows in B. In the controls and in the IC contralateral to cochlear ablation, DNLL axonal branching is refined and maintained within banded compartments. In the IC ipsilateral to the cochlear ablation, DNLL axonal branching proliferates into adjacent spaces and obliterates the banded pattern.
As expected, our results do show a loss of banding in the IC ipsilateral to the ablation. Surprisingly, however, that loss of banding is not due to a retraction of the inactivated DNLL axons into adjoining spaces, but rather to an expansion of the target zone of those axons. In addition, we observed no change in the banded pattern of input from the unaffected DNLL in the IC contralateral to the ablation. These data are consistent with the observation that MNTB arbors inactivated by cochlear removal do not demonstrate the normal refinement of their projections within the LSO (Sanes and Takacs, 1993).
A Hebbian mechanism for developmental refinement of projections implies a “fire together wire together” principle. The challenge has been to consider how inputs that are inhibitory and thus prevent generation of the postsynaptic action potential are refined. It is presently understood that GABA and glycine evoke depolarization of postsynaptic cells in embryonic and postnatal animals (Ben-Ari, 2002). One possible resolution to the challenge then is that the co-release of glutamate and GABA, into the synaptic cleft of developing inhibitory synapse in conjunction with NMDA receptors mediates the stabilization of inhibitory synapses (Gillespie et al., 2005). In the present paradigm, the anatomical refinement of GABAergic inputs from DNLL to the IC, between P4 and P9, is correlated temporally with the developmental period during which GABA produces a hyperpolarizing response (Kandler and Friauf, 1995; Kotak et al., 1998; Kullmann and Kandler, 2001). While the expression of the potassium chloride cotransporter (KCC2) that mediates the depolarizing affect of GABA appears to be unaffected by ablation, the functional state of this cotransporter may be affected thus disrupting the developmental switch from depolarization to hyperpolarization (Vale et al., 2003).
After unilateral cochlear ablation we suggest that the crossed DNLL inputs to IC ipsilateral to the ablation are deactivated by the ablation but increase the extent of their distribution due to a disruption of coordinating signals for axonal growth and refinement. Indeed stimulation of the lateral lemniscus ipsilateral to the ablation reveals diminished inhibition in the IC on that side due to impaired paired pulse facilitation, a potential mechanism of synapse stabilization (Vale et al., 2004).
After unilateral ablation excitatory stimulation of the crossed DNLL input to IC contralateral to the ablation should remain intact. Although inhibition in the IC contralateral to the ablation is compromised due to decreased inhibitory strength, paired pulse facilitation is still present (Vale et al., 2004). These observations suggest that DNLL inputs to the IC contralateral to the ablation are able to undergo normal segregation into bands because the inputs to the DNLL ipsilateral to the ablation are intact.
It is important to keep in mind that the relationship among banded projections from different auditory nuclei, including those from the DNLL, to the IC is unclear. For example, physiological evidence suggests that the convergence of LSO and DNLL projections in the IC is vital for interaural processing in the IC (Pollak and Park, 1993; Kelly et al., 1996, 1998; Kelly and Li, 1997; Pollak et al., 2003) suggesting that banded projections from these two nuclei overlap. Consequently, when considering Hebbian mechanisms as determining the refinement of specific auditory projections in the IC, it is essential to consider that the competitive interactions involved in band formation are more diverse and, thus, are far more complex than simply ipsilateral and contralateral populations from a single auditory nucleus.
CONCLUSION
In conclusion, in the present study, the cochlea was ablated on P9 in order to evaluate the effect of ablation on DNLL bands after they had already segregated in the IC. DiI-labeled DNLL banded projections in the IC ipsilateral to the ablation were disrupted compared with the IC contralateral to the ablation and to DNLL bands in controls. Although it is not possible to identify a cellular mechanism from our data, we speculate that the increased anatomical territory of DNLL fibers in the IC ipsilateral to the ablation together with the appropriate refinement of DNLL projections to the IC contralateral to the ablation is due to a disruption in the signals for axonal growth and stabilization and supports the hypothesis that the cochlea is required for the maintenance of the banded pattern of DNLL projections in the IC before the onset of hearing.
Abbreviations
- CN
cochlear nucleus
- DiI
1,1′-dioctodecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- DNLL
dorsal nucleus of the lateral lemniscus
- IC
inferior colliculus
- MNTB
medial nucleus of the trapezoid body
- P
postnatal day
- SOC
superior olivary complex
References
- Adams JC, Mugnaini E. Dorsal nucleus of the lateral lemniscus: a nucleus of GABAergic projection neurons. Brain Res Bull. 1984;13:585–590. doi: 10.1016/0361-9230(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Bajo VM, Merchan MA, Lopez DE, Rouiller EM. Neuronal morphology and efferent projections of the dorsal nucleus of the lateral lemniscus in the rat. J Comp Neurol. 1993;334:241–262. doi: 10.1002/cne.903340207. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bledsoe SC, Jr, Nagase S, Miller JM, Altschuler RA. Deafness-induced plasticity in the mature central auditory system. Neuroreport. 1995;7:225–229. [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Henkel CK. Development of auditory afferents to the central nucleus of the inferior colliculus. In: Winer JA, Schreiner CE, editors. The inferior colliculus. New York: Springer; 2005. pp. 537–558. [Google Scholar]
- Brunso-Bechtold JK, Thompson GC, Masterton RB. HRP study of the organization of auditory afferents ascending to central nucleus of inferior colliculus in cat. J Comp Neurol. 1981;197:705–722. doi: 10.1002/cne.901970410. [DOI] [PubMed] [Google Scholar]
- Cant NB. Structural development of the mammalian central auditory pathways. In: Rubel EW, Popper AN, Fay RR, editors. Development of the auditory system. New York: Springer; 1998. pp. 315–413. [Google Scholar]
- Cant NB, Benson CG. Organization of the inferior colliculus of the gerbil (Meriones unguiculatus): differences in distribution of projections from the cochlear nuclei and the superior olivary complex. J Comp Neurol. 2006;495:511–528. doi: 10.1002/cne.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Kelly JB, Wu SH. The commissure of Probst as a source of GABAergic inhibition. Hear Res. 1999;138:106–114. doi: 10.1016/s0378-5955(99)00156-2. [DOI] [PubMed] [Google Scholar]
- Crowley JC, Katz LC. Ocular dominance development revisited. Curr Opin Neurobiol. 2002;12:104–109. doi: 10.1016/s0959-4388(02)00297-0. [DOI] [PubMed] [Google Scholar]
- Friauf E. Tonotopic order in the adult and developing auditory system of the rat as shown by c-fos immunocytochemistry. Eur J Neurosci. 1992;4:798–812. doi: 10.1111/j.1460-9568.1992.tb00190.x. [DOI] [PubMed] [Google Scholar]
- Friauf E, Lohmann C. Development of auditory brainstem circuitry. Activity-dependent and activity-independent processes. Cell Tissue Res. 1999;297:187–195. doi: 10.1007/s004410051346. [DOI] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK. Plasticity in the development of afferent patterns in the inferior colliculus of the rat after unilateral cochlear ablation. J Neurosci. 2000a;20:6939–6949. doi: 10.1523/JNEUROSCI.20-18-06939.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriele ML, Brunso-Bechtold JK, Henkel CK. Development of afferent patterns in the inferior colliculus of the rat: projection from the dorsal nucleus of the lateral lemniscus. J Comp Neurol. 2000b;416:368–382. doi: 10.1002/(sici)1096-9861(20000117)416:3<368::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Gillespie DC, Kim G, Kandler K. Inhibitory synapses in the developing auditory system are glutamatergic. Nat Neurosci. 2005;8:332–338. doi: 10.1038/nn1397. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Mantolan-Sarmiento B, Gonzalez-Gonzalez B, Perez-Gonzalez H. Sources of GABAergic input to the inferior colliculus of the rat. J Comp Neurol. 1996;372:309–326. doi: 10.1002/(SICI)1096-9861(19960819)372:2<309::AID-CNE11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Hardie NA, Shepherd RK. Sensorineural hearing loss during development: morphological and physiological response of the cochlea and auditory brainstem. Hear Res. 1999;128:147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Hashisaki GT, Rubel EW. Effects of unilateral cochlea removal on anteroventral cochlear nucleus neurons in developing gerbils. J Comp Neurol. 1989;283:5–73. doi: 10.1002/cne.902830402. [DOI] [PubMed] [Google Scholar]
- Henkel CK, Fuentes-Santamaria V, Alvarado JC, Brunso-Bechtold JK. Quantitative measurement of afferent layers in the ferret inferior colliculus: DNLL projections to sublayers. Hear Res. 2003;177:32–42. doi: 10.1016/s0378-5955(02)00794-3. [DOI] [PubMed] [Google Scholar]
- Henkel CK, Gabriele ML, McHaffie JG. Quantitative assessment of developing afferent patterns in the cat inferior colliculus revealed with calbindin immunohistochemistry and tract tracing methods. Neuroscience. 2005;136:945–955. doi: 10.1016/j.neuroscience.2005.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri S, Erzurumlu RS, Crossin K. Barrel construction in rodent neocortex: role of thalamic afferents versus extracellular matrix molecules. Proc Natl Acad Sci U S A. 1991;88:4489–4493. doi: 10.1073/pnas.88.10.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Jones SM, Paggett KC. Primordial rhythmic bursting in embryonic cochlear ganglion cells. J Neurosci. 2001;21:8129–8135. doi: 10.1523/JNEUROSCI.21-20-08129.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K. Activity-dependent organization of inhibitory circuits: lessons from the auditory system. Curr Opin Neurobiol. 2004;14:96–104. doi: 10.1016/j.conb.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci. 1995;15:6890–6904. doi: 10.1523/JNEUROSCI.15-10-06890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandler K, Gillespie DC. Developmental refinement of inhibitory sound-localization circuits. Trends Neurosci. 2005;28:290–296. doi: 10.1016/j.tins.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Li L. Two sources of inhibition affecting binaural evoked responses in the rat’s inferior colliculus: the dorsal nucleus of the lateral lemniscus and the superior olivary complex. Hear Res. 1997;104:112–126. doi: 10.1016/s0378-5955(96)00182-7. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Li L, van Adel B. Sound localization after kainic acid lesions of the dorsal nucleus of the lateral lemniscus in the albino rat. Behav Neurosci. 1996;110:1445–1455. doi: 10.1037//0735-7044.110.6.1445. [DOI] [PubMed] [Google Scholar]
- Kelly JB, Liscum A, van Adel B, Ito M. Projections from the superior olive and lateral lemniscus to tonotopic regions of the rat’s inferior colliculus. Hear Res. 1998;116:43–54. doi: 10.1016/s0378-5955(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Kim G, Kandler K. Elimination and strengthening of glycinergic/GABAergic connections during tonotopic map formation. Nat Neurosci. 2003;6:282–290. doi: 10.1038/nn1015. [DOI] [PubMed] [Google Scholar]
- Kitzes LM. Some physiological consequences of neonatal cochlear destruction in the inferior colliculus of the gerbil, Meriones unguiculatus. Brain Res. 1984;306:171–178. doi: 10.1016/0006-8993(84)90366-4. [DOI] [PubMed] [Google Scholar]
- Kitzes LM, Kageyama GH, Semple MN, Kil J. Development of ectopic projections from the ventral cochlear nucleus to the superior olivary complex induced by neonatal ablation of the contralateral cochlea. J Comp Neurol. 1995;353:341–363. doi: 10.1002/cne.903530303. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Sanes DH. Synaptically evoked prolonged depolarizations in the developing auditory system. J Neurophysiol. 1995;74:1611–1620. doi: 10.1152/jn.1995.74.4.1611. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Korada S, Schwartz IR, Sanes DH. A developmental shift from GABAergic to glycinergic transmission in the central auditory system. J Neurosci. 1998;18:4646–4655. doi: 10.1523/JNEUROSCI.18-12-04646.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M. Projections of the nuclei of the lateral lemniscus in the cat: an autoradiographic study. Brain Res. 1981;221:57–69. doi: 10.1016/0006-8993(81)91063-5. [DOI] [PubMed] [Google Scholar]
- Kullmann PH, Kandler K. Glycinergic/GABAergic synapses in the lateral superior olive are excitatory in neonatal C57Bl/6J mice. Brain Res Dev Brain Res. 2001;131:143–147. doi: 10.1016/s0165-3806(01)00271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Snyder RL, Hradek GT. Postnatal refinement of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2002;448:6–27. doi: 10.1002/cne.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Hradek GT, Chair L, Snyder RL. Neonatal deafness results in degraded topographic specificity of auditory nerve projections to the cochlear nucleus in cats. J Comp Neurol. 2006;497:13–31. doi: 10.1002/cne.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Chen S. Endogenously generated spontaneous spiking activities recorded from postnatal spiral ganglion neurons in vitro. Brain Res Dev Brain Res. 2000;119:297–305. doi: 10.1016/s0165-3806(99)00169-8. [DOI] [PubMed] [Google Scholar]
- Lippe WR. Rhythmic spontaneous activity in the developing avian auditory system. J Neurosci. 1994;14:1486–1495. doi: 10.1523/JNEUROSCI.14-03-01486.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippe WR. Relationship between frequency of spontaneous bursting and tonotopic position in the developing avian auditory system. Brain Res. 1995;703:205–213. doi: 10.1016/0006-8993(95)01096-3. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Martin RL, Mossop JE, Moore DR. Response properties of neurons in the inferior colliculus of the monaurally deafened ferret to acoustic stimulation of the intact ear. J Neurophysiol. 1997;78:767–779. doi: 10.1152/jn.1997.78.2.767. [DOI] [PubMed] [Google Scholar]
- Meister M, Wong RO, Baylor DA, Shatz CJ. Synchronous bursts of action potentials in ganglion cells of the developing mammalian retina. Science. 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- Merchan MA, Saldana E, Plaza I. Dorsal nucleus of the lateral lemniscus in the rat: concentric organization and tonotopic projection to the inferior colliculus. J Comp Neurol. 1994;342:259–278. doi: 10.1002/cne.903420209. [DOI] [PubMed] [Google Scholar]
- Moore DR, Irvine DR. The development of some peripheral and central auditory responses in the neonatal cat. Brain Res. 1979;163:49–59. doi: 10.1016/0006-8993(79)90150-1. [DOI] [PubMed] [Google Scholar]
- Moore DR, Kitzes LM. Projections from the cochlear nucleus to the inferior colliculus in normal and neonatally cochlea-ablated gerbils. J Comp Neurol. 1985;240:180–195. doi: 10.1002/cne.902400208. [DOI] [PubMed] [Google Scholar]
- Moore DR, Kowalchuk NE. Auditory brainstem of the ferret: effects of unilateral cochlear lesions on cochlear nucleus volume and projections to the inferior colliculus. J Comp Neurol. 1988;272:503–515. doi: 10.1002/cne.902720405. [DOI] [PubMed] [Google Scholar]
- Moore DR, Rogers NJ, O’Leary SJ. Loss of cochlear nucleus neurons following aminoglycoside antibiotics or cochlear removal. Ann Otol Rhinol Laryngol. 1998;107:337–343. doi: 10.1177/000348949810700413. [DOI] [PubMed] [Google Scholar]
- Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear Res. 2000;147:183–187. doi: 10.1016/s0378-5955(00)00054-x. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Killackey HP, Kitzes LM. Ascending projections to the inferior colliculus following unilateral cochlear ablation in the neonatal gerbil, Meriones unguiculatus. J Comp Neurol. 1983;214:144–153. doi: 10.1002/cne.902140204. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Shneiderman A. An EM study of the dorsal nucleus of the lateral lemniscus: inhibitory, commissural, synaptic connections between ascending auditory pathways. J Neurosci. 1989a;9:967–982. doi: 10.1523/JNEUROSCI.09-03-00967.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver DL, Shneiderman A. The anatomy of the inferior colliculus: a cellular basis for integration of monaural and binaural information. In: Altschuler RA, Bobbin RP, Clopton BM, Hoffman DW, editors. Neurobiology of hearing: the central auditory system. New York: Raven Press; 1989b. pp. 195–222. [Google Scholar]
- Penn AA, Riquelme PA, Feller MB, Shatz CJ. Competition in retinogeniculate patterning driven by spontaneous activity. Science. 1998;279:2108–2112. doi: 10.1126/science.279.5359.2108. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Park TJ. The effects of GABAergic inhibition on monaural response properties of neurons in the mustache bat’s inferior colliculus. Hear Res. 1993;65:99–117. doi: 10.1016/0378-5955(93)90205-f. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Burger RM, Klug A. Dissecting the circuitry of the auditory system. Trends Neurosci. 2003;26:33–39. doi: 10.1016/s0166-2236(02)00009-7. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Hyson RL. Afferent influences on brain auditory system development. Brain Dysfunction. 1992;5:65–93. [Google Scholar]
- Rubel EW, Cramer KS. Choosing axonal real estate: location, location, location. J Comp Neurol. 2002;448:1–5. doi: 10.1002/cne.10255. [DOI] [PubMed] [Google Scholar]
- Ryan AF, Woolf NK, Sharp FR. Tonotopic organization in the central auditory pathway of the Mongolian gerbil: a 2-deoxyglucose study. J Comp Neurol. 1982;207:369–380. doi: 10.1002/cne.902070408. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Siverls V. Development and specificity of inhibitory terminal arborizations in the central nervous system. J Neurobiol. 1991;22:837–854. doi: 10.1002/neu.480220805. [DOI] [PubMed] [Google Scholar]
- Sanes DH, Takacs C. Activity-dependent refinement of inhibitory connections. Eur J Neurosci. 1993;5:570–574. doi: 10.1111/j.1460-9568.1993.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Shatz CJ, Stryker MP. Prenatal tetrodotoxin infusion blocks segregation of retinogeniculate afferents. Science. 1988;242:87–89. doi: 10.1126/science.3175636. [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Henkel CK. Banding of lateral superior olivary nucleus afferents in the inferior colliculus: a possible substrate for sensory integration. J Comp Neurol. 1987;266:519–534. doi: 10.1002/cne.902660406. [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system? J Comp Neurol. 1988;276:188–208. doi: 10.1002/cne.902760204. [DOI] [PubMed] [Google Scholar]
- Shneiderman A, Chase MB, Rockwood JM, Benson CG, Potashner SJ. Evidence for a GABAergic projection from the dorsal nucleus of the lateral lemniscus to the inferior colliculus. J Neurochem. 1993;60:72–82. doi: 10.1111/j.1471-4159.1993.tb05824.x. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Leake PA. Topography of spiral ganglion projections to cochlear nucleus during postnatal development in cats. J Comp Neurol. 1997;384:293–311. doi: 10.1002/(sici)1096-9861(19970728)384:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Suneja SK, Benson CG, Potashner SJ. Glycine receptors in adult guinea pig brain stem auditory nuclei: regulation after unilateral cochlear ablation. Exp Neurol. 1998;154:473–488. doi: 10.1006/exnr.1998.6946. [DOI] [PubMed] [Google Scholar]
- Tierney TS, Russell FA, Moore DR. Susceptibility of developing cochlear nucleus neurons to deafferentation-induced death abruptly ends just before the onset of hearing. J Comp Neurol. 1997;378:295–306. doi: 10.1002/(sici)1096-9861(19970210)378:2<295::aid-cne11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Trune DR. Influence of neonatal cochlear removal on the development of mouse cochlear nucleus: II. Dendritic morphometry of its neurons. J Comp Neurol. 1982;209:425–434. doi: 10.1002/cne.902090411. [DOI] [PubMed] [Google Scholar]
- Tucci D, Cant NB, Durham D. Conductive hearing loss results in changes in cytochrome oxidase activity in gerbil central auditory system. J Assoc Res Otolaryngol. 2002;3:89–106. doi: 10.1007/s101620010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Sanes DH. Afferent regulation of inhibitory synaptic transmission in the developing auditory midbrain. J Neurosci. 2000;20:1912–1921. doi: 10.1523/JNEUROSCI.20-05-01912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Sanes DH. The effect of bilateral deafness on excitatory and inhibitory synaptic strength in the inferior colliculus. Eur J Neurosci. 2002;16:2394–2404. doi: 10.1046/j.1460-9568.2002.02302.x. [DOI] [PubMed] [Google Scholar]
- Vale C, Schoorlemmer J, Sanes DH. Deafness disrupts chloride transporter function and inhibitory synaptic transmission. J Neurosci. 2003;23:7516–7524. doi: 10.1523/JNEUROSCI.23-20-07516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale C, Juiz JM, Moore DR, Sanes DH. Unilateral cochlear ablation produces greater loss of inhibition in the contralateral inferior colliculus. Eur J Neurosci. 2004;20:2133–2140. doi: 10.1111/j.1460-9568.2004.03679.x. [DOI] [PubMed] [Google Scholar]
- van Adel BA, Kidd SA, Kelly JB. Contribution of the commissure of Probst to binaural evoked responses in the rat’s inferior colliculus: interaural time differences. Hear Res. 1999;130:115–130. doi: 10.1016/s0378-5955(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J. Postnatal development of auditory nerve and cochlear nucleus neuronal responses in kittens. Hear Res. 1987;28:97–116. doi: 10.1016/0378-5955(87)90157-2. [DOI] [PubMed] [Google Scholar]
- Zook JM, Casseday JH. Convergence of ascending pathways at the inferior colliculus of the mustache bat, Pteronotus parnellii. J Comp Neurol. 1987;261:347–361. doi: 10.1002/cne.902610303. [DOI] [PubMed] [Google Scholar]