Abstract
Recombinant vaccinia viruses have shown promise as vaccine vectors. However, their effectiveness is markedly reduced by pre-existing anti-vaccinia immunity. The possibility of new vaccinia immunizations in the event of a bioterror-related smallpox release poses an additional negative impact on the utility of vaccinia-based vectors. Thus, we aimed to design a vaccinia vector that would enhance the immune response to an expressed foreign protein in a pre-immune animal model. To do this, we made use of the finding that most neutralizing antibodies against the extracellular form of vaccinia virus are directed against the B5 protein. We found that mice immunized with vaccinia, primed with Gag plasmid DNA, and boosted with a recombinant vaccinia virus lacking the majority of the B5 ectodomain expressing a test antigen, HIV Gag, had stronger anti-Gag immune responses than mice that were boosted with a wild type virus-expressing Gag. These findings are particularly striking given the more attenuated phenotype of this virus, as compared to its wild type counterpart. Importantly, we found that vaccination with a B5R deletion virus, followed by boosting with the Gag-expressing virus lacking the majority of the B5 ectodomain, resulted in poorer anti-Gag immune responses. Thus, recombinant vaccinia viruses lacking the B5 ectodomain may serve as vaccine vectors in DNA prime-vaccinia boost vaccinations of individuals with pre-existing immunity against vaccinia. These data open the possibility of extending the potential benefit of replication competent recombinant vaccinia virus vectors to a larger population.
Keywords: vaccinia virus, immunity, B5 protein, recombinant vector, vaccines, DNA, mice
Introduction
Recombinant vaccinia viruses are appealing vaccine vectors for several reasons: they generate potent antibody and T-cell responses, their large cloning capacity allows for the expression of multiple foreign genes, they have a wide host and cell range, and they form stable recombinants (Moroziewicz and Kaufman, 2005). Vaccinia-based vectors have been constructed as vaccine candidates against a number of infectious diseases including HIV, tuberculosis, and malaria, and have been used in immunotherapies against cancer (e.g., (Ami et al., 2005; Mastrangelo, Maguire, and Lattime, 2000; Rochlitz et al., 2003; Shen and Nemunaitis, 2005)). Official approval has been granted for the use of a vaccinia-based vaccine to protect wildlife against the rabies virus (Pastoret and Brochier, 1996). Importantly, the use of vaccinia vectors to combat various diseases has illustrated that the virus is relatively well tolerated. Attenuated strains of vaccinia, such as modified vaccinia virus Ankara (MVA), have been employed to further promote the safety of vaccinia-based vectors (Shen and Nemunaitis, 2005). To enhance immune responses to vaccinia virus vectors, a strategy of priming with DNA prior to poxvirus boosting has been employed (Gilbert et al., 2005; Hanke et al., 1999; McShane et al., 2001; Sullivan et al., 2000).
While the potent immune responses elicited by vaccinia virus are helpful for the development of memory T- and B-cells specific for a foreign antigen, there is a drawback to the immunogenicity elicited by this virus. Until the World Health Organization successfully eradicated smallpox in the 1970s, the smallpox vaccine was used as a standard childhood vaccine (Mahalingam, Damon, and Lidbury, 2004). As demonstrated by Hammarlund et al., antiviral immunity against vaccinia remains high for several decades post vaccination (Hammarlund et al., 2003). This long-lasting immunity to the smallpox vaccine greatly reduces the number of people who could fully benefit from a vaccinia-based vaccine therapy (e.g., (Cooney et al., 1991)). A further hindrance to the future use of vaccinia-based vectors will arise if mass vaccination is reinstated as a countermeasure against bioterrorism with smallpox (Halloran et al., 2002).
There are two infectious forms of vaccinia virus: mature virus (MV) and extracellular virus (EV). MV represents the majority of progeny virus formed during an infection and EV, though less abundant, is critical for dissemination and spread within the infected host (Smith, Vanderplasschen, and Law, 2002). Neutralizing antibodies against both virus forms are required for optimal protection from poxvirus infections (Appleyard and Andrews, 1974; Turner and Squires, 1971). The EV-specific B5 protein was recently shown to be the primary target of EV-neutralizing antibodies (Bell et al., 2004). Indeed, a mutant vaccinia virus lacking the majority of the B5 ectodomain, but making nearly wild type levels of EV, is resistant to neutralization by vaccinia immune globulin (Bell et al., 2004). Based on this information, we hypothesized that a virus lacking the B5 ectodomain would be less readily cleared by a vaccinia-experienced immune system, making it a more efficient vector to express a foreign protein than a wild type (WT) virus expressing the same foreign antigen. In this study, pre-immune mice were given a single HIV-1 Gag DNA prime and then boosted with a WT or B5-mutant virus that each expressed the same foreign protein, HIV-1 Gag. Importantly, we found that while the virus lacking the majority of the B5-ectodomain is attenuated in vivo, it elicits a more potent anti-Gag immune response than its WT counterpart. This proof of concept finding should help pave the way for the development of replication competent vaccinia-based vector approaches that can be utilized in individuals with pre-existing immunity to vaccinia.
Results
After DNA prime, boosting of pre-immune mice with vvΔB5-gag induced a stronger anti-Gag immune response than vvWT-gag
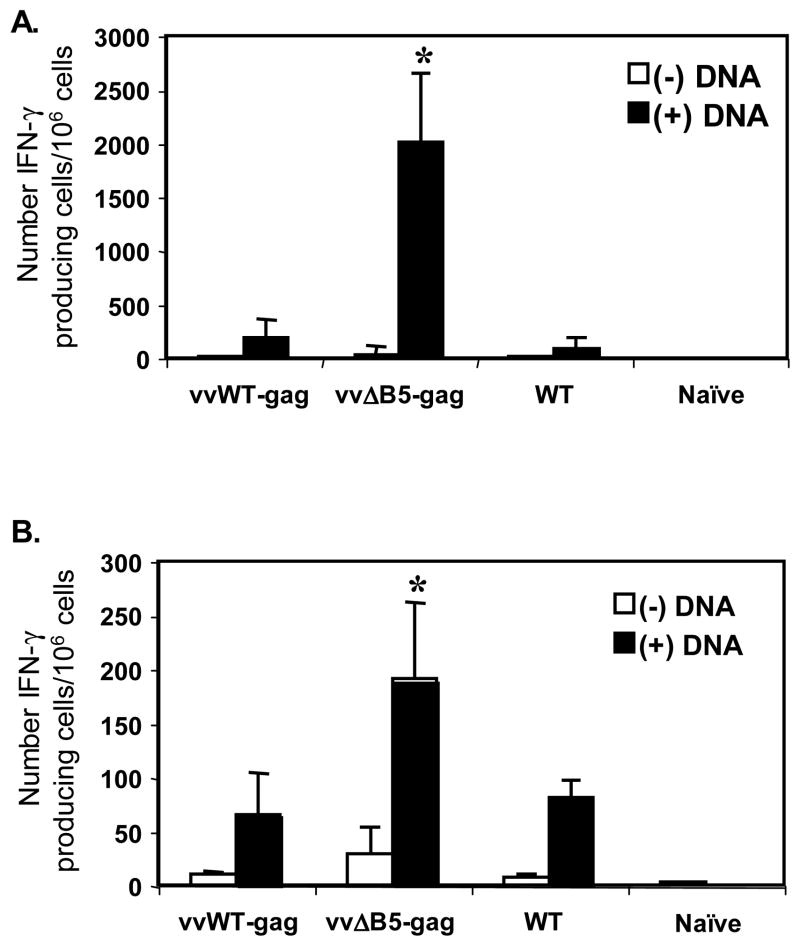
Because pre-existing vaccinia virus immunity blunts the immune response to foreign proteins expressed in recombinant vaccinia viruses, we sought to test a vaccinia vector that might still generate responses. DNA priming has been show to enhance the immune response to poxvirus vectors (Gilbert et al., 2005; Hanke et al., 1999; McShane et al., 2001; Sullivan et al., 2000), and has even been used to enhance the immune responses of vaccinia-immune hosts (Yang et al., 2003). However, this strategy requires multiple DNA treatments (Yang et al., 2003). Thus, as a proof of concept, we sought to design a vaccinia vector that could work after a single DNA prime. This new vector design was based on evidence that the major EV-neutralizing activity is directed against the B5-protein (Bell et al., 2004). Our aim was to determine if boosting of pre-immune mice with vvΔB5-gag could promote enhanced expression of the Gag protein due to escape of this virus from pre-existing anti-B5 neutralizing antibodies. Thus, we first immunized mice with wild type vaccinia virus to generate vaccinia-immune mice. Two weeks later, we primed some groups of mice with a single injection of an early generation Gag-expressing DNA vaccine (Qiu et al., 1999). Four weeks after the initial vaccinia virus immunization, we boosted the mice with vvWT-gag, vvΔB5-gag, or WT control virus. We quantitated the number of Gag-specific IFN-γ producing CD4+ and CD8+ T cells two weeks post boost. As shown in Figure 1, little to no response was measured in vaccinia-immune mice in the absence of Gag DNA priming. Vaccinia-immune mice primed with DNA and then boosted with vvΔB5-gag had significantly higher CD8+ and CD4+ Gag responses than mice boosted with vvWT-gag (Figure 1A and B). Indeed, vvWT-gag failed to stimulate a measurable response over background IFN-γ production induced by the WT vaccinia boost of pre-immune mice. We hypothesized that pre-existing anti-B5 immunity in vaccinia-immune mice resulted in more rapid clearance of the vvWT-gag after challenge, thus preventing establishment of an immune response to Gag. vvΔB5-gag, in contrast, is able to establish enough of an infection to allow anti-Gag responses to develop.
Figure 1.
Gag-specific T-cell activation after boosting of vaccinia-immune mice with vvWT-gag, vvΔB5-gag, or WT viruses. All mice were initially immunized with WT vaccinia virus. Two weeks later, groups of mice (5 mice/group) were either treated (black bar) or untreated (white bar) with Gag DNA, and then boosted 2 weeks later with vvWT-gag, vvΔB5-gag, or WT viruses. Splenocytes were stimulated for 24 h in the presence of control peptides, or (A) a class I Gag epitope, or (B) a class II Gag epitope. Gag-specific IFN-γ producing cells were quantitated by ELISpot. The number of positive cells in wells receiving control peptide was subtracted from those receiving Gag. Shown are the means ± SEM of mice in each group for each condition. This experiment was performed three times with similar results (*, p < 0.05 compared with vvWT-gag or WT virus).
vvWT-gag and vvΔB5-gag are both highly attenuated in vivo
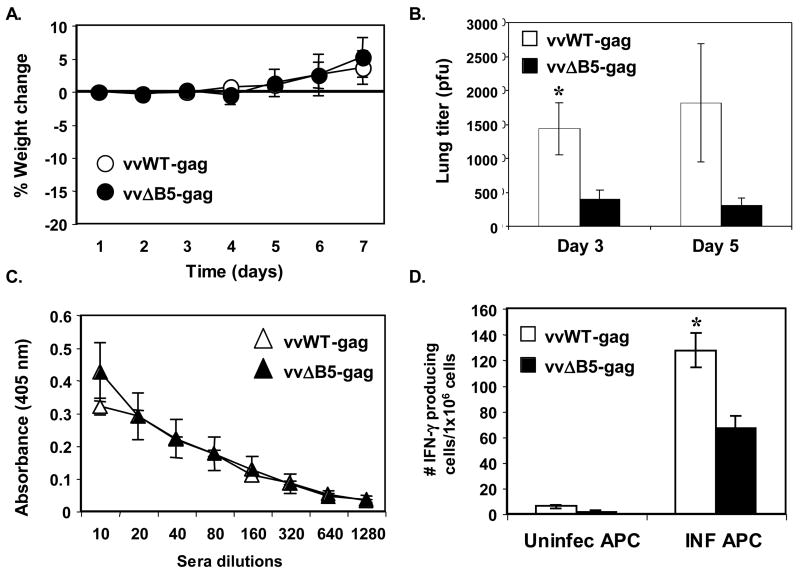
To insure that differences in the strength of the anti-Gag T-cell responses mounted against infection with vvWT-gag versus vvΔB5-gag did not result from discrepancies in the virulence and/or inherent immunogenicity of these two recombinants, we infected groups of naïve mice with either virus and assessed their virulence by measuring weight and quantitating viral titers in organs. As shown in Figure 2A, groups of mice infected intranasally with a high titer of vvWT-gag or vvΔB5-gag failed to lose weight. These mice appeared healthy and did not show any signs of illness. This is not surprising considering that both viruses are TK-minus, which is known to result in severe attenuation in mice (Buller et al., 1985; Phillpotts et al., 2000). Livers and spleens harvested from infected mice at various times post infection failed to show evidence of viral dissemination, consistent with the severe attenuation of these viruses. However, we were able to titer virus out of the lungs at days three and five post infection (Figure 2B). Importantly, vvWT-gag infection resulted in consistently higher viral titers than vvΔB5-gag infection at both time points. While the mutant virus lacking the majority of the B5 ectodomain makes nearly wild type levels of EV in tissue culture (Herrera et al., 1998; Mathew et al., 1998), the highly conserved ectodomain of B5 in orthopoxviruses indicates that the deleted region may have other important in vivo functions that results in the further attenuation we found. Thus, vvΔB5-gag provides enhanced anti-Gag immune responses, but is even more attenuated then vvWT-gag. We next examined the anti-vaccinia virus immune responses generated in these groups of mice. While vvWT-gag and vvΔB5-gag induced equivalent anti-vaccinia antibody responses after infection (Figure 2C), vvWT-gag produced a stronger cellular immune response to vaccinia (Figure 2D). Our finding that vvWT-gag is, if anything, more virulent and leads to better anti-vaccinia virus cellular immune responses than vvΔB5-gag lends more credence to our hypothesis that the anti-Gag response elicited by vvΔB5-gag is the result of delayed clearance in the absence of anti-B5 antibodies.
Figure 2.
Pathogenesis and vaccinia-specific immune responses to vvWT-gag and vvΔB5-gag. Groups of 12 mice were infected intranasally with 5×106 pfu of vvWT-gag or vvΔB5-gag and (A) percent weight change was measured over time. (B) Three mice in each group were sacrificed at days three and five and virus titers were quantitated in the lungs. (C) Five mice in each group were sacrificed six weeks after intra-nasal infection and anti-vaccinia virus antibody titers were measured by ELISA. (D) Splenocytes from these sacrificed mice were co-cultured with naïve antigen presenting cells (APC) that had been infected with WT virus (INF APC) or left uninfected (Uninfect APC) and IFN-γ producing cells were quantitated by ELISpot. Shown are the means ± SEM of mice in each group for each condition. These experiments were performed two times with similar results (*, p < 0.05 compared with vvΔB5-gag).
Primary vaccination with vvB5R-KO virus prevents induction of a strong anti-Gag immune response upon boosting with vvΔB5-gag
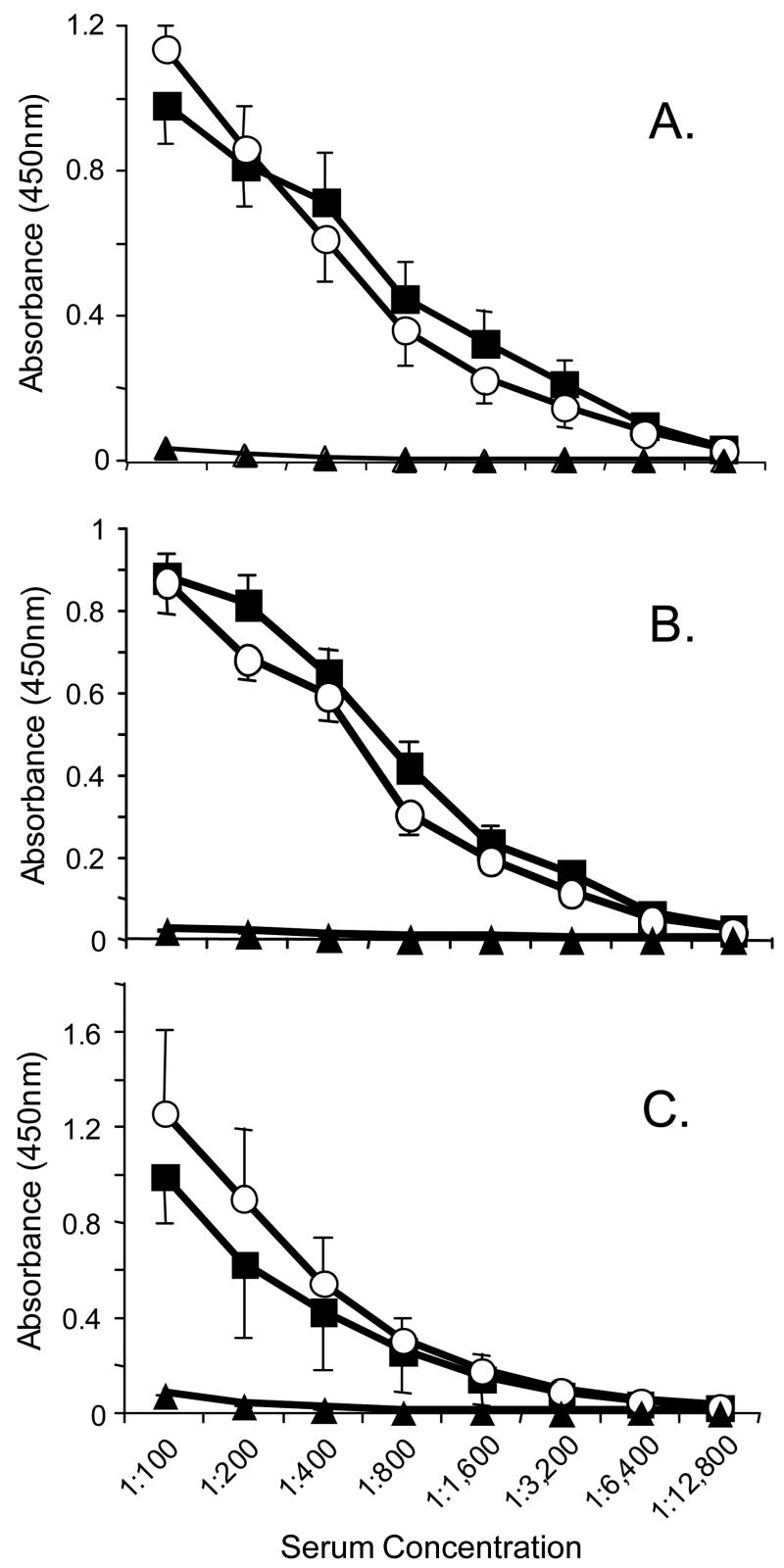
To provide further evidence that the pre-existing anti-B5 antibodies had a greater effect on Gag immunogenicity generated by vvWT-gag than by vvΔB5-gag, we examined the Gag-specific immune responses generated in pre-immune mice that were immunized with a virus in which the B5R gene is completely deleted (Wolffe, Isaacs, and Moss, 1993). We hypothesized that since the B5R-deletion virus expresses all other viral proteins, it should generate vaccinia-immune responses except ones directed at the B5-protein. Since the B5R-deletion virus is highly attenuated (Wolffe, Isaacs, and Moss, 1993), we wanted to ensure that with the dose given, mice vaccinated by scarification with the B5R-deletion virus could generate a similar level of anti-vaccinia virus immune responses as the wild type virus. We vaccinated groups of mice, bled them one month later, and measured antibody responses to whole vaccinia virus lysate and individual MV and EV surface proteins. We found similar anti-vaccinia antibody responses to whole infected cell lysate (Figure 3A), baculovirus expressed L1 (Figure 3B), and baculovirus expressed A33 (Figure 3C). This finding of comparable immune responses to WT and the full B5R-deletion viruses is similar to what was reported by Jackson et al in their studies of immune responses to a vaccinia virus vector that contained a B5R deletion and its parental virus (Jackson et al., 2005).
Figure 3.
Anti-vaccinia virus antibody responses in mice vaccinated with wild type virus or vvB5R-KO. Groups of four mice were immunized with WT (closed squares) or vvB5R-KO (open circles) by tail scarification and anti-vaccinia immune responses were measured by ELISA one month after vaccination. Antibody responses to (A) whole vaccinia virus infected cell lysate, (B) vaccinia virus MV protein L1, (C) vaccinia virus EV protein A33 were determined. Shown are the means ± SD of sera from individual mice in each group at each dilution. Closed triangles represent sera from naïve mice.
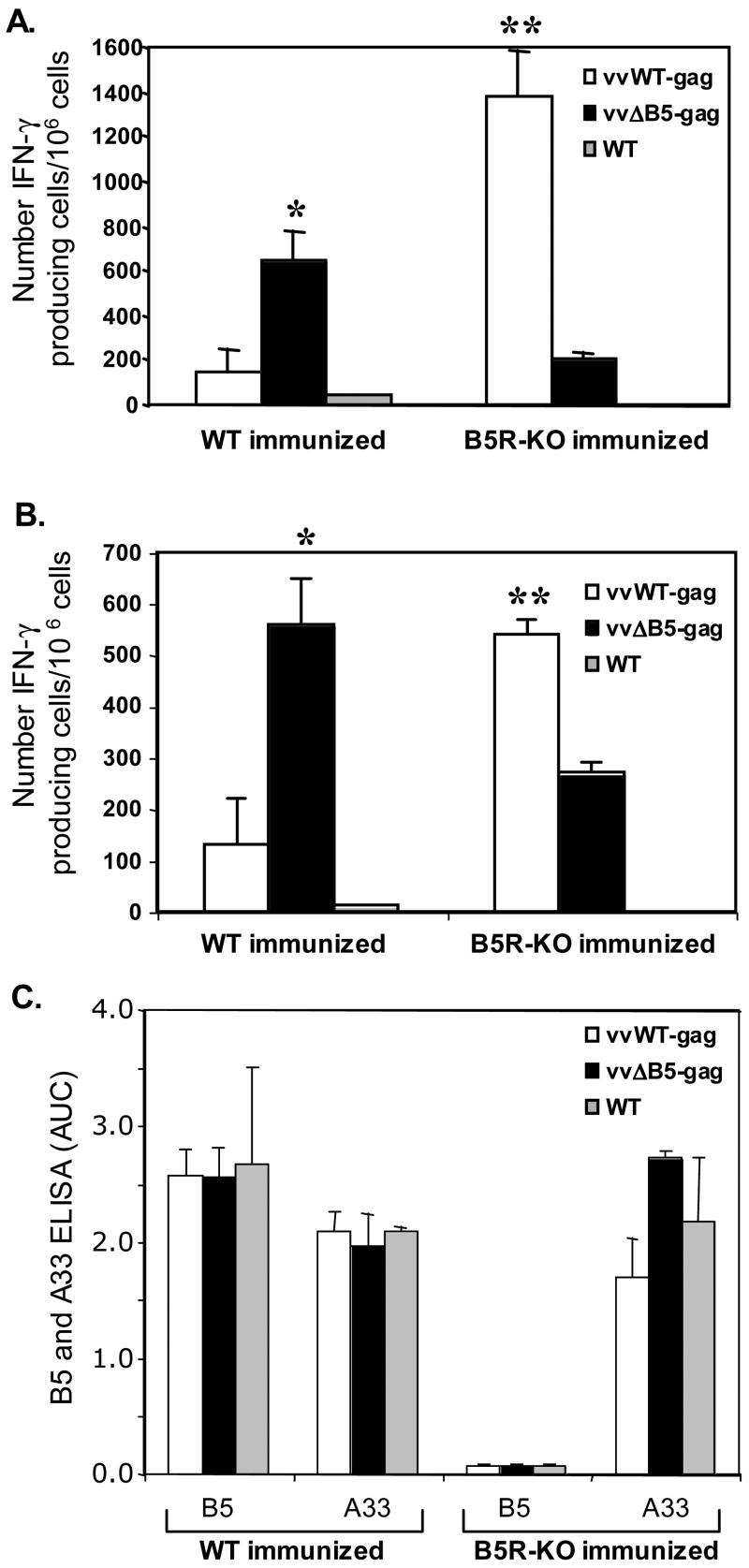
To test differences in anti-Gag immune responses generated by vvWT-gag and vvΔB5-gag in pre-immune mice, we primed groups with Gag DNA two weeks after the initial vaccinia virus vaccinations. Four weeks following the initial vaccinia immunization, we boosted the mice with vvWT-gag, vvΔB5-gag, or WT control virus, and quantitated the number of Gag-specific IFN-γ producing CD8+ and CD4+ T cells two weeks later. In contrast to the mice with pre-existing immunity to vaccinia virus after WT-virus vaccination, mice immunized with vvB5R-KO and boosted with vvΔB5-gag had lower Gag-specific responses than mice boosted with vvWT-gag (Figure 4A and B). Furthermore, we observed the opposite trend in mice boosted with vvWT-gag (Figure 4A and B). Since vvWT-gag is more virulent than vvΔB5-gag, it is possible that in the absence of a potent anti-B5 antibody response, this virus has an opportunity to generate better anti-Gag responses. As further support of our hypothesis that the lack of anti-B5 immunity is responsible for enhanced Gag-specific immunity in mice immunized with vvΔB5-gag, we also examined the antibody responses two weeks after boosting with the Gag-expressing viruses. As shown in Figure 4C, while animals vaccinated with WT virus and boosted with the Gag-expressing viruses had robust anti-B5 antibody responses, mice vaccinated with the B5R-deletion virus and boosted with the Gag-expressing viruses generated little to no anti-B5 antibody.
Figure 4.
Gag-specific T-cell activation and anti-vaccinia antibody responses in mice immunized with WT or B5R-KO viruses and boosted with vvWT-gag, vvΔB5-gag, or WT viruses. Groups of five mice were immunized with the indicated virus, primed with Gag DNA, and then boosted with vvWT-gag, vvΔB5-gag, or WT viruses. Splenocytes were stimulated for 24h in the presence of control peptides, (A) a class I Gag epitope, or (B) a class II Gag epitope. Gag-specific IFN-γ producing cells were quantitated by ELISpot. The number of positive cells in wells receiving control peptide was subtracted from those receiving Gag. Shown are the means ± SEM of mice in each group for each condition. These experiments were performed three times with similar results (*, p < 0.05 compared with vvWT-gag boosted; **, p < 0.05 compared with vvΔB5-gag boosted). (C) Sera taken from groups of mice at the time of splenocyte harvest were analyzed for anti-B5 and anti-A33 IgG antibody responses by ELISA. Shown are the means (± SEM) of the calculated area under the curve (AUC) for six serial, 3-fold dilutions from 1:50 to 1:12,150.
Discussion
Laboratory studies of vaccinia virus are important because they provide an increased understanding of its role as a vaccine against smallpox, and lead to the development of new strategies for its use as a vaccine vector. The potential future use of vaccinia as a vaccine vector is at risk of being thwarted in this new age of biodefense. In general, people born prior to the early 1970’s likely had routine smallpox vaccination and thus have immunity against vaccinia virus. In response to the threat of bioterrorism with smallpox, there is now the potential that smallpox vaccination will again become widespread. Given the promise shown by vaccinia-based vaccines and therapies against other infectious diseases and cancers, it would be unfortunate to have to abandon this approach. Because prior smallpox vaccination does not generate sterilizing immunity, many strategies will need to rely on the use of replication competent vaccinia virus vectors (i.e., not MVA) to generate optimal immune responses to the foreign antigen. Based on our knowledge of the importance of anti-B5 antibodies in EV neutralization, we tested the hypothesis as a proof of concept that vaccinia vectors could be manipulated so that they could still be utilized in a DNA prime-vaccinia boost strategy in hosts with pre-existing vaccinia immunity. We found that infection of pre-immune mice with a virus lacking the majority of the ectodomain of B5, vvΔB5-gag, was able to induce an anti-Gag specific immune response after a single DNA prime not achieved by a wild type virus expressing the same Gag antigen. The anti-Gag responses elicited by this virus are likely the result of an enhanced ability to spread in the absence of pre-existing anti-B5 antibodies. Importantly, while vvΔB5-gag may have a slight advantage in the setting of pre-existing immunity, the absence of the B5 ectodomain and the TK region used to express Gag, attenuates this virus’ ability to grow and spread compared to vvWT-gag, which is also TK-minus, but has a fully intact B5. Thus in addition to being a vector that can enhance immune responses to a foreign protein in those with pre-existing immunity to vaccinia, it also is a vaccinia-based vector with reduced virulence. We focused on cellular immune responses because the portion of Gag we cloned and expressed in the recombinant vaccinia viruses lacks its natural N-terminus and thus Gag particles are not released from infected cells, so little to no anti-Gag antibody responses can be detected (data not shown). It is important to point out that our results do not contradict the previously published findings of Jackson et al (Jackson et al., 2005). In that study, a panel of recombinant vaccinia viruses lacking various genes (including one that contained a B5R deletion) and expressing the HIV envelope did not improve the capacity of the viruses to augment immune responses to the expressed foreign protein when compared to the parental virus expressing the same foreign protein. However they were examining a very different question than the one we posed in our study. They were not looking at pre-existing vector immunity, but rather the effect of the absence of various vaccinia virus genes on immune responses to a foreign protein after a prime and boost (Jackson et al., 2005).
Numerous studies have examined methods for enhancing the immune response to foreign proteins that are expressed by viral vectors. One effective and commonly used technique is to delete immune modulatory proteins made by the viral vector. For example, deletion of the vaccinia virus anti-inflammatory genes, SPI-1 and SPI-2, is shown to increase both the humoral and T-helper responses to a foreign protein while simultaneously attenuating the vector (Legrand et al., 2004). In addition, herpes simplex virus type-1 vectors constructed to lack a viral gene that interferes with MHC class I expression are found to exhibit enhanced anti-tumor T-cell activity (Todo et al., 2001). Another important method for enhancing the immune response to a foreign protein produced by a viral vector is by suppressing the antiviral immune response. Lieber et al. show that infection with a recombinant adenovirus vector expressing an IκBα supersuppresor results in reduced production of antiviral cytokines and enhanced immune responses to the transgene product (Lieber et al., 1998). Transient deletion of CD4+ T cells is also found to allow establishment of an adenovirus vector within its host (Kolls et al., 1996). Most recently, studies have sought to specifically bias the immune system so as to maximize the response to a viral vector-induced protein. These have included viral expression of immune modulatory cytokines, chemokines, and costimulatory molecules (Ahlers, Belyakov, and Berzofsky, 2003; Kikuchi et al., 2005; Klas et al., 2006). While each of these strategies has proven to be effective in naïve animals, it is difficult to assess how successful they would be in the context of pre-existing immunity against the viral vector. In addition, administration of exogenous immune suppressors, or activators, into humans is accompanied by questions of safety and tolerance. An important benefit to our method of challenging vaccinia-immune animals with a viral vector lacking B5 ectodomain is that no manipulation of the host immune response is required.
Our findings that vvΔB5-gag induces a better anti-Gag immune response than vvWT-gag in pre-immune mice lends further support to the importance of B5 as a target of EV-neutralizing antibodies (Bell et al., 2004). Our results imply that after immunization with a WT vaccinia virus, vvΔB5-gag is not readily controlled by anti-B5 responses, thus allowing expression of Gag to occur. Vaccination with a B5R-KO virus, in contrast, results in development of an anti-vaccinia immune response that does not rely on an anti-B5 response. It is only in this context that vvΔB5-gag is more readily cleared, while the vvWT-gag virus, with an intact B5 protein, is able to spread and generate a better anti-Gag immune response. This report provides the initial groundwork for the extension of vaccinia-based vector therapies that rely on replication competent vaccinia virus to individuals with pre-existing immunity against vaccinia. The consecutive inactivation of additional genes encoding target proteins for neutralizing antibodies may further extend the usefulness of such vectors.
Materials and Methods
Recombinant vaccinia viruses
Two recombinant viruses (strain IHDJ) expressing a fragment of Gag from the HXB2 clone of prototype HIV strain 3B (from amino acid 29 to 498) under control of a synthetic strong early/late vaccinia promoter (Chakrabarti, Sisler, and Moss, 1997) were used in the study. The wild type virus with the Gag gene inserted into the thymidine kinase gene (TK) (vSIAA-34) was previously described (Kwak et al., 2004). For this study, we refer to that virus as vvWT-gag. A B5-mutant virus expressing the same antigen in the TK region was generated using as the parental virus a previously constructed virus, vSI-26 (Herrera et al., 1998), which lacks 80% of the B5-ectodomain. To construct this virus, plasmid pSI-1724 was used to direct homologous recombination of the foreign gene into the TK gene, and the recombinant virus was plaque purified on a TK-minus cell line in the presence of bromo-deoxyuridine and x-gal. The isolated virus, vSIHK-36, is referred to in this report as vvΔB5-gag. Using a rabbit polyclonal antiserum to Gag (Steimer et al., 1986), western blots of infected cell lysates showed that Gag is equally expressed by both viruses (data not shown). A previously described recombinant vaccinia virus, vSI-14 (Wolffe, Isaacs, and Moss, 1993), which has the entire B5R open reading frame deleted was also used and is referred to as vvB5R-KO in this study.
Mice
Female BALB/c (H-2d) were purchased from Charles River Laboratories and maintained in a pathogen-free microisolator environment. Six to eight week old female mice were used in this study.
Vaccinations
Mice were immunized once by tail scarification with approximately 5×106 pfu of WT or 2×107 pfu of vvB5R-KO viruses. We used this slightly higher dose of the B5R-deletion virus because this virus is attenuated due to its inability to efficiently make EV and we wanted to ensure generation of good anti-vaccinia virus immune responses (see below). Two weeks later, some groups of mice were primed with 100 μg of HIV-1 p37gag DNA plasmid (Qiu et al., 1999) intramuscularly. After two additional weeks, mice were intraperitoneally boosted with approximately 2×106 pfu of vvWT-gag, vvΔB5-gag, or WT virus. To analyze the primary immune response to Gag, we sacrificed mice two weeks after the boost (see below).
Gag-specific IFN-γ ELISpot
Two weeks after boosting with vvWT-gag, vvΔB5-gag, or WT, mice were sacrificed and splenocyte suspensions prepared. Red blood cells were lysed and splenocytes resuspended in complete tissue culture media (DMEM supplemented with 10% FBS (Hyclone), 2 mM-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 50 μM β-mercaptoethanol). IFN-γ ELISpot was performed as previously described (Kwak et al., 2004) using a murine IFN-γ ELISpot kit (R&D Systems). In brief, 2.5×105 splenocytes were plated into each well in duplicate and incubated with either Gag class I epitope (1 μM), Gag class II epitope (10 μM), control peptide LL0 91–99 (1 μM), or control peptide pigeon cytochrome c (10 μM) at 37°C. All peptides were synthesized and described previously (Mata and Paterson, 1999; Mata et al., 1998; Pamer, Harty, and Bevan, 1991; Shen et al., 2002). Briefly, the Kd-restricted class I Gag peptide is sequence 197–205 (AMQMLKETI) (Mata et al., 1998) and the H-2d restricted class II Gag peptide is sequence 253–272 (NPPIPVGEIYKRWIILGLNK) (Mata and Paterson, 1999). After 24 h, plates were washed and IFN-γ producing cells were stained with biotinylated anti-IFN-γ antibody, followed by alkaline phosphatase conjugated streptavidin and its substrate, BCIP/NBT chromogen. Plates were washed, and the number of colored spots quantitated in blinded fashion using a dissection microscope. The number of positive cells in wells that received control peptide was subtracted from those that received Gag peptides.
Pathogenesis of recombinant viruses and vaccinia-specific immune responses
Mice were infected intranasally with 5×106 pfu of vvWT-gag or vvΔB5-gag and weight was followed over time. Groups of three mice infected with each virus were sacrificed at days three and five, and virus titers were quantitated in the lung, spleen, and liver. Harvested organs were homogenized, and freeze-thawed three times. Samples were sonicated and titered in duplicate by serial dilution. Dilutions were added to monolayers of BSC-1 cells and incubated for 2 h at 37°C. The inoculum was suctioned off, the wells overlaid with media containing 2.5% FBS and 1% carboxymethylcellulose, and the plates incubated for two days. The wells were then stained with 0.1% crystal violet and the plaques counted.
To measure vaccinia-specific immune responses, five mice from each group were sacrificed six weeks post infection. Serum was harvested by cardiac puncture for humoral immune measurements and spleens were harvested to determine cellular immune responses. For humoral immune responses, 96-well flat-bottomed ELISA plates were coated overnight at 4°C using whole cell vaccinia-infected cell lysates in bicarbonate coating buffer as previously described (Hammarlund et al., 2003; Viner and Isaacs, 2005). Serial three-fold dilutions of sera were incubated for 1 h at room temperature. Plates were washed and incubated with horseradish peroxidase (HRP) -conjugated rabbit anti-mouse IgGAM (1:2000; Zymed, San Francisco, CA). After 1 h, OPD (Acros Organics, NJ) was added and the reaction was stopped with 1 M HCl and absorbance measured using an ELISA reader at 490 nm. For vaccinia-specific IFN-γ production splenocyte suspensions were prepared from harvested spleens. Cells were enriched for lymphocytes by plating in six-well plates at 2×106 cells/well, incubating at 37°C, and harvesting only the non-adherent cells. After 3 h, cells were resuspended in complete tissue culture media and IFN-γ ELISpot was performed using an IFN-γ ELISpot kit. Briefly, 2×105 splenocytes were plated into each well and co-cultured with 5×104 antigen-presenting cells (APCs). APCs were naïve splenocytes infected for 1 h at a multiplicity of infection of 3 pfu/cell with vaccinia virus (strain WR) and gamma-irradiated with ~1000 rad prior to plating. Uninfected APCs were used to quantitate nonspecific responses. After 24 h, wells were washed and plates were incubated overnight with a biotinylated anti-IFN-γ mAb. IFN-γ producing cells were detected with alkaline phosphate-streptavidin and BCIP/NBT chromogen substrate. Positive cells were quantitated using a dissection microscope.
Vaccinia-specific antibody responses after scarification with wild type virus or the B5R-deletion virus
To examine the anti-vaccinia virus humoral immune responses after vaccination with the WT or vvB5R-KO viruses, groups of mice were bled one month after the initial vaccinia vaccination and a direct ELISA using vaccinia-infected cell lysates was performed as described above. We also tested sera against individual MV and EV proteins. 96-well plates were coated with baculovirus-expressed and purified L1 protein (Aldaz-Carroll et al., 2005b) and A33 protein (Earl et al., 2004) at 5 μg/ml. Plates were washed, blocked, probed, and developed as described above, except that HRP-conjugated goat anti-mouse IgG (1:4000) was used as a secondary antibody, and TMB (3,3,5,5-tetramethylbenzidene, BD Biosciences) was used as the substrate. Absorbance was measured at 450 nm. To further examine the effect of antibody responses in mice initially vaccinated with the WT or vvB5R-KO viruses and then boosted with the recombinant-Gag expressing viruses, groups of mice were bled two weeks after the boost and a direct ELISA using baculovirus-expressed and purified A33 (Earl et al., 2004) and B5 proteins (Aldaz-Carroll et al., 2005a) was performed as described above.
Statistical analysis
Statistical differences between groups were found using the Student’s paired t test, and a p value of <0.05 was considered significant.
Acknowledgments
We thank Edward Alexander for technical assistance, David B. Weiner and Sandra A. Calarota for insights and help with the DNA vaccinations, and Gary H. Cohen and Roselyn J. Eisenberg for the purified baculovirus expressed A33, B5, and L1 proteins. The animal research included in this study has complied with all relevant federal guidelines and institutional policies. This work was supported by Public Health Service grant AI-47237 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlers JD, Belyakov IM, Berzofsky JA. Cytokine, chemokine, and costimulatory molecule modulation to enhance efficacy of HIV vaccines. Curr Mol Med. 2003;3(3):285–301. doi: 10.2174/1566524033479843. [DOI] [PubMed] [Google Scholar]
- Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Hirao L, Isaacs SN, Moss B, Eisenberg RJ, Cohen GH. Epitope-mapping studies define two major neutralization sites on the vaccinia virus extracellular enveloped virus glycoprotein B5R. J Virol. 2005a;79(10):6260–71. doi: 10.1128/JVI.79.10.6260-6271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaz-Carroll L, Whitbeck JC, Ponce de Leon M, Lou H, Pannell LK, Lebowitz J, Fogg C, White CL, Moss B, Cohen GH, Eisenberg RJ. Physical and immunological characterization of a recombinant secreted form of the membrane protein encoded by the vaccinia virus L1R gene. Virology. 2005b;341(1):59–71. doi: 10.1016/j.virol.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Ami Y, Izumi Y, Matsuo K, Someya K, Kanekiyo M, Horibata S, Yoshino N, Sakai K, Shinohara K, Matsumoto S, Yamada T, Yamazaki S, Yamamoto N, Honda M. Priming-boosting vaccination with recombinant Mycobacterium bovis bacillus Calmette-Guerin and a nonreplicating vaccinia virus recombinant leads to long-lasting and effective immunity. J Virol. 2005;79(20):12871–9. doi: 10.1128/JVI.79.20.12871-12879.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleyard G, Andrews C. Neutralizing activities of antisera to poxvirus soluble antigens. J Gen Virol. 1974;23:197–200. doi: 10.1099/0022-1317-23-2-197. [DOI] [PubMed] [Google Scholar]
- Bell E, Shamim M, Whitbeck JC, Sfyroera G, Lambris JD, Isaacs SN. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology. 2004;325(2):425–31. doi: 10.1016/j.virol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Buller RML, Smith GL, Cremer K, Notkins AL, Moss B. Decreased virulence of recombinant vaccinia virus expression vectors is associated with a thymidine kinase-negative phenotype. Nature. 1985;317:813–815. doi: 10.1038/317813a0. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S, Sisler JR, Moss B. Compact, synthetic, vaccinia virus early/late promoter for protein expression. Biotechniques. 1997;23:1094–7. doi: 10.2144/97236st07. [DOI] [PubMed] [Google Scholar]
- Cooney EL, Collier AC, Greenberg PD, Coombs RW, Zarling J, Arditti DE, Hoffman MC, Hu SL, Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991;337:567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- Earl PL, Americo JL, Wyatt LS, Eller LA, Whitbeck JC, Cohen GH, Eisenberg RJ, Hartmann CJ, Jackson DL, Kulesh DA, Martinez MJ, Miller DM, Mucker EM, Shamblin JD, Zwiers SH, Huggins JW, Jahrling PB, Moss B. Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox. Nature. 2004;428:182–185. doi: 10.1038/nature02331. [DOI] [PubMed] [Google Scholar]
- Gilbert SC, Moorthy VS, Andrews L, Pathan AA, McConkey SJ, Vuola JM, Keating SM, Berthoud T, Webster D, McShane H, Hill AV. Synergistic DNA-MVA prime-boost vaccination regimes for malaria and tuberculosis. Vaccine. 2005;5:5. doi: 10.1016/j.vaccine.2005.08.048. [DOI] [PubMed] [Google Scholar]
- Halloran ME, Longini IM, Jr, Nizam A, Yang Y. Containing bioterrorist smallpox. Science. 2002;298(5597):1428–32. doi: 10.1126/science.1074674. [DOI] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9(9):1131–7. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Hanke T, Neumann VC, Blanchard TJ, Sweeney P, Hill AV, Smith GL, McMichael A. Effective induction of HIV-specific CTL by multi-epitope using gene gun in a combined vaccination regime. Vaccine. 1999;17(6):589–96. doi: 10.1016/s0264-410x(98)00238-2. [DOI] [PubMed] [Google Scholar]
- Herrera E, Lorenzo MM, Blasco R, Isaacs SN. Functional analysis of vaccinia virus B5R protein: essential role in virus envelopment is independent of a large portion of the extracellular domain. J Virol. 1998;72:294–302. doi: 10.1128/jvi.72.1.294-302.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SS, Ilyinskii P, Philippon V, Gritz L, Yafal AG, Zinnack K, Beaudry KR, Manson KH, Lifton MA, Kuroda MJ, Letvin NL, Mazzara GP, Panicali DL. Role of genes that modulate host immune responses in the immunogenicity and pathogenicity of vaccinia virus. J Virol. 2005;79(10):6554–9. doi: 10.1128/JVI.79.10.6554-6559.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Andarini S, Xin H, Gomi K, Tokue Y, Saijo Y, Honjo T, Watanabe A, Nukiwa T. Involvement of fractalkine/CX3CL1 expression by dendritic cells in the enhancement of host immunity against Legionella pneumophila. Infect Immun. 2005;73(9):5350–7. doi: 10.1128/IAI.73.9.5350-5357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klas SD, Lavine CL, Whitt MA, Miller MA. IL-12-assisted immunization against Listeria monocytogenes using replication-restricted VSV-based vectors. Vaccine. 2006;24(9):1451–61. doi: 10.1016/j.vaccine.2005.05.046. Epub 2005 Sep 23. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Lei D, Odom G, Nelson S, Summer WR, Gerber MA, Shellito JE. Use of transient CD4 lymphocyte depletion to prolong transgene expression of E1-deleted adenoviral vectors. Hum Gene Ther. 1996;7(4):489–97. doi: 10.1089/hum.1996.7.4-489. [DOI] [PubMed] [Google Scholar]
- Kwak H, Mustafa W, Speirs K, Abdool AJ, Paterson Y, Isaacs SN. Improved protection conferred by vaccination with a recombinant vaccinia virus that incorporates a foreign antigen into the extracellular enveloped virion. Virology. 2004;322(2):337–48. doi: 10.1016/j.virol.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Legrand FA, Verardi PH, Jones LA, Chan KS, Peng Y, Yilma TD. Induction of potent humoral and cell-mediated immune responses by attenuated vaccinia virus vectors with deleted serpin genes. J Virol. 2004;78(6):2770–9. doi: 10.1128/JVI.78.6.2770-2779.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber A, He CY, Meuse L, Himeda C, Wilson C, Kay MA. Inhibition of NF-kappaB activation in combination with bcl-2 expression allows for persistence of first-generation adenovirus vectors in the mouse liver. J Virol. 1998;72(11):9267–77. doi: 10.1128/jvi.72.11.9267-9277.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam S, Damon IK, Lidbury BA. 25 years since the eradication of smallpox: why poxvirus research is still relevant. Trends Immunol. 2004;25(12):636–9. doi: 10.1016/j.it.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Mastrangelo MJ, Maguire HC, Lattime EC. Intralesional vaccinia/GM-CSF recombinant virus in the treatment of metastatic melanoma. Adv Exp Med Biol. 2000;465:391–400. doi: 10.1007/0-306-46817-4_34. [DOI] [PubMed] [Google Scholar]
- Mata M, Paterson Y. Th1 T cell responses to HIV-1 Gag protein delivered by a Listeria monocytogenes vaccine are similar to those induced by endogenous listerial antigens. J Immunol. 1999;163:1449–56. [PubMed] [Google Scholar]
- Mata M, Travers PJ, Liu Q, Frankel FR, Paterson Y. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J Immunol. 1998;161:2985–93. [PubMed] [Google Scholar]
- Mathew E, Sanderson CM, Hollinshead M, Smith GL. The extracellular domain of vaccinia virus protein B5R affects plaque phenotype, extracellular enveloped virus release, and intracellular actin tail formation. J Virol. 1998;72:2429–38. doi: 10.1128/jvi.72.3.2429-2438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane H, Brookes R, Gilbert SC, Hill AV. Enhanced immunogenicity of CD4(+) t-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect Immun. 2001;69(2):681–6. doi: 10.1128/IAI.69.2.681-686.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroziewicz D, Kaufman HL. Gene therapy with poxvirus vectors. Curr Opin Mol Ther. 2005;7(4):317–25. [PubMed] [Google Scholar]
- Pamer EG, Harty JT, Bevan MJ. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991;353(6347):852–5. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastoret PP, Brochier B. The development and use of a vaccinia-rabies recombinant oral vaccine for the control of wildlife rabies; a link between Jenner and Pasteur. Epidemiol Infect. 1996;116(3):235–40. doi: 10.1017/s0950268800052535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillpotts RJ, Lescott T, Gates AJ, Jones L. The immune response to vaccinia virus is significantly reduced after scarification with TK- recombinants as compared to wild-type virus. Acta Virol. 2000;44(3):151–6. [PubMed] [Google Scholar]
- Qiu JT, Song R, Dettenhofer M, Tian C, August T, Felber BK, Pavlakis GN, Yu XF. Evaluation of novel human immunodeficiency virus type 1 Gag DNA vaccines for protein expression in mammalian cells and induction of immune responses. J Virol. 1999;73(11):9145–52. doi: 10.1128/jvi.73.11.9145-9152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochlitz C, Figlin R, Squiban P, Salzberg M, Pless M, Herrmann R, Tartour E, Zhao Y, Bizouarne N, Baudin M, Acres B. Phase I immunotherapy with a modified vaccinia virus (MVA) expressing human MUC1 as antigen-specific immunotherapy in patients with MUC1-positive advanced cancer. J Gene Med. 2003;5(8):690–9. doi: 10.1002/jgm.397. [DOI] [PubMed] [Google Scholar]
- Shen X, Wong SB, Buck CB, Zhang J, Siliciano RF. Direct priming and cross-priming contribute differentially to the induction of CD8+ CTL following exposure to vaccinia virus via different routes. J Immunol. 2002;169(8):4222–9. doi: 10.4049/jimmunol.169.8.4222. [DOI] [PubMed] [Google Scholar]
- Shen Y, Nemunaitis J. Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Mol Ther. 2005;11(2):180–95. doi: 10.1016/j.ymthe.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Smith GL, Vanderplasschen A, Law M. The formation and function of extracellular enveloped vaccinia virus. J Gen Virol. 2002;83(12):2915–2931. doi: 10.1099/0022-1317-83-12-2915. [DOI] [PubMed] [Google Scholar]
- Steimer KS, Puma JP, Power MD, Powers MA, George-Nascimento C, Stephans JC, Levy JA, Sanchez-Pescador R, Luciw PA, Barr PJ, et al. Differential antibody responses of individuals infected with AIDS- associated retroviruses surveyed using the viral core antigen p25gag expressed in bacteria. Virology. 1986;150:283–90. doi: 10.1016/0042-6822(86)90289-8. [DOI] [PubMed] [Google Scholar]
- Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408(6812):605–9. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- Todo T, Martuza RL, Rabkin SD, Johnson PA. Oncolytic herpes simplex virus vector with enhanced MHC class I presentation and tumor cell killing. Proc Natl Acad Sci U S A. 2001;98(11):6396–401. doi: 10.1073/pnas.101136398. Epub 2001 May 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GS, Squires EJ. Inactivated smallpox vaccine: immunogenicity of inactivated intracellular and extracellular vaccinia virus. J Gen Virol. 1971;13:19–25. doi: 10.1099/0022-1317-13-1-19. [DOI] [PubMed] [Google Scholar]
- Viner KM, Isaacs SN. Activity of vaccinia virus-neutralizing antibody in the sera of smallpox vaccinees. Microbes Infect. 2005;7(4):579–83. doi: 10.1016/j.micinf.2005.02.004. Epub 2005 Mar 22. [DOI] [PubMed] [Google Scholar]
- Wolffe EJ, Isaacs SN, Moss B. Deletion of the vaccinia virus B5R gene encoding a 42-kilodalton membrane glycoprotein inhibits extracellular virus envelope formation and dissemination. J Virol. 1993;67:4732–4741. doi: 10.1128/jvi.67.8.4732-4741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B, Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol. 2003;77(1):799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]