Abstract
The phytochrome (phy) family of sensory photoreceptors (phyA–phyE in Arabidopsis thaliana) induces changes in target-gene expression upon light-induced translocation to the nucleus, where certain members interact with selected members of the constitutively nuclear basic helix-loop-helix transcription factor family, such as PHYTOCHROME-INTERACTING FACTOR3 (PIF3). Previous evidence indicates that the binding of the photoactivated photoreceptor molecule to PIF3 induces rapid phosphorylation of the transcription factor in the cell prior to its degradation via the ubiqitin-proteosome system. To investigate whether this apparent primary signaling mechanism can be generalized to other phy-interacting partners, we have examined the molecular behavior of a second related phy-interacting member of the basic helix-loop-helix family, PIF5, during early deetiolation, immediately following initial exposure of dark-grown seedlings to light. The data show that red light induces very rapid phosphorylation and subsequent degradation (t1/2 < 5 min) of PIF5 via the proteosome system upon irradiation. Photobiological and genetic evidence indicates that the photoactivated phy molecule acts within 60 s to induce this phosphorylation of PIF5, and that phyA and phyB redundantly dominate this process, with phyD playing an apparently minor role. Collectively, the data support the proposal that the rapid phy-induced phosphorylation of PIF3 and PIF5 may represent the biochemical mechanism of primary signal transfer from photoactivated photoreceptor to binding partner, and that phyA and phyB (and possibly phyD) may signal to multiple, shared partners utilizing this common mechanism.
The phytochrome (phy) family of sensory photoreceptors (phyA–phyE in Arabidopsis [Arabidopsis thaliana]) monitors informational light signals from the environment and directs growth and developmental responses commensurate with the ambient conditions (Schäfer and Nagy, 2006; Whitelam and Halliday, 2007). Perception of these signals resides in the photoreceptor's capacity to switch reversibly between the biologically inactive Pr and the biologically active Pfr conformers upon sequential absorption of red (R) and far-red (FR) photons (Quail, 2002; Tu and Lagarias, 2005). Considerable progress has been made in recent years in defining the mechanisms by which the perceived light signals are transduced by the phy molecule to the photoresponsive nuclear genes that direct the plant's photomorphogenic behavior (Chen et al., 2004; Nagatani, 2004; Schepens et al., 2004; Duek and Fankhauser, 2005; Quail, 2006, 2007a, 2007b; Jiao et al., 2007), but much remains to be learned.
Current data indicate that R-induced Pfr formation leads to rapid translocation of the phy molecule from the cytoplasm into the nucleus (Sakamoto and Nagatani, 1996; Yamaguchi et al., 1999; Huq et al., 2003; Nagatani, 2004), where it interacts with factors such as members of the basic helix-loop-helix (bHLH) transcription factor superfamily, called phy-interacting factors (PIFs; Duek and Fankhauser, 2005; Quail, 2006, 2007a), and induces changes in expression of a spectrum of target genes, detectable as early as 5 min after initial exposure to light (Lissemore and Quail, 1988; Monte et al., 2004; Tepperman et al., 2004, 2006; Jiao et al., 2005; Ma et al., 2005; Quail, 2007b). Several members of subfamily 15 of the Arabidopsis bHLH family (Bailey et al., 2003; Toledo-Ortiz et al., 2003; Khanna et al., 2004), designated PIF1 (also called PIF3-LIKE5 [PIL5]; Yamashino et al., 2003; Huq et al., 2004; Oh et al., 2004, 2006, 2007; Shen et al., 2005), PIF3 (Ni et al., 1998, 1999; Martinez-Garcia et al., 2000; Kim et al., 2003; Bauer et al., 2004; Monte et al., 2004; Park et al., 2004; Al-Sady et al., 2006; Shin et al., 2007), PIF4 (Huq and Quail, 2002), PIF5(PIL6) (Fujimori et al., 2004; Khanna et al., 2004; R. Khanna, Y. Shen, and P. Quail, unpublished data), PIF6(PIL2) (Khanna et al., 2004), and PIF7 (P. Leivar, E. Monte, and P. Quail, unpublished data), have been shown to interact selectively with the Pfr form of phyA and/or phyB, and have been investigated to varying degrees for involvement in phy signaling. Each of these factors carries a short motif in its N-terminal domain (called APB for active phyB binding) shown to be necessary and sufficient for phyB binding (Khanna et al., 2004). PIF3 also carries a separate, unrelated motif (APA for active phyA binding) that is necessary for phyA binding (Al-Sady et al., 2006), but no evidence for an APA domain has been reported thus far for the other PIFs. The emerging evidence indicates that each PIF may have individualized as well as overlapping biological functions with the other PIFs in controlling seed germination and early seedling development during the deetiolation process (Huq and Quail, 2002; Kim et al., 2003; Huq et al., 2004; Oh et al., 2004, 2006, 2007; Khanna et al., 2004; Monte et al., 2004; R. Khanna, Y. Shen, and P. Quail, unpublished data; P. Leivar, E. Monte, and P. Quail, unpublished data).
The molecular mechanism by which photoactivated phy induces altered expression of target genes is unknown, but there is evidence that intranuclear binding of the photoreceptor to PIF1 and PIF3 leads to rapid degradation of these bHLH proteins via the ubiquitin-proteosome system (Bauer et al., 2004; Monte et al., 2004; Park et al., 2004; Shen et al., 2005; Al-Sady et al., 2006). These observations suggest the possibility that phy may regulate expression by altering the abundance of these transcription factors at the promoters of target genes. In the case of PIF3, the data indicate that phy binding induces very rapid, intranuclear phosphorylation of the factor prior to its degradation, suggesting that this covalent modification represents the signal-transfer transaction from photoreceptor to signaling partner, and that it tags the bHLH protein for ubiquitination and degradation (Al-Sady et al., 2006). However, the question of whether this postulated signal transfer mechanism is specific for PIF3 or occurs more generally across other target proteins has remained unanswered. Here, we address this question for a second member of the bHLH family, PIF5. Evidence from studies with pif5 mutants and transgenic overexpressors of PIF5 indicate that this factor functions in phyB-imposed inhibition of seedling hypocotyl elongation in response to R light (Fujimori et al., 2004; R. Khanna, Y. Shen, and P. Quail, unpublished data).
RESULTS AND DISCUSSION
R Light Induces Rapid PIF5 Degradation through the Proteosome System
Preliminary analysis indicated a rapid decline of epitope-tagged PIF5 levels to an apparent lower steady-state level, within the first 10 min of exposure of dark-grown seedlings to continuous R (Rc; Fig. 1A). To determine whether the endogenous, native PIF5 protein behaves similarly and to investigate the initial kinetics of the light-induced decline, we generated affinity-purified antibodies against a nonconserved region of PIF5 and performed early time-course measurements following R exposure of dark-grown seedlings. Initial western-blot analysis indicated that PIF5 is a very low abundance protein but detectable with care in seedling extracts (Supplemental Fig. S1). Using this antibody, we observed a very rapid decrease in endogenous PIF5 after initial R exposure, with a t1/2 of less than 5 min, and leveling off at an apparent new steady-state level, about 10-fold lower than the initial dark level (Fig. 1B). Epitope (hemagglutinin [HA])-tagged PIF5, driven by the 35S cauliflower mosaic virus promoter, exhibited similarly rapid degradation kinetics to those of the native protein (Fig. 1C), indicating that this overexpressed molecule reflects the behavior of endogenous PIF5. In addition, because expression driven by the 35S promoter is constitutive (Monte et al., 2004), the data suggest that the R-induced effect is exerted predominantly posttranscriptionally. Epitope-tagged, overexpressed PIF5 was very recently reported to decline in light-grown, diurnally entrained plants exposed to light after a dark period (Nozue et al., 2007). However, neither the speed of this response to light nor the possible molecular signaling mechanism that induces this degradation process was investigated in that study.
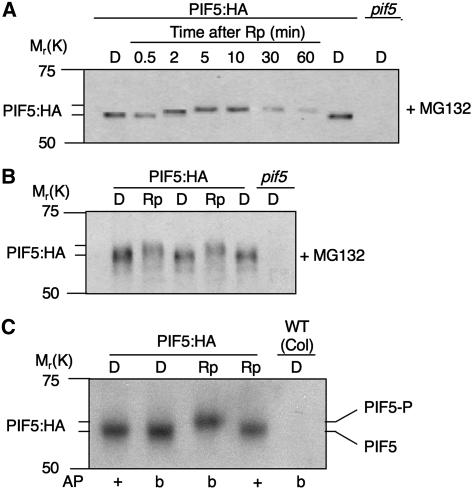
Figure 1.
R light induces rapid PIF5 degradation through the 26S proteasome system. A, PIF5 protein in transgenic seedlings overexpressing PIF5:HA is degraded rapidly over a time course of 1 h in Rc. Four-day-old dark-grown (D) PIF5:HA seedlings were treated with Rc (10 μmol m−2 s−1) over a time course of 1 h. The period of Rc irradiation before extraction is indicated in minutes. Seedlings were extracted directly into hot denaturing buffer and subjected to western-blot analysis using anti-HA or anti-tubulin antibody. One sample was preincubated in the dark with the proteasome inhibitor MG132 as indicated (+) for 4 h before transfer to Rc for 60 min. Protein samples extracted from dark-grown wild-type (Col) seedlings (Col D) are shown as a negative control. B, Top, Rapid degradation of endogenous PIF5. Shown is the western-blot analysis of endogenous PIF5 levels over a 1-h time course in darkness following exposure of dark-grown wild-type (Col ecotype) seedlings to a brief, saturating Rp of 7,500 μmol m−2 in 30 s. The time of extraction after the initial Rp is indicated. pif5 null mutants were included as a negative control. Seedlings were extracted directly into hot denaturing buffer, proteins were separated on 8.0% SDS-PAGE, and blots were probed with an affinity-purified antibody against a nonconserved region of PIF5 or anti-tubulin as a loading control. B, Bottom, Quantification of PIF5 levels from western blots of three biological replicates of the time course. Relative PIF5 levels were quantified from the western ECL signal, normalized to the tubulin signal, and expressed as a percentage of the PIF5 level in dark-control seedlings. Error bars represent the sd, n = 3. D = dark-grown, unirradiated controls. C, Top, Rapid R-induced degradation of epitope-tagged PIF5 (PIF5:HA) is mediated through the 26S proteasome system. Time course of PIF5:HA degradation in transgenic seedlings overexpressing PIF5:HA (PIF5:HAOX), pretreated for 4 h in darkness with (+) or without (−) the proteasome inhibitor MG132 before exposure to a saturating Rp as in B. Seedlings were treated, extracted, and samples electrophoresed as in B, and blotted samples were probed with anti-HA or anti-tubulin antibody. A pif5 null mutant was included as a negative control. C, Bottom, Quantification of PIF5:HA levels from western blots of three biological replicates of the time course as in B. Error bars represent the sd, n = 3. D = dark-grown, unirradiated controls.
To begin to define the pathway by which PIF5 is degraded, we examined the effect of the proteosomal inhibitor MG132 on PIF5 degradation. The data show that the inhibitor retarded the R-induced decline in PIF5 levels (Fig. 1, A and C), indicating that the ubiquitin-proteosome system is likely responsible for the degradation process.
R Light Induces Rapid Phosphorylation of PIF5 in Vivo Preceding Degradation
To begin to investigate the molecular mechanism by which R light initiates PIF5 degradation, we examined the protein for rapid changes in molecular behavior immediately following R exposure. Using gel conditions designed to detect changes in apparent molecular mass and MG132 to inhibit degradation, we observed a small shift in electrophoretic mobility, generated in vivo, within 2 min of seedling exposure to a saturating R-light pulse (Rp), preceding the major phase of degradation (Fig. 2, A and B). This in vivo-induced mobility shift was reversed by in vitro treatment of the extracted PIF5 with alkaline phosphatase (AP), but not if the phosphatase was first boiled to inactivate its enzyme activity (Fig. 2C). These data suggest that R light rapidly induces phosphorylation of PIF5 in the cell prior to degradation, in a manner similar to PIF3 (Al-Sady et al., 2006).
Figure 2.
R light induces rapid PIF5 phosphorylation in vivo prior to degradation. A, Time course of PIF5 electrophoretic-mobility shift in darkness after a Rp treatment (7,500 μmol m−2 in 30 s) of dark-grown, PIF5:HA-overexpressing seedlings. Seedlings were pretreated with MG132 as in Figure 1C to reduce the degradation rate over the 1-h time course, and then subjected to irradiation, extraction, and western-blot analysis, using the anti-HA antibody, as in Figure 1C, except that protein samples were separated on a 6.5% SDS-PAGE gel as described in “Materials and Methods” to permit higher resolution detection of small changes in mobility. The time-resolved decrease in mobility following the Rp can be visualized by comparison with the unirradiated (D) controls. A pif5 null mutant was included as negative control. B, Direct comparison of dark-control (D) and Rp-treated samples in repeated alternate gel tracks confirms reliability of observed R-induced mobility shift of PIF5. The Rp sample was extracted following 5 min in darkness from the initial Rp treatment (7,500 μmol m−2 in 30 s). C, The R-induced PIF5 mobility shift is due to PIF5 phosphorylation. PIF5:HA seedlings were either kept in darkness (D) or treated with a Rp (7,500 μmol m−2 in 30 s) and returned to darkness for a total of 5 min (Rp). Samples were then extracted on ice into nondenaturing buffer and immunoprecipitated by using anti-HA antibody prior to AP treatment and western-blot analysis. Heat-denatured AP was added to the control samples as described in the “Materials and Methods.” Blots were probed with anti-HA antibody. +, Active AP was added. b, Heat-denatured (boiled) AP was added. PIF5-P designates the presumptive phosphorylated PIF5:HA band.
Photoactivated Phy Acts within 60 s in Vivo to Induce PIF5 Phosphorylation and Degradation
To examine the role of the phy family in mediating the R-induced phosphorylation and degradation responses, we used both photobiological and genetic strategies. The data show that a long-wavelength FR-light pulse (FRp), given immediately after an inductive Rp, partially blocked the subsequent degradation of PIF5 over the next 1 h in darkness (Fig. 3A). A similar FRp after a brief inductive Rp was found to completely abrogate the induction of the subsequent mobility shift observed at 5 min after the Rp (Fig. 3B). These data provide evidence that one or more phys is responsible for the R induction of phosphorylation and degradation of PIF5. The lack of full FR reversal of the 1-h degradation response was also observed for PIF3, and appears to be due to the residual (0.1%) phyA-Pfr that is established by this treatment, being sufficient to induce partial degradation over this extended period (Al-Sady et al., 2006). However, this very low level of Pfr is apparently insufficient to induce a detectable mobility shift in the short-term phosphorylation assays for either PIF3 or PIF5.
Figure 3.
R-light induction of endogenous PIF5 phosphorylation and degradation can be photoreversibly abrogated. A, R-induced PIF5 degradation is partially reversed by a saturating long-wavelength FRp. Dark-grown wild-type (Col) seedlings were exposed to a saturating Rp, FRp, or a Rp followed immediately by FRp (Rp/FRp; 5,000 μmol m−2 in 10 s for both Rp and FRp), followed by return to darkness for 1 h before extraction. Protein samples were separated on an 8% SDS-PAGE gel and subjected to western-blot analysis using the anti-PIF5 or anti-tubulin antibody. B, Induction of PIF5 phosphorylation by a Rp is abrogated by an immediately subsequent FRp. Dark-grown wild-type (Col) seedlings were exposed to Rp, FRp, or Rp/FRp (5,000 μmol m−2 in 10 s for both Rp and FRp), followed by return to darkness for 5 min before extraction. Seedlings were incubated in MG132 for 4 h before light treatment to maintain the mobility-shifted product. Protein samples were extracted into hot denaturing buffer, separated on a 6.5% SDS-PAGE gel, and subjected to western-blot analysis using the anti-PIF5 antibody as described in the “Materials and Methods.” C, Induction of PIF5 phosphorylation by a Rp escapes rapidly from photoreversal by a subsequent FRp. Dark-grown wild-type (Col) seedlings were exposed to a Rp (5,000 μmol m−2 in 10 s) followed by a FRp (5,000 μmol m−2 in 10 s), either immediately or after a brief dark period of 10, 30, 60, or 90 s from the end of the Rp to the beginning of the FRp (Rp-D-FRp), followed then by return to darkness for a total of 5 min from the initial Rp before extraction. Protein samples were extracted into hot denaturing buffer, separated on a 6.5% SDS-PAGE gel, and subjected to western-blot analysis using the anti-PIF5 antibody as described in the “Materials and Methods.” D = dark-grown, unirradiated controls. A pif5 null mutant is shown as negative control.
To define the earliest window during which Pfr can act to induce phosphorylation of PIF5 in the cell, we performed a so-called “escape-from-FR-reversibility” experiment. In this experiment, Pfr was presented to the cell by a short, high-intensity Rp, and allowed to remain in this form for increasing periods of darkness before being removed by a subsequent high-intensity FRp. The data show that the phy molecule needs to be in the cell for as little as 60 s in the Pfr form in order to induce detectable phosphorylation (Fig. 3C). The same pattern is observed for both endogenous (Fig. 3) and overexpressed (Supplemental Fig. S2) PIF5, indicating that the system is not saturated by increasing the abundance of the target protein and that the behavior of the overexpressed protein appears to accurately reflect that of the endogenous PIF5.
phyA and phyB Dominate Induction of PIF5 Phosphorylation and Degradation in Response to R Light
To determine which members of the phy family are responsible for R-induced phosphorylation and degradation of PIF5, we examined these responses in selected single, double, and triple null mutants of phyA, phyB, and/or phyD. The data show that neither monogenic phyA nor phyB null mutant shows reduced PIF5 degradation (Fig. 4A). The phyAphyB double mutant does exhibit a markedly reduced rate of PIF5 degradation, but a degree of residual degradation is still observed over the 1-h experimental period (Fig. 4, B–D). By contrast, the phyAphyBphyD triple mutant exhibits no detectable PIF5 degradation over the 1-h period (Fig. 4, C and E). These data indicate that phyA, phyB, and phyD act qualitatively redundantly in this process, with phyA and phyB dominating quantitatively. Either phyA or phyB appears sufficient to induce maximal PIF5 degradation, with phyD compensating only weakly and partially for the simultaneous absence of both phyA and phyB. There is no evidence that phyC or phyE participate in this process. Consistent with these data, a PIF5 construct mutated in the APB domain, such that binding to phyB is eliminated (Khanna et al., 2004; Al-Sady et al., 2006), exhibits normal R-induced degradation (Supplemental Fig. S3). This result implies that PIF5, like PIF3 (Al-Sady et al., 2006), appears likely to have a binding site necessary for phyA action that is independent of the APB phyB binding site. We have not yet attempted to define this putative binding site in PIF5. Overall the roles of the different phy family members in regulating PIF5 abundance closely resemble the role originally reported for PIF3 by Bauer et al. (2004).
Figure 4.
phyA, phyB, and phyD act qualitatively redundantly to induce PIF5 degradation. A, PIF5 is degraded in phyA or phyB monogenic mutant seedlings, similar to that in wild-type (Col) seedlings, in response to a Rp. Dark-grown seedlings (D) were exposed to a saturating Rp (7,500 μmol m−2 in 30 s), followed by return to darkness for the indicated time before extraction (R10 = 10 min; R60 = 60 min from initial Rp) and western-blot analysis. B, Rate of Rp-induced PIF5 degradation is significantly reduced in phyAB double mutant seedlings compared to that in the wild type (Col). Experimental design is as in A. C, Residual Rp-induced PIF5 degradation in the phyAphyB double mutant is abolished in the phyAphyBphyD triple mutant. Because the phyAphyBphyD mutant is in the Ler ecotype, the wild type (Ler) is included for comparison. Experimental design is as in A. D, Time course of Rp-induced PIF5 degradation in phyAphyB double mutant seedlings compared to that in wild-type (Col) seedlings. Dark-grown seedlings were exposed to a saturating Rp (7,500 μmol m−2 in 30 s), followed by return to darkness for the indicated time before extraction and western-blot analysis. E, Time course of Rp-induced PIF5 degradation in phyAphyBphyD triple mutant seedlings compared to that in wild-type (Ler) seedlings. Experimental design is as in D. All protein samples in this figure were extracted directly into hot denaturing buffer, separated on an 8% SDS-PAGE gel, and subjected to western-blot analysis using the anti-PIF5 or anti-tubulin antibody as described in the “Materials and Methods.” D = dark-grown, unirradiated controls. A pif5 null mutant is shown as negative control.
Examination of the R-induced mobility shift of PIF5 in the same phy mutants as described above showed that the shift occurs for endogenous PIF5 in both the monogenic phyA and phyB mutants similar to that in wild type, but is not observed in the phyAphyB double null mutant (Fig. 5). This result indicates that PIF5, like PIF3 (Al-Sady et al., 2006), is phosphorylated equally well when phyA or phyB are present, but that no phyD-induced phosphorylation is detectable by this assay. Consistent with the above data, the overexpressed PIF5 construct that is blocked in phyB binding through mutation of its APB motif exhibited normal R-induced phosphorylation (Supplemental Fig. S4). This result, together with the behavior of the phyB mutant, is consistent with the proposal that PIF5 may have a phyA binding site, independent of the APB domain, through which phyA can interact with and induce phosphorylation of the transcription factor in vivo, in a manner similar to PIF3 (Al-Sady et al., 2006).
Figure 5.
phyA and phyB act redundantly to induce PIF5 phosphorylation in vivo. Rp-induced phosphorylation of endogenous PIF5 occurs in phyA or phyB monogenic mutant seedlings as efficiently as in the wild type (Col), but is absent in the phyAphyB double mutant. Dark-grown seedlings were pretreated for 4 h in the dark with MG132 and exposed to a Rp (7,500 μmol m−2 in 30 s), followed by return to darkness for 5 min before extraction into hot denaturing buffer. Protein samples were separated on a 6.5% SDS-PAGE gel and subjected to western-blot analysis using anti-PIF5 or anti-tubulin antibody as described in the “Materials and Methods.” D = dark-grown, unirradiated controls. A pif5 null mutant is shown as negative control.
PIF5 Interacts Selectively with Photoactivataed phyB in ex Planta Binding Assays
To examine the capacity of PIF5 to physically bind to the individual phy family members, we used both in vitro pull-down and yeast two-hybrid interaction assays. As reported previously (Khanna et al., 2004), PIF5 binds strongly and conformer specifically to the Pfr form of phyB in pull-down assays (Fig. 6A). However, no conformer-specific binding to the other phys was detected. Whether the equally low level of binding to both conformers of the other phys (in turn equivalent to that of the Pr form of phyB) is significant has not been determined. Because of the indications of phyA-PIF5 interactions in vivo described above and because photoactivated phyA binding to PIF3 is markedly weaker than that of phyB (Zhu et al., 2000; Huq and Quail, 2002), we used a more sensitive light-switchable, yeast two-hybrid system (Shimizu-Sato et al., 2002) to assay for weak phyA-PIF5 interactions as described previously for PIF3 (Al-Sady et al., 2006). However, although this system detected clear Pfr-specific interactions of phyB with both PIF3 and PIF5 (Fig. 6B) and of phyA with PIF3 (Fig. 6C), as expected, no specific interaction of phyA with PIF5 was observed (Fig. 6C). Similarly, no specific binding of phyC or phyD to PIF5 was observed with this assay (Supplemental Fig. S5). The reason for the apparent discrepancy between the behavior of phyA (and phyD) in the living plant cell and in the interaction assays is unclear, but one possibility is that the interactions are so weak and/or transient as to be unstable under these assay conditions. On the other hand, another possibility is that phyA does not interact directly with PIF5 in the cell, but rather induces PIF5 phosphorylation through one or more intermediary factors. This would require a complexity whereby the mechanism by which phyA induces the phosphorylation of PIF5 would differ from that by which phyA induces the phosphorylation of PIF3, where the necessity of direct in vivo interaction between these two binding partners has been clearly established (Al-Sady et al., 2006). Similarly, the apparently similar mechanisms employed by both phyA and phyB to redundantly induce PIF3 phosphorylation would stand in contrast to the possibly different mechanisms these two phy family members would use to redundantly phosphorylate PIF5. While such molecular complexity is certainly possible, it would appear to be highly unusual.
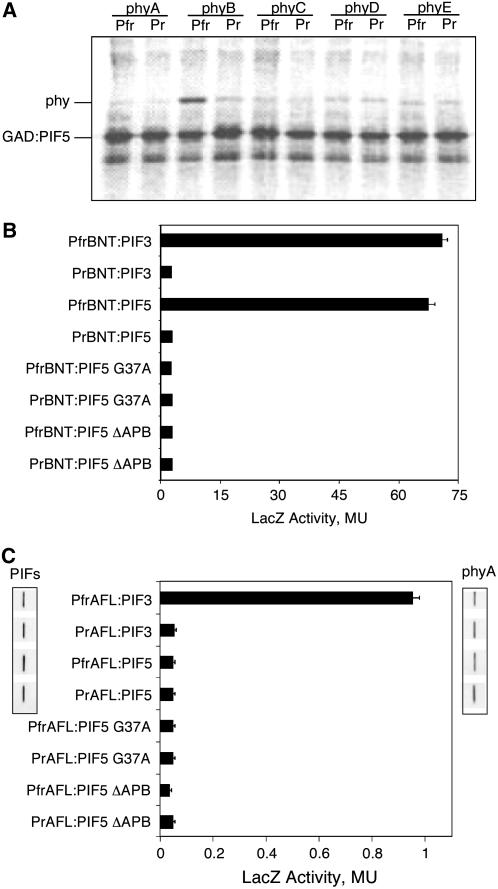
Figure 6.
PIF5 interacts specifically with photoactivated phyB via its APB motif. A, PIF5 interacts specifically with photoactivated phyB (Pfr) in in vitro coimmunoprecipitation assays. [35S]Met-labeled recombinant GAD:PIF5 (bait) and the five phys (phyA–phyE; prey), similarly [35S]Met labeled, were expressed separately in vitro using the TnT system and used in in vitro pull-down assays, with each phy in the Pr or Pfr form, as described previously (Khanna et al., 2004). The samples were separated by SDS-PAGE and the gel processed for autoradiography. B, The unmutated PIF5 protein (PIF5) interacts specifically with the photoactivated phyB N-terminal domain (PfrBNT), in the light-switchable yeast assay. The constructs and the assay used are described in “Materials and Methods.” The mutation in the APB motif of PIF5 (PIF5 G37A) abolishes the specific interaction between PIF5 and PfrBNT, as does the deletion mutant lacking the APB domain of PIF5 (PIF5 ΔAPB). The PIF3 and PfrBNT interaction is included as a positive control. MU, Miller units. C, PIF5 does not display an observable interaction with full-length photoactivated phyA (PfrAFL) in the light-switchable yeast assay. The PIF3 and PfrAFL interaction is shown as a positive control. The protein expression levels of PIF5 and PIF3 from the indicated yeast assays, as well as that of phyA, were analyzed and compared by the western-blot analysis (small boxes) as described (Al-Sady et al., 2006).
CONCLUSION
The data presented here identify PIF5 as a second member of the phy-interacting subfamily of Arabidopsis bHLH transcription factors that is subject to rapid phosphorylation upon presumptive intranuclear interaction with photoactivated phys in the cell prior to proteosomal degradation. The evidence suggests that this molecular tag may represent the recognition signal for ubiquitination and degradation of the protein (Lipford and Deshaies, 2003; Al-Sady et al., 2006). It is therefore possible that induced phosphorylation represents a general mechanism of signal transfer from the photoactivated phy molecule to this class of phy-interacting bHLH proteins, and perhaps other potential targets of phy signaling. The speed of the phosphorylation (within 60 s) and degradation (t1/2 < 5 min) processes following phy photoactivation is apparently even more rapid for PIF5 than for PIF3 (Al-Sady et al., 2006) and is consistent with phy-induced transphosphorylation being the primary signaling transaction of the photoreceptor molecule. However, the central question of whether the phy molecule is itself a protein kinase, autonomously responsible for this activity (Montgomery and Lagarias, 2002), is still an open (Al-Sady et al., 2006).
The data indicate that, as for PIF3, phyA and phyB act redundantly in inducing PIF5 phosphorylation and degradation in response to R-light activation, with phyD playing a minor role. Although we were unable to detect any specific physical binding of photoactivated phyA to PIF5 by interaction assay, overall the data suggest that such an interaction may occur in the plant cell via a binding site in the PIF5 molecule that is independent of the phyB (APB) binding site, although other more complex possibilities are not ruled out. Collectively, the evidence suggests the possibility that phyA and phyB may have multiple, shared primary signaling partners that are targets of a common biochemical signal transfer mechanism.
The mechanism by which the reductions in the abundance of these transcription factors might alter gene expression is as yet unclear. However, recent evidence suggests that PIF3 functions as a positive, constitutive transcriptional activator that together with another factor(s) provides a brief window of transient induction of responsive genes upon initial light exposure until its levels drop through degradation (Al-Sady et al., 2006; B. Al-Sady, E. Kikis, and P. Quail, unpublished data).
MATERIALS AND METHODS
Plant Material and Growth Conditions
Wild-type Arabidopsis (Arabidopsis thaliana), ecotype Columbia (Col) or Landsberg erecta (Ler), was used in these experiments. The phyA, phyB, and phyAphyB mutants in Col and the phyAphyBphyD mutant in Ler were obtained from G. Whitelam as described previously (Al-Sady et al., 2006). THE pif5 T-DNA insertional line (Salk_072306) was obtained from the Arabidopsis Biological Resource Center. The PIF5 transgenic lines were generated as described (R. Khanna and P. Quail, unpublished data): Arabidopsis (Col-0) plants were transformed with PIF5 cDNA driven by the 35S promoter, 35S∷PIF5, to obtain PIF5OX; 35S∷PIF5 G37A to obtain PIF5OX-mAPB; and 35S∷PIF5:HA to obtain PIF5:HA. Seedlings were grown in darkness for 4 d at 21°C before R- or long-wavelength FR (RG9)-light treatments as described previously (Ni et al., 1999; Al-Sady et al., 2006). For MG132 treatment, MG132 (dissolved in dimethyl sulfoxide [DMSO]) or equal volume of DMSO was mixed with 0.5 mL of 0.1% plating agar solution and applied to the root area of 4-d-old dark-grown seedlings for 4 h before light treatments. The final concentration of MG132 in the GM medium was 30 μm.
PIF5 Antibody Production
A nonconserved fragment of the PIF5 protein including amino acids 101 to 252 was expressed in Escherichia coli with a 6XHis affinity tag and purified using the ProBond purification system (Invitrogen). Antisera were produced (Covance) and the crude serum was affinity purified using a GST-PIF5 (PIF5 fragment with GST fusion) affinity column and an AminoLink Plus immobilization kit (Pierce) according to the manufacturer's instruction. Antibody elution was achieved with 4 m MgCl2 solution and fractions were checked via western-blot analysis against the antigen. The best fraction was dialyzed against 1× phosphate buffered saline (PBS) buffer before storage at −20°C. This affinity-purified antibody showed no detectable cross-reactivity with other closely related members of the Arabidopsis bHLH family by western blot, including the well-characterized PIF3, and showed the absence of any band corresponding to PIF5 in the pif5 mutant.
Protein Extraction and Western-Blot Analysis
Four-day-old dark-grown seedlings were either kept in darkness or treated with different light conditions as indicated. The treated seedlings were dried with paper towel and immediately extracted into boiling denaturing extraction buffer (100 mm Tris-HCl, pH 7.8, 4 m urea, 5% SDS, 15% glycerol, 10 mm β-MeOH) at a ratio of 2:1 (v/w). A mixture of protease inhibitors, including 2 μg/L aprotinin, 3 μg/L leupeptin, 1 μg/L pepstatin, 2 mm phenylmethylsulfonyl fluoride, and 30 μL protease inhibitor cocktail (Sigma, St. Louis) per mL of plant extract, was added right before extraction. The plant extract was boiled for 4 min and cleared by centrifugation at 15,000g for 15 min at room temperature. Total protein was quantified using a RC-DC protocol (Bio-Rad), and the obtained protein concentration was checked for tubulin controls in a preliminary western-blot analysis against tubulin. For the purpose of PIF5 protein degradation and quantification, 30 to 60 μg of total protein was separated on an 8% SDS-PAGE gel (10 × 12 cm; Amersham) and the electrophoresis was stopped before the dye ran out of the gel. For the purpose of investigating PIF5 protein modification, 40 to 80 μg of total protein was separated on a 6.5% SDS-PAGE gel and the electrophoresis was stopped before the 37-kD protein standards (Bio-Rad) ran out of the gel. The immunoblot was probed by specific antibody in an overnight incubation at 4°C. The antibody used for endogenous, native PIF5 was as described above. The antibody used for PIF5:HA fusion protein detection was from Santa Cruz Biotech. The anti-tubulin antibody was from Sigma. For western-blot band detection, SuperSignal West Femto substrate (Pierce) or Visualizer substrate (Upstate) was used to obtain enhanced chemiluminescence (ECL).
Quantification of PIF5 Protein Level
Protein samples extracted in a time course of 1 h were separated on an 8% SDS-PAGE gel as described above and probed with either anti-PIF5 or anti-tubulin (Sigma) antibodies. A dilution series of PIF5 protein standards was made using the dark-control protein extract, including 100%, 50%, 25%, 12.5%, 6.25%, and 3.125% of the total protein amount, and was probed in parallel with the individual time points from the respective time course on western blots. The ECL signals from each blot were recorded on x-ray film with multiple exposures and analyzed using National Institutes of Health imaging software. The linearity between ECL signals of each band and the corresponding PIF5 protein standards in the dilution series was analyzed in each exposure and the best linear fit was chosen to calculate relative PIF5 protein levels. The same approach was used to analyze relative tubulin protein levels in each protein extract as loading controls. The unit of PIF5 protein level in each sample was determined as a percentage of PIF5 protein amount in darkness after normalization using tubulin controls. Mean values and sd were calculated from three biological replicates.
Immunoprecipitation and AP Treatment
Four-day-old dark-grown seedlings expressing 35S∷PIF5:HA were treated with MG132 as described above. Seedlings, either kept in darkness or treated with R light, were extracted on ice into nondenaturing buffer (50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 1 mm EDTA, 25 mm NaF, 10 mm sodium orthovanadate, 1% Triton X-100, 1% DMSO, 5 μm β-MeOH, 100 nm calyculin A, 2 μg/L aprotinin, 3 μg/L leupeptin, 1 μg/L pepstatin, 2 mm phenylmethylsulfonyl fluoride, and 1× Complete protease inhibitor cocktail [Roche]) with a ratio of 3:1 (v/w) and cleared by centrifugation at 15,000g for 10 min at 4°C. PIF5:HA was immunoprecipitated from supernatants with anti-HA antibody (Santa Cruz Biotech) and Protein A-agarose beads (Santa Cruz Biotech) following 2-h incubation at 4°C. The pellets were washed two times with 1× PBS wash buffer, followed by two times with calf intestinal AP (CIAP) buffer (100 mm Tris, pH 9.0, 50 mm MgCl2, 100 mm NaCl, and 1× Complete protease inhibitor cocktail [Roche]). The pellets were resuspended in 50 μL of CIAP buffer and then treated for 15 min at 30°C with either 100 units of CIAP (Roche) or the same amount of heat-inactivated CIAP enzyme. Pellets were boiled in 1× SDS sample buffer and subjected to western-blot analysis.
In Vitro Coimmunoprecipitation Assay
The PIF5 cDNA was cloned into the pET17b in vitro expression vector (Invitrogen) and PIF5 protein was expressed as a GAD fusion protein at the N terminus of PIF5 as described (Khanna et al., 2004). The in vitro expression construct used for phyA to phyE was the same as described (Ni et al., 1999). All proteins were expressed in vitro using the TnT transcription/translation system (Promega) in the presence of [35S]Met. The bait was prepared by incubating GAD-PIF5 fusion protein with monoclonal anti-GAD antibody (Santa Cruz, Biotech) and Protein A-agarose beads (Santa Cruz Biotech) for 2 h at 4°C. The bait was washed three times with 1× PBS wash buffer, pH 7.2, containing 0.1% (v/v) Tergitol Nonidet P-40 (Sigma) and 1× Complete protease inhibitor cocktail (Roche). The prey (phy) was prepared by incubating with phycocyanobilin for 1 h at 4°C in the dark, followed by a saturating 10-min Rp of 350 μmol m−2 s−1 (for conversion to the Pfr conformer). One-half of this R-treated prey was further treated with a saturating 5-min FRp of 297 μmol m−2 s−1 (for reconversion to the Pr conformer). The prepared bait and prey were incubated together in 1× PBS binding buffer (1× PBS wash buffer including 0.1% bovine serum albumin) for 2 h at 4°C, and the pellets were washed three times in the dark with the wash buffer, separated on SDS-PAGE, and processed for autoradiography.
Yeast Two-Hybrid Assay
Quantitative yeast two-hybrid assays (light-switchable assays) with phyAFL:DBD, phyBNT:DBD, phyCFL:DBD, phyDFL:DBD, phyDNT:DBD, and PIF5:GAD were cloned and performed as described (Shimizu-Sato et al., 2002). PIF5 wild type, PIF5 APB mutant (G37A), and PIF5 APB deletion mutant (ΔAPB) were constructed as GAD fusion proteins using forward primers containing EcoRI sites and reverse primers containing BamHI sites and cloned into pGAD424 (CLONTECH). Yeast extracts for western-blot analysis were obtained as described (CLONTECH; Yeast Protocols Handbook) and analyzed by western blot using anti-GAD antibody (Santa Cruz Biotech) and anti-phyA antibody (Hirschfeld et al., 1998).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Detection of endogenous PIF5 protein in crude seedling extracts using affinity-purified anti-PIF5 antibody.
Supplemental Figure S2. R-light induction of transgenically overexpressed PIF5 (PIF5OX) phosphorylation and degradation can be photoreversibly abrogated.
Supplemental Figure S3. Mutation in the APB motif of PIF5 does not alter R-induced PIF5OX degradation.
Supplemental Figure S4. Mutation in the APB motif of PIF5 does not alter R-induced PIF5OX electrophoretic mobility shift.
Supplemental Figure S5. Investigation of PIF5 interaction with phyC and phyD in the light-switchable yeast assays.
Supplementary Material
Acknowledgments
We thank Garry Whitelam for phy mutants, Bassem Al-Sady for helpful discussions and valuable comments on the manuscript, and Jim Tepperman for his help with the preparation of the figures.
This work was supported by the National Institutes of Health (grant no. GM–47475), the U.S. Department of Energy (grant no. DEFG03–87ER13742), and the U.S. Department of Agriculture/Agricultural Research Service Current Research Information System (grant no. 5335–21000–017–00D).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Peter H. Quail (quail@nature.berkeley.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Al-Sady B, Ni W, Kircher S, Schäfer E, Quail PH (2006) Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome mediated degradation. Mol Cell 4 439–446 [DOI] [PubMed] [Google Scholar]
- Bailey PC, Martin C, Toledo-Ortiz G, Quail PH, Huq E, Heim MA, Jakoby M, Werber M, Weisshaar B (2003) Update on the basic helix-loop-helix transcription factor gene family in Arabidopsis thaliana. Plant Cell 15 2497–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Viczian A, Kircher S, Nobis T, Nitschke R, Kunkel T, Panigrahi KC, Adam E, Fejes E, Schäfer E, et al (2004) Constitutive photomorphogenesis 1 and multiple photoreceptor control degradation of phytochrome interacting 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell 16 1433–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Chory J, Fankhauser C (2004) Light signal transduction in higher plants. Annu Rev Genet 38 87–117 [DOI] [PubMed] [Google Scholar]
- Duek PD, Fankhauser C (2005) bHLH class transcription factors take centre stage in phytochrome signaling. Trends Plant Sci 10 51–54 [DOI] [PubMed] [Google Scholar]
- Fujimori T, Yamashino T, Kato T, Mizuno T (2004) Circadian-controlled basic/helix-loop-helix factor, PIL6, implicated in light-signal transduction in Arabidopsis thaliana. Plant Cell Physiol 45 1078–1086 [DOI] [PubMed] [Google Scholar]
- Hirschfeld M, Tepperman JM, Clack T, Quail PH, Sharrock RA (1998) Coordination of phytochrome levels in phyB mutants of Arabidopsis as revealed by apoprotein-specific monoclonal antibodies. Genetics 149 523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq E, Al-Sady B, Hudson M, Kim C, Apel K, Quail PH (2004) Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305 1937–1941 [DOI] [PubMed] [Google Scholar]
- Huq E, Al-Sady B, Quail PH (2003) Nuclear translocation of the photoreceptor phytochrome B is necessary for its biological function in seedling photomorphogenesis. Plant J 35 660–664 [DOI] [PubMed] [Google Scholar]
- Huq E, Quail PH (2002) PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J 21 2441–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Lau OS, Deng XW (2007) Light-regulated transcriptional networks in higher plants. Nat Rev Genet 8 217–230 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Ma L, Strickland E, Deng XW (2005) Conservation and divergence of light-regulated genome expression patterns during seedling development in rice and Arabidopsis. Plant Cell 17 3239–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna R, Huq E, Kikis EA, Al-Sady B, Lanzatella C, Quail PH (2004) A novel molecular recognition motif necessary for targeting photoactivated phytochrome signaling to specific basic helix-loop-helix transcription factors. Plant Cell 16 3033–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yi H, Choi G, Shin B, Song PS, Choi G (2003) Functional characterization of phytochrome interacting factor 3 in phytochrome-mediated light signal transduction. Plant Cell 15 2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipford JR, Deshaies RJ (2003) Diverse roles for ubiquitin-dependent proteolysis in transcriptional activation. Nat Cell Biol 5 845–850 [DOI] [PubMed] [Google Scholar]
- Lissemore JL, Quail PH (1988) Rapid transcriptional regulation by phytochrome of the genes for phytochrome and chlorophyll a/b-binding protein in Avena sativa. Mol Cell Biol 8 4840–4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Chen C, Liu X, Jiao Y, Su N, Li L, Wang X, Cao M, Sun N, Zhang X, et al (2005) A microarray analysis of the rice transcriptome and its comparison to Arabidopsis. Genome Res 15 1274–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Garcia JF, Huq E, Quail PH (2000) Direct targeting of light signals to a promoter element-bound transcription factor. Science 288 859–863 [DOI] [PubMed] [Google Scholar]
- Monte E, Tepperman JM, Al-Sady B, Kaczorowski KA, Alonso JM, Ecker JR, Li X, Zhang Y, Quail PH (2004) The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc Natl Acad Sci USA 101 16091–16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery BL, Lagarias JC (2002) Phytochrome ancestry: sensors of bilins and light. Trends Plant Sci 7 357–366 [DOI] [PubMed] [Google Scholar]
- Nagatani A (2004) Light-regulated nuclear localization of phytochromes. Curr Opin Plant Biol 7 708–711 [DOI] [PubMed] [Google Scholar]
- Ni M, Tepperman JM, Quail PH (1998) PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95 657–667 [DOI] [PubMed] [Google Scholar]
- Ni M, Tepperman JM, Quail PH (1999) Binding of phytochrome B to its nuclear signaling partner PIF3 is reversibly induced by light. Nature 400 781–784 [DOI] [PubMed] [Google Scholar]
- Nozue K, Covington M, Duek PD, Lorrain S, Fankhauser C, Harmer SL, Maloof JN (2007) Rhythmic growth explained by coincidence between internal and external cues. Nature 448 358–361 [DOI] [PubMed] [Google Scholar]
- Oh E, Kim J, Park E, Kim JI, Kang C, Choi G (2004) PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16 3045–3058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, Lee HS, Sun TP, Kamiya Y, Choi G (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19 1192–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Kamiya Y, Bae G, Chung WI, Choi G (2006) Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J 47 124–139 [DOI] [PubMed] [Google Scholar]
- Park E, Kim J, Lee Y, Shin J, Oh E, Chung WI, Liu JR, Choi G (2004) Degradation of phytochrome interacting factor 3 in phytochrome-mediated light signaling. Plant Cell Physiol 45 968–975 [DOI] [PubMed] [Google Scholar]
- Quail PH (2002) Phytochrome photosensory signaling networks. Nat Rev Mol Cell Biol 3 85–93 [DOI] [PubMed] [Google Scholar]
- Quail PH (2006) Phytochrome signal transduction network. In E Schäfer, F Nagy, eds, Photomorphogenesis in Plants and Bacteria. Springer, Dordrecht, The Netherlands, pp 335–356
- Quail PH (2007. a) Phytochrome interacting factors. In G Whitelam, K Halliday, eds, Light and Plant Development. Blackwell Publishing, Oxford, pp 81–105
- Quail PH (2007. b) Phytochrome-regulated gene expression. J Integr Plant Biol 49 11–20 [Google Scholar]
- Sakamoto K, Nagatani A (1996) Nuclear localization activity of phytochrome B. Plant J 10 859–868 [DOI] [PubMed] [Google Scholar]
- Schäfer E, Nagy F, editors (2006) Photomorphogenesis in Plants and Bacteria. Springer, Dordrecht, The Netherlands
- Schepens I, Duek P, Fankhauser C (2004) Phytochrome-mediated light signaling in Arabidopsis. Curr Opin Plant Biol 7 564–569 [DOI] [PubMed] [Google Scholar]
- Shen H, Moon J, Huq E (2005) PIF1 is regulated by light-mediated degradation through the ubiquitin-26S proteasome pathway to optimize photomorphogenesis of seedlings in Arabidopsis. Plant J 44 1023–1035 [DOI] [PubMed] [Google Scholar]
- Shimizu-Sato S, Huq E, Tepperman JM, Quail PH (2002) A light-switchable gene promoter system. Nat Biotechnol 20 1041–1044 [DOI] [PubMed] [Google Scholar]
- Shin J, Park E, Choi G (2007) PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J 49 981–994 [DOI] [PubMed] [Google Scholar]
- Tepperman JM, Hudson ME, Khanna R, Zhu T, Chang SH, Wang X, Quail PH (2004) Expression profiling of phyB mutant demonstrates substantial contribution of other phytochromes to red-light-regulated gene expression during seedling de-etiolation. Plant J 38 725–739 [DOI] [PubMed] [Google Scholar]
- Tepperman JM, Hwang YS, Quail PH (2006) phyA dominates in transduction of red-light signals to rapidly responding genes at the initiation of Arabidopsis seedling de-etiolation. Plant J 48 728–742 [DOI] [PubMed] [Google Scholar]
- Toledo-Ortiz G, Huq E, Quail PH (2003) The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell 15 1749–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu SL, Lagarias JC (2005) The phytochromes. In WR Briggs, J Spudich, eds, Handbook of Photosensory Receptors. Wiley-VCH, Weinheim, Germany, pp 121–145
- Whitelam G, Halliday K, editors (2007) Light and Plant Development. Blackwell Publishing, Oxford
- Yamaguchi R, Nakamura M, Mochizuki N, Kay SA, Nagatani A (1999) Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J Cell Biol 145 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashino T, Matsushika A, Fujimori T, Sato S, Kato T, Tabata S, Mizuno T (2003) A link between circadian-controlled bHLH factors and the APRR1/TOC1 quintet in Arabidopsis thaliana. Plant Cell Physiol 44 619–629 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Tepperman JM, Fairchild CD, Quail PH (2000) Phytochrome B binds with greater apparent affinity than phytochrome A to the basic helix-loop-helix factor PIF3 in a reaction requiring the PAS domain of PIF3. Proc Natl Acad Sci USA 97 13419–13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.