Abstract
Increasing experimental evidence suggests that the tricarboxylic acid cycle in plants is of greater importance in illuminated photosynthetic tissues than previously thought. In this study, transgenic tomato (Solanum lycopersicum) plants expressing a fragment of the β-subunit of succinyl-coenzyme A ligase in either the antisense orientation or using the RNA interference approach, however, revealed little alteration in either photosynthesis or plant growth despite exhibiting dramatic reductions in activity. Moreover, the rate of respiration was only moderately affected in the transformants, suggesting that this enzyme does not catalyze a crucial step in mitochondrial respiration. However, metabolite and transcript profiling of these lines alongside enzyme and label redistribution experiments revealed that, whereas considerable activity of this enzyme appears to be dispensable, the reason for such a mild phenotype in extremely inhibited lines was an up-regulation of an alternative pathway for succinate production—that offered by the γ-aminobutyric acid shunt. When taken together, these data highlight the importance both of succinate production for mitochondrial metabolism and the interplay between various routes of its production. The results are discussed in the context of current models of plant respiration in mitochondrial and cellular metabolism of the illuminated leaf.
As in all eukaryotes, the plant tricarboxylic acid (TCA) cycle comprises the second stage of the process of cellular respiration and dominates mitochondrial metabolism through its essential role in linking the pathway of glycolysis with that of the electron transport chain. In plants, the presence and location of enzymes capable of catalyzing the complete TCA cycle were demonstrated decades ago (Beevers, 1961); however, the precise function of the constituent enzymes remains far from clear (Hill, 1997; Siedow and Day, 2000; Carrari et al., 2003). The TCA cycle functions to form both ATP and reducing equivalents, as well as to provide carbon skeletons for a wide range of important cellular constituents, including amino and fatty acids, flavonoids, alkaloids, and isoprenoids (Douce and Neuberger, 1989; Mackenzie and McIntosh, 1999; Bender-Machado et al., 2004; Fatland et al., 2005). However, with the exception of the convincing demonstration that the mitochondrial pyruvate dehydrogenase complex is reversibly inactivated by phosphorylation in the light (Budde and Randall, 1990; Tovar-Mendez et al., 2003) and the demonstration that TCA cycle intermediates are rapidly exported out of the mitochondria (Hanning and Heldt, 1993; Atkin et al., 2000), little is known concerning the metabolic regulation of this important pathway in plants. Even fundamental questions, such as whether the reactions of the TCA cycle that are not associated with nitrate assimilation are active in illuminated photosynthetic tissue and whether they contribute to the energy requirements of photosynthetic Suc synthesis, remain controversial (Krömer, 1995; Padmasree et al., 2002; Carrari et al., 2003; Fernie et al., 2004a; Tcherkez et al., 2005). In addition to this potential role in meeting the energy and carbon skeleton demands of the cell the TCA cycle has been implicated to play important roles in the regulation of cellular redox (Scheibe et al., 2005) and control of the carbon/nitrogen balance (Noguchi and Terashima, 2006; Dutilleul et al., 2005).
Despite the fact that a recent NMR-based study of Tcherkez suggested that the TCA cycle was almost completely inhibited in the light (Tcherkez et al. 2005), results from our laboratory revealed that reduction in the activity of aconitase or the mitochondrial malate dehydrogenase resulted in substantial reductions in both dark and light respiration (Carrari et al., 2003; Nunes-Nesi et al., 2005). Furthermore, detailed investigation revealed that the activity of the TCA cycle is likely considerably higher in illuminated leaves than suggested by Tcherkez and coworkers (Nunes-Nesi et al., 2007b). In addition to exhibiting reduced rates of respiration, these lines were demonstrated to display improved photosynthetic performance (Carrari et al., 2003; Nunes-Nesi et al., 2005). When taken together, these lines of evidence suggest an important role of the TCA cycle in the illuminated leaf and, moreover, one that is not merely restricted to those reactions that have been well characterized to play an important function in nitrate assimilation (Hodges et al., 2003). Whereas the exact reason for the increased photosynthetic performance in the TCA cycle-deficient genotypes is yet to be elucidated, it seems probable that it is in some way linked to the enhanced ascorbate content that they exhibit (Urbanczyk-Wochniak et al., 2006). These studies thus add further support to the growing body of evidence that suggests an important role for both the TCA cycle and the mitochondrial electron transport chain in the maintenance of optimal rates of photosynthesis (Carrari et al., 2003; Dutilleul et al., 2003; Raghavendra and Padmasree, 2003; Bartoli et al., 2005; Nunes-Nesi et al., 2005). In this context, an interesting reaction to study is that catalyzed by succinyl-CoA ligase (SCoAL) because this reaction provides succinate as substrate to the respiratory chain and preliminary studies suggest it to be allosterically regulated in vivo (Studart-Guimarães et al., 2005). In plants, as in animals, SCoAL consists of two types of subunits, the α-subunit, which is believed to be catalytic, and the β-subunit, which is supposed to be regulatory. In tomato (Solanum lycopersicum), there are two genes encoding the α-subunit, but only one gene encoding the β-subunit (Studart-Guimarães et al., 2005). Thus, to continue our ongoing project to determine the function of the TCA cycle in the illuminated leaf, we here describe the generation of transgenic plants exhibiting decreased expression of the β-subunit of SCoAL and displaying dramatic decreases in the activity of this enzyme. The resultant lines were comprehensively characterized at the biochemical, physiological, and transcriptional levels. Data obtained are discussed in the context of energy metabolism and its regulation in the illuminated leaf.
RESULTS
Antisense Inhibition of the β-Subunit of Succinyl-CoA Results in a Dramatic Decrease in the Activity of the Enzyme, But Relatively Mild Effects on Growth and Development
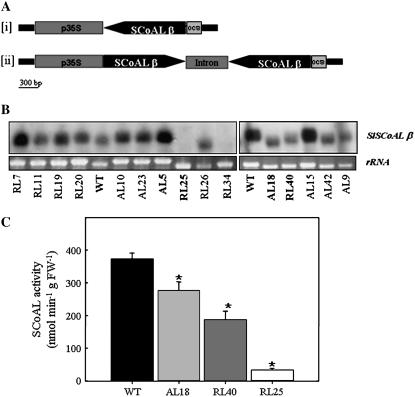
A 1,427-bp (full-length) fragment of the cDNA encoding the β-subunit of SCoAL (Studart-Guimarães et al., 2005) was cloned either in the antisense orientation into the transformation vector pK2WG7 (Karimi et al., 2002) between the cauliflower mosaic virus (CaMV) promoter and the ocs terminator (Fig. 1A) or using the RNA interference (RNAi) design (pK7GWIWG2[I]; Karimi et al., 2002; Fig. 1A). We then transferred 60 transgenic plants obtained by Agrobacterium tumefaciens-mediated transformation to the greenhouse. Screening of the lines on the level of expression of this (single-copy) gene facilitated the identification of several lines displaying a reduction in this parameter (Fig. 1B). To confirm this result and to assess the impact of the reduction of expression of the β-subunit, we next assessed the succinyl-CoA activity in three of the transformants exhibiting reduced expression by means of a recently established cycling assay. These determinations revealed dramatic reductions in activity of 26%, 50%, and 91% in lines AL18 (antisense), RL40, and RL25 (RNAi), respectively (Fig. 1C). That the reduction was so dramatic was somewhat surprising in light of the fact that the β-subunit is generally considered to be regulatory, whereas the α-subunit is considered to be catalytic (e.g. Lambeth et al., 2004). In an additional survey of SCoAL reduced lines, we isolated a third RNAi line (RL34) displaying even greater reduction of enzymatic activity (down to 5% of that observed in wild type).
Figure 1.
Constructs and screening of tomato plants deficient in SCoAL activity. A, Construction of a chimeric gene for expression of the β-subunit of SCoAL antisense RNA (subsection i) or RNAi (subsection ii) consisting of a 540-bp fragment encoding the CaMV 35S promoter and a 1,017-bp (antisense) or two 1,017-bp tandem fragments separated by a stem loop and the ocs terminator. B, Northern analysis of leaves of transgenic plants with altered expression of SCoAL as compared to the wild type (WT). C, SCoAL activity in 6-week-old leaves taken from fully expanded source leaves of transgenic plants with altered expression of SCoAL as compared to wild type. Values are presented as mean ± se of determination on six individual plants per line; an asterisk indicates values that were determined by the t test to be significantly different (P < 0.05) from the wild type. AL, Antisense lines; RL, RNAi lines.
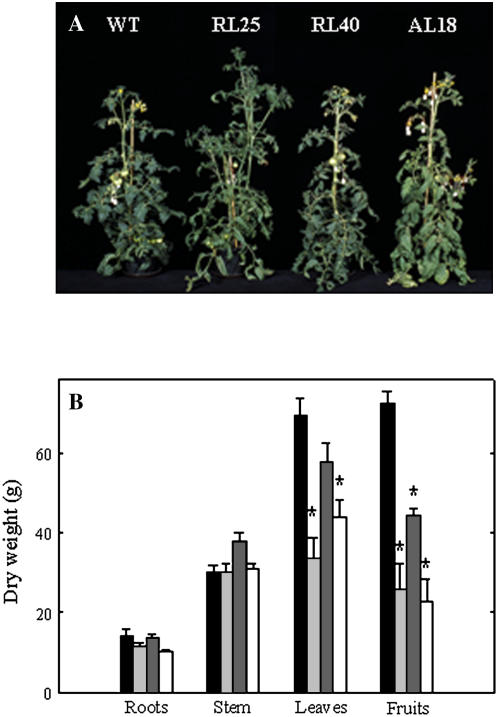
For phenotypic characterization, plants were propagated in vitro to obtain at least six plants per line and transferred to the greenhouse where they were grown alongside wild-type control plants. The transgenic lines were indistinguishable from wild type at early developmental stages, but by 8 weeks after transfer to the greenhouse, the transgenics tended to be taller as a consequence of exhibiting higher internodal length (significantly so in the case of RL25; Fig. 2A). Close inspection of the transformants revealed that they had lower production of leaves and fruits—a fact reflected in dry-weight determination (Fig. 2B). Transgenic plants also produced smaller fruits. A similar phenotype was also observed in line RL34 (data not shown).
Figure 2.
Growth phenotype of SCoAL transgenic tomato plants. Transgenic plants showed reduced fruit yield and slightly reduced leaf biomass. A, Photograph showing representative plants after 8 weeks of growth. B, Biomass (in g/DW) of various plant organs on plant maturity. Wild type, Black bar; AL18, light gray bar; RL40, dark gray bar; RL25, white bar. Values are presented as mean ± se of determination on six individual plants per line; an asterisk indicates values that were determined by the t test to be significantly different (P < 0.05) from the wild type. SCoAL activity of the lines determined 2 weeks prior to these experiments were 162.4 ± 10.7, 134.6 ± 3.4, 139.7 ± 7.5, and 12.4 ± 2.9 nmol−1 min−1 g−1 FW for wild type, AL18, RL40, and RL25, respectively.
Reduction of SCoAL Activity Has Little Consequence on Photosynthetic Rates
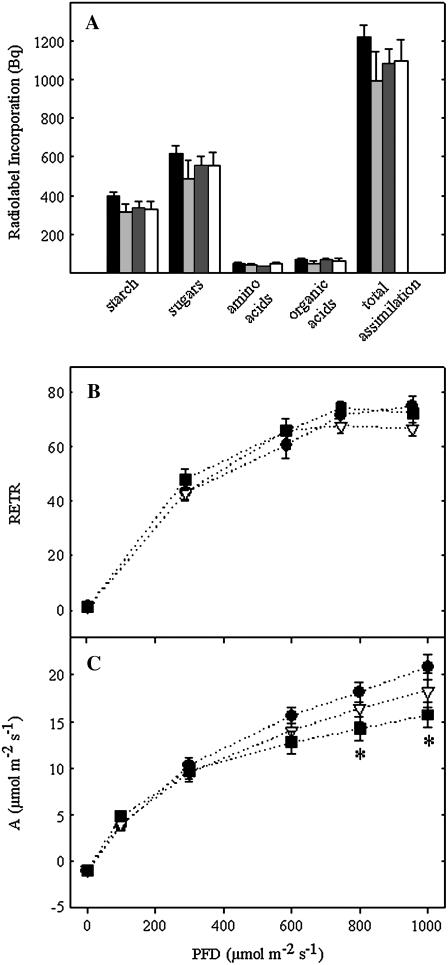
Given the fact that, at later stages of development, plants were characterized by decreased aerial biomass and that tomato genotypes deficient in other enzymes of the TCA cycle have previously been documented to exhibit altered rates of photosynthesis (Carrari et al., 2003; Nunes-Nesi et al., 2005), we next turned our attention to evaluating photosynthetic parameters of SCoAL plants. As a first experiment, we studied the metabolism of 14CO2 by leaf discs excised from wild-type and transformant plants. Notably, there was a consistent decrease in the rate of assimilation and a subsequent decrease in the amount of radiolabel recovered in the primary products of photosynthesis; however, these changes were very minor and not statistically significant (Fig. 3A). In vivo fluorescence emission was next measured across a range of photon flux densities (PFDs), from 0 to 1,000 μmol m−2 s−1, using a pulse amplitude modulation (PAM) fluorometer to estimate the relative electron transport rates of the chloroplast. However, the transgenics exhibited rates comparable to wild type at all light intensities evaluated (Fig. 3B). The deduction of further parameters, such as the rates of photochemical and nonphotochemical quenching, revealed that these were also invariant in the transformants (data not shown). Analysis of gas exchange revealed a minor decrease in assimilation rate under conditions of high irradiance (800 and 1,000 μmol m−2 s−1); however, statistical analysis revealed that this difference was confined to a single transgenic line (RL25; Fig. 3C) and notably there was no alteration in the rate of photosynthesis under the PFD under which plants were grown. We further measured the rate of carbon fixation by feeding 14CO2 to leaf discs (Supplemental Table S4). Data obtained suggested that neither the three lines described above nor the third RNAi line displayed altered carbon assimilation under saturating CO2 concentrations, thus suggesting lines were not compromised in their capacity for photosynthesis.
Figure 3.
Effect of decreased SCoAL activity on photosynthesis. A, Photosynthetic carbon assimilation and partitioning at the onset of illumination. Leaf discs were cut from six separate plants of each genotype, after 6 weeks of growth, at the end of the night and illuminated at 150 μmol photons m−2 s−1 of photosynthetically active radiation in an oxygen electrode chamber containing air saturated with 14CO2. After 30 min, the leaf discs were extracted and fractionated. Wild type, Black bars; AL18, light gray bars; RL40, dark gray bars; RL25, white bars. B, In vivo fluorescence emission was measured as an indicator of the electron transport rates by use of a PAM fluorometer at PFDs ranging from 0 to 1,000 μmol photons m−2 s−1. C, Assimilation rate as a function of PFD. Wild type, Black circles; RL40, white triangles; RL25, black squares. Values are presented as mean ± se of determinations on six individual plants per line. Asterisk indicates values that were determined by the t test to be significantly different (P < 0.05) from the wild type. SCoAL activity of the lines determined in parallel to these experiments was identical to that described in the legend for Figure 2.
Inhibition of SCoAL Activity Results in Slightly Reduced Rates of Respiration
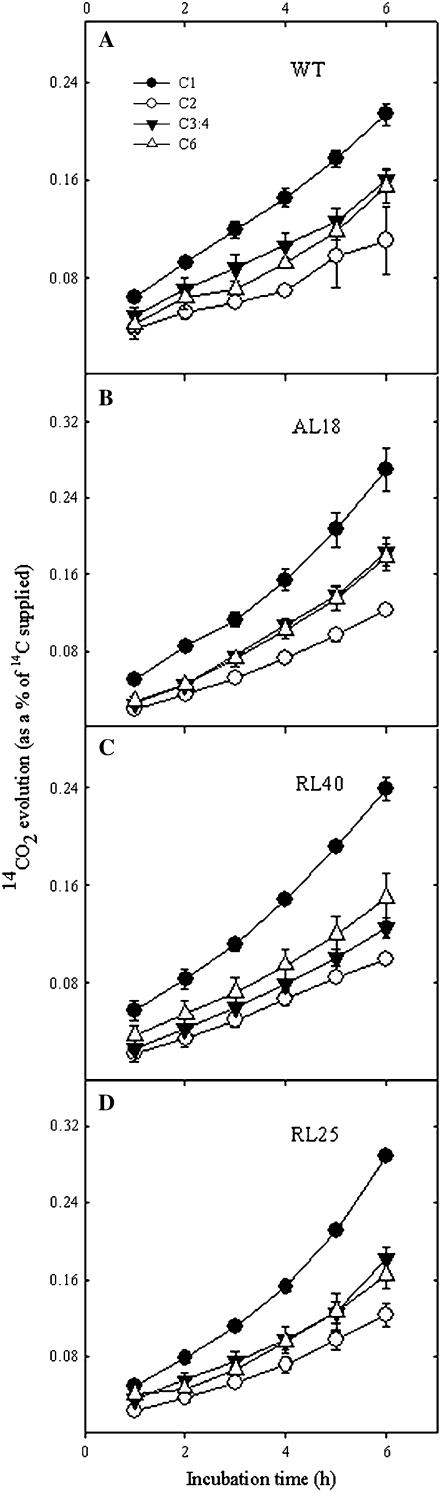
Close examination of gas exchange in the dark revealed an inhibition of dark respiration of approximately 30% in the strongest line (RL25). For more direct evidence of an alteration in the rate of flux through the TCA cycle, the relative rates of carbohydrate oxidation were evaluated in the transformants. For this purpose, the evolution of 14CO2 following incubation of leaf discs in positionally labeled 14C-Glc molecules was recorded. Leaf discs taken from plants in the light were incubated and supplied with [1-14C], [2-14C], [3:4-14C], or [6-14C]Glc over a period of 6 h. During this time, the 14CO2 evolved was collected at hourly intervals. CO2 can be released from the C1 position by the action of enzymes that are not associated with mitochondrial respiration, but CO2 evolution from the C3:4 positions of Glc cannot (ap Rees and Beevers, 1960). Thus, the ratio of CO2 evolution from the C1 and C3:4 positions of Glc provides an indication of the relative rate of other processes of carbohydrate oxidation with respect to the rate of the TCA cycle (Nunes-Nesi et al., 2005). The rate of 14CO2 evolution from leaves incubated in [1-14C]Glc was always highest; however, the absolute rate of CO2 evolution from the C1 position of the transgenic lines was in excess when compared to the wild type (Fig. 4). The release from C3:4 positions was not much lower in the transgenic plants than in the wild type in absolute terms, but in relative terms the C1/C3:4 ratios in the transgenic plants were much higher (after 6 h, wild type, 0.422 ± 0.13; AL18, 0.665 ± 0.05; RL40, 0.717 ± 0.06; RL25, 0.693 ± 0.03). Therefore, these results indicate a lower proportion of carbohydrate oxidation is carried out by the TCA cycle in the transgenic lines. CO2 evolution from C2 and C6 positions was relatively similar across the transgenic lines and the wild type, suggesting that the operation of carbohydrate oxidation in the chloroplast was not grossly affected in these lines. Given that assessment of dark respiration in the illuminated leaf is notoriously difficult (see Villar et al., 1994), we compared the transgenics to the wild type in two further aspects. First, we evaluated the relative rate of organic acid and amino acid production in the leaf, which provides an indirect measure of the rate of flux through the TCA cycle (Nunes-Nesi et al., 2007b). This approach revealed there was no relationship between these fluxes and the activity of SCoAL in the transformants (data not shown). If anything, there was an increase in the flux to organic acids; however, this change was confined to a single line, which only exhibited relatively minor reduction in SCoAL activity. Second, and most directly, we measured gas exchange over a range of limited PDFs. Determination of the rate of dark respiration (Rd) following the method of Häusler et al. (1999) revealed that this was invariant between the transformant and wild-type genotypes (Supplemental Fig. S1; Supplemental Table S1). The fact that all measures are in broad agreement gives us confidence in our conclusion that the rate of respiration is largely unaffected in the transformants.
Figure 4.
Respiratory parameters in leaves of the SCoAL transgenic lines. 14CO2 evolution from isolated leaf discs, after 6 weeks of growth in the light. Leaf discs were taken from 10-week-old plants and were incubated in 10 mm MES-KOH solution, pH 6.5, 0.3 mm Glc supplemented with [1-14C], [2-14C], [3:4-14C], or [6-14C]Glc (at a specific activity of 7.5 MBq mmol−1). The 14CO2 liberated was captured, at hourly intervals, in a KOH trap and subsequently quantified by liquid scintillation counting. Values are presented as means ± se of six determinants per line. SCoAL activity of the lines determined in parallel to these experiments was identical to that described in the legend for Figure 2.
Photosynthetic Carbon Metabolism and Metabolite Levels in SCoAL Transformants
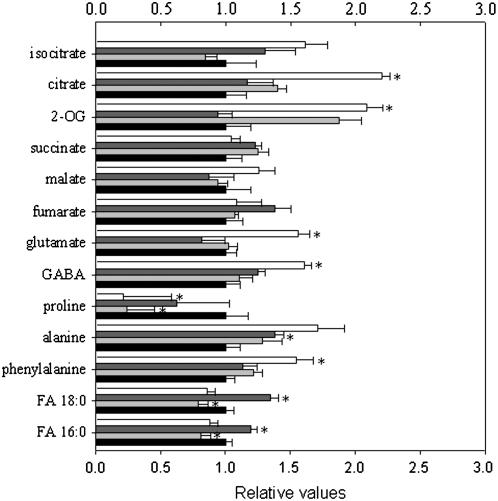
Analysis of the carbohydrate content of leaves from 7-week-old plants during a diurnal cycle revealed no significant change in Suc, Glc, Fru, or starch (data not shown) in the transformants. However, it is important to note that, under the light conditions used here, the diel in the content of these metabolites was small and their natural variance was great (as previously observed in leaves of this species; Laporte et al., 1997; Carrari et al., 2003; Nunes-Nesi et al., 2005). Despite this fact, we decided to carry out a broader metabolite profile to characterize the metabolic consequences of perturbing SCoAL activity. First, the levels of adenylates and uridinylates were determined in trichloracetic acid extracts from leaves by HPLC (Supplemental Table S2). The levels of these metabolites were also unaltered in the transgenics. In the same extracts, we determined the levels of a range of phosphorylated intermediates using previously established highly sensitive cycling assays (Gibon et al., 2002). These assays revealed significantly higher levels of Glc-6-P, but no significant differences in Glc-1-P, Fru-6-P, or inorganic phosphate in the transformants. Considering that the SCoAL reaction involves transfer of CoA, we next measured the levels of acetyl-CoA and reduced CoA, but there were no differences in the levels of these metabolites. As a second procedure to examine the consequence of altering the activity of SCoAL on the TCA cycle, we evaluated the levels of many intermediates of the TCA cycle and other pathways utilizing an established gas chromatography (GC)-mass spectrometry (MS) protocol for metabolic profiling (Fernie et al., 2004b). These studies revealed an increase in TCA cycle intermediates upstream from succinate (isocitrate, citrate, and 2-oxoglutarate [2-OG]; Fig. 5; Supplemental Table S3), but only in the most strongly repressed lines (Fig. 5; Supplemental Table S4). In this experiment, no differences were observable in the levels of other intermediates of the cycle, not even in succinate in spite of it being one of the reaction products of SCoAL. When amino acids were analyzed, Glu, γ-aminobutyric acid (GABA), and Ala increased in the strongest line, potentially suggesting activation of the GABA shunt (see Fig. 8). Interestingly, a significant decrease in Pro content was additionally observed in lines AL18 and RL25. In a second experiment, using the dramatically inhibited line RL34, similar trends were observed in the metabolites of the TCA cycle. We measured a 3- to 4-fold increase in 2-OG, the upstream intermediate of succinate, and the levels of GABA were also significantly higher (Supplemental Table S4). However, reductions were observed in the levels of metabolites downstream of the reaction catalyzed by SCoAL (Supplemental Table S4), possibly emphasizing the high degree of SCoAL inhibition.
Figure 5.
Relative metabolite content of fully expanded leaves from 6-week-old plants of the SCoAL transgenic lines. Metabolites were determined as described in the “Materials and Methods.” The full dataset can be accessed at our Web site (www.mpimp-golm.mpg.de/fernie). Data are normalized with respect to the mean response calculated for the wild type. Values are presented as mean ± se of determinations on six individual plants per line. Asterisk indicates values that were significantly different from wild type when assessed by t tests (P < 0.05). Wild type, Black bars; AL18, light gray bars; RL40, dark gray bars; RL25, white bars. SCoAL activity of the lines determined in parallel to these experiments was identical to that described in the legend for Figure 2.
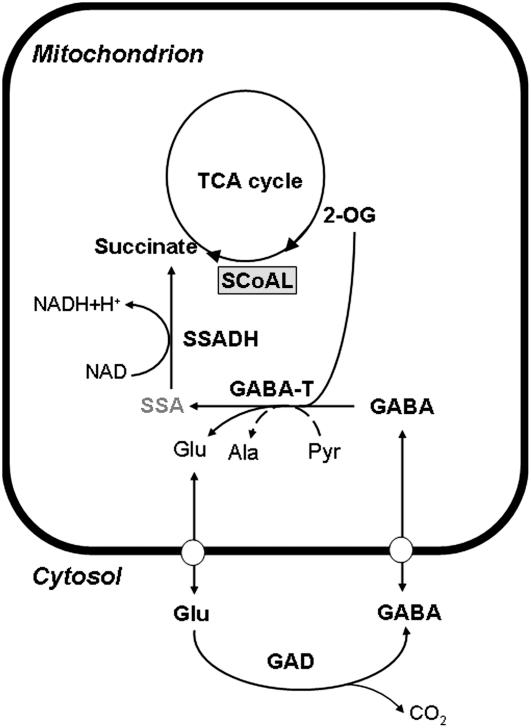
Figure 8.
Metabolic scheme of the GABA shunt and the bypass of SCoAL. Glu is decarboxylated in the cytosol to GABA, which is subsequently transported to the mitochondria and metabolized to SSA and eventually to succinate, which reenters the TCA cycle. SSA, Succinic semialdehyde; SSADH, succinic semialdehyde dehydrogenase.
Evaluation of Changes in Transcript Level between Wild-Type and SCoAL-Deficient Tomato Leaves
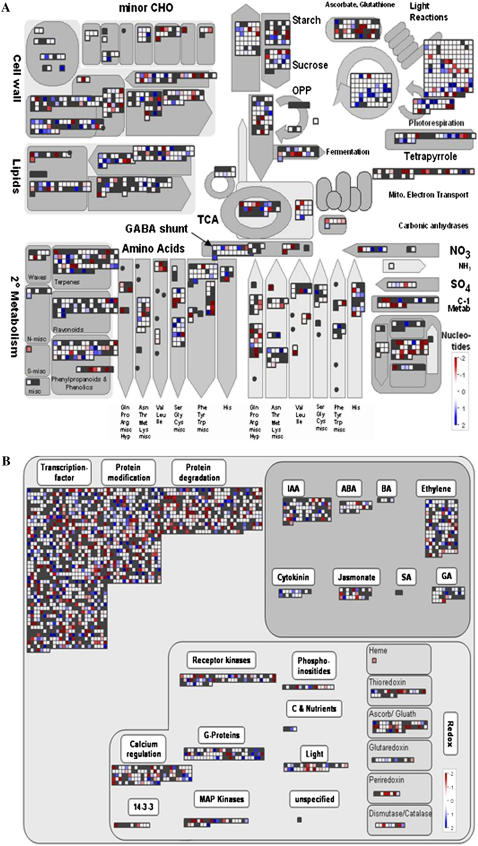
To identify genes that were differentially expressed in the tomato wild type and in the transformed line with strongest decrease in SCoAL activity, we next used DNA microarray technology, hybridizing labeled cDNAs against the commercially available TOM1 chip (Alba et al., 2004) and using the recently modified MapMan software (Urbanczyk-Wochniak et al., 2006) for visualization. The SCoAL transgenic RL25 was characterized by a large number of changes in transcripts for genes associated with nucleotide metabolism, followed by the Calvin cycle, amino acid metabolism, secondary metabolism, mitochondrial electron transport and redox, ascorbate, and glutathione-associated genes (Fig. 6A). In general, the Aco1 mutant (Carrari et al., 2003) and the well-characterized malate dehydrogenase antisense line AL8 (Nunes-Nesi et al., 2005) revealed a similar pattern of change in transcription to that observed in SCoAL line RL25 (Urbanczyk-Wochniak et al., 2006). However, the SCoAL line displayed a markedly reduced proportion of changes in photosynthetic light reactions and in the TCA cycle. At the level of individual genes, the majority of the genes involved in the Calvin cycle had increased transcript levels as well as most genes for encoding proteins of the photosynthetic light reactions, photorespiration, and flavonoid metabolism (Fig. 6A). A trend for decrease in transcription levels of genes involved in amino acid synthesis was also observed, but this also holds true for genes involved in their degradation. In contrast, almost all genes involved in the mitochondrial electron transport chain displayed a decrease in transcript level, and there is a clear trend for decreased transcript levels of genes involved in nucleotide metabolism and the TCA cycle (Fig. 6A). Interestingly, most of the genes annotated for Glu decarboxylase (GAD; Fig. 6A), which is an integral enzyme of the GABA shunt, showed increased transcription in the transgenic line (with MapMan genes 4.3.17.21, 4.1.5.9, 1.2.14.18, and 6.4.19.6 all being expressed between 1.4- and 1.5-fold of the level observed in wild type). This most likely reflects up-regulation of utilization of Glu metabolism to support the TCA cycle to compensate for the restriction of succinate biosynthesis via SCoAL. For completeness, genes associated with regulatory processes are provided in Figure 6B. The huge number of genes exhibiting altered transcript levels in these categories was somewhat surprising considering that the transformants did not exhibit strong visual or metabolic phenotype. Comparison of the transcription changes following modulation in the expression of SCoAL with previously reported data for the other genotypes (Urbanczyk-Wochniak et al., 2006) revealed some commonality, but also many differences, as would be expected, because at a gross phenotypic level the SCoAL plants were not as strongly affected as the Aco1 mutant and the malate dehydrogenase antisense lines. These entire datasets and all data point annotations are best viewed at http://gabi.rzpd.de/projects/MapMan.
Figure 6.
Differences in transcript levels between leaves of the well-characterized SCoAL line RL25 and wild type for genes associated with metabolism (A) and regulation (B). Both sets of material were harvested after 6 weeks of growth in the middle of the day. Red and blue represent a decrease and an increase of expression, respectively, in the SCoAL transgenic line with respect to the wild type. The color scale used is reproduced in the figure. This figure and all data point annotations are best viewed at http://gabi.rzpd.de/projects/MapMan (see “Materials and Methods”). SCoAL activity of the lines determined in parallel to these experiments was identical to that described in the legend for Figure 2.
Measurement of Key Enzyme Activity of Carbon Metabolism
Analysis of maximal catalytic activity of a broad range of enzymes revealed no changes in important enzymes of photosynthesis (Rubisco and Calvin cycle enzymes) or of the key starch synthetic enzyme ADP-Glc pyrophosphorylase or of pyruvate kinase (Table I). However, an increase in the activity of the ATP-dependent phosphofructokinase (PFK; in line AL18) and of phosphoribulokinase (in lines AL18 and RL40) was observed and a strong decrease in the activities of UGPase and NADP-GAPDH was also observed (in lines AL18 and RL40). An increase in the activity of PFK was previously reported in lines exhibiting reduced expression of the mitochondrial malate dehydrogenase (Nunes-Nesi et al., 2005) and may indicate compensation for reduced flux through the TCA cycle. However, it should be noted that the changes documented here are generally minor and confined to a subset of the transgenic lines tested. Furthermore, the other changes reported here are distinct from those in previous screens of enzymes in leaves of plants deficient in TCA cycle activity (Carrari et al., 2003; Nunes-Nesi et al., 2005). Given that both the metabolite and transcript data hint to induction in GABA shunt activity in the transformants, especially in the case of the most severely repressed line, we next expanded our survey of enzymatic activities to include constituent enzymes of this pathway. For this purpose, we chose to assay GAD, which catalyzes the conversion of Glu to GABA, shown to be a key regulator in GABA biosynthesis. Both lines with strong inhibition of SCoAL, RL25 and RL34, had significant increase in GAD activity (Table I; Supplemental Table S4). The activity of an alternative enzyme of Glu catabolism, namely, Glu dehydrogenase, responsible for the catalysis of the reductive amination of 2-OG, was also found to be significantly higher in activity in line RL25 (Table I).
Table I.
Enzyme activity in SCoAL transgenic lines
Activity was determined in 6-week-old fully expanded source leaves. Data presented are mean ± se of determinations on six individual plants per genotype. Values set in bold type were determined by the t test to be significantly different (P < 0.05) from the wild type. –, Not measured; AGPase, ADP-Glc pyrophosphorylase; UGPase, UDP-Glc pyrophosphorylase; GDH, Glu dehydrogenase.
| Enzyme | Activity
|
|||
|---|---|---|---|---|
| Wild Type | AL18 | RL40 | RL25 | |
| nmol min−1 g−1 FW | ||||
| AGPase | 507 ± 68 | 591 ± 64 | 792 ± 177 | 602 ± 61 |
| UGPase | 8.48 ± 1.2 | 8.64 ± 0.9 | 4.81 ± 1.2 | 3.97 ± 1.0 |
| PFK ATP dependent | 69.9 ± 6.1 | 94.9 ± 7.7 | 78.7 ± 7.9 | 65.5 ± 2.0 |
| PFK PPi dependent | 0.21 ± 0.05 | 0.19 ± 0.04 | 0.28 ± 0.03 | 0.16 ± 0.03 |
| NADP-GAPDH | 0.83 ± 0.07 | 0.54 ± 0.03 | 1.02 ± 0.15 | 0.30 ± 0.12 |
| Pyruvate kinase | 1.55 ± 0.15 | 1.45 ± 0.1 | 1.67 ± 0.18 | 1.46 ± 0.12 |
| Rubisco | 3.67 ± 0.55 | 2.31 ± 0.48 | 2.79 ± 0.33 | 2.94 ± 0.80 |
| Transketolase | 4.14 ± 0.45 | 3.40 ± 0.49 | 3.42 ± 0.62 | 3.43 ± 0.81 |
| Phosphoribulokinase | 1.62 ± 0.18 | 2.29 ± 0.22 | 2.79 ± 0.23 | 2.02 ± 0.26 |
| Phosphoglyceratekinase | 0.87 ± 0.18 | 0.99 ± 0.12 | 1.29 ± 0.11 | 0.60 ± 0.11 |
| GDHa | 313.6 ± 41.6 | – | 413.3 ± 87.2 | 556.5 ± 109.2 |
| GADb | 0.99 ± 0.09 | – | 1.23 ± 0.13 | 1.60 ± 0.07 |
Rubisco and GDH activity values are presented as μmol min−1 g−1 FW.
GAD activity values are presented as 14CO2 (as a % of 14C supplied) min−1 mg−1 protein.
Direct Evaluation of Flux through the GABA Shunt
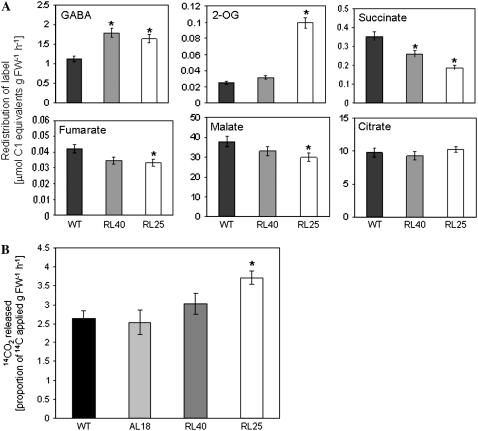
When taken together, the above data strongly suggest that the reason that respiration is barely compromised in these plants is an up-regulation of the GABA shunt to provide an alternative source of succinate for the mitochondrial electron transport chain. To evaluate whether this is indeed the case, we next evaluated the relative isotope redistribution in leaves excised from wild-type and transformant plants using a combination of feeding 13C-labeled substrate to the leaf via the transpiration stream and a recently adapted GC-MS protocol that facilitates the estimation of intracellular fluxes (Roessner-Tunali et al., 2003). To assess the relative rates of the normal reactions of the TCA cycle and of the GABA shunt, we supplied 15 mm 13C-Glu and evaluated the redistribution of 13C to metabolites associated with these pathways. Glu could theoretically enter the TCA cycle either through the GABA shunt (Fig. 8) or by transamination of Glu in the cytosol and uptake of 2-OG by the mitochondria, or by entering the mitochondria via Glu transporters and being converted to 2-OG within this compartment. Following incubation of isolated leaves in 13C-Glu, a significantly increased incorporation of label into GABA (Fig. 7A) and 2-OG (Fig. 7A) was observed for line RL25 in comparison to that observed for the wild type. Nevertheless, the transformants were characterized by a slight, yet significant, reduction in the label incorporation into succinate (Fig. 7A). These results are consistent with the inhibition of SCoAL and with the slight reduction in respiration observed in the SCoAL lines. Nonetheless, the redistribution of label to other organic acids, as exemplified by fumarate, malate, and citrate (Fig. 7A), was minor or unaltered. In an alternate approach, we fed positionally labeled [1-14C]Glu to isolated leaf discs and analyzed the release of radiolabeled CO2, which is liberated through the decarboxylation reaction of GAD in the GABA shunt (Turano and Fang, 1998). The results obtained clearly show a higher fraction of labeled CO2 released by the most strongly inhibited transgenic lines (Fig. 7B) and thus corroborate the results obtained from 13C-Glu incubation.
Figure 7.
Estimation of GABA shunt activity. A, Redistribution of label following feeding of leaves [U-13C]Glu via the petiole (see “Materials and Methods”). Values are presented as the accumulation of micromolar C1 equivalents per hour per gram FW. B, Flux through the GABA shunt was determined by measuring 14CO2 release from leaf discs incubated in [1-14C]Glu. Incubation was performed as described in “Materials and Methods.” Values are presented as means ±se of six determinants per line. Wild type, Black bars; AL18, light gray bars; RL40, dark gray bars; RL25, white bars. For experiment A, SCoAL activity of the lines was identical to that described in the legend for Figure 2. For experiment B, the measurements of additional lines, RL34 and AL10, as well as SCoAL activity of the lines are provided in Supplemental Table S4.
DISCUSSION
Effect of Reduction of SCoAL Activity on Rate of Photosynthesis, Respiration, and Plant Growth
Previous studies have revealed that the functions of several enzymes of the TCA cycle are intricately related to efficient photosynthetic function (Carrari et al., 2003; Nunes-Nesi et al., 2005, 2007a). Both the Aco1 mutant of Solanum pennellii and transgenic plants of S. lycopersicum, in which the mitochondrial malate dehydrogenase was inhibited using antisense technology, displayed enhanced photosynthetic performance (Carrari et al., 2003; Nunes-Nesi et al., 2005). In contrast, tomato plants exhibiting decreased expression of fumarase were greatly impaired in photosynthesis due to a restriction in stomatal function; however, the precise mechanism by which this is achieved has not yet been clarified (Nunes-Nesi et al., 2007a). Given these findings, alongside previous pharmacological demonstrations of the importance of respiration to photosynthesis (Raghavendra and Padmasree, 2003), it was initially surprising that the lines we created here did not exhibit altered photosynthesis. Detailed biochemical characterization in previous studies revealed that those genotypes exhibiting elevated rates of photosynthesis were also characterized by increased levels of ascorbate (Carrari et al., 2003; Nunes-Nesi et al., 2005). Several lines of experimental evidence suggest that the increased ascorbate is a consequence of the fact that, via the action of galactonolactone dehydrogenase, the cytochrome pathway appears to be capable of utilizing galactonolactone as an alternative substrate (detailed in Nunes-Nesi et al., 2005; Bartoli et al., 2006). Interestingly, plants reduced in either fumarase (Nunes-Nesi et al., 2007a) or SCoAL activity (this study) displayed neither an increase in ascorbate nor an increase in photosynthetic efficiency. Indeed, whereas the more strongly inhibited SCoAL lines showed a decrease in leaf biomass and fruit production, their phenotype was relatively mild when compared to plants deficient in the activity of aconitase, the mitochondrial malate dehydrogenase, or fumarase (Carrari et al., 2003; Nunes-Nesi et al., 2005, 2007a). Analysis of photosynthetic metabolism of the SCoAL transformants revealed that the carbon assimilation rate was largely invariant and that the photochemical efficiency of PSII was unimpaired. In keeping with this, 14CO2 assimilation studies carried out under saturating concentrations of CO2 revealed no alteration in photosynthetic capacity. Soluble sugars and starch were additionally measured through a diurnal cycle, but no differences were observed, with similarly little change in metabolites that are classically associated with either metabolic (Stitt, 1997) or redox (Scheibe et al., 2005) regulation of photosynthesis. Furthermore, although there was a clear buildup of metabolites of the TCA cycle that lie upstream of SCoAL in the most severely affected transformants, this was coupled to only a minor depletion in those that lie downstream of SCoAL, suggesting that the depletion in the activity did not overly perturb succinate production. In keeping with this observation, the relative reduction in the rates of dark respiration and flux through the TCA cycle in the illuminated leaf were not as severe as noted previously following perturbation of other enzymes of the cycle in which reductions of down to 30% of wild-type respiration were observed (Carrari et al., 2003; Nunes-Nesi et al., 2005). This comparison is particularly intriguing given that the reduction in activity of the SCoAL is far greater than that achieved for the previously studied enzymes. This suggests that the SCoAL lines, despite exhibiting a slight reduction in succinate production, are able to produce enough succinate to support normal rates of respiration. That such a dramatic reduction was seen on inhibition of expression of the regulatory subunit is of note and suggests that future studies on the structure-function relationships of the subunits are probably merited.
Up-Regulation of Alternative Pathways for Succinate Production
The surprisingly slight effect of deficiency in SCoAL activity could be due to one of two facts: either (1) succinate production is not important for normal mitochondrial function; or (2) the cell has alternate routes for producing succinate to support respiration and these are up-regulated in the transformants to compensate for the reduced production by SCoAL. If the latter hypothesis were correct, this would suggest a more critical function of the production of succinate than the former. All the data obtained from this study point toward complementation of succinate production by alternative pathways, although it should be noted that the residual activity observed in all but the most dramatic antisense line may be sufficient to furnish the succinate requirements of the cell under normal conditions. Such redundancy has previously been noted in the primary metabolism of plants and it has been suggested that the presence of multiple routes to the same end allows the plant greater flexibility (ap Rees and Hill, 1994; Rontein et al., 2002; Plaxton and Podesta, 2006). Surveying the levels of a wide range of primary metabolites revealed that the most strongly inhibited lines were characterized not only by increases of the metabolites upstream of SCoAL, but also in the closely associated Glu and GABA, suggesting that SCoAL transgenic plants may be increasing carbon partitioning through the GABA shunt. This pathway bypasses two of the steps of the TCA cycle—conversion of 2-OG to succinyl-CoA, catalyzed by 2-OG dehydrogenase, and the subsequent conversion of succinyl-CoA to succinate (see Fig. 8). The GABA shunt produces GABA from Glu and metabolizes it to produce succinate (Satyanarayan and Nair, 1990; Bouché et al., 2003a). It is composed of three enzymes: cytosolic GAD and the mitochondrial enzymes GABA transaminase (GABA-T) and succinic semialdehyde dehydrogenase. Glu dehydrogenase and Glu decarboxylase were increased in activity in the transformants; when taken together with the increase in Glu and GABA levels, this provided reasonable evidence for the conversion of 2-OG to Glu and activation of the GABA shunt in the transformants. However, to assess that this was the case, we demonstrated that the capacity to mobilize Glu as substrate for the TCA cycle via GABA was elevated in the transformants with approximately 51% and 67% of wild-type succinate production being met in lines RL25 and RL40, respectively. Direct analysis of the flux through the GABA shunt obtained by analyzing the in vivo activity of GAD revealed that this was significantly increased only in lines exhibiting dramatic decreases in SCoAL activity and only marginally affected in the other lines.
From these studies, we can speculate that up-regulation of the GABA shunt provides the cytochrome pathway of the transformants with sufficient succinate as substrate. That said, other alternative routes for the conversion of succinyl-CoA to succinate must also be considered because an alternative contribution, albeit a minor one, cannot as yet be formally ruled out. One such putative route is via the glyoxylate cycle, in which the enzyme isocitrate lyase catalyzes the conversion of isocitrate to glyoxylate and succinate in the peroxisome, and in plants it is debated to serve an anaplerotic function (Eastmond and Graham, 2001; Smith, 2002). The succinate produced could in theory be transported into the mitochondrion through the succinate-fumarate translocator (Catoni et al., 2003), where it could reenter the TCA cycle to be further oxidized by succinate dehydrogenase. In plants, the glyoxylate cycle operates mainly during mobilization of seed triglycerides for seedling growth, where it has a key role in conversion of acetyl-CoA from fatty acid β-oxidation into oxaloacetate, and subsequently into sugar. That said, it is, at best, questionable whether it represents an important source of succinate in the illuminated tomato leaf. Another possible bypass of the SCoAL reaction could be provided by CoA transferases, which catalyze the transfer of CoA moieties from one compound to another. One such transferase present in plant mitochondria, acetyl-CoA hydrolase (Zeiher and Randall, 1990), has broad substrate specificity for different thioesters, including propionyl-CoA, butyryl-CoA, acetyl-CoA, and palmytoyl-CoA, with lower affinity for oleolyl-CoA, succinyl-CoA, and crotonyl-CoA (Zeiher and Randall, 1990).
CONCLUSION
In this article, we have shown that repression of SCoAL activity in tomato exhibits only relatively minor effects on respiration, photosynthesis, and growth. It is also conceivable that whatever change in growth was observed is due to an effect of altered metabolism at night, a scenario we have not explored in this article. We have, however, provided evidence that once SCoAL activity is reduced beyond a certain threshold, up-regulation of the GABA shunt that is mediated at the level of transcription occurs. We believe that this up-regulation allows maintenance of normal rates of respiration and normal cellular function in the illuminated leaf. Our results provide strong evidence for the significance of GABA metabolism as a functional bypass of 2-OG to the succinate section of the TCA cycle in plants. Interestingly, surveying the literature and publicly available transcript resources reveals that SCoAL and enzymes of the GABA shunt are, by and large, differentially regulated, depending on cellular circumstance. This is apparent both at the level of gene expression (see Genevestigator; Zimmermann et al., 2004), protein abundance (Sweetlove et al., 2002), and allosteric regulation of the enzyme activity (Busch and Fromm, 1999; Studart-Guimarães et al., 2005). Presumably, this allows succinate production to be more tightly regulated, depending on both prevailing environmental conditions and energetic demands imposed by cellular metabolism. These data thus highlight the importance of succinate production in the mitochondria and reveal a potential mechanism by which the plant can maintain this even in situations in which succinyl-CoA activity is compromised. Future experiments should focus on understanding the relative fluxes of the pathways of succinate production under a range of environmental conditions and on elucidating the details underpinning the mechanism by which GAD is up-regulated in the transgenic lines.
MATERIALS AND METHODS
Materials
Tomato (Solanum lycopersicum) ‘Moneymaker’ was obtained from Meyer Beck. Plants were handled as described in the literature (Carrari et al., 2003; Nunes-Nesi et al., 2005). All chemicals and enzymes used in this study were obtained from Roche Diagnostics, with the exception of radiolabeled sodium bicarbonate and d-[1-14C], d-[3:4-14C], and d-[6-14C]Glc, which were from Amersham International; d-[2-14C]Glc was from American Radiolabeled Chemicals. [U-13C]Glu and [U-13C]acetate were purchased from Cambridge Isotope Laboratories.
cDNA Cloning and Expression
A 1,427-bp full-length cDNA fragment of the β-subunit of SCoAL was cloned in antisense orientation into the vector pK2WG7 (Karimi et al., 2002) or using the RNAi (vector pK7GWIWG2[I]) approach, between the CaMV 35S promoter and the ocs terminator. This construct was introduced into plants by an Agrobacterium-mediated transformation protocol and plants were selected and maintained as described in the literature (Obiadalla-Ali et al., 2004). Initial screening of 60 lines was carried out using the assay described by Studart-Guimarães et al., (2005), and lines RL25 and RL40 (RNAi) and AL18 (antisense) were chosen on the basis of the reduction in expression and activity that they exhibited for detailed physiological and biochemical analyses.
Northern-Blot Analysis
Total RNA was isolated using the commercially available TRIzol kit (Gibco-BRL) according to the manufacturer's suggestions for extraction from plant material. Hybridization using standard conditions was carried out using a 1.0-kb fragment of the gene encoding the SCoAL β-subunit.
Microarray Analysis
Glass slides containing arrayed tomato ESTs were obtained directly from the Center for Gene Expression Profiling (CGEP) at the Boyce Thompson Institute, Cornell University, The Geneva Agricultural Experiment Station, and the U.S. Department of Agriculture Federal Plant and Nutrition Laboratory. The tomato array contains 13,440 spots randomly selected from cDNA libraries isolated from a range of tissues, including leaf, root, fruit, and flowers, and representing a broad range of metabolic and developmental processes. Technical details of the spotting are provided as MIAME (http://www.mpimp-golm.mpg.de/fernie). Further annotation of this file was carried out to provide gene identities and putative functions for the ESTs described on the Solanaceae Genomics Network (http://soldb.cit.cornell.edu) Web site. Fluorescent probe preparation and microarray hybridization were exactly as described previously (Urbanczyk-Wochniak et al., 2006). Detailed information is included into MIAME (http://www.mpimpgolm.mpg.de/fernie). Microarray experiment slides were normalized with print-tip loess and moving minimum background subtraction using the Bioconductor limma package framework (Gentleman et al., 2004). Microarray slides were subsequently scale normalized, adjusting the log ratios to have the same median absolute deviation across arrays (Yang et al., 2002; Smyth and Speed, 2003). Moderated t statistics were used to detect any genes likely to be differentially expressing (Smyth, 2004). MapMan files were constructed from resulting analysis log2-fold change values, where any poor-quality spots created during the experimental process were down-weighted. Moreover, only spots that had detectable signals in both channels over all arrays were averaged for display in MapMan.
Analysis of Enzyme Activity
Enzyme extracts were prepared as described previously (Tauberger et al., 2000). All activity was determined as described by Carrari et al. (2003) with the exception of PFK and phosphoglucomutase activities, which were determined as defined by Fernie et al. (2001); SCoAL, which was determined using the assay described by Studart-Guimarães et al. (2005); Glu decarboxylase as described by Turano and Fang (1998); and Glu dehydrogenase activity as described by Purnell et al. (2005).
Determination of Metabolite Levels
Leaf samples were taken at the time point indicated, immediately frozen in liquid nitrogen, and stored at −80°C until further analysis. Extraction was performed by rapid grinding of tissue in liquid nitrogen and immediate addition of the appropriate extraction buffer. The levels of starch, Suc, Fru, and Glc in the leaf tissue were determined exactly as described previously (Fernie et al., 2001). Levels of glycolytic intermediates, nucleotides, and nucleosides were measured as described by Gibon et al. (2002), whereas phosphate was determined using the protocol described by Sharkey and Vanderveer (1989). The levels of all other metabolites was quantified by GC-MS following the protocol described by Roessner et al. (2001), with the exception that the peak identification was optimized to tomato tissues (Roessner-Tunali et al., 2003) and the metabolites studied included recent additions to our mass spectral libraries (Geigenberger et al., 2004; Schauer et al., 2005). Pigments were measured exactly as described by Bender-Machado et al. (2004).
Measurements of Photosynthetic Parameters
The 14C-labeling pattern of Suc, starch, and other cellular constituents was performed by illuminating leaf discs (10-mm diameter) in a leaf-disc oxygen electrode (Hansatech) in saturating 14CO2 at a PFD of 250 μmol m−2 s−1 of photosynthetically active radiation at 20°C for 30 min, and subsequent fractionation was performed exactly as detailed by Lytovchenko et al. (2002). Fluorescence emission was measured in vivo using a PAM fluorometer (Walz) on 9-week-old plants maintained at fixed irradiance (250 and 700 mmol photons m−2 s−1) for 30 min prior to measurement of chlorophyll fluorescence yield and relative electron transport rates, which were calculated using the WinControl software package (Walz). Gas-exchange measurements were performed in a special custom-designed open system (Lytovchenko et al., 2002).
Measurement of Respiratory Parameters
Dark respiration was measured using the same gas-exchange system as defined above. Estimation of the TCA cycle flux on the basis of 14CO2 evolution was carried out following incubation of isolated leaf discs in 10 mm MES-KOH, pH 6.5, containing 2.32 kBq mL−1 [1-14C], [2-14C],[3:4-14C], or [6-14C]Glc. 14CO2 evolved was trapped in KOH and quantified by liquid scintillation counting. The results were interpreted following ap Rees and Beevers (1960). Rd was determined using a graphic method as described by Häusler et al. (1999). CO2 assimilation rates were measured at 22°C, relative humidity of 50%, and limited PFDs (40, 80, and 120 μmol m−2 s−1) under below-ambient CO2 concentrations(50, 80, 100, and 200 μmol mol−1). For statistical analysis of each determination, a nonlinear curve was fitted (second polynomial regression) to all three individual A/Ci curves. Intercepts of individual curve pairs were determined for all possible combinations of curve pairs and mean values and sds were calculated. Measurements were performed on fully developed source leaves of 4- to 5-week-old plants of identical developmental stages.
13C and 14C Feeding Experiments
For 13C feeding experiments, leaves were excised from 6-week-old plants and labeled substrate was fed into the transpiration stream via the petiole as described by Morcuende et al. (1998). The petiole was placed in a solution containing 10 mm MES-KOH (pH 6.5) and 15 mm [U-13C]Glu, and the incubation was left to proceed for 6 h. At the end of this time period, a leaf disc was cut (always from an equivalent part of the leaf), washed carefully, weighted, and frozen in liquid nitrogen and stored at −80°C until further analysis. Samples were then extracted and evaluated exactly as described by Roessner-Tunali et al. (2004), and label redistribution was determined as described by Giege et al. (2003) and Tieman et al. (2006). Estimation of the GABA shunt flux on the basis of 14CO2 evolution was carried out following incubation of isolated discs of 6-week-old plants in a solution containing 10 mm MES-KOH (pH 6.5) containing 2.32 kBq mL−1 [1-14C]Glu. 14CO2 evolved was trapped in KOH and quantified by liquid scintillation counting.
Statistical Analysis
t tests were performed using the algorithm embedded into Microsoft Excel. The term significant is used in the text only when the change in question has been confirmed to be significant (P < 0.05) with the t test.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Representative A/Ci curves for determination of Rd in SCoAL transgenic lines RL25 (A) and RL40 (B), and the wild type (C).
Supplemental Table S1. Rd and values of CO2 compensation point (γ).
Supplemental Table S2. Nucleotides, phosphorylated intermediates, and acetyl-CoA levels in SCoAL lines.
Supplemental Table S3. Relative metabolite content of fully expanded leaves from 8-week-old plants of SCoAL transgenic lines.
Supplemental Table S4. Parameters measured on a different set of plants incorporating a second strongly reduced line (RL34).
Supplementary Material
Acknowledgments
We are very grateful to Dr. Joachim Fisahn for help in organization of gas-exchange measurements and discussion of the results and to Helga Kulka for excellent care of the plants.
This work was supported by a Konrad Adanauer fellowship (to C.S.-G.), Minerva and Alexander von Humboldt fellowships (to A.F.), the German Plant Genomics Research Program of the Bundesministerium für Bildung und Forschung (to B.U.), and Max-Planck-Gesellschaft (to F.C., A.N.-N., and A.R.F.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Alisdair R. Fernie (fernie@mpimp-golm.mpg.de).
The online version of this article contains Web-only data.
References
- Alba R, Fei Z, Payton P, Liu Y, Moore SL, Debbie P, Cohn J, D'Ascenzo GJS, Rose JKC, Martin G, et al (2004) ESTs, cDNA microarrays, and gene expression profiling: tools for dissecting plant physiology and development. Plant J 39 697–714 [DOI] [PubMed] [Google Scholar]
- ap Rees T, Beevers H (1960) Pathways of glucose dissimilation in carrot slices. Plant Physiol 35 830–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ap Rees T, Hill SA (1994) Metabolic control analysis of plant metabolism. Plant Cell Environ 17 587–599 [Google Scholar]
- Atkin OK, Evans JR, Ball MC, Lambers H, Pons TL (2000) Leaf respiration of snow gum in the light and dark: interactions between temperature and irradiance. Plant Physiol 122 915–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoli CG, Gomez F, Gergoff G, Guiamet JJ, Puntarulo S (2005) Up-regulation of the mitochondrial alternative oxidase pathway enhances photosynthetic electron transport under drought conditions. J Exp Bot 56 1269–1276 [DOI] [PubMed] [Google Scholar]
- Bartoli CG, Yu J, Gomez F, Fernandez L, McIntosh L, Foyer CH (2006) Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J Exp Bot 57 1621–1631 [DOI] [PubMed] [Google Scholar]
- Beevers H (1961) Respiratory Metabolism in Plants. Row, Peterson, Everson, UK
- Bender-Machado L, Bäuerlein M, Carrari F, Schauer N, Lytovchenko A, Gibon Y, Kelly AA, Ehlers Loureiro M, Müller-Röber B, Willmitzer L, et al (2004) Expression of a yeast acetyl CoA hydrolase in the mitochondrion of tobacco plants inhibits growth and restricts photosynthesis. Plant Mol Biol 55 645–662 [DOI] [PubMed] [Google Scholar]
- Bouché N, Fait A, Bouchez D, Møller SG, Fromm H (2003. a) Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc Natl Acad Sci USA 100 6843–6848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budde RJA, Randall DD (1990) Pea leaf mitochondrial pyruvate dehydrogenase complex is inactivated in vivo in a light dependent manner. Proc Natl Acad Sci USA 87 673–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch KB, Fromm H (1999) Plant succinic semialdehyde dehydrogenase. Cloning, purification, localization in mitochondria, and regulation by adenine nucleotides. Plant Physiol 121 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrari F, Nunes-Nesi A, Gibon Y, Lytovchenko A, Ehlers-Loureiro M, Fernie AR (2003) Reduced expression of aconitase results in an enhanced rate of photosynthesis and marked shifts in carbon partitioning in illuminated leaves of wild species tomato. Plant Physiol 133 1322–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catoni E, Schwab R, Hilpert M, Desimone M, Schwacke R, Flügge U-I, Schumacher K, Frommer WB (2003) Identification of an Arabidopsis mitochondrial succinate-fumarate translocator. FEBS Lett 534 87–92 [DOI] [PubMed] [Google Scholar]
- Douce R, Neuberger M (1989) The uniqueness of plant mitochondria. Annu Rev Plant Physiol Plant Mol Biol 40 371–414 [Google Scholar]
- Dutilleul C, Driscoll S, Cornic G, De Paepe R, Foyer CH, Noctor G (2003) Functional mitochondrial complex I is required for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol 131 264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutilleul C, Lelarge C, Prioul JL, De Paepe R, Foyer CH, Noctor G (2005) Mitochondria-driven changes in leaf NAD status exert a crucial influence on the control of nitrate assimilation and the integration of carbon and nitrogen metabolism. Plant Physiol 139 64–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastmond PJ, Graham IA (2001) Re-examining the role of glyoxylate cycle in oilseeds. Trends Plant Sci 6 72–77 [DOI] [PubMed] [Google Scholar]
- Fatland BL, Nikolau BJ, Wurtele ES (2005) Reverse genetic characterisation of cytosolic acetyl CoA generation by ATP-citrate lyase in Arabidopsis. Plant Cell 17 182–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie AR, Carrari F, Sweetlove L (2004. a) Respiratory metabolism: glycolysis, the TCA cycle and mitochondrial electron transport chain. Curr Opin Plant Biol 7 254–261 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Roscher A, Ratcliffe RG, Kruger NJ (2001) Fructose 2,6-bisphosphate activates pyrophosphate: fructose-6-phosphate 1-phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta 212 250–263 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Trethewey RN, Krotzky A, Willmitzer L (2004. b) Metabolic profiling: from diagnostics to systems biology. Nat Rev Mol Cell Biol 5 763–769 [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Regierer B, Lytovchenko A, Leisse A, Schauer N, Springer F, Kossmann J, Fernie AR (2004) Heterologous expression of a ketohexokinase in potato plants leads to inhibited rates of photosynthesis, severe growth retardation and abnormal leaf development. Planta 218 569–578 [DOI] [PubMed] [Google Scholar]
- Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, et al (2004) Bioconductor: open software development for computational biology and bioinformatics. Genome Biol 5 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel cycling system. Plant J 30 221–235 [DOI] [PubMed] [Google Scholar]
- Giege P, Heazlewood JL, Roessner-Tunali U, Millar AH, Fernie AR, Levaer CJ, Sweetlove LJ (2003) Enzymes of glycolysis are functionally associated with the mitochondrion in Arabidopsis cells. Plant Cell 15 2140–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanning I, Heldt HW (1993) On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea L.) leaves: partitioning between respiration and export of redox equivalents and precursors for nitrate assimilatory products. Plant Physiol 103 1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häusler RE, Kleines M, Uhrig H, Hirsch H-J, Smets H (1999) Overexpression of phosphoenolpyruvate carboxylase from Corynebacterium glutamicum lowers the CO2 compensation point (Γ*) and enhances dark and light respiration in transgenic potato. J Exp Bot 50 1231–1242 [Google Scholar]
- Hill SA (1997) Carbon metabolism in mitochondria. In DT Dennis, DH Turpin, DD Lefebvre, DB Layzell, eds, Plant Metabolism. Longman, Harlow, UK
- Hodges M, Flesch V, Galvez S, Bismuth E (2003) Higher plant NADP(+)-dependent isocitrate dehydrogenases, ammonium assimilation and NADPH production. Plant Physiol Biochem 41 577–585 [Google Scholar]
- Karimi M, Inzé D, Depicker A (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7 193–195 [DOI] [PubMed] [Google Scholar]
- Krömer S (1995) Respiration during photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 46 45–70 [Google Scholar]
- Lambeth DO, Tews KN, Adkins S, Frohlich D, Milavetz I (2004) Expression of two succinyl-CoA synthetases with different nucleotide specificities in mammalian tissues. J Biol Chem 279 36621–36624 [DOI] [PubMed] [Google Scholar]
- Laporte MM, Galagan JA, Shapiro JA, Boersig MR, Shewmaker CK, Sharkey TD (1997) Sucrose-phosphate synthase activity and yield analysis of tomato plants transformed with maize sucrose-phosphate synthase. Planta 203 253–259 [DOI] [PubMed] [Google Scholar]
- Lytovchenko A, Sweetlove LJ, Pauly M, Fernie AR (2002) The influence of cytosolic phosphoglucomutase on photosynthetic carbohydrate metabolism. Planta 215 1013–1021 [DOI] [PubMed] [Google Scholar]
- Mackenzie S, McIntosh L (1999) Higher plant mitochondria. Plant Cell 11 571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcuende R, Krapp A, Hurry V, Stitt M (1998) Sucrose-feeding leads to increased rates of nitrate assimilation, increased rates of alpha-oxoglutarate synthesis, and increased synthesis of a wide spectrum of amino acids in tobacco leaves. Planta 206 394–409 [Google Scholar]
- Noguchi K, Terashima I (2006) Responses of spinach leaf mitochondria to low N availability. Plant Cell Environ 29 710–719 [DOI] [PubMed] [Google Scholar]
- Nunes-Nesi A, Carrari F, Gibon Y, Sulpice R, Lytovchenko A, Fisahn J, Graham J, Ratcliffe RG, Sweetlove LJ, Fernie AR (2007. a) Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J 50 1093–1106 [DOI] [PubMed] [Google Scholar]
- Nunes-Nesi A, Carrari F, Lytovchenko A, Smith AMO, Ehlers-Loureiro M, Ratcliffe RG, Sweetlove LJ, Fernie AR (2005) Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol 137 611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Nesi A, Sweetlove LJ, Fernie AR (2007. b) The influence of the tricarboxylic acid cycle on photosynthetic metabolism of the illuminated leaf. Physiol Plant 129 45–56 [Google Scholar]
- Obiadalla-Ali H, Fernie AR, Kossmann J, Lloyd JR (2004) Developmental analysis of carbohydrate metabolism in tomato (Lycopersicum esculentum cv. Micro-Tom) fruits. Physiol Plant 120 196–204 [DOI] [PubMed] [Google Scholar]
- Padmasree K, Padmavathi L, Raghavendra AS (2002) Essentiality of mitochondrial oxidative metabolism for photosynthesis: optimization of carbon assimilation and protection against photoinhibition. Crit Rev Biochem Mol Biol 37 71–119 [DOI] [PubMed] [Google Scholar]
- Plaxton WC, Podesta FE (2006) The functional organisation and control of plant respiration. CRC Crit Rev Plant Sci 25 159–198 [Google Scholar]
- Purnell MP, Skopelitis DS, Roubelakis-Angelakis KA, Botella JR (2005) Modulation of higher-plant NAD(H)-dependent glutamate dehydrogenase activity in transgenic tobacco via alteration of beta subunit levels. Planta 222 167–180 [DOI] [PubMed] [Google Scholar]
- Raghavendra AS, Padmasree K (2003) Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci 8 546–553 [DOI] [PubMed] [Google Scholar]
- Roessner U, Luedemann A, Brust D, Fiehn O, Linke T, Willmitzer L, Fernie AR (2001) Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13 11–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner-Tunali U, Hegeman B, Lytovchenko A, Carrari F, Bruedigam C, Granot D, Fernie AR (2003) Metabolic profiling of transgenic tomato plants overexpressing hexokinase reveals that the influence of hexose phosphorylation diminishes during fruit development. Plant Physiol 133 84–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roessner-Tunali U, Liu J, Leisse A, Balbo I, Perez-Melis A, Willmitzer L, Fernie AR (2004) Kinetics of labelling of organic and amino acids in potato tubers by gas-chromatography mass-spectrometry following incubation in 13C labelled isotopes. Plant J 39 669–679 [DOI] [PubMed] [Google Scholar]
- Rontein D, Dieuaide-Noubhani M, Dufourc EJ, Raymond P, Rolin D (2002) The metabolic architecture of plant cell: stability of central metabolism and flexibility of anabolic pathways during the growth cycle of tomato cells. J Biol Chem 277 43948–43960 [DOI] [PubMed] [Google Scholar]
- Satyanarayan V, Nair PM (1990) Metabolism, enzymology and possible roles of 4-aminobutyrate in higher plants. Phytochemistry 29 367–375 [Google Scholar]
- Schauer N, Steinhauser D, Strelkov S, Schomburg D, Allison G, Moritz T, Lundgren K, Roessner-Tunali U, Forbes MG, Willmitzer L, et al (2005) GC-MS libraries for the rapid identification of metabolites in complex biological samples. FEBS Lett 579 1332–1337 [DOI] [PubMed] [Google Scholar]
- Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S (2005) Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot 56 1481–1489 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Vanderveer PJ (1989) Stromal phosphate concentration is low during feedback limited photosynthesis. Plant Physiol 91 679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedow J, Day DA (2000) Respiration and photorespiration. In BB Buchanen, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Biologists, Rockville, MD, pp 676–728
- Smith SM (2002) Does the glyoxylate cycle have an anaplerotic function in plants? Trends Plant Sci 7 12–13 [DOI] [PubMed] [Google Scholar]
- Smyth GK (2004) Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3 3–26 [DOI] [PubMed] [Google Scholar]
- Smyth GK, Speed TP (2003) Normalization of cDNA microarray data. Methods 31 265–273 [DOI] [PubMed] [Google Scholar]
- Stitt M (1997) The flux of carbon between the chloroplast and the cytoplasm. In DT Dennis, DH Turpin, DD Lefebre, DN Layzelle, eds, Plant Metabolism. Longman, Harlow, UK, pp 382–400
- Studart-Guimarães S, Gibon Y, Frankel N, Wood CC, Zanor MI, Fernie AR, Carrari F (2005) Identification and characterisation of the α and β-subunits of succinyl CoA of tomato. Plant Mol Biol 59 781–791 [DOI] [PubMed] [Google Scholar]
- Sweetlove LJ, Heazlewood JL, Herald V, Hotzapffel R, Day DA, Leaver CJ, Millar AH (2002) The impact of oxidative stress on Arabidopsis mitochondria. Plant J 32 891–904 [DOI] [PubMed] [Google Scholar]
- Tauberger E, Fernie AR, Emmermann M, Renz A, Kossman J, Willmitzer L, Trethewey RN (2000) Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose 6-phosphate. Plant J 23 43–53 [DOI] [PubMed] [Google Scholar]
- Tcherkez G, Cornic G, Bligny R, Gout E, Ghashghaie J (2005) In vivo respiratory metabolism of illuminated leaves. Plant Physiol 138 1596–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieman D, Taylor M, Schauer N, Fernie AR, Hanson AD, Klee HJ (2006) Tomato aromatic amino acid decarboxylases participate in the synthesis of the flavor volatiles 2-phenylethanol and 2-phenylacetaldehyde. Proc Natl Acad Sci USA 103 8287–8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Mendez A, Miernyk JA, Randall DD (2003) Regulation of pyruvate dehydrogenase complex activity in plant cells. Eur J Biochem 270 1043–1049 [DOI] [PubMed] [Google Scholar]
- Turano FJ, Fang TK (1998) Characterization of two glutamate decarboxylase cDNA clones from Arabidopsis. Plant Physiol 117 1411–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanczyk-Wochniak E, Usadel B, Thimm O, Nunes-Nesi A, Carrari F, Davy M, Bläsing O, Kowalczyk M, Weicht D, Polinceusz A, et al (2006) Conversion of MapMan to allow the analysis of transcript data from Solanaceous species: effects of genetic and environmental alterations in energy metabolism in the leaf. Plant Mol Biol 60 773–792 [DOI] [PubMed] [Google Scholar]
- Villar R, Held AA, Merino J (1994) Comparison of methods to estimate dark respiration in light in leaves of two woody species. Plant Physiol 105 167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP (2002) Normalisation for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiher CA, Randall DD (1990) Identification and characterization of mitochondrial acetyl-coenzyme A hydrolase from Pisum sativum L. seedlings. Plant Physiol 94 20–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.