Abstract
The plastid terminal oxidase (PTOX) is a plastoquinol oxidase whose absence in tomato (Solanum lycopersicum) results in the ghost (gh) phenotype characterized by variegated leaves (with green and bleached sectors) and by carotenoid-deficient ripe fruit. We show that PTOX deficiency leads to photobleaching in cotyledons exposed to high light primarily as a consequence of reduced ability to synthesize carotenoids in the gh mutant, which is consistent with the known role of PTOX as a phytoene desaturase cofactor. In contrast, when entirely green adult leaves from gh were produced and submitted to photobleaching high light conditions, no evidence for a deficiency in carotenoid biosynthesis was obtained. Rather, consistent evidence indicates that the absence of PTOX renders the tomato leaf photosynthetic apparatus more sensitive to light via a disturbance of the plastoquinone redox status. Although gh fruit are normally bleached (most likely as a consequence of a deficiency in carotenoid biosynthesis at an early developmental stage), green adult fruit could be obtained and submitted to photobleaching high light conditions. Again, our data suggest a role of PTOX in the regulation of photosynthetic electron transport in adult green fruit, rather than a role principally devoted to carotenoid biosynthesis. In contrast, ripening fruit are primarily dependent on PTOX and on plastid integrity for carotenoid desaturation. In summary, our data show a dual role for PTOX. Its activity is necessary for efficient carotenoid desaturation in some organs at some developmental stages, but not all, suggesting the existence of a PTOX-independent pathway for plastoquinol reoxidation in association with phytoene desaturase. As a second role, PTOX is implicated in a chlororespiratory mechanism in green tissues.
The plastid terminal oxidase (PTOX) is a nucleus-encoded plastid-located plastoquinone (PQ)-O2 oxidoreductase (plastoquinol oxidase) whose absence gives rise to the immutans phenotype in Arabidopsis (Arabidopsis thaliana) and in the ghost (gh) phenotype in tomato (Solanum lycopersicum; Carol et al., 1999; Wu et al., 1999; Josse et al., 2000; Carol and Kuntz, 2001; Rodermel, 2001; Aluru et al., 2006). These phenotypes are characterized by variegated leaves consisting of green and bleached sectors and, in addition in tomato, by a yellow-orangey ripe fruit. The latter is characterized by reduced carotenoid content (Barr et al., 2004). In addition, bleached leaf sectors accumulate the carotenoid precursor phytoene, indicating that PTOX functions as a cofactor for the carotenoid dehydrogenases, namely, phytoene desaturase (PDS) and, most likely, ζ-carotene desaturase (ZDS). These conclusions are consistent with the known involvement of quinone as a cofactor for PDS (Mayer et al., 1990; Norris et al., 1995). PDS and ZDS sequentially catalyze the conversion of the colorless phytoene to lycopene (the main pigment in red tomato fruit), which, in turn, is converted to various photoprotective carotenoids. Thus, the phenotype observed in the absence of PTOX can be explained by reduced ability to synthesize carotenoids leading to photobleaching of green tissues.
PTOX shares sequence similarity with the alternative oxidase found in mitochondria of a number of species (Berthold and Stenmark, 2003). In the presence of plastoquinol, PTOX is able to divert the electron flow to O2 in Escherichia coli membranes when the cytochrome path is inhibited by cyanide (Josse et al., 2003). PTOX behaves as an intrinsic membrane protein in the thylakoid lamellae (Lennon et al., 2003). Thus, PTOX possesses all the characteristics expected for a terminal oxidase involved in chlororespiration (Peltier and Cournac, 2002; Kuntz, 2004). Such a role for PTOX is not incompatible with a role in carotenoid desaturation because the latter process is dependent on a redox pathway (Morstadt et al., 2002). Evidence was obtained that seems to confirm a chlororespiratory role for PTOX in algae (Cournac et al., 2000, 2002) and in tobacco (Nicotiana tabacum) overexpressing a PTOX gene (Joet et al., 2002). Furthermore, data indicate induction of the PTOX gene and/or an accumulation of the protein under stress conditions in tobacco lack-ing both ascorbate peroxidase and catalase (Rizhsky et al., 2002) or deficient in PSII (Baena-Gonzalez et al., 2003). Strikingly, the high mountain plant Ranunculus glacialis, which has been shown to contain alternative sinks to dissipate photosynthetic electrons, was also found to contain high levels of PTOX (Streb et al., 2005). In contrast, using Arabidopsis lines either deficient in or overexpressing PTOX, Rosso et al. (2006) concluded that this enzyme does not act as a stress-induced safety valve involved in the protection of the photosynthetic apparatus. These authors stressed that high PTOX levels may simply be correlative and not indicative of an energy-dissipating role for this enzyme.
In this article, various tomato organs were used under experimental conditions leading to photobleaching to examine in the absence of PTOX whether this phenomenon is linked to abnormal carotenoid content or to abnormal photosynthetic electron transport. A potential influence of PTOX on the redox status of the PQ pool was also examined.
RESULTS
Effect of PTOX Deficiency in gh Tomato Cotyledons
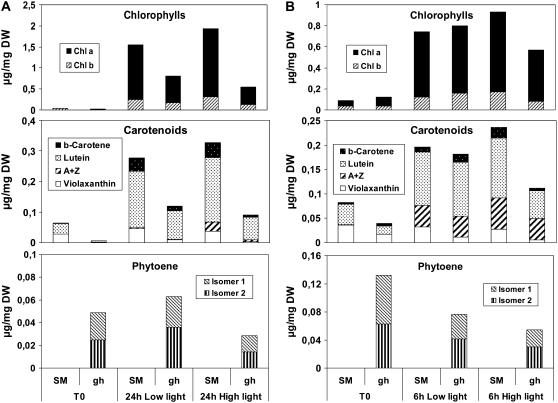
‘San Marzano’ (SM) control and gh seedlings were grown in darkness for 4 d after seed imbibition (time point T0). These white seedlings were then transferred to either low or high light conditions for 24 h. As shown in Figure 1A, at T0, cotyledons from both SM and gh were almost devoid of chlorophyll and carotenoid (mainly lutein and violaxanthin) levels were low, but slightly higher in SM than in gh. Within the low light period, both cotyledon types accumulated substantial amounts of chlorophyll a/b and carotenoids (including β-carotene and traces of other compounds). All pigment levels in gh were reduced to about one-half the amount found in SM.
Figure 1.
Pigment content of cotyledons from SM and gh tomato lines. Seedlings were grown in darkness for 4 and 6 d after seed imbibition to obtain white (A) and pale yellow (B) cotyledons (T0 time point), respectively. Seedlings were then transferred to either low light (20 μmol m−2 s−1 PAR) or to a higher light intensity (200 μmol m−2 s−1 PAR). Samples were analyzed after 24 h of light for white cotyledons and after 6 h of light for yellow cotyledons. Eight cotyledons were pooled for a given measurement. Each measurement was repeated three times with a different pool of cotyledons. ses of the mean values were below 12% (data not shown). Whether changes are statistically significant is discussed in the “Results” section. PAR, Photosynthetically active radiation.
Under high light conditions (Fig. 1A), a slightly higher level (not statistically significant) of pigments was observed in SM (including the xanthophyll cycle pigments antheraxanthin and zeaxanthin; A + Z) when compared to low light, whereas this increase was not observed in gh (chlorophyll content is even reduced in gh under high light compared to low light).
The carotenoid precursor phytoene was not detected in SM under any condition, but was present (as two isomers) in gh at T0 (Fig. 1A). A rise in phytoene level was observed in gh under low light but was not statistically significant. Accumulation of phytoene is most likely the result of two parameters: (1) the activity of the whole biosynthetic pathway; and (2) the limiting rate of PDS activity in the absence of PTOX. However, phytoene levels in gh were lower under high light than under low light (statistically significant; P < 0.01), despite the fact that the carotenoid biosynthetic pathway seems active under high light (as deduced from the high levels of colored carotenoid in SM). Because lower levels of colored carotenoids and chlorophyll are also found in gh under high light versus low light, the simplest explanation for these observations is increased photodestruction of all these compounds.
Seedlings grown for a longer period (6 d) in darkness (Fig. 1B, time point T0) showed a faint yellow color. Significantly higher carotenoid levels were present in SM than in gh. The same low or high light conditions were then applied during 6 h. Low light led to an increase in chlorophyll and carotenoid levels, with no significant difference between SM and gh. Compared to low light, high light triggered again a slight increase (not statistically significant) in pigment levels in SM. In gh, significantly lower pigment levels were observed under high light compared to SM. In addition, its phytoene levels under high light were lower than at T0 (statistically significant; P < 0.01), which can be explained in part by photodestruction. The fact that lower levels of phytoene were also observed in gh under low light at 6 h than at T0 (statistically significant; P < 0.05) is more surprising and may suggest that carotenoid biosynthesis is less active (because almost-normal carotenoid content was reached; see Fig. 1B).
Taken together, these data suggest that PTOX deficiency leads to photobleaching in cotyledons exposed to high light primarily as a consequence of reduced ability to synthesize colored carotenoids in the mutant, which is fully consistent with the known role of PTOX as a PDS cofactor.
Effect of PTOX Deficiency in Stressed Tomato Leaves
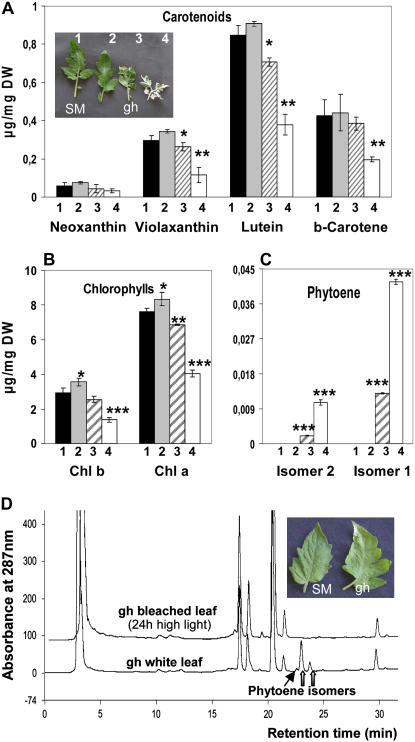
When grown under standard growth chamber conditions, SM and gh plants produce green and variegated leaves, respectively. The color aspect of gh leaves is decided at a very early leaf developmental stage. Depending on light intensities at an early seedling developmental stage (see “Materials and Methods”), gh leaves, which are mainly green, mainly white, or variegated, could be obtained (Fig. 2A, inset). Mainly white leaves (of adult size) showed clear reduction in chlorophyll a/b and carotenoids (neoxanthin, violaxanthin, lutein, and β-carotene) when compared to green leaves, whereas this reduction was not as pronounced in variegated leaves (Fig. 2, A and B), as expected. A slight (but significant) trend for higher chlorophyll content was observed in fully green leaves from gh versus SM. It is accompanied by a slight increase in carotenoid content (although it does not appear to be statistically significant for all carotenoids). Such an increase was also reported in green sectors of variegated leaves from the immutans mutant (Baerr et al., 2005) and is accompanied by an increase in the rate of carbon assimilation (Aluru et al., 2007). These observations were interpreted as the necessity for green sectors to compensate for the limited photosynthetic and metabolic activities of adjacent white sectors. The latter data cannot be compared directly with ours, which were obtained with entirely green tomato leaves. In the gh mutant, phytoene was detected in white, but not in green, leaves (Fig. 2C). This latter observation suggests that, despite the absence of PTOX, PDS activity is not limiting carotenoid biosynthesis in green leaves from gh.
Figure 2.
Bleaching of SM and gh adult leaves. A, Carotenoid content in SM (1) and different types of gh leaves (2–4) as shown in the inserted photograph: 2, green gh; 3, variegated; 4, mainly white. For details, see “Materials and Methods.” Statistically significant differences are indicated compared to SM leaves: *, P < 0.05; **, P < 0.01; ***, P < 0.001. B, Chlorophyll content in the four leaf types. C, Phytoene content in the four leaf types. D, HPLC elution profiles of pigments (A287). The top trace shows an extract from sectors bleaching after 24 h on green gh leaves submitted to high light conditions (1,000 μmol m−2 s−1), as shown in the inset photograph. The bottom trace shows an extract from mainly white gh leaves, as shown in A. Thick arrows point to the two major phytoene isomers that are quantified in C; the thin arrow points to a minor phytoene isomer.
Detached fully green leaves (with no visible deficit in pigments, as shown in Fig. 2A, sample 2) were then exposed to high light (1,000 μmol m−2 s−1) for long incubation periods. Leaves from both SM and gh showed early bleaching symptoms in some sectors after 20 to 24 h. This trend appears earlier and is stronger in gh (Fig. 2D, inset) and extends to larger leaf sectors with longer incubation time. Pigments were extracted separately from green and bleaching sectors. As expected, these leaves showed accumulation of the xanthophyll cycle pigments A + Z (data not shown) and bleaching sectors contained reduced chlorophyll and carotenoid content. This reduction was more pronounced in gh than in SM. However, an unexpected observation was that phytoene was not detected in any tissues in contrast to what was observed in cotyledons (Fig. 1) or mainly white or variegated leaves (Fig. 2C). Figure 2D compares typical HPLC elution profiles from a bleached sector after 24 h of high light and from a mainly white leaf whose aspect is determined early during leaf development (as shown in Fig. 2A).
To shed more light on changes occurring in chloroplasts under these experimental conditions, a series of experiments were performed at earlier time points (before bleaching was visible). First, pigment analyses (Table I) were performed using plants grown under low light (60 μmol m−2 s−1) conditions (T0), and then green leaves were detached and incubated either under low light (60 μmol m−2 s−1) or high light (1,000 μmol m−2 s−1) for 6 h (T6h) or 16 h (T16h). At T6h under low light, only marginal changes in pigment content were observed with respect to T0 in both SM and gh leaves, with the exception of an increase in the levels of xanthophyll A + Z for both SM and gh. This increase was unexpected because this light intensity is identical to that experienced by the plants before these experiments and is most likely due to the detachment of leaves. At T6h under high light conditions, higher A + Z levels were observed, as expected, and, interestingly, with a stronger increase in SM versus gh. No other significant change in pigment content was observed at T6h under high light, with the exception of a decrease in violaxanthin, the precursor of A + Z during activation of the xanthophyll cycle. Compared to T6h, at T16h under high light conditions, the most notable change was an increase in A + Z levels in gh, which equaled the levels in SM (data not shown). No phytoene was detected in any sample.
Table I.
Pigment content of SM and gh green leaves
Plants were grown under low light (60 μmol m−2 s−1). At T0, leaves were detached and incubated 6 h under either low (60 μmol m−2 s−1) or high (1,000 μmol m−2 s−1) light. Values (given as μg mg−1 dry weight) are the mean of four measurements. sd is given in brackets. Statistically significant differences under high light compared to low light are indicated: **, P < 0.01; ***, P < 0.001. V, Violaxanthin; Chl, chlorophyll; n.d., not detected.
| T0
|
Low Light
|
High Light
|
||||
|---|---|---|---|---|---|---|
| SM | gh | SM | gh | SM | gh | |
| Neoxanthin | 0.13 (0.02) | 0.13 (0.02) | 0.12 (0.02) | 0.17 (0.03) | 0.09 (0.01) | 0.14 (0.02) |
| Violaxanthin | 0.53 (0.03) | 0.66 (0.13) | 0.56 (0.05) | 0.61 (0.06) | 0.35 (0.03)*** | 0.55 (0.02) |
| Z + A | n.d. | n.d. | 0.17 (0.01) | 0.16 (0.02) | 0.39 (0.08)** | 0.24 (0.03)** |
| Lutein | 1.60 (0.10) | 1.94 (0.17) | 1.91 (0.10) | 2.21 (0.17) | 1.72 (0.22) | 2.07 (0.11) |
| β-Carotene | 0.74 (0.04) | 0.86 (0.05) | 0.72 (0.03) | 0.93 (0.08) | 0.68 (0.04) | 0.85 (0.03) |
| Total carotenoids | 2.90 (0.23) | 3.61 (0.35) | 3.49 (0.17) | 4.09 (0.29) | 3.23 (0.34) | 3.85 (0.13) |
| (Z + A)/(Z + A + V) | 0 | 0 | 23.58 (2.49) | 21.14 (0.80) | 53.16 (3.61)*** | 30.03 (1.71)*** |
| Chl b | 6.52 (0.71) | 7.55 (0.30) | 6.94 (0.44) | 8.51 (0.72) | 6.65 (0.74) | 8.14 (0.50) |
| Chl a | 13.86 (0.70) | 14.67 (0.11) | 13.76 (0.73) | 15.86 (1.71) | 12.84 (0.82) | 15.47 (0.77) |
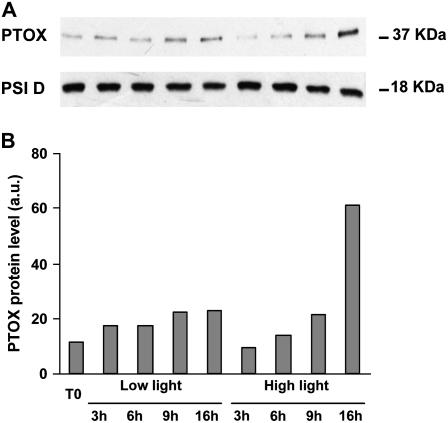
Proteins were extracted from leaves at T0 and over a time course up to T16h, under either low or high light. Immunodetection did not reveal any signal for PTOX in gh (data not shown), as expected, but a 37-kD band was visible in SM (Fig. 3A). High light conditions led to a gradual rise in PTOX level in SM (which is particularly evident at T16h). Under low light, only a slight increase with time is apparent (most likely due to leaf detachment). The PSI-D polypeptide level was not found to change during this time course and can therefore be considered an internal control to ensure equal gel loading. Figure 3B shows the densitometric scanning of the PTOX band from Figure 3A after normalization using the PSI-D band as a standard.
Figure 3.
PTOX protein levels in SM leaves. A, Immunodetection of PTOX and PSI-D (a PSI protein used as a control for equal gel loading). Plants were grown under low light (60 μmol m−2 s−1) and at T0 detached leaves were incubated during a 16-h time course under low (60 μmol m−2 s−1) or high (1,000 μmol m−2 s−1) light. Leaf protein samples were separated by gel electrophoresis and immunodetected as described in “Materials and Methods.” B, Densitometric scanning values of the PTOX band in A after normalization using the PSI-D values as a standard.
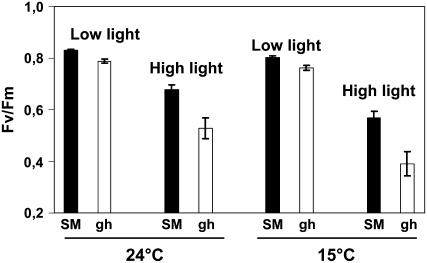
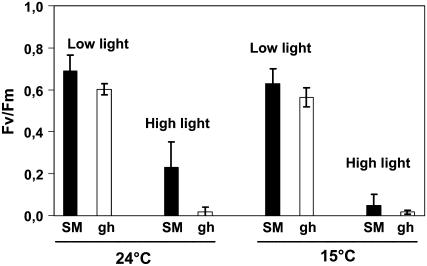
The maximal quantum yield of PSII was estimated from the Fv/Fm ratio. At T6h, under high light (Fig. 4), the Fv/Fm ratio was found to decrease, as expected, under these photoinhibitory conditions in both SM and gh leaves (but more in gh). The same observations were made when the temperature was shifted to 15°C instead of 24°C: Again, gh leaves showed a lower Fv/Fm ratio than SM leaves under high light (Fig. 4). At T16h, the further decrease in Fv/Fm was also stronger in gh than in SM leaves (data not shown).
Figure 4.
Photoinhibition of SM and gh fully green leaves. The maximal quantum yield of PSII is expressed as the Fv/Fm ratio. SM and gh leaves were incubated at 24°C or 15°C for 6 h under either low (60 μmol m−2 s−1) or high light (1,000 μmol m−2 s−1) and then kept for 30 min in the dark before chlorophyll fluorescence measurement. Mean values of six measurements (i.e. six different leaves) are shown.
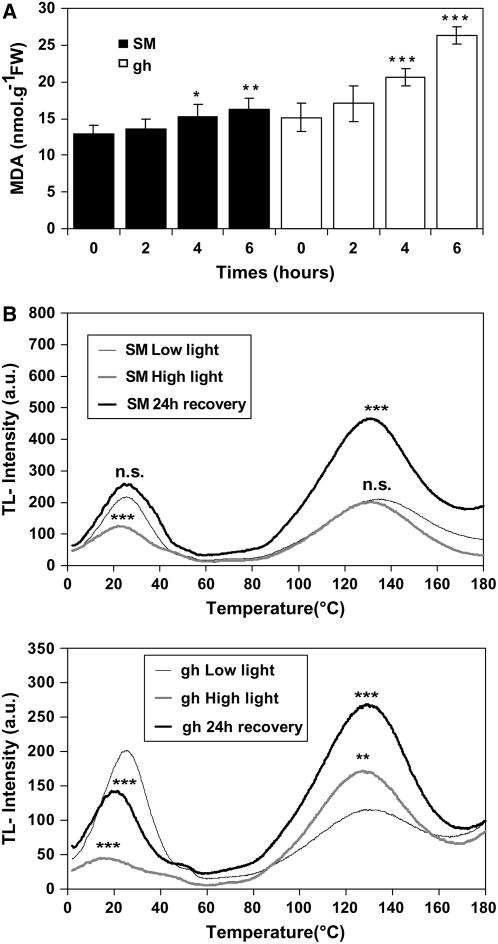
Lipid peroxidation was estimated using the malondialdehyde (MDA) method over a 6-h time course after transfer of leaves to high light. These data (Fig. 5A) suggest an increase in lipid peroxidation in both gh and SM, but greater in gh.
Figure 5.
Damage to PSII and oxidative stress in SM and gh fully green leaves. A, Lipid peroxidation in SM (black columns) and gh (white columns) leaves. Plants were exposed to high light stress (1,000 μmol m−2 s−1) for 6 h. Lipid peroxidation was estimated using the MDA method. Data are mean values of a minimum of five experiments. Statistically significant differences are indicated compared to T0: *, P < 0.05; **, P < 0.01; ***, P < 0.001. B, Thermoluminescence (TL) measurements in SM (top) and gh (bottom) leaf discs from control leaves (low light) or leaves treated for 6 h under high light (1,000 μmol m−2 s−1) or leaves treated under the same high light conditions but allowed to recover for 24 h under the light conditions used for the control leaves. Samples were then treated as described in “Materials and Methods,” cooled to 2°C, and flash illuminated to induce charge separation. The samples were then heated to induce charge recombination of PSII radical pairs (2°C–60°C; the B band represents the thermoinduced light emission from the recombination of S2QB− radical pairs after one light flash) and chemiluminescent reactions (70°C–180°C) representing the chemical stress status (HTL2 band). n.s., Not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared to control). TL measurements were performed under N2 atmosphere to avoid autooxidation at high temperatures. There was no influence on the B band by N2 flushing compared to air (data not shown).
Thermoluminescence measurements were performed on attached green leaves directly after 6 h of high light stress and after an additional 24-h recovery period under low light conditions. As controls, leaves maintained for 6 h under low light were used. Thermoluminescence glow curves (Ducruet, 2003) show a B band peaking at 25°C and a high temperature band peaking at 130°C to 140°C (HTL2; representing the oxidative stress of leaves). As shown in Figure 5B (left), the B band is shifted after light stress to a position corresponding to a lower temperature with a 43% decrease in light-stressed SM leaves, indicating photoinhibition and damage to PSII. After 24 h of recovery, the B band resumes its initial amplitude and peak position. In light-stressed gh leaves, a decrease of 78% is observed for the B band, also accompanied by a shift to lower temperature, but after 24 h of recovery the B band regenerated only to 70% compared to control leaves and the peak shift was still visible (Fig. 5B, right).
No increase in the HTL2 band was visible after 6 h of light stress in SM leaves (Fig. 5B). In contrast, the HTL2 band was 150% higher in light-stressed gh leaves compared to control leaves (significant at P < 0.01). After 24 h of recovery, a further increase was observed in gh, but an increase was also observed in SM at that time point in comparison to 6 h of light stress (see “Discussion”).
It therefore appears that the absence of PTOX renders the tomato leaf photosynthetic apparatus more sensitive to extreme conditions (provided here by a transfer to excessive light). This increased sensitivity is observed in the absence of any detectable accumulation of phytoene or decrease in carotenoid content.
Effects of PTOX on the Redox State of the PQ Pool
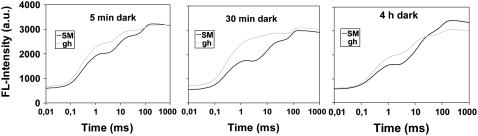
Fast fluorescence kinetics (OJIP, also called OI1I2P; Schreiber, 2004; Strasser et al., 2004) were performed after 5 min, 30 min, or 4 h of dark adaptation and recorded during a light pulse of 1 s (Fig. 6). The O level (0.01 ms), also reflecting F0, is increased significantly in the gh mutant versus SM, after 5 or 30 min of dark adaptation, but not after a longer dark adaptation of 4 h. In addition, for all dark adaptation times, the fluorescence at 2 ms (J level) is strongly increased during the light pulse in gh compared to SM. An increased J level is an excellent indicator of a more reduced PQ pool and a more pronounced primary electron acceptor of PSII (QA−) accumulation under light excitation (Haldimann and Strasser, 1999). Whereas the J level decreases continuously with increasing dark adaptation time in SM, it increases in gh between 5 to 30 min of dark adaptation and strongly decreases again after 4 h. In parallel to the J level, the I level is also significantly increased after 5 and 30 min of dark adaptation in gh versus SM. Strikingly, after 4 h of dark adaptation, the P level in gh remains at a similar level compared to that after 5 and 30 min, whereas a significant increase is observed in SM (Fig. 6). These observations are discussed below.
Figure 6.
Fast fluorescence kinetics (OJIP) for fully green leaves from gh and SM plants pulse illuminated after different times of dark adaptation. The O level at 0.01 ms represents a fluorescence value close to F0. The fluorescence rise from the O to the J level (2 ms) indicates the reduction of QA. The fluorescence rise from the J to the I level (30 ms) indicates the reduction of QB and partially of the PQ pool. The rise from the I to the P level (200–300 ms) indicates the electron flow through PSI (PQ pool fully reduced at P level).
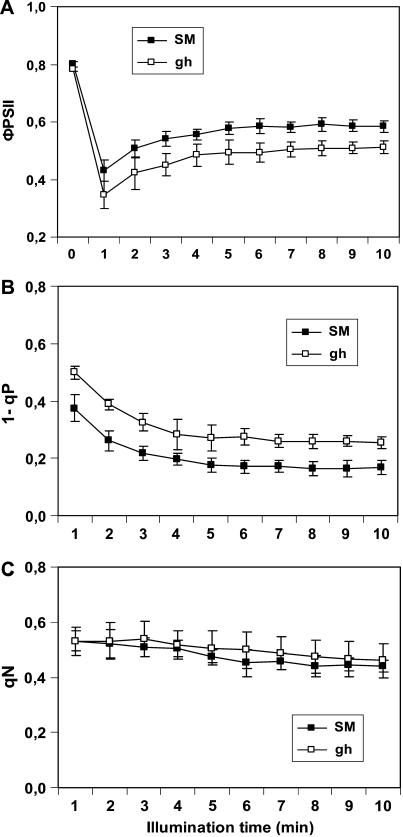
In another set of experiments, induction kinetics of chlorophyll fluorescence were performed over a longer time range after 30-min incubation of leaves in the dark followed by exposure to actinic light (200 μmol m−2 s−1) for 10 min (Fig. 7). In this time window, the Kautsky kinetic describes the drop of the fluorescence from the Fm level (P level) to the steady-state level (Fs) and is characterized by the complex superposition of processes, including the light-induced activation of PSI, the Calvin cycle, alternative electron transfer (e.g. Mehler reaction), and buildup of ΔpH-dependent nonphotochemical quenching (qN). Consistent results were obtained showing that the effective quantum yield of PSII (ΦPSII) is lower in gh versus SM and that the relative reduction state of QA (estimated as 1 − photochemical quenching parameter [qP]) is higher in gh versus SM. 1 − qP also reflects the overall reduction state of the electron transport chain and therefore the quinone pool. When this parameter was expressed as 1 − qL (Kramer et al., 2004), the same result was obtained (data not shown). In contrast, qN was not found to differ significantly between both leaf types.
Figure 7.
Induction kinetics for the chlorophyll fluorescence parameters ΦPSII (A), 1 − qP (B), and qN (C). SM and green gh leaves were dark adapted 30 min and then illuminated with actinic light of 200 μmol m−2 s−1 for 10 min. Mean values (n = 6) were used.
Effect of PTOX Deficiency in Tomato Fruit
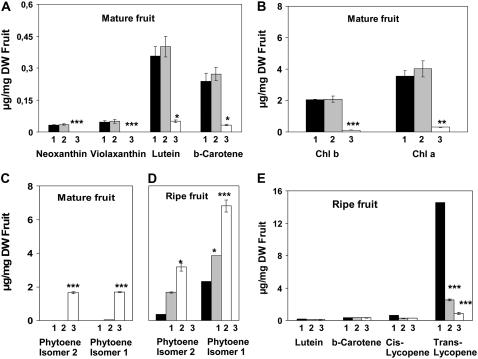
Young tomato fruit bleach in the gh line under standard greenhouse conditions or show a variegated green/white color under shaded conditions (Barr et al., 2004). Using controlled growth chamber conditions, we produced white fruit (termed “gh white” in the subsequent experiments) as well as fully green fruit (termed “gh green”) for gh. The latter were obtained by keeping fruit under low light. To avoid plant etiolation under these conditions, only the fruit were kept under low light, which was achieved by wrapping the fruit in a cloth as soon as they started to develop. During ripening, gh white fruit become yellow (a color predominantly due to flavonoids), whereas gh green fruit become orangey. Under both light conditions, SM fruit are green before ripening and become fully red during ripening. As shown in Figure 8, A and B, SM and gh green fruit at the mature green stage (adult size) show only nonsignificant variation in pigment content. As expected, these pigment levels are severely reduced in gh white fruit. Whereas phytoene is present in gh white fruit, only trace amounts of this pigment are found in gh green fruit (Fig. 8C). During ripening, phytoene is present in all fruit types, including SM, but at the highest level in fruit that ripened from the gh white type (Fig. 8D). During ripening, the level of the newly accumulating pigments (all-trans lycopene and low amounts of its cis-isomers) is dramatically affected in the gh white fruit type and reduced to about 17% of their level in SM in the gh green fruit type (Fig. 8E). The latter observation contrasts with the normal carotenoid content of gh fruit at the green stage (Fig. 8, A and B).
Figure 8.
Pigment content of mature and ripe SM and gh fruits. The colored carotenoid (A), chlorophyll (B), and phytoene (C) contents were analyzed in mature green (adult size) fruit. The phytoene (D) and colored carotenoid (E) contents were analyzed in ripe fruit (10 d after the breaker stage). Samples were taken from SM green and red fruit (histogram columns indicated by 1), gh green and their derived orangey fruit (2), and gh bleached and their derived yellow fruit (3). Statistically significant differences are indicated compared to SM fruit: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Explants from mature green fruit were incubated under 1,000 μmol m−2 s−1 for up to 48 h at 24°C. Bleaching symptoms were observed after approximately 24 h in both SM and gh explants, but were stronger in gh (data not shown). Analysis of their carotenoid content at various time points (6, 18, 30, and 42 h) revealed no phytoene (data not shown), indicating that the absence of PTOX does not limit carotenoid biosynthesis in these gh explants. Estimation of the maximal quantum yield of PSII after 6 h of incubation (Fig. 9) showed that gh fruit are more sensitive to photoinhibition than SM fruit under this high light condition at 24°C. At 15°C, both fruit types are fully photoinhibited under these light conditions.
Figure 9.
Photoinhibition of SM and gh green fruit. The maximal quantum yield of PSII is expressed as the Fv/Fm ratio. Fruit pericarp of SM and gh were incubated at 24°C or 15°C for 6 h under either low (60 μmol m−2 s−1) or high light (1,000 μmol m−2 s−1) and then kept 30 min in the dark before chlorophyll fluorescence measurement. Mean values are shown (n = 3).
DISCUSSION
Fully Greened Tissues Can Desaturate Carotenoids without PTOX
Our data show that, in the absence of PTOX, the lower capacity to desaturate carotenoids is primarily responsible for the phenotype in cotyledons of dark-grown tomato gh seedlings and when these seedlings are transferred to light. These tissues have reduced colored carotenoid levels and accumulate their precursor phytoene. Considering the well-known photoprotective role of carotenoids, this pigment deficiency will lead to bleaching in tissues where assembly of the photosynthetic apparatus is initiated upon transfer to light. Although not studied here, it can be speculated that such a pigment deficit is also (at least partially) responsible for the variegated leaf phenotype that is initiated at an early chloroplast developmental stage (when optimal carotenoid biosynthesis is likely to be important). Consistent with this view, bleached sectors in variegated leaves contain phytoene. However, an additional role for PTOX in photosynthetic electron transport (see below) during the buildup of the photosynthetic apparatus cannot be excluded.
In contrast, when normal chloroplast biogenesis was not impaired (i.e. when excessive light was avoided at an early stage), different observations were made in gh green adult leaves: We were unable to detect phytoene (nor a deficit in carotenoid content) even under such excessive light ultimately leading to bleaching. This is highly surprising because the carotenoid biosynthetic pathway is likely to be very active under such conditions to compensate for carotenoid turnover and photodestruction (Simkin et al., 2003). This contrasts with the accumulation of phytoene in adult tomato leaves when PDS is inhibited by the bleaching herbicide norflurazon (Simkin et al., 2003). Thus, the absence of phytoene in leaves bleaching under strong light is not due to its photodegradation, but rather to the fact that the carotenoid biosynthetic pathway is not limited by the lack of PTOX. In other words, PTOX is dispensable as a carotenoid desaturase cofactor in tissues with fully functional photosynthetic apparatus. Because optimal carotenoid desaturation is obtained in the range of the midpoint potential of the PQ/plastoquinol redox couple (Nievelstein et al., 1995), this redox control may be obtained for PQs in the vicinity of the desaturases by a mechanism provided by photosynthetic membranes. Chemical reoxidation of plastoquinol (Khorobrykh and Ivanov, 2002) may also participate.
gh Leaves Are Affected in Photosynthetic Electron Transport under Light Constraints
Our data consistently suggest that PTOX plays a specific role in the protection of the fully assembled photosynthetic apparatus under light constraints. We used adult leaves subjected to severe light conditions, which led to photobleaching after prolonged incubation (24 h) in both gh and control SM lines. Such prolonged light exposure and provoked photobleaching are not normal phenomena in nature but were used here to ensure that the applied conditions were creating extreme constraints. We then used earlier time points compatible with a normal photoperiod for detailed investigation. These indicated higher PSII photoinhibition in gh versus SM leaves after 6 h (Fig. 4) and 16 h of light stress (data not shown), as well as higher damage level of PSII in gh than in SM and only partial recovery in gh (thermoluminescence B band; Fig. 5B). High-temperature thermoluminescence measurements (HTL2 band; Vavilin and Ducruet 1998; Vavilin et al., 1998) also point to reduced potential to prevent oxidative damage in gh versus SM leaves after 6 h of light stress. Estimation of lipid peroxidation by MDA level measurements (Fig. 5A) is consistent with the latter observations. The observed delayed oxidative damages (occurring poststress; HTL2 band) may appear surprising, but progress of lipid peroxidation (MDA levels) after stress relief has also been observed under low-temperature photoinhibition (Hegedüs et al., 2004) and drought stress (Olsson et al., 1996). What is clear from our observation on this poststress phenomenon (Fig. 5B) is that it affects both the gh and control lines and therefore seems independent of PTOX.
In vitro, PTOX is a plastoquinol oxidase (Josse et al., 2000, 2003). Consistent in vivo data were obtained here suggesting that PTOX affects the redox status of PQ in leaves. Under 10 min of actinic illumination, induction kinetics of chlorophyll fluorescence show higher 1 − qP values (lower photochemical quenching) in gh consistent with reduced operational quantum yield of PSII. This is indicative of the more reduced status of PSII acceptors (QA) in gh leaves compared to control SM leaves (Fig. 7), which most likely indicates that the PQ pool is more reduced. Unlike 1 − qP values, the qN parameter was not significantly different in gh and control leaves, suggesting that the extent of qN reached similar levels. The higher reduction state of the electron transport chain under steady-state illumination in gh versus SM leaves suggests that the lack of PTOX leads to less efficient utilization of photosynthetic and alternative electron sinks (Ort and Baker, 2002).
In addition, fast fluorescence induction kinetics (Fig. 6) show that PTOX regulates the reduction state of the PQ pool in the dark. The increased amplitude of the J level indicates that a more strongly reduced PQ pool is present in gh, the extent of which depends on the duration of the dark incubation prior to pulse illumination. The simplest way to interpret these results is to assume that, in the absence of PTOX, the NAD(P)H dehydrogenase (NDH) complex strongly reduces PQs in the first 30 min in the dark. The decrease in the J level after 4 h in gh suggests that, over a long period of time, reoxidation of reduced PQs does occur in gh, either by pure chemical oxidation with molecular oxygen (Khorobrykh and Ivanov, 2002) or by an alternative mechanism. Inactivation of the NDH complex after long dark adaptation might also contribute to PQH2 reoxidation. The J-I phase describes the reduction of the PQ pool (Schreiber et al., 1989). Because the PQ pool is more reduced in gh than in SM after dark adaptation, the amplitude of this phase is significantly lower in gh than in SM (Fig. 6). Recent results indicate that the I-P phase in higher plants is governed by electron transfer beyond the cytochrome b6/f complex and a transient block at the acceptor side of PSI due to inactive ferredoxin-NADP+-oxidoreductase (Schansker et al., 2005). The increase of the P level after 4 h of dark adaptation in SM indicates the release of yet-unexplained quenching of fluorescence. These observations need further investigation before they can be unambiguously interpreted.
Role of PTOX in Photosynthesis: Safety Valve or Regulatory Adjustment?
Correlative evidence for PTOX as a bona fide safety valve (i.e. allowing the transfer of excess electrons to O2) is essentially provided for higher plants by gene expression/protein accumulation data (Rizhsky et al., 2002; Baena-Gonzalez et al., 2003; Quiles, 2006). However, PTOX remains a minor constituent of photosynthetic membranes (Lennon et al., 2003) with the notable exception of alpine plants such as Geum montanum and R. glacialis (Streb et al., 2005), which may have selected natural overexpressers as an additional protection mechanism. We could show here that PTOX protein levels do increase unambiguously (in wild-type control plants) under strong light conditions, but only after a long period (especially after 16 h; Fig. 3). In contrast, after 6 h of illumination, no increase in PTOX level was observed, although we could demonstrate clear involvement of PTOX in photoprotection at that time point. Therefore, it seems difficult to propose a role for PTOX merely as a safety valve allowing massive photosynthetic electron flow toward O2.
Acclimation of plants to environment and its constantly changing conditions implies a highly sophisticated network of regulation, which seems to involve chlororespiration (Rumeau et al., 2007). Quiles (2006) has shown that in vitro chlororespiratory activity (NADH dehydrogenase and a PTOX-like activity) is stimulated when thylakoids are isolated from heat and high light-treated oat (Avena sativa) plants. PTOX activity, rather than directly compensating for an over-reduced PQ status, could be viewed as a fine-tuning device. Together with the NDH complexes and the ferredoxin/PGR5-dependent PQ reductase, PTOX might provide photosynthetic regulatory mechanisms (Rumeau et al., 2007; Shikanai, 2007). A regulatory function for PTOX is suggested by our present data. After 6 h of photostress (but not after 16 h when PTOX protein levels are at their highest in SM), lower levels of de-epoxidated xanthophyll cycle pigments (A + Z; Table I) were observed in gh versus SM. Furthermore, the more strongly reduced electron transport chains when dark-to-light transitions are applied to gh leaves (Fig. 7) indicate a lack of fine tuning for optimal allocation of electrons to photosynthetic and nonphotosynthetic sinks. This is important in the first minutes of illumination when light activation of the Calvin cycle and the development of ΔpH-dependent qN are critical. The more reduced state of QA during this time might also render PSII more sensitive to photobleaching because photoinhibition or photodamage to PSII strongly depends on the redox state of PSII and membrane energization (Ögren, 1991).
A regulatory role for PTOX is also suggested by the slightly higher pigment content of green leaves in gh versus SM (Fig. 2, A and B). However, this slight increase in both chlorophyll and carotenoid content is unlikely to be the causal link between the lack of PTOX and the various effects on photosynthetic electron transport described here. For instance, in gh the influence of dark adaptation time on fast fluorescence kinetics (Fig. 6) or delay in full activation of the xanthophyll cycle during long incubation under severe light conditions (Table I) must result from more complex regulatory mechanisms. In addition, a rise in chlorophyll content was observed in quite similar proportions when wild-type and immutans Arabidopsis were compared (Rosso et al., 2006), whereas these authors did not report effects on the photosynthetic electron chain for this species. This apparent discrepancy between tomato and Arabidopsis is also, in our opinion, more in favor of a regulatory role for PTOX: In a multicomponent regulatory network, it is conceivable that the relative importance of a given component differs from species to species. This is even more plausible because, within one single species (tomato; our present data), the relative importance of the dual role of PTOX (related to carotenoid biosynthesis and to photosynthesis) differs from organ to organ and also within a given organ (leaf, fruit) from one developmental stage to another.
Changes in PTOX Function during Tomato Fruit Development
As in leaves, bleaching of young gh fruit is determined at an early developmental stage. The presence of phytoene in white gh fruit suggests it is linked (at least partially) to a deficit in carotenoid biosynthesis. In contrast, the sensitivity to high light conditions of mature gh fruit that were maintained green could not be linked to a deficit in carotenoid biosynthesis. We found gh green fruit to be more susceptible to photoinhibition than SM control fruit, which again is similar to our observation in adult green gh leaves.
In ripening gh fruit derived from green fruit, the lycopene-synthesizing capacity of fruit is reduced compared to wild type, but is not zero, which may suggest that, in the absence of PTOX, another cofactor of carotenoid desaturase replaces PTOX. The identity of this cofactor (Carol and Kuntz, 2001) and whether it is identical to the one that operates in green or etiolated (dark-grown cotyledons) tissues remain to be determined. Alternatively, chemical reoxidation of plastoquinol may be sufficient for the low level of phytoene desaturation in gh ripening fruit as well as in dark-grown cotyledons.
Bleaching at the green stage in gh fruit influences the capacity to synthesize carotenoids during fruit ripening: The amount of lycopene present in ripe fruit derived from white gh fruit is decreased 3-fold compared to ripe fruit derived from green gh fruit (Fig. 8E). Because carotenoid biosynthesis is catalyzed by membrane-bound enzymes and because bleached gh fruit are affected in their plastid ultrastructure (Barr et al., 2004), it seems reasonable to consider that irreversible damage to membranes in bleached tissues will affect this biosynthetic pathway. Because fruit ripening is characterized by the disassembly of photosynthetic membranes to resynthesize new (achlorophyllous) ones, membrane disintegration at an early stage will affect this biosynthetic pathway during ripening. It is also likely that photobleaching will have additional pleiotropic effects that will affect the carotenoid biosynthetic pathway.
MATERIALS AND METHODS
Plant Materials
Tomato (Solanum lycopersicum) of SM genotype and its monogenic gh mutant (LA0259; Tomato Genetic Stock Center) were grown at 24°C with a 16-h photoperiod (white light, 60 μmol m−2 s−1). Seedlings were first grown at low light intensity (10 μmol m−2 s−1 for 1 week and then 20 μmol m−2 s−1 for 4 weeks) and then placed under the above-mentioned light conditions to obtain adult plants with green leaves for gh. Both white and variegated gh leaves were obtained by incubation of seedlings directly at 60 μmol m−2 s−1.
For experiments with cotyledons, SM and gh seedlings were grown in darkness for 4 and 6 d after seed imbibition to obtain white and pale yellow cotyledons, respectively. Seedlings were then transferred to either low light (20 μmol m−2 s−1) or higher light intensities (200 μmol m−2 s−1).
For light stress experiments, young (fully elongated) leaves or mature green fruit were harvested 3 to 4 h after the beginning of the photoperiod and incubated at 24°C under 60 μmol m−2 s−1 (control) and 1,000 μmol m−2 s−1 (stress).
Chlorophyll Fluorescence
Chlorophyll a fluorescence was measured with a pulse-modulated fluorometer (Walz) at room temperature. Leaves and fruits were kept in the dark for 30 min prior to the measurements. Variable fluorescence (Fv) was calculated as Fm − F0, where F0 is minimal fluorescence (under a weak measuring beam) and Fm maximal fluorescence (determined after an 800-ms saturating pulse of white light at 2,500 μmol m−2 s−1). Prior to fluorescence measurements, photoinhibitory conditions were obtained by exposing plant samples to an irradiance of 1,000 μmol m−2 s−1 for 6 or 16 h at 15°C or 24°C.
For experiments using prolonged illumination, actinic white light (200 μmol m−2 s−1) was used and saturating pulses were applied at 1-min intervals for 10 min to determine maximal fluorescence (F′m), steady-state fluorescence (Fs), and, after actinic light was switched off and a brief far-red pulse was applied, to measure minimal fluorescence (F′0). The coefficient qP was calculated as (F′m − Fs)/(F′m − F′0) (Schreiber et al., 1989), qN as 1 − (F′m − F′0)/(Fm − F0), and ΦPSII as (F′m − Fs)/F′m.
Fluorescence induction kinetic curves (OJIP) were measured with a Handy-PEA fluorometer (Hansatech) according to Strasser et al. (2004). Plant leaves were dark adapted for 5 min, 30 min, and 4 h in a leaf clip prior to illumination with saturating light of 3,500 μmol m−2 s−1 from three red LEDs (duration 1 s).
Thermoluminescence and Lipid Peroxidation Measurements
Thermoluminescence measurements were performed on 6-mm leaf discs as described previously (Gilbert et al., 2004). Briefly, leaf discs were preilluminated by two single turnover flashes at 20°C and dark adapted for 5 min to establish a defined ratio of S0/S1 states in the water-splitting complex and a defined ratio of QB/QB− at the acceptor side of PSII. Samples were then cooled to 2°C and illuminated with one single turnover flash to induce charge separation. Then the samples were heated from 2°C to 180°C at a rate of 20°C min−1. All thermoluminescence steps were performed under N2 atmosphere to reduce autooxidation by O2. A change in the intensity of the B band (S2QB−) peaking at 25°C points to damage of PSII. The amplitude of the 130°C thermoluminescence band (HTL2 band) was used as an index of lipid peroxidation.
MDA assays were performed according to Hodges et al. (1999) and Sairam and Srivastava (2001). About 100 mg of fresh tissue were ground in 1 mL of chilled reagent (0.25% [w/v] thiobarbituric acid in 10% [w/v] TCA). After incubation at 95°C for 20 min, extracts were cooled at room temperature and then centrifuged. Thiobarbituric acid reactivity was determined in the supernatant by measuring the A532. Nonspecific turbidy was determined at 600 nm.
Extraction and Immunodetection of Total Proteins
Total protein was extracted from frozen and ground material using the Hurkman and Tanaka method (Hurkman and Tanaka, 1986). Protein samples were fractionated by SDS-PAGE and electroblotted onto nitrocellulose. Membranes were blocked in 5% milk and immunodetected with antibodies. PSI-D antibodies were purchased from Agrisera. Immunodetection was performed using the horseradish peroxidase conjugate substrate kit (Bio-Rad) and ECL western-blotting kit (Amersham) as recommended by the suppliers. Densitometric analysis of the immunoblots was performed using Image J software (Wayne Rasband, National Institute of Mental Health).
Pigment Analysis
Pigments were extracted from lyophilized samples (5-mg leaves or cotyledons, 10-mg fruit pericarp) using methanol (neutralized with 5 mm Tris-HCl, pH 7.0). In addition, fruit methanolic extracts were added with 1 volume of water and then phase partitioned with a volume of chloroform. The aqueous phase was re-extracted twice with chloroform and the pigments dried from the pooled chloroform phases. The HPLC method used to analyze and quantify phytoene, carotenoids, and chlorophylls has been detailed by Fraser et al. (2000). Identification of carotenoids was achieved by comparing retention times and spectral properties of authentic standards. Quantification was achieved from standard curves.
Acknowledgments
We are grateful to Prof. Dr. C. Wilhelm and Dr. T. Jakob (Leipzig) and Dr. S. Lobréaux (Grenoble) for helpful discussions, and to J.P. Alcaraz (Grenoble) for expert technical assistance. M.S. was supported by the Iranian Ministry of Science, Research, and Technology (Ph.D. scholarship).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Marcel Kuntz (marcel.kuntz@ujf-grenoble.fr).
References
- Aluru MR, Stessman DJ, Spalding MH, Rodermel SR (2007) Alterations in photosynthesis in Arabidopsis lacking IMMUTANS, a chloroplast terminal oxidase. Photosynth Res 91 11–23 [DOI] [PubMed] [Google Scholar]
- Aluru MR, Yu F, Fu A, Rodermel S (2006) Arabidopsis variegation mutants: new insights into chloroplast biogenesis. J Exp Bot 57 1871–1881 [DOI] [PubMed] [Google Scholar]
- Baena-Gonzalez E, Allahverdiyeva Y, Svab Z, Maliga P, Josse EM, Kuntz M, Maenpaa P, Aro EM (2003) Deletion of the tobacco plastid psbA gene triggers an upregulation of the thylakoid-associated NAD(P)H dehydrogenase complex and the plastid terminal oxidase (PTOX). Plant J 35 704–716 [DOI] [PubMed] [Google Scholar]
- Baerr JN, Thomas JD, Taylor BG, Rodermel SR, Gray GR (2005) Differential photosynthetic compensatory mechanisms exist in the immutans mutant of Arabidopsis thaliana. Physiol Plant 124 390–402 [Google Scholar]
- Barr J, White WS, Chen L, Bae CH, Rodermel S (2004) The GHOST terminal oxidase regulates developmental programming in tomato fruit. Plant Cell Environ 27 840–852 [Google Scholar]
- Berthold DA, Stenmark P (2003) Membrane-bound diiron carboxylate proteins. Annu Rev Plant Biol 54 497–517 [DOI] [PubMed] [Google Scholar]
- Carol P, Kuntz M (2001) A plastid terminal oxidase comes to light: implications for carotenoid biosynthesis and chlororespiration. Trends Plant Sci 6 31–36 [DOI] [PubMed] [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breittenbach J, Sandmann G, Mache R, Coupland G, Kuntz M (1999) Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac L, Latouche G, Cerovic Z, Redding K, Ravenel J, Peltier G (2002) In vivo interactions between photosynthesis, mitorespiration, and chlororespiration in Chlamydomonas reinhardtii. Plant Physiol 129 1921–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cournac L, Redding K, Ravenel J, Rumeau D, Josse EM, Kuntz M, Peltier G (2000) Electron flow between photosystem II and oxygen in chloroplasts of photosystem I-deficient algae is mediated by a quinol oxidase involved in chlororespiration. J Biol Chem 275 17256–17262 [DOI] [PubMed] [Google Scholar]
- Ducruet JM (2003) Chlorophyll thermoluminescence of leaf discs: simple instruments and progress in signal interpretation open the way to new ecophysiological indicators. J Exp Bot 54 2419–2430 [DOI] [PubMed] [Google Scholar]
- Fraser PD, Pinto ME, Holloway DE, Bramley PM (2000) Technical advance: application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J 24 551–558 [DOI] [PubMed] [Google Scholar]
- Gilbert M, Wagner H, Weingart I, Skotnica J, Nieber K, Tauer G, Bergmann F, Fischer H, Wilhelm C (2004) A new type of thermoluminometer: a highly sensitive tool in applied photosynthesis research and plant stress physiology. J Plant Physiol 161 641–651 [DOI] [PubMed] [Google Scholar]
- Haldimann P, Strasser RJ (1999) Effects of anaerobiosis as probed by the polyphasic chlorophyll a fluorescence rise kinetic in pea (Pisum sativum L.). Photosynth Res 62 67–83 [Google Scholar]
- Hegedüs A, Erdei S, Janda T, Tóth E, Horváth G, Dudits D (2004) Transgenic tobacco plants overproducing alfalfa aldose/aldehyde reductase show higher tolerance to low temperature and cadmium stress. Plant Sci 166 1329–1333 [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207 604–611 [DOI] [PubMed] [Google Scholar]
- Hurkman WJ, Tanaka CK (1986) Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol 81 802–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joet T, Genty B, Josse EM, Kuntz M, Cournac L, Peltier G (2002) Involvement of a plastid terminal oxidase in plastoquinone oxidation as evidenced by expression of the Arabidopsis thaliana enzyme in tobacco. J Biol Chem 277 31623–31630 [DOI] [PubMed] [Google Scholar]
- Josse EM, Alcaraz JP, Laboure AM, Kuntz M (2003) In vitro characterization of a plastid terminal oxidase (PTOX). Eur J Biochem 270 3787–3794 [DOI] [PubMed] [Google Scholar]
- Josse EM, Simkin AJ, Gaffe J, Laboure AM, Kuntz M, Carol P (2000) A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol 123 1427–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorobrykh SA, Ivanov BN (2002) Oxygen reduction in a plastoquinone pool of isolated pea thylakoids. Photosynth Res 71 209–219 [DOI] [PubMed] [Google Scholar]
- Kramer D, Johnson G, Kiirats O, Edwards G (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79 209–218 [DOI] [PubMed] [Google Scholar]
- Kuntz M (2004) Plastid terminal oxidase and its biological significance. Planta 218 896–899 [DOI] [PubMed] [Google Scholar]
- Lennon AM, Prommeenate P, Nixon PJ (2003) Location, expression and orientation of the putative chlororespiratory enzymes, Ndh and IMMUTANS, in higher-plant plastids. Planta 218 254–260 [DOI] [PubMed] [Google Scholar]
- Mayer MP, Beyer P, Kleinig H (1990) Quinone compounds are able to replace molecular oxygen as terminal electron acceptor in phytoene desaturation in chromoplasts of Narcissus pseudonarcissus L. Eur J Biochem 191 359–363 [DOI] [PubMed] [Google Scholar]
- Morstadt L, Graber P, De Pascalis L, Kleinig H, Speth V, Beyer P (2002) Chemiosmotic ATP synthesis in photosynthetically inactive chromoplasts from Narcissus pseudonarcissus L. linked to a redox pathway potentially also involved in carotene desaturation. Planta 215 134–140 [DOI] [PubMed] [Google Scholar]
- Nievelstein V, Vanderkerckhove J, Tadros MH, von Lintig J, Nitschke W, Beyer P (1995) Carotene desaturation is linked to a redox pathway in Narcissus pseudonarcissus chromoplast membranes. Involvement of a 23 kDa oxygen evolving complex like protein. Eur J Biochem 233 864–872 [DOI] [PubMed] [Google Scholar]
- Norris SR, Barrette TR, DellaPenna D (1995) Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 7 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ögren E (1991) Prediction of photoinhibition of photosynthesis from measurements of fluorescence quenching components. Planta 184 538–544 [DOI] [PubMed] [Google Scholar]
- Olsson M, Nilsson K, Liljenberg C, Hendry GAF (1996) Drought stress in seedlings: lipid metabolism and lipid peroxidation during recovery from drought in Lotus comiculatrus and Cerastium fontanum. Physiol Plant 96 577–584 [Google Scholar]
- Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis. Curr Opin Plant Biol 5 193–198 [DOI] [PubMed] [Google Scholar]
- Peltier G, Cournac L (2002) Chlororespiration. Annu Rev Plant Biol 53 523–550 [DOI] [PubMed] [Google Scholar]
- Quiles MJ (2006) Stimulation of chlororespiration by heat and high light intensity in oat plants. Plant Cell Environ 29 1463–1470 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, Inze D, Mittler R (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J 32 329–342 [DOI] [PubMed] [Google Scholar]
- Rodermel S (2001) Pathways of plastid-to-nucleus signaling. Trends Plant Sci 6 471–478 [DOI] [PubMed] [Google Scholar]
- Rosso D, Ivanov AG, Fu A, Geisler-Lee J, Hendrickson L, Geisler M, Stewart G, Krol M, Hurry V, Rodermel SR, et al (2006) IMMUTANS does not act as a stress-induced safety valve in the protection of the photosynthetic apparatus of Arabidopsis during steady-state photosynthesis. Plant Physiol 142 574–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumeau D, Peltier G, Cournac L (2007) Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ 30 1041–1051 [DOI] [PubMed] [Google Scholar]
- Sairam RK, Srivastava GC (2001) Water stress tolerance of wheat (Triticum aestivum L.): variations in hydrogen peroxide accumulation and antioxidant activity in tolerant and susceptible genotypes. J Agron Crop Sci 186 63–70 [Google Scholar]
- Schansker G, Tóth SZ, Strasser RJ (2005) Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim Biophys Acta 1706 250–261 [DOI] [PubMed] [Google Scholar]
- Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In C Papageorgiou, Govindjee, eds, Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer, Dordrecht, The Netherlands, pp 217–319
- Schreiber U, Neubauer C, Klughammer C (1989) Devices and methods for room-temperature fluorescence analysis. Philos Trans R Soc Lond B Biol Sci 323 241–251 [Google Scholar]
- Shikanai T (2007) Cyclic electron transport around photosystem I: genetic approaches. Annu Rev Plant Biol 58 199–217 [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Laboure AM, Kuntz M, Sandmann G (2003) Comparison of carotenoid content, gene expression and enzyme levels on tomato (Lycopersicon esculentum L) leaves. Z Naturforsch [C] 58c: 371–380 [DOI] [PubMed]
- Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll a fluorescence transient. In C Papageorgiou, Govindjee, eds, Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer, Dordrecht, The Netherlands, pp 321–362
- Streb P, Josse EM, Gallouet E, Baptist F, Kuntz M, Cornic G (2005) Evidence for alternative electron sinks to photosynthetic carbon assimilation in the high mountain plant species Ranunculus glacialis. Plant Cell Environ 28 1123–1135 [Google Scholar]
- Vavilin DV, Ducruet JM (1998) The origin of 115–130°C thermoluminescence bands in chlorophyll-containing material. Photochem Photobiol 68 191–198 [Google Scholar]
- Vavilin DV, Ducruet JM, Matorin DN, Venediktov PS, Rubin AB (1998) Membrane lipid peroxidation, cell viability and photosystem II activity in the green alga Chlorella pyrenoidosa subjected to various stress conditions. J Photochem Photobiol B 42 233–239 [Google Scholar]
- Wu DY, Wright DA, Wetzel C, Voytas DF, Rodermel S (1999) The immutans variegated locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell 11 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]