In plant as well as in animal systems, extracellular ATP (eATP) regulates a broad range of physiological processes. In animals, downstream signaling events regulated by eATP have been described before. Among the second messengers reported to be involved in eATP signaling, there are inositol phosphates, Ca2+, cAMP, and nitric oxide (NO; Abbracchio and Burnstock, 1998; Shen et al., 2005), but in plants this study is in its infancy. It has been shown that Ca2+ and superoxide are required for ATP-induced responses (Demidchik et al., 2003; Song et al., 2006). Here, we report that exogenous ATP induces NO production in plant cells via P2-like receptors accordingly to what has been revealed in animals (Shen et al., 2005, 2006). Even though the data are consistent with the presence of P2-like receptors in plants, they have not been identified yet. Thus, our results suggest that eATP-mediated production of NO is present in different biological systems.
In animals, eATP is implicated in many physiological functions ranging from neurotransmission to cell death (Fredholm et al., 1994; Burnstock and Williams, 2000). eATP exerts its effect through purinergic receptors, including ligand-gated ion channels (P2X) and G protein-coupled receptors (P2Y; Ralevic and Burnstock, 1998). Both P2X and P2Y receptors affect directly or indirectly intracellular calcium signaling, resulting in a variety of downstream cellular responses. Even though the presence of plant purinergic receptors has been suggested (Song et al., 2006), the molecular components involved in eATP signaling pathway are poorly known. eATP has been implicated in different plant developmental processes, e.g. the inhibition of root gravitropism, polar auxin transport (Tang et al., 2003), pollen germination (Steinebrunner et al., 2003), and cell viability (Chivasa et al., 2005), and for the regulation of growth in Arabidopsis (Arabidopsis thaliana; Wu et al., 2007). It has also been found that eATP induces an increase in the cytosolic Ca2+ concentration (Demidchik et al., 2003), accumulation of reactive oxygen species, and up-regulation of gene expression involved in plant responses to wounding (Jeter et al., 2004; Song et al., 2006). Reactive oxygen species and Ca2+ are implicated as second messengers in NO-regulated stress responses (Garcia-Mata et al., 2003; Desikan et al., 2004; Lamotte et al., 2005; Laxalt et al., 2007). Considering that NO is a downstream component of eATP perception in animals (Shen et al., 2005), we hypothesize that NO is a component of eATP-mediated plant responses.
NO is an unstable gas and a diffusible multifunctional molecule involved in numerous physiological processes in phylogenetically distant species (Gow and Ischiropoulos, 2001). In the last decade, the role of NO in plants has received much attention (Wendehenne et al., 2006). Initial investigations on NO functions demonstrated that in plants NO is a component of the signaling pathways, remarkably similar to those found in animals. This suggested that NO-regulated transduction pathways could be common in plants and animals (Wendehenne et al., 2001).
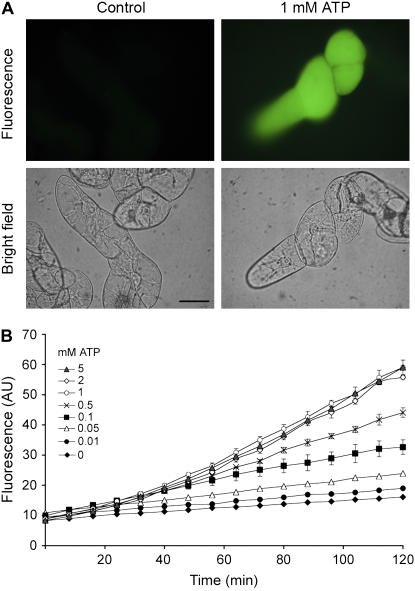
In this work, we investigated the effect of exogenous ATP on NO production in tomato (Solanum lycopersicum ‘Money Maker’; line Msk8) suspension-cultured cells. Suspension-cultured cells were grown at 24°C in the dark at 125 rpm in Murashige and Skoog medium (Duchefa) supplemented with 5.4 μm naphthylacetic acid, 1 μm 6-benzyladenine, and vitamins (Duchefa). Cultured cells of 4 to 5 d old were exposed to different treatments in microwells for fluorometric measurements for different time periods. Thus, tomato cell suspensions were pretreated for 30 min with the NO-specific fluorophore diaminofluorescein-FM diacetate (DAF-FM DA) and subsequently incubated in the presence or absence of ATP for 60 min. Figure 1A shows that 1 mm ATP induced high levels of NO (observed as green fluorescence), as compared to control cells. A dose-response experiment was quantified using a fluorometer. Cells were treated with different ATP concentrations and adjusted at pH 5.6 in the presence of DAF-FM DA. Fluorescence was measured during 120 min (Fig. 1B). NO production increased as ATP concentration augmented (0.05 mm to 1 mm), reaching the maximum production at 1 mm. The production of NO was rapid and sustained at least over 120 min (Fig. 1B). In the absence of exogenous ATP, DAF-FM DA fluorescence weakly increased, indicating that a basal level of NO production occurred in the tomato cell suspensions (Fig. 1B). At the same concentrations, ATP was also able to induce NO production in tobacco (Nicotiana tabacum) BY-2 cells (data not shown). ATP doses used did not modify cell viability measured at 120 min or overnight (data not shown). The NO-specific scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide diminished ATP-induced NO production in a dose-dependent manner, indicating that the fluorescence is caused by NO production (Fig. 2). Our results indicate that exogenous applications ranging from micromolar to millimolar ATP unequivocally induce NO production. Physiologically, eATP concentrations could increase depending on the situation (Jeter et al., 2004). During wounding, the cell membrane is broken and millimolar ATP concentrations could be released into the extracellular space (Jeter et al., 2004). In addition, other stress conditions like mechanical stimuli and osmotic stress induce ATP release (Jeter et al., 2004). More recently, eATP levels were closely correlated with regions of active growth and cell expansion (Kim et al., 2006). Remarkably, an increase of NO production has been reported in the above-mentioned eATP-mediated processes (Gould et al., 2003; Correa-Aragunde et al., 2004; Huang et al., 2004; Paris et al., 2007). We hypothesized that NO may give rise to a component of the eATP signaling pathway involved in different physiological processes as reported in animals.
Figure 1.
Exogenous ATP induces NO production in tomato cells. Suspension-cultured cells (‘Money Maker’ tomato; line Msk8) of 4 to 5 d old were exposed to ATP (previously adjusted to pH 5.7) in the presence of the NO-specific fluorescent probe DAF-FM DA. Experimental procedures are described in detail by Laxalt et al. (2007). A, NO production is visualized as green fluorescence in tomato cells. Pictures were taken 30 min after 1 mm ATP treatment and show general phenomena, representative for at least six individual experiments. Approximately 50% of viable cells show a response to eATP according to the microscopy data. A bright-field image for each treatment is shown below the fluorescent image. Bar = 5 μm. B, Dose- and time-response curves of NO production. Fluorescence was determined using a microwell fluorometer over a 120-min period and expressed as arbitrary units (AU). Error bars represent se of means. A representative graph of three independent experiments is shown. Note that nontreated cells show no significant fluorescence increase.
Figure 2.
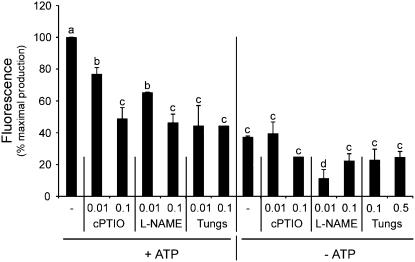
NO levels are reduced by a specific NO scavenger and NOS and NR inhibitors. Cells were preincubated for 30 min with different concentrations (mm) of the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), the NOS inhibitor l-NAME, or the NR inhibitor tungstate, and treated with 1 mm ATP (+ATP) or not treated (−ATP). NO levels were measured as relative fluorescence determined as in Figure 1B and expressed as a percentage (100% corresponding to the gradient of NO production of ATP-treated cells in the absence of inhibitors). The fluorescence was measured in a 120-min time interval. Error bars represent se of means (n = 3). Means denoted with the same letter do not significantly differ at P < 0.005 according to one-way ANOVA.
NO production in mammals mainly relies on the activity of NO synthase, named NOS (EC 1.14.13.39). Even if the presence of a plant NOS gene has not been yet identified, the Arg → NO + citrulline NOS-dependent pathway seems to be operative in plants (del Rio et al., 2004). In plants, another well-described source of NO is nitrate reductase (NR), although other NO sources exist (del Rio et al., 2004). To examine putative sources of NO production in ATP-treated tomato cells, the ability of a nonhydrolyzable analog of Arg, l-ω-nitro-Arg-methyl-ester (l-NAME), was used as an inhibitor of NO production via NOS activity. Tungstate was used as a NR inhibitor. Figure 2 shows that in ATP-stimulated cells the presence of either l-NAME or tungstate decreased NO production in a dose-dependent manner. These data indicate that both NOS and NR activities are implicated in ATP-induced NO production in tomato cells.
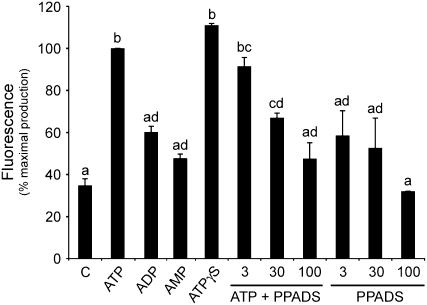
Many animal purinergic receptors have broad nucleotide specificity (Ralevic and Burnstock, 1998), and it has been demonstrated that higher plants exhibit cellular responsiveness to nucleotides in a similar manner. Like animals, plants respond to eATP and ADP by increasing cytosolic Ca2+ concentration and showed no response to AMP or pyrimidine base (Jeter et al., 2004). In Arabidopsis, ATP and ADP, but not AMP, induce superoxide production (Song et al., 2006). Figure 3 shows NO production by tomato cell suspensions treated with different adenosine phosphates. ADP and AMP slightly induced NO production, but none of them attained the induction produced by ATP treatment (Fig. 3). CTP and UDP did not induce NO production (data not shown). Tomato cells were treated with the nonhydrolyzable ATP (ATP-γ-S) as an agonist of P2-like receptor. Figure 3 shows that ATP-γ-S clearly stimulated NO production, reaching the same level that was observed in ATP-stimulated cells. Thus, the agonist specificity (Fig. 3) and the ATP concentration dependency (Fig. 1B) suggest a receptor mediation of ATP-induced NO production. Subsequently, the involvement of P2 receptors in ATP-induced NO production was tested using an antagonist. Figure 3 shows that the nonspecific P2 receptor antagonist pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) significantly inhibited the stimulatory effect of ATP on NO production in a dose-dependent manner. The inhibition by PPADS demonstrates that ATP-induced NO production is mediated by P2 purinergic-like receptors in tomato cells.
Figure 3.
ATP mediates NO production via P2-like receptors. Shown are NO levels in cells treated with 1 mm ATP, ADP, AMP, or ATP-γ-S. Doses effects of the P2 antagonist PPADS (μm) on 1 mm ATP-induced NO production were measured. NO levels were measured as in Figure1B and expressed as a percentage (100% corresponding to the gradient of NO production of ATP-treated cells in the absence of inhibitors). Error bars represent se of means (n = 3). Means denoted with the same letter do not significantly differ at P < 0.005 according to one-way ANOVA.
As more data become available, it is evident that NO integrates different signaling transduction pathways. It regulates Ca2+ fluxes, cGMP increases, protein kinases, and phospholipase C activation (Wendehenne et al., 2006; Laxalt et al., 2007). The fact that both eATP (Chivasa et al., 2005; Song et al., 2006) and NO (Lamattina et al., 2003; Delledonne et al., 2005) have been postulated as stress and growth signals allows us to suggest that they probably are participating together to regulate different physiological processes during plant growth and in response to environmental stimuli. Functional studies will be directed to evaluate the participation of NO on eATP-mediated processes using mutants unpaired in NO production. More precise information about the nature of the mechanisms that mediate eATP-induced NO production will contribute to understanding the role of this signaling pathway in plants.
Acknowledgments
A.M.L., C.A.C., and L.L. are researchers from Consejo Nacional de Investigaciones Científicas y Técnicas. C.V.T. is supporting staff from Comisión de Investigaciones Científicas, Argentina.
This work was supported by Universidad Nacional de Mar Del Plata, Consejo Nacional de Investigaciones Científicas y Técnicas, and Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Lorenzo Lamattina (lolama@mdp.edu.ar).
References
- Abbracchio MP, Burnstock G (1998) Purinergic signaling: pathophysiological roles. Jpn J Pharmacol 78 113–145 [DOI] [PubMed] [Google Scholar]
- Burnstock G, Williams M (2000) P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther 295 862–869 [PubMed] [Google Scholar]
- Chivasa S, Ndimba BK, Simon WJ, Lindsey K, Slabas AR (2005) Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell 17 3019–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Aragunde N, Graziano M, Lamattina L (2004) Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218 900–905 [DOI] [PubMed] [Google Scholar]
- del Rio LA, Corpas FJ, Barroso JB (2004) Nitric oxide and nitric oxide synthase activity in plants. Phytochemistry 65 783–792 [DOI] [PubMed] [Google Scholar]
- Delledonne M (2005) No news is good news for plants. Curr Opin Plant Biol 8 390–396 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Nichols C, Oliynyk M, Dark A, Glover BJ, Davies JM (2003) Is ATP a signaling agent in plants? Plant Physiol 133 456–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cells. J Exp Bot 55 205–212 [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Lindstrom K, Wallman-Johansson A (1994) Propentofylline and other adenosine transport inhibitors increase the efflux of adenosine following electrical or metabolic stimulation of rat hippocampal slices. J Neurochem 62 563–573 [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl− channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 100 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KS, Lamotte O, Klinguer A, Pugin A, Wendehenne D (2003) Nitric oxide production in tobacco leaf cells: a generalized stress response? Plant Cell Environ 26 1851–1862 [Google Scholar]
- Gow AJ, Ischiropoulos H (2001) Nitric oxide chemistry and cellular signaling. J Cell Physiol 187 277–282 [DOI] [PubMed] [Google Scholar]
- Huang X, Stettmaier K, Michel C, Hutzler P, Mueller M, Durner J (2004) Nitric oxide is induced by wounding and influences jasmonic acid signaling in Arabidopsis thaliana. Planta 218 938–946 [DOI] [PubMed] [Google Scholar]
- Jeter CR, Tang W, Henaff E, Butterfield T, Roux SJ (2004) Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 16 2652–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Sivaguru M, Stacey G (2006) Extracellular ATP in plants. Visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol 142 984–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamattina L, Garcia-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54 109–136 [DOI] [PubMed] [Google Scholar]
- Lamotte O, Courtois C, Barnavon L, Pugin A, Wendehenne D (2005) Nitric oxide in plants: the biosynthesis and cell signaling properties of a fascinating molecule. Planta 221 1–4 [DOI] [PubMed] [Google Scholar]
- Laxalt AM, Raho N, Have AT, Lamattina L (2007) Nitric oxide is critical for inducing phosphatidic acid accumulation in xylanase-elicited tomato cells. J Biol Chem 282 21160–21168 [DOI] [PubMed] [Google Scholar]
- Paris R, Lamattina L, Casalongue CA (2007) Nitric oxide promotes the wound-healing response of potato leaflets. Plant Physiol Biochem 45 80–86 [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50 413–492 [PubMed] [Google Scholar]
- Shen J, Harada N, Nakazawa H, Kaneko T, Izumikawa M, Yamashita T (2006) Role of nitric oxide on ATP-induced Ca2+ signaling in outer hair cells of the guinea pig cochlea. Brain Res 1081 101–112 [DOI] [PubMed] [Google Scholar]
- Shen J, Harada N, Nakazawa H, Yamashita T (2005) Involvement of the nitric oxide-cyclic GMP pathway and neuronal nitric oxide synthase in ATP-induced Ca2+ signaling in cochlear inner hair cells. Eur J Neurosci 21 2912–2922 [DOI] [PubMed] [Google Scholar]
- Song CJ, Steinebrunner I, Wang X, Stout SC, Roux SJ (2006) Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol 140 1222–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinebrunner I, Wu J, Sun Y, Corbett A, Roux SJ (2003) Disruption of apyrases inhibits pollen germination in Arabidopsis. Plant Physiol 131 1638–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Brady SR, Sun Y, Muday GK, Roux SJ (2003) Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiol 131 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Pugin A, Klessig DF, Durner J (2001) Nitric oxide: comparative synthesis and signaling in animal and plant cells. Trends Plant Sci 6 177–183 [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Courtois C, Besson A, Gravot A, Buchwalter A, Pugin A, Lamotte O (2006) NO-based signaling in plants. In L Lamattina, JC Polacco, eds, Nitric Oxide in Plant Growth, Development and Stress Physiology, Vol 6. Springer-Verlag, Berlin, pp 35–51
- Wu J, Steinebrunner I, Sun Y, Butterfield T, Torres J, Arnold D, Gonzalez A, Jacob F, Reichler S, Roux SJ (2007) Apyrases (nucleoside triphosphate-diphosphohydrolases) play a key role in growth control in Arabidopsis. Plant Physiol 144 961–975 [DOI] [PMC free article] [PubMed] [Google Scholar]