Abstract
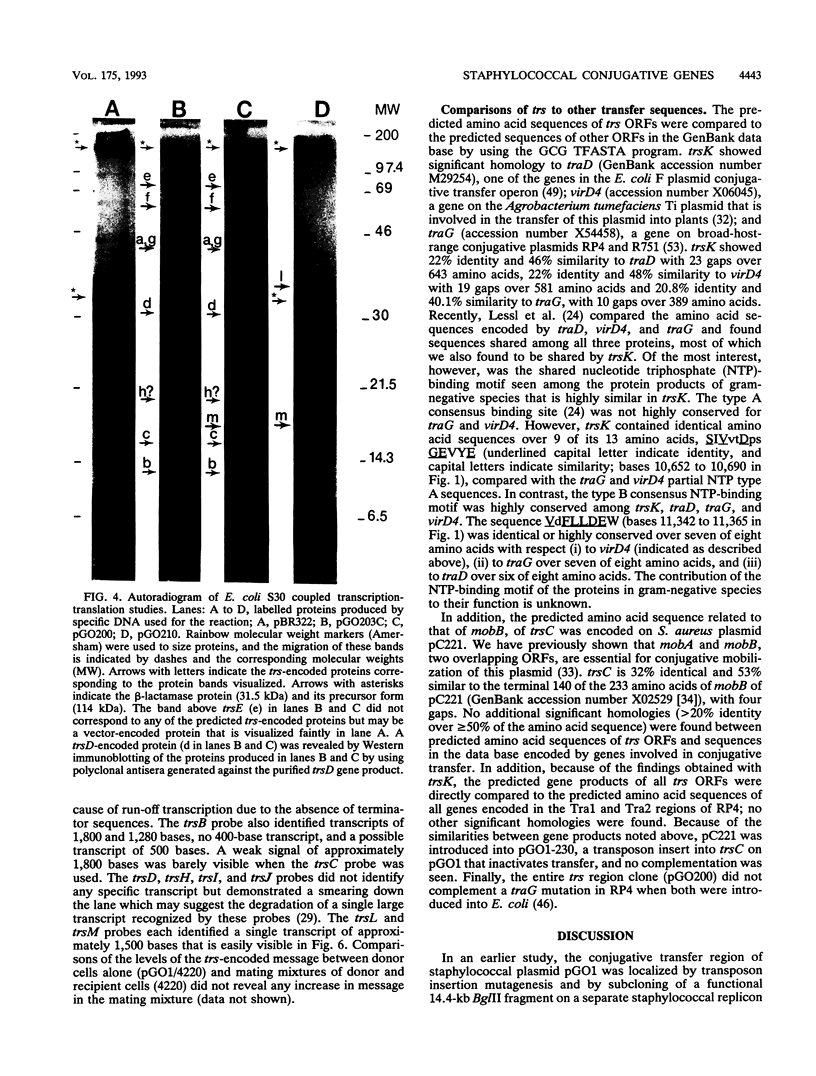
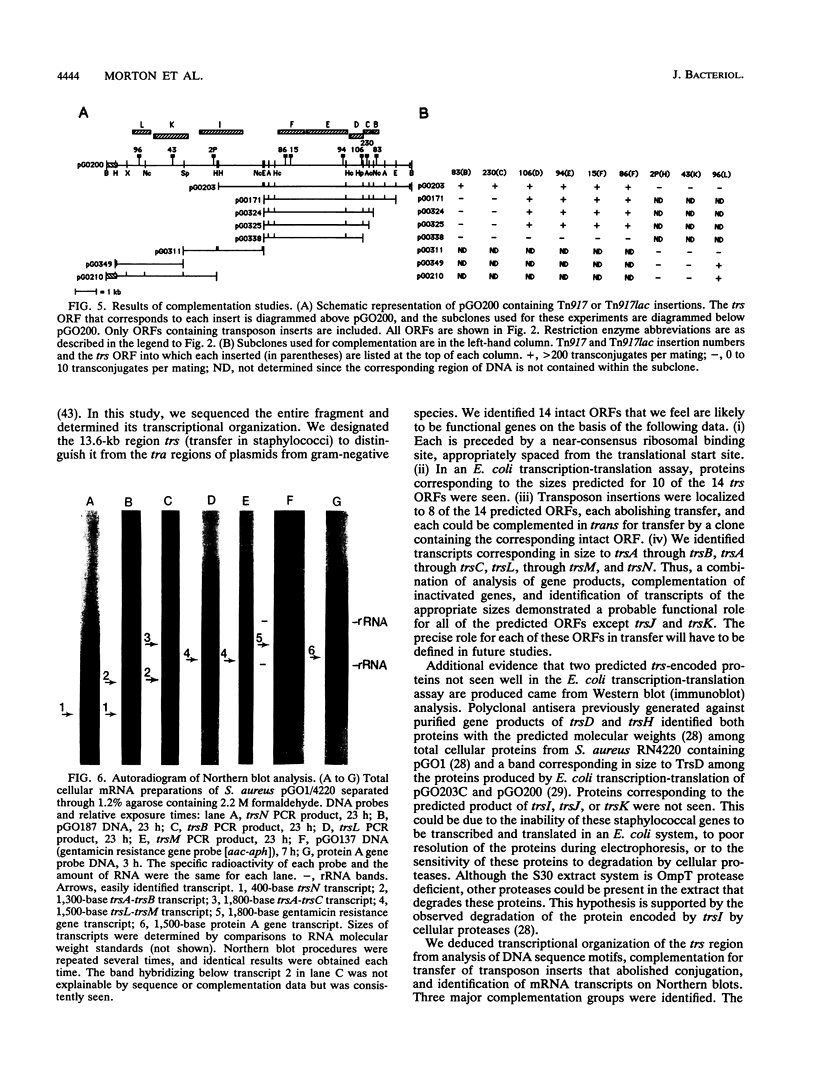
The conjugative transfer genes of 52-kb staphylococcal R plasmid pGO1 were localized to a single BglII restriction fragment and cloned in Escherichia coli. Sequence analysis of the 13,612-base transfer region, designated trs, identified 14 intact open reading frames (ORFs), 13 of which were transcribed in the same direction. Each ORF identified was preceded by a typical staphylococcal ribosomal binding sequence, and 10 of the 14 proteins predicted to be encoded by these ORFs were seen when an E. coli in vitro transcription-translation system was used. Functional transcription units were identified in a Staphylococcus aureus host by complementation of Tn917 inserts that abolished transfer and by Northern (RNA) blot analysis of pGO1 mRNA transcripts. These studies identified three complementation groups (trsA through trsC, trsD through trsK, and trsL-trsM) and four mRNA transcripts (trsA through trsC [1.8 kb], trsA-trsB [1.3 kb], trsL-trsM [1.5 kb], and trsN [400 bases]). No definite mRNA transcript was seen for the largest complementation group, trsD through trsK (10 kb). Comparison of predicted trs-encoded amino acid sequences to those in the data base showed 20% identity of trsK to three related genes necessary for conjugative transfer of plasmids in gram-negative species and 32% identity of trsC to a gene required for conjugative mobilization of plasmid pC221 from staphylococci.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Coughter J. P., Johnston J. L. Plasmid-encoded trimethoprim resistance in staphylococci. Antimicrob Agents Chemother. 1986 May;29(5):733–740. doi: 10.1128/aac.29.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Dietrick D. R., Johnston J. L. Molecular epidemiology of transmissible gentamicin resistance among coagulase-negative staphylococci in a cardiac surgery unit. J Infect Dis. 1985 Feb;151(2):243–251. doi: 10.1093/infdis/151.2.243. [DOI] [PubMed] [Google Scholar]
- Archer G. L., Johnston J. L. Self-transmissible plasmids in staphylococci that encode resistance to aminoglycosides. Antimicrob Agents Chemother. 1983 Jul;24(1):70–77. doi: 10.1128/aac.24.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer G. L., Scott J. Conjugative transfer genes in staphylococcal isolates from the United States. Antimicrob Agents Chemother. 1991 Dec;35(12):2500–2504. doi: 10.1128/aac.35.12.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis-Maino L., Berger-Bächi B., Weber H., Beck W. D., Kayser F. H. IS431, a staphylococcal insertion sequence-like element related to IS26 from Proteus vulgaris. Gene. 1987;59(1):107–113. doi: 10.1016/0378-1119(87)90271-x. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Byrne M. E., Gillespie M. T., Skurray R. A. Molecular analysis of a gentamicin resistance transposonlike element on plasmids isolated from North American Staphylococcus aureus strains. Antimicrob Agents Chemother. 1990 Nov;34(11):2106–2113. doi: 10.1128/aac.34.11.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes B. A., Schaberg D. R. Transfer of resistance plasmids from Staphylococcus epidermidis to Staphylococcus aureus: evidence for conjugative exchange of resistance. J Bacteriol. 1983 Feb;153(2):627–634. doi: 10.1128/jb.153.2.627-634.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froggatt J. W., Johnston J. L., Galetto D. W., Archer G. L. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1989 Apr;33(4):460–466. doi: 10.1128/aac.33.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetto D. W., Johnston J. L., Archer G. L. Molecular epidemiology of trimethoprim resistance among coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987 Nov;31(11):1683–1688. doi: 10.1128/aac.31.11.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Ruff E. A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983 Sep;24(3):450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman R. K., Levy S. B. Evidence that TET protein functions as a multimer in the inner membrane of Escherichia coli. J Bacteriol. 1988 Apr;170(4):1715–1720. doi: 10.1128/jb.170.4.1715-1720.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsters M., Silva B., Van Vliet F., Genetello C., De Block M., Dhaese P., Depicker A., Inzé D., Engler G., Villarroel R. The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid. 1980 Mar;3(2):212–230. doi: 10.1016/0147-619x(80)90110-9. [DOI] [PubMed] [Google Scholar]
- Jaffe H. W., Sweeney H. M., Nathan C., Weinstein R. A., Kabins S. A., Cohen S. Identity and interspecific transfer of gentamicin-resistance plasmids in Staphylococcus aureus and Staphylococcus epidermidis. J Infect Dis. 1980 Jun;141(6):738–747. doi: 10.1093/infdis/141.6.738. [DOI] [PubMed] [Google Scholar]
- Kasatiya S. S., Baldwin J. N. Nature of the determinant of tetracycline resistance in Staphylococcus aureus. Can J Microbiol. 1967 Aug;13(8):1079–1086. doi: 10.1139/m67-144. [DOI] [PubMed] [Google Scholar]
- Kornblum J. S., Projan S. J., Moghazeh S. L., Novick R. P. A rapid method to quantitate non-labeled RNA species in bacterial cells. Gene. 1988;63(1):75–85. doi: 10.1016/0378-1119(88)90547-1. [DOI] [PubMed] [Google Scholar]
- Krah E. R., 3rd, Macrina F. L. Genetic analysis of the conjugal transfer determinants encoded by the streptococcal broad-host-range plasmid pIP501. J Bacteriol. 1989 Nov;171(11):6005–6012. doi: 10.1128/jb.171.11.6005-6012.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lessl M., Balzer D., Pansegrau W., Lanka E. Sequence similarities between the RP4 Tra2 and the Ti VirB region strongly support the conjugation model for T-DNA transfer. J Biol Chem. 1992 Oct 5;267(28):20471–20480. [PubMed] [Google Scholar]
- Lessl M., Pansegrau W., Lanka E. Relationship of DNA-transfer-systems: essential transfer factors of plasmids RP4, Ti and F share common sequences. Nucleic Acids Res. 1992 Nov 25;20(22):6099–6100. doi: 10.1093/nar/20.22.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell R. W., Sweeney H. M., Cohen S. Conjugational transfer of gentamicin resistance plasmids intra- and interspecifically in Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1983 Jan;23(1):151–160. doi: 10.1128/aac.23.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. J., Krishnapillai V. Tn7 and Tn501 Insertions into Pseudomonas aeruginosa plasmid R91-5: mapping of two transfer regions. J Bacteriol. 1982 Jan;149(1):276–283. doi: 10.1128/jb.149.1.276-283.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Porter S. G., Yanofsky M. F., Nester E. W. Molecular characterization of the virD operon from Agrobacterium tumefaciens. Nucleic Acids Res. 1987 Sep 25;15(18):7503–7517. doi: 10.1093/nar/15.18.7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projan S. J., Archer G. L. Mobilization of the relaxable Staphylococcus aureus plasmid pC221 by the conjugative plasmid pGO1 involves three pC221 loci. J Bacteriol. 1989 Apr;171(4):1841–1845. doi: 10.1128/jb.171.4.1841-1845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Projan S. J., Kornblum J., Moghazeh S. L., Edelman I., Gennaro M. L., Novick R. P. Comparative sequence and functional analysis of pT181 and pC221, cognate plasmid replicons from Staphylococcus aureus. Mol Gen Genet. 1985;199(3):452–464. doi: 10.1007/BF00330758. [DOI] [PubMed] [Google Scholar]
- Ranelli D. M., Jones C. L., Johns M. B., Mussey G. J., Khan S. A. Molecular cloning of staphylococcal enterotoxin B gene in Escherichia coli and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5850–5854. doi: 10.1073/pnas.82.17.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees C. E., Bradley D. E., Wilkins B. M. Organization and regulation of the conjugation genes of IncI1 plasmid colIb-P9. Plasmid. 1987 Nov;18(3):223–236. doi: 10.1016/0147-619x(87)90065-5. [DOI] [PubMed] [Google Scholar]
- Schenk S., Laddaga R. A. Improved method for electroporation of Staphylococcus aureus. FEMS Microbiol Lett. 1992 Jul 1;73(1-2):133–138. doi: 10.1016/0378-1097(92)90596-g. [DOI] [PubMed] [Google Scholar]
- Shaw J. H., Clewell D. B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985 Nov;164(2):782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Brose E. C., Kwan S., Yan W. Mapping of transfer regions within incompatibility group HI plasmid R27. J Bacteriol. 1985 Jun;162(3):1221–1226. doi: 10.1128/jb.162.3.1221-1226.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. D., Jr, Archer G. L. Identification and cloning of the conjugative transfer region of Staphylococcus aureus plasmid pGO1. J Bacteriol. 1989 Feb;171(2):684–691. doi: 10.1128/jb.171.2.684-691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenesch F., Kornblum J., Novick R. P. A temporal signal, independent of agr, is required for hla but not spa transcription in Staphylococcus aureus. J Bacteriol. 1991 Oct;173(20):6313–6320. doi: 10.1128/jb.173.20.6313-6320.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters V. L., Strack B., Pansegrau W., Lanka E., Guiney D. G. Mutational analysis of essential IncP alpha plasmid transfer genes traF and traG and involvement of traF in phage sensitivity. J Bacteriol. 1992 Oct;174(20):6666–6673. doi: 10.1128/jb.174.20.6666-6673.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K. E., Clewell D. B. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J Bacteriol. 1988 Sep;170(9):4343–4352. doi: 10.1128/jb.170.9.4343-4352.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Walker G. C. Conjugal transfer system of the IncN plasmid pKM101. J Bacteriol. 1985 Jan;161(1):402–410. doi: 10.1128/jb.161.1.402-410.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter R. B., Gold L. Overproduction of bacteriophage Q beta maturation (A2) protein leads to cell lysis. Cell. 1983 Jul;33(3):877–885. doi: 10.1016/0092-8674(83)90030-2. [DOI] [PubMed] [Google Scholar]
- Ziegelin G., Pansegrau W., Strack B., Balzer D., Kröger M., Kruft V., Lanka E. Nucleotide sequence and organization of genes flanking the transfer origin of promiscuous plasmid RP4. DNA Seq. 1991;1(5):303–327. doi: 10.3109/10425179109020786. [DOI] [PubMed] [Google Scholar]