Abstract
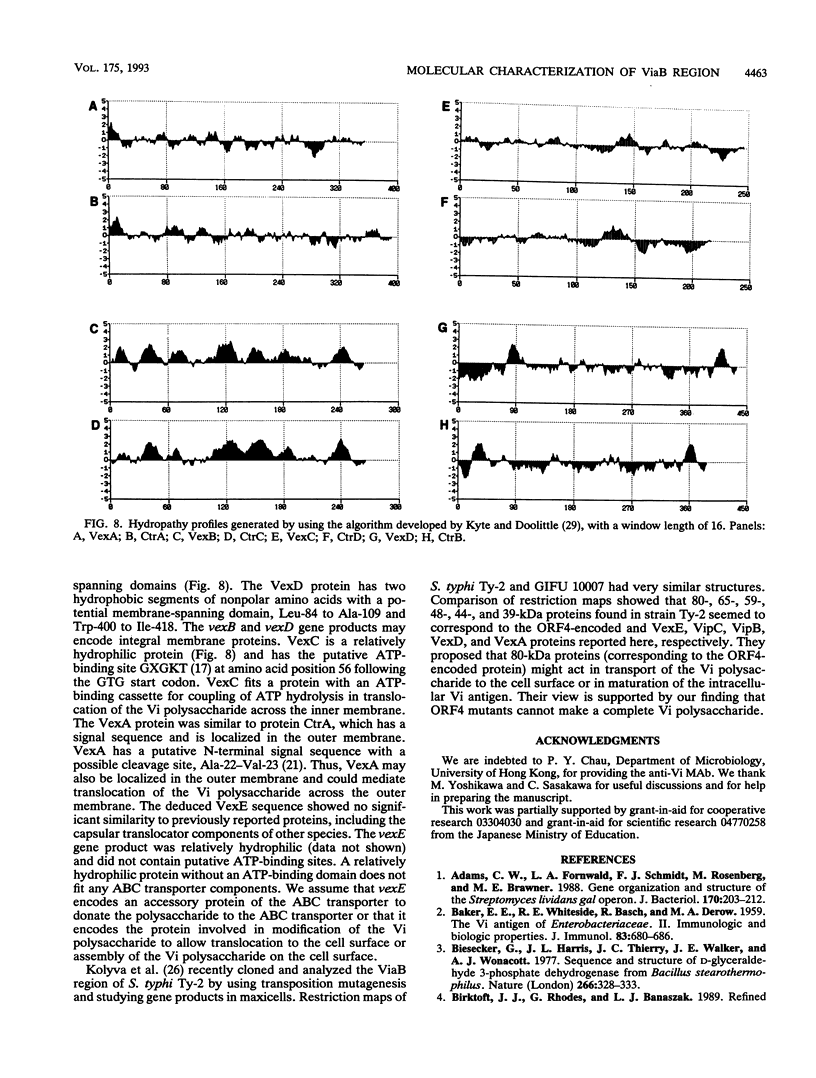
Plasmid pGBM124, which contains a 14-kb Salmonella typhi chromosomal DNA fragment capable of producing the Vi antigen in Escherichia coli HB101 and ViaB-deleted S. typhi GIFU 10007-3, was studied. We determined the complete nucleotide sequence of this fragment and found 11 open reading frames. Mutagenesis, subcloning, and complementation analysis showed that three genes (vipA, vipB, and vipC) are involved in biosynthesis of the Vi polysaccharide. The putative primary amino acid sequence suggests that both vipA and vipB encode the NAD- or NADP-dependent enzymes to synthesize the nucleotide sugar for the Vi polysaccharide. Five genes (vexA, vexB, vexC, vexD, and vexE) may be involved in translocation of the Vi polysaccharide. Proteins VexA, VexB, VexC, and VexD had moderate similarities to components of group II capsule transporters, and the VexC protein had a putative ATP-binding site. These data indicate that the transport system for the Vi polysaccharide belongs to the ATP-binding cassette transporters. By using the isogenic Vi+ and Vi- strains constructed in this study, we reconfirmed that the Vi antigen is necessary for the serum resistance of S. typhi.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Fornwald J. A., Schmidt F. J., Rosenberg M., Brawner M. E. Gene organization and structure of the Streptomyces lividans gal operon. J Bacteriol. 1988 Jan;170(1):203–212. doi: 10.1128/jb.170.1.203-212.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER E. E., WHITESIDE R. E., BASCH R., DEROW M. A. The VI antigens of the Enterobacteriaceae. I. Purification and chemical properties. J Immunol. 1959 Dec;83:680–696. [PubMed] [Google Scholar]
- Biesecker G., Harris J. I., Thierry J. C., Walker J. E., Wonacott A. J. Sequence and structure of D-glyceraldehyde 3-phosphate dehydrogenase from Bacillus stearothermophilus. Nature. 1977 Mar 24;266(5600):328–333. doi: 10.1038/266328a0. [DOI] [PubMed] [Google Scholar]
- Boulnois G. J., Roberts I. S. Genetics of capsular polysaccharide production in bacteria. Curr Top Microbiol Immunol. 1990;150:1–18. doi: 10.1007/978-3-642-74694-9_1. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron B. A., Donelson J. E. Sequence of the Saccharomyces GAL region and its transcription in vivo. J Bacteriol. 1984 Apr;158(1):269–278. doi: 10.1128/jb.158.1.269-278.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch M., Edwards U., Bousset K., Krausse B., Weisgerber C. Evidence for a common molecular origin of the capsule gene loci in gram-negative bacteria expressing group II capsular polysaccharides. Mol Microbiol. 1991 May;5(5):1251–1263. doi: 10.1111/j.1365-2958.1991.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Gottesman S., Stout V. Regulation of capsular polysaccharide synthesis in Escherichia coli K12. Mol Microbiol. 1991 Jul;5(7):1599–1606. doi: 10.1111/j.1365-2958.1991.tb01906.x. [DOI] [PubMed] [Google Scholar]
- Hall M. D., Levitt D. G., Banaszak L. J. Crystal structure of Escherichia coli malate dehydrogenase. A complex of the apoenzyme and citrate at 1.87 A resolution. J Mol Biol. 1992 Aug 5;226(3):867–882. doi: 10.1016/0022-2836(92)90637-y. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Ezaki T., Li N., Yamamoto H. Molecular cloning of the ViaB region of Salmonella typhi. FEMS Microbiol Lett. 1991 Dec 15;69(1):53–56. doi: 10.1016/0378-1097(91)90645-q. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Gallagher M. P., Hyde S. C., Mimmack M. L., Pearce S. R. Periplasmic binding protein-dependent transport systems: the membrane-associated components. Philos Trans R Soc Lond B Biol Sci. 1990 Jan 30;326(1236):353–365. doi: 10.1098/rstb.1990.0017. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Hornick R. B., Greisman S. E., Woodward T. E., DuPont H. L., Dawkins A. T., Snyder M. J. Typhoid fever: pathogenesis and immunologic control. 2. N Engl J Med. 1970 Oct 1;283(14):739–746. doi: 10.1056/NEJM197010012831406. [DOI] [PubMed] [Google Scholar]
- Houng H. S., Noon K. F., Ou J. T., Baron L. S. Expression of Vi antigen in Escherichia coli K-12: characterization of ViaB from Citrobacter freundii and identity of ViaA with RcsB. J Bacteriol. 1992 Sep;174(18):5910–5915. doi: 10.1128/jb.174.18.5910-5915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde S. C., Emsley P., Hartshorn M. J., Mimmack M. M., Gileadi U., Pearce S. R., Gallagher M. P., Gill D. R., Hubbard R. E., Higgins C. F. Structural model of ATP-binding proteins associated with cystic fibrosis, multidrug resistance and bacterial transport. Nature. 1990 Jul 26;346(6282):362–365. doi: 10.1038/346362a0. [DOI] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E. M., KRAUSKOPF B., BARON L. S. GENETIC MAPPING OF VI AND SOMATIC ANTIGENIC DETERMINANTS IN SALMONELLA. J Bacteriol. 1965 Aug;90:302–308. doi: 10.1128/jb.90.2.302-308.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann B., Jann K. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol Immunol. 1990;150:19–42. doi: 10.1007/978-3-642-74694-9_2. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Baron L. S. Genetic transfer of the Vi antigen from Salmonella typhosa to Escherichia coli. J Bacteriol. 1969 Jul;99(1):358–359. doi: 10.1128/jb.99.1.358-359.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörnvall H. Horse liver alcohol dehydrogenase. On the primary structures of the isoenzymes. Eur J Biochem. 1970 Sep;16(1):41–49. doi: 10.1111/j.1432-1033.1970.tb01050.x. [DOI] [PubMed] [Google Scholar]
- Kolyva S., Waxin H., Popoff M. Y. The Vi antigen of Salmonella typhi: molecular analysis of the viaB locus. J Gen Microbiol. 1992 Feb;138(2):297–304. doi: 10.1099/00221287-138-2-297. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Hopkins I., Moxon E. R. Capsule loss in H. influenzae type b occurs by recombination-mediated disruption of a gene essential for polysaccharide export. Cell. 1988 May 6;53(3):347–356. doi: 10.1016/0092-8674(88)90155-9. [DOI] [PubMed] [Google Scholar]
- Kroll J. S., Loynds B., Brophy L. N., Moxon E. R. The bex locus in encapsulated Haemophilus influenzae: a chromosomal region involved in capsule polysaccharide export. Mol Microbiol. 1990 Nov;4(11):1853–1862. doi: 10.1111/j.1365-2958.1990.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lemaire H. G., Müller-Hill B. Nucleotide sequences of the gal E gene and the gal T gene of E. coli. Nucleic Acids Res. 1986 Oct 10;14(19):7705–7711. doi: 10.1093/nar/14.19.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maitra U. S., Ankel H. Uridine diphosphate-4-keto-glucose, an intermediate in the uridine diphosphate-galactose-4-epimerase reaction. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2660–2663. doi: 10.1073/pnas.68.11.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher R. B., Tait R. C., Betlach M., Boyer H. W. Protein expression in E. coli minicells by recombinant plasmids. Cell. 1977 Mar;10(3):521–536. doi: 10.1016/0092-8674(77)90039-3. [DOI] [PubMed] [Google Scholar]
- Ohlsson I., Nordström B., Brändén C. I. Structural and functional similarities within the coenzyme binding domains of dehydrogenases. J Mol Biol. 1974 Oct 25;89(2):339–354. doi: 10.1016/0022-2836(74)90523-3. [DOI] [PubMed] [Google Scholar]
- Pavelka M. S., Jr, Wright L. F., Silver R. P. Identification of two genes, kpsM and kpsT, in region 3 of the polysialic acid gene cluster of Escherichia coli K1. J Bacteriol. 1991 Aug;173(15):4603–4610. doi: 10.1128/jb.173.15.4603-4610.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Royer T. J., Mainzer S. E., Schmidt B. F. Carbohydrate utilization in Streptococcus thermophilus: characterization of the genes for aldose 1-epimerase (mutarotase) and UDPglucose 4-epimerase. J Bacteriol. 1990 Jul;172(7):4037–4047. doi: 10.1128/jb.172.7.4037-4047.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. D., Robbins J. B. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984 Sep;150(3):436–449. doi: 10.1093/infdis/150.3.436. [DOI] [PubMed] [Google Scholar]
- Roderick S. L., Banaszak L. J. The three-dimensional structure of porcine heart mitochondrial malate dehydrogenase at 3.0-A resolution. J Biol Chem. 1986 Jul 15;261(20):9461–9464. [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Roychoudhury S., May T. B., Gill J. F., Singh S. K., Feingold D. S., Chakrabarty A. M. Purification and characterization of guanosine diphospho-D-mannose dehydrogenase. A key enzyme in the biosynthesis of alginate by Pseudomonas aeruginosa. J Biol Chem. 1989 Jun 5;264(16):9380–9385. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. N., Boulnois G. J., Roberts I. S. Molecular analysis of the Escherichia coli K5 kps locus: identification and characterization of an inner-membrane capsular polysaccharide transport system. Mol Microbiol. 1990 Nov;4(11):1863–1869. doi: 10.1111/j.1365-2958.1990.tb02035.x. [DOI] [PubMed] [Google Scholar]
- Taylor S. S. Amino acid sequence of dogfish muscle lactate dehydrogenase. J Biol Chem. 1977 Mar 10;252(5):1799–1806. [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Tsang R. S., Chau P. Y. Production of Vi monoclonal antibodies and their application as diagnostic reagents. J Clin Microbiol. 1987 Mar;25(3):531–535. doi: 10.1128/jcm.25.3.531-535.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga R. K., Terpstra P., Hol W. G. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986 Jan 5;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Wyk P., Reeves P. Identification and sequence of the gene for abequose synthase, which confers antigenic specificity on group B salmonellae: homology with galactose epimerase. J Bacteriol. 1989 Oct;171(10):5687–5693. doi: 10.1128/jb.171.10.5687-5693.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeschnigk M., von Wilcken-Bergmann B., Starzinski-Powitz A. cDNA from rat cells with reconstitutive galactose-epimerase activity in E. coli. Nucleic Acids Res. 1990 Sep 11;18(17):5289–5289. doi: 10.1093/nar/18.17.5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]