Abstract
Background & Aims:
Controversy exists as to whether patients with inflammatory bowel disease have an underlying immunodeficiency. We have focused on a murine model of the Wiskott-Aldrich syndrome, an immunodeficiency in which autoimmunity can manifest in the form of an inflammatory bowel disease-like illness. Wiskott-Aldrich syndrome protein (WASP) deficiency in mice results in similar clinical features. We characterized the colitis in WASP-deficient mice.
Methods:
WASP-deficient mice were followed clinically and histologically. Immunological studies were performed to determine the pathogenic cell population(s), the predominant cytokine expression pattern, and the role of cytokine(s) in colitis pathogenesis.
Results:
All WASP-deficient mice develop colitis by six months of age. Lymphocytes are required for disease induction, and CD4+ T cells from WASP-deficient mice are sufficient to induce disease in lymphocyte-deficient hosts. Lamina propria preparations from WASP-deficient mice demonstrated elevations in IFN-γ, IL-4, and IL-13 levels but decreased IL-6 and no difference in IL-17 expression in comparison to WT controls. Treatment with neutralizing antibody to IL-4, but not to IFN-γ, abrogated colitis development. However, mice deficient in both WASP and IL-4, showed no difference in histologic colitis scores at 24 weeks of age compared to WASP-deficient mice.
Conclusions:
These results demonstrate a critical role for lymphocytes and a relative Th2 cytokine predominance in the colitis associated with WASP-deficient mice. This is the only model of colitis with elevated Th2 cytokines and aberrant natural Treg function and is unique in having a human disease counterpart with similar defects.
Introduction
The precise abnormalities that lead to inflammatory bowel disease remain unknown. A dysregulated innate and/or adaptive immune response to the commensal bacterial flora plays a central role in disease pathogenesis, as highlighted by a wide variety of animal models1-5. Most animal models of colitis have implicated T cells, especially CD4+ cells, as the mediators of inflammation, whether through an activated effector T cell population reactive to normal intestinal flora, regulatory T cell dysfunction, or an imbalance between pro- and anti-inflammatory cytokine production or function.
The inflammation associated with most IBD models appears to be associated and/or mediated, at least in part, by Th1 (i.e. IFN-γ, IL-12, TNF-α, and IL-2)3-5 or Th17 cytokines (i.e. IL-17 and IL-23)6-10. Inhibition of Th1 cytokine function or production has been shown to abrogate colitis development in several Th1-mediated models11-13. No similar increase in IL-4 has been observed in these models.
Indeed, out of almost 40 murine models of IBD, only a handful have been associated with a Th2 pattern of cytokine expression14-21. In these models, IL-4 is upregulated, frequently with elevations of IL-13 and/or IL-5. Treatment with anti-IL-4 antibody (Ab) has been shown to abrogate disease, at least in part, in some models16, 22, 23.
We have generated a model of IBD that results from the deletion of the gene that encodes for the Wiskott-Aldrich syndrome protein (WASP)24. WASP is a signaling molecule that integrates surface-receptor signals to the actin cytoskeleton and is altered or absent in patients with Wiskott-Aldrich syndrome (WAS)25. This rare X-linked immunodeficiency is characterized by eczema, thrombocytopenia, lymphoreticular malignancies, and recurrent infections26 with up to 70% of patients developing autoimmune diseases, including an inflammatory bowel disease-like colitis27-31. Also as in humans, WASP deficiency in mice is associated with lymphopenia, mild thrombocytopenia, profound T cell signaling defects24, 32, and a decrease in natural Treg number and function33-36. Hematopoietic cells from WKO mice, like human WAS cells, have defects in migration37, 38. Importantly, the majority of WKO mice develop colitis that is characterized by a neutrophilic and lymphocytic infiltrate into the colonic lamina propria24.
In this study, we investigated the pathogenic processes essential to the induction of colitis in WKO mice. We characterized the natural history of colonic inflammation, the leukocyte populations that are critical for disease induction, and the cytokine milieu associated with disease activity. Our findings have relevance not only to the study of inflammatory bowel disease but also to those focusing on the pathogenesis and treatment of primary immunodeficiencies and autoimmunity.
Materials and Methods
Mice
WASP KO (WKO) mice were generated on a 129 SvEv background24. Wildtype (WT) and RAG-2 KO mice were obtained from Taconic (Hudson, NY) on a 129 SvEv background. WASP/RAG double KO (WRDKO) mice were generated by crossing WKO mice with RAG-2 KO mice. WASP/IL-4 double KO mice were generated by crossing WKO mice with IL-4 KO mice (C57BL6 background) and backcrossed onto 129 for five generations. Mice were maintained in specific pathogen free (SPF) animal facilities at Massachusetts General Hospital (Boston, MA). All experiments were conducted upon approval and according to regulations of the Subcommittee on Research Animal Care (SRAC) of the Massachusetts General Hospital.
Clinical, histologic, and immunohistochemical analysis
Please see Supplemental Information
Cell isolation and CD4+ T cell purification
Please see Supplemental Information
Flow Cytometric Analysis
Please see Supplemental Information.
Analysis of cytokine production
Please see Supplemental Information.
Antibody treatment
Seven to eight WKO mice were administered weekly intraperitoneal injections of 1mg/mouse of either rat anti-mouse IL-4 antibody or control rat IgG antibodies diluted in PBS starting at 10 weeks of age for eight weeks, following a modified protocol16, 22. Rat anti-mouse IL-4 antibody was purified from the 11B11 hybridoma cell line as described23. Seven to eleven WKO mice were administered weekly intraperitoneal injections of 2mg/mouse of either rat anti-mouse IFN-γ antibody39 or control rat IgG antibodies diluted in PBS starting at 10 weeks of age for eight weeks as described13. Rat anti-mouse IFN-γ antibody was purified from the XMG1.2 hybridoma cell line39. Rat IgG antibodies were obtained from Antibodies Incorporated (Davis, CA). Mice were weighed weekly, and sacrificed one week after the last injection.
Adoptive Cell Transfer of CD4+ T cells
Please see Supplemental Information
Bone Marrow Transplantation
Please see Supplemental Information
Intracellular Cytokine Staining
Please see Supplemental Information
Th1 and Th2 skewing of CD4+ T cells
CD4+ T cells from mesenteric and peripheral lymph node cells and spleens were isolated from 10-week-old female WT (n = 3) and WKO (n = 3) mice, and after RBC lysis, Th polarization was performed as previously described40 with a few modifications (please see Supplemental Information for details). On day 6, cells were split into two groups, one for intracellular cytokine staining and one for cytokine secretion analysis via ELISA. Cells that were cultured for ELISA were stimulated with PMA (1.25ng/ml) and ionomycin (250ng/ml) for 48 hours, after which, the supernatants were collected and analyzed by ELISA.
Statistical analysis
All comparisons were done using two-tailed Student's t-test.
Results
WKO mice develop an IBD-like disease limited to the colon by four months of age
Our initial studies of WKO mice revealed frequent signs of colitis, including wasting, rectal prolapse, diarrhea, and sometimes early death24. In the current study, we characterized, in detail, the colonic inflammation of WKO mice.
Gross examination of the gastrointestinal tract demonstrated a normal small intestine but a substantial thickening of the colon (Figure 1A, upper left panel) throughout its entire length. Some mice developed rectal prolapse. Severe disease was often associated with shortening of the colon, adhesion to neighboring tissues, and extensive enlargement of mesenteric lymph nodes. The spleen was often enlarged (Figure 1A), usually correlating with disease activity, where WKO spleens weighed, on average, three times as much as WT spleens (220mg in 6-month old WKO mouse [n=4] compared to 61mg in 6-month old WT mice [n = 4], p < 0.0005).
Figure 1. A majority of WKO mice develop spontaneous colitis and splenomegaly.

(A) Upper left panel shows normal WT (top) compared to thickened WKO (bottom) colons. H&E staining reveals crypt elongation and a large inflammatory cell infiltrate into the lamina propria of WKO colon (right, objective: 10x) compared to normal WT colon (lower left, objective: 10x). Splenomegaly is often associated with colitis in WKO mice (upper middle panel). Bar represents 1cm. (B) Prevalence of clinical (weight loss, diarrhea, rectal prolapse, or death) and microscopic (wall thickening, LP infiltration, crypt abscess) signs of colitis by age. Note, none of the 11 WT mice followed concurrently demonstrated any clinical or histologic signs of colitis.
To quantify the incidence and severity of colitis development, 11 WT and 30 WKO mice were followed for four months starting at two months of age. Weekly observations of clinical signs (i.e. diarrhea, weight loss, rectal prolapse, death) revealed disease prevalence in WKO mice to be 10% by three months and approximately 50% by five months with no WT mice developing clinical disease (Figure 1B). Almost half of those affected had severe or fatal colitis. Course of disease was progressive; death started to occur at four months of age.
Epithelial hyperplasia and leukocyte infiltration characterize the colonic mucosa in WKO mice
Microscopic evidence of colitis in WKO mice occurred earlier than the development of clinical signs. Six to eight WKO mice in each group were sacrificed at various ages to determine the prevalence of disease at specific time points. The results demonstrated that 71% of WKO mice at 3 months of age showed microscopic evidence of colonic inflammation with an overall average microscopic colitis score of 2.4 out of 8 (Figure 1B). By 6 months of age, 100% of WKO mice had signs of colitis with an average microscopic score of 5.7. None of the 11 WT mice at 6 months of age showed any macroscopic or microscopic evidence of colitis.
Histologically, the colitis was characterized by crypt elongation, epithelial hyperplasia, and an extensive infiltration of inflammatory cells into the lamina propria (LP - Figure 1A, right panel) with occasional crypt abscesses. Goblet cells were often depleted, and mucosal architecture was lost in severe cases, but granulomas were never observed. Correlating with mucosal thickening, WKO colons have an almost ten-fold increase in colonic LP cells per colon compared with WT colons (9.55×106 cells/colon, n = 18 vs. 1.15×106 cells per colon, n = 78, respectively). Immunohistochemical staining demonstrated the LP infiltrate to consist of CD4+ T cells24, CD8+ T cells, F4/80+ macrophages, and CD11c+ dendritic cells (Figure 2). The presence of neutrophils in the colonic LP was reported previously24. In addition, clusters of plasma cells are found scattered throughout the lamina propria (data not shown).
Figure 2. WKO colonic LP is infiltrated with several distinct leukocyte subsets.

Note an increase of macrophages (F4/80+ cells, black arrowheads), CD8+ cells (black arrows), and dendritic cells (CD11c+ cells, black curved arrows) in WKO colonic lamina propria. Data are representative of stainings of 2-4 WT and WKO samples each. Staining was performed on WKO colons with severe colitis (age > 6 months). All micrographs were taken with 10x objective.
Activated T cells are expanded in both WKO LP and peripheral lymphoid organs
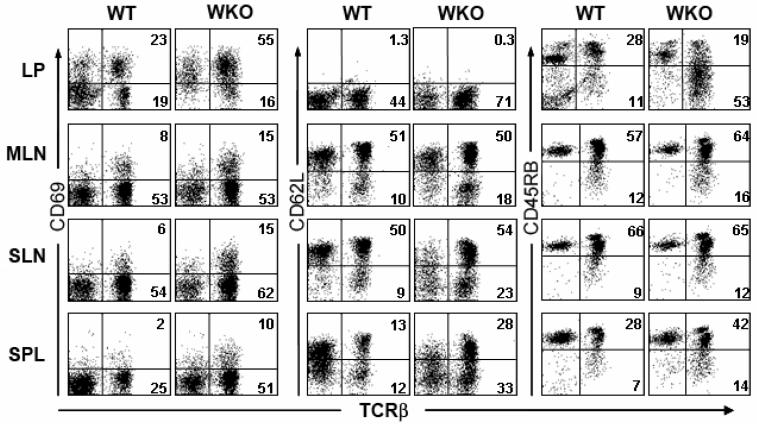
Next, we examined the immunophenotype of the WKO LP T cells from 3-4 month old mice. We observed that the majority of these cells were activated (CD69+, CD62Llo, CD45RBlo, Figure 3). This expanded population of activated T cells consists of both CD4+ cells and CD8+ cells (data not shown). We then investigated whether the expansion of activated T cells in WKO mice is unique to the LP compartment. In mesenteric and subcutaneous lymph nodes and spleen, there is an increase in activated CD4+ T cells (CD69+, CD62Llo, Figure 3) in WKO mice compared to WT. However, in contrast to the activated cells in the LP, these cells express a higher level of CD45RB (Figure 3).
Figure 3. WKO colonic LP contains activated T cells.
Lymphocytes from WT and WKO colonic lamina propria (LP), mesenteric lymph nodes (MLN), subcutaneous lymph nodes (SLN), and spleen (SPL) were analyzed for activation markers. WKO LP is comprised of a higher percentage of T cells (i.e., TCRβ+) than WT. There is marked expansion of activated T cells (as evidenced by CD69+ and CD62L− staining) in the LP and less striking expansion in other lymphoid compartments in WKO mice compared to WT mice. Data are representative examples of at least three individual experiments. Mice used in the experiment were 3-4 months old.
WKO lymphocytes are essential and T cells are sufficient for initiating colitis
Since WASP is expressed predominantly in hematopoietic cells, we expected that the pathogenic cell population(s) must originate from this lineage. To confirm this assumption, bone marrow transplant experiments were performed using total bone marrow cells (BMCs). All mice receiving WKO BMCs (n = 4) developed macroscopic and microscopic signs of colitis; whereas there was no evidence of colitis in recipients of WT BMCs (n = 5). Representative sections of each group are shown in Figure 4A.
Figure 4. WKO lymphocytes are required and CD4+ T cells are sufficient for colitis induction.

(A) Representative cross sections of colons from WKO or WT bone marrow cells (BMC) → RAG-2 KO transfer recipients (objective: 4x). Note the absence of colitis in recipients that received only WT BMCs compared to the markedly thickened colon of recipients of WKO BMCs. (B) WRDKO mice do not develop colitis (objective: 4x). (C) Macroscopic (upper panels) and microscopic analyses (lower panels, objective: 10x) demonstrated that CD4+ T cells from WKO (right) but not WT mice (center) can transfer colitis to RAG-2 KO recipients. RAG-2 KO control mouse colons are shown on the left.
We next examined whether lymphocytes were required for colitis development. To address this issue, we generated WASP and RAG-2 double KO mice (WRDKO), which lack T and B lymphocytes while other hematopoietic lineages remain intact. Initial observations of approximately 30 WRDKO mice have found them to be viable with normal development but a shorter lifespan in a small percentage of mice. Of note, none of the 30 mice had diarrhea, and analyses of WRDKO intestines, both macroscopically and microscopically, did not reveal any sign of colitis (Figure 4B). Three out of 14 WRDKO mice aged 4-6 months developed minimal thickening of colonic crypts without inflammatory cell infiltration (overall average microscopic colitis score: 0.25, p < 0.0001 compared to 4-month old WKO mice whose scores averaged 3.8) in comparison to 87% of WKO mice of the same age with average microscopic colitis scores ranging from 3.8 to 5.7 (Figure 1C). These data indicate that lymphocytes are essential for colitis development in WKO mice.
To determine whether CD4+ WKO T cells are sufficient to cause disease, CD4+ T cells derived from either WT or WKO mesenteric lymph nodes (MLN) were adoptively transplanted into RAG-2 KO recipient mice. Analysis ten weeks post cell transfer revealed significant colitis (average microscopic colitis score 3.4, Figure 4C) in all recipients of WKO CD4+ cells. RAG-2 KO control mice without cell transfer and RAG-2 KO recipients of WT CD4+ cells did not develop colitis (Figure 4C), with a p < 0.01 comparing colitis scores of WKO CD4+ cell recipient colons versus those of WT CD4+ cell recipient colons. To assess whether CD4+ T cells from young, healthy (2-month-old) WKO mice were also colitogenic, we performed a second set of experiments comparing recipients of CD4+ T cells from either non-colitic (young) or colitic (older) WKO mice. Recipient animals of both groups developed colitis with no significant difference in their colitogenic potential (data not shown). These results demonstrate the pathogenic potential of WKO CD4+ T cells, even from young, pre-colitic mice.
WKO colitis is associated with an increase in Th2 cytokines
The Th2 cytokines IL-4 and IL-13 and the Th1-cytokine IFN-γ were found to be markedly elevated in WKO colonic LP cells compared to WT LP cells (Figure 5A). Levels of IL-4 and IL-13 elevation correlated with the extent of gross colonic thickening (data not shown). Similar results were seen after stimulation with anti-CD3 + anti-CD28 antibodies (data not shown). In contrast, there was no increase in levels of IL-5, IL-12p70, or IL-17 (Figure 5A). Expression of IL-10 was mildly elevated and IL-6 decreased to approximately 50% of levels seen in WT mice (Figure 5A). IL-2 expression was significantly elevated in WKO LP cells stimulated with PMA and ionomycin (Figure 5A) but not when cells were stimulated with anti-CD3 and anti-CD28 Ab (data not shown), consistent with our previous observations in peripheral WKO T cells24. Lastly, stimulated LP cells show elevated levels of TNF-α in most but not all WKO mice (Figure 5A). Overall, the profound elevations in IL-4 and IL-13, even with some elevation of Th1 cytokines, indicates a relative Th2 skewing.
Figure 5. Relative Th2 skewing associated with colitis in WKO mice.

(A) WKO LP cells secrete markedly elevated levels of IL-4 and IL-13 as well as IFN-γ, TNF-α, and IL-2 with a decrease in IL-6. Results shown are means ± SEM pooling from two to four experiments, *p < 0.05 between WT and WKO stimulated with P+I. (B) Only IL-4 and IL-10 are mildly elevated in WKO MLN compared to WT. Results shown are means ± SEM pooling from two to four experiments, *p<0.05 between WT and WKO stimulated with P+I. (C) Shown are representative sections of two independent experiments of WT (left panel, objective: 10x) and inflamed (5-month-old) WKO (right panel, objective: 10x; inset, objective: 40x) colonic sections stained for IL-4 by immunohistochemistry. US = unstimulated; P+I = stimulated with PMA and ionomycin.
We next investigated whether the same cytokine expression pattern existed in the MLN and spleen in WKO mice. Only IL-4 and IL-10 were elevated in MLN from WKO mice compared to WT mice (Figure 5B). There was no elevation of any cytokines tested (IL-4, IL-10, IL-13, IL-5, and IFN-γ) in the spleen (data not shown). Consistent with the increase in Th2 cytokines in WKO LP in vitro, immunoperoxidase staining revealed IL-4 protein expression in five-month old WKO, but not WT, colon (Figure 5C).
IFN-γ Neutralization Does not Alleviate WKO Colitis
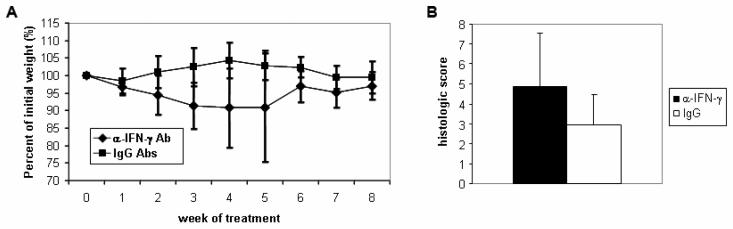
Given the elevation in IFN-γ levels of WKO mouse colonic LP cells, we investigated the role of IFN-γ in colitis pathogenesis by the administration of anti-IFN-γ antibody. The anti-IFN-γ Ab has been used previously for neutralizing IFN-γ39 and, as shown below, is effective at inducing a Th2 phenotype. Anti-IFN-γ Ab-treated mice (n = 7) weighed less than control IgG Ab-treated mice (n = 11) throughout the treatment course (Figure 6A), and consistent with this finding, at 9 weeks after initiation of antibody treatment, histologic colitis scores were slightly higher in anti-IFN-γ Ab-treated mice than control IgG Ab-treated mice (4.9 versus 3.0, p = 0.07; Figure 6B). These results demonstrate that IFN-γ does not play a major pro-inflammatory role in colitis development in WKO mice.
Figure 6. Neutralization of IFN-γ does not alleviate colitis in WKO mice.
(A) Mice treated with weekly injections of anti-IFN-γ antibody (n = 7), but not mice treated with control IgG antibodies (n = 11), lost weight. (B) Average histologic score of anti-IFN-γ Ab-treated mice compared to control IgG Ab-treated mice one week after final antibody treatment (p = 0.07).
WKO colitis is ameliorated by IL-4 neutralization but is not entirely dependant on IL-4
Given the marked elevation in IL-4 expression by WKO colonic LP cells, we next determined the importance of this cytokine in colitis development by a similar neutralizing antibody administration as that above. IL-4 neutralization attenuated the development of colitis in WKO mice (Figure 7A-7B). The majority of mice treated with anti-IL-4 antibody, but not control IgG antibodies, gained weight (Figure 7A, p < 0.05 one week after last injection). Given the young age of these mice, weight gain at this period is expected; the failure to gain weight suggests a pathologic process. In addition, microscopic colitis scores on necropsy one week after the last injection demonstrated moderate to severe colitis in three out of seven mice treated with control rat IgG (overall average microscopic colitis score: 2.2), while only one out of eight mice treated with anti-IL-4 Ab had minimal signs of colitis (overall average microscopic score 0.1, p < 0.05). Representative sections of colons from mice treated with anti-IL-4 Ab and mice treated with control IgG Ab that developed colitis are shown in Figure 7B.
Figure 7. Neutralization of IL-4 attenuates WKO colitis.

(A) Mice treated with weekly injections of anti-IL-4 antibody, but not mice treated with control IgG antibodies, gained weight. (B) Representative sections from mice treated with anti-IL-4 antibody and mice treated with control IgG antibodies that developed colitis (objective: 10x). (C) LP cells from mice treated with anti-IL-4 antibody secreted negligible amounts of cytokines. In contrast, LP cells from mice treated with control rat IgG antibodies that developed severe colitis secreted a moderate amount of IL-10 and a large amount of IL-4, IL-13, and IFN-γ, *p < 0.05 between cells stimulated with P+I from mice treated with anti-IL-4 antibody and mice treated with control IgG antibodies that developed colitis. Shown are means ± SEM pooling from two to four mice in each group. US = unstimulated; P+I = stimulated with PMA and ionomycin. (D) WASP/IL-4 DKO mice demonstrated higher weights and lower clinical colitis scores at 18 weeks of age than WKO counterparts that were followed concurrently.
We confirmed the efficacy of the IL-4 neutralization by analyzing IL-4 secretion in LP cells (Figure 7C). IL-4 expression was elevated in IgG antibody-treated mice with colitis, up to the level seen in untreated colitic WKO mice. In contrast, IL-4 levels were normalized in anti-IL-4 antibody-treated WKO mice. LP cells from IgG antibody-treated WKO mice without colitis had similar IL-4 expression as WKO mice treated with anti-IL-4 antibody or WT mice. As seen in untreated colitic WKO mice (Figure 5A), LP cells from colitic mice treated with control IgG antibodies had marked elevations in IL-4, IL-13, and IFN-γ and mild elevation in IL-10 (Figure 7C).
To further examine the role of IL-4 in the development of colitis in WKO mice, we generated WASP/IL-4 double KO (DKO) mice and followed them concurrently with WKO mice over a six-month period of time. As shown in Figure 7D, at 18 weeks of age, DKO mice had higher average weight (represented by the average of the percentage of initial weight, p < 0.01) and lower clinical scores than the WKO mice. By six months of age, this delayed development of disease was no longer apparent and there was no clear difference in histologic scores between WKO (n = 26) and WASP/IL-4 DKO (n = 27) mice (data not shown).
WKO CD4+ T Cells Are Able to be Skewed to Th1 or Th2 Phenotypes
Given the markedly elevated levels of IL-4 and IL-13 in WKO colonic LP, we explored whether WKO CD4+ T cells express IL-4 at baseline and their relative ability to be skewed in vitro to either Th1 or Th2 cytokine secreting cells. CD4+ T cells from age-matched, gender-matched WT and pre-colitic WKO mice were cultured in non-polarizing (Th0) or either Th1 or Th2 polarizing conditions. Cells were stained for intracellular cytokine production (Figure 8) and protein levels determined by ELISA (data not shown). Analogous to WT cells, WKO cells were capable of being skewed to either Th1 (i.e., IFN-γ-producing) or Th2 (i.e. IL-4 producing) cells (Figure 8). Furthermore, there was no difference in baseline IL-4 production in WKO Th0 cells when compared with WT cells (Figure 8).
Figure 8. CD4+ T cells from WKO can be skewed to secrete Th1 or Th2 cytokines.
(A) Representative flow cytometry plots of intracellular IL-4 and IFN-γ staining in WT (n = 3) and WKO (n = 3) CD4+ T cells polarized to Th0, Th1, or Th2 by appropriate stimulations. (B) Graph representing the mean percentage ± SD of CD4+ T cells expressing IL-4 or IFN-γ in cells isolated from WT (n = 3) and WKO (n = 3) mice polarized in Th0, Th1, or Th2 conditions.
Discussion
Murine models of IBD have permitted intense investigation into the pathogenesis of mucosal inflammation3-5. The colitis observed in WKO mice is unique because of both the presence of disease in human patients with the same genetic defect and the relative Th2 cytokine skewing. WKO mice develop spontaneous colitis starting at three months of age with 100% of mice affected by six months of age. The disease is progressive, eventually leading to early death in most cases. A similar observation of colitis, although milder and without further characterization, has recently been reported in WKO mice on the C57BL/6 background34.
Most murine models of IBD require CD4+ T cells for disease induction3, 4. Similarly, we demonstrated that WKO lymphocytes are required for disease initiation and CD4+ T cells are sufficient to induce disease. CD4+ T cells from both young, non-colitic as well as older, colitic WKO mice induce colitis in lymphocyte-deficient recipients. For further categorization of CD4+ T cell-associated disease, animal models of IBD have been classified into type 1 models where an abnormal effector cell population is present, and type 2 models where a lack and/or dysfunction of regulatory cells appears to be the triggering event3. Although our data are not yet sufficient to fully classify the colitis in WKO mice into one of these two categories, we and others have recently described aberrant generation and function of natural Tregs in WKO mice34-36, an observation consistent with that found in humans WAS patients34-36. Thus, abnormal production and/or survival and/or function of regulatory T cells may be one mechanism leading to colitis associated with WASP deficiency. Although we have demonstrated that CD4+ T cells from WKO mice are able to transfer disease to immunodeficient hosts, it remains to be determined whether an aberrant non-T cell population is required for the priming of dysregulated T cells.
Overall, the cytokine produced by stimulated LP cells isolated from inflamed WKO mice exhibit a mixed Th1/Th2 pattern with an unique Th2 predominance. WKO LP cells produce elevated levels of IFN-γ, IL-4, and IL-13 in vitro, and increased IL-4 expression was demonstrated in situ. There was no difference in IL-17 production, and IFN-γ is not pathogenic, since IFN-γ neutralization failed to alleviate disease. In contrast, IL-4 neutralization attenuated disease development, suggesting an important role of IL-4 in colitis initiation. However, although weight and clinical colitis scores were attenuated at 18 weeks of age in DKO mice when compared to WKO mice, this delay in disease development was no longer apparent at six months of age suggesting that, in the absence of IL-4, other cytokines can drive colitis development in this model. Although the underlying mechanism responsible for the relative Th2 cytokine skewing in WKO LP remains unknown, aberrant expression of Th1 associated transcription factors may be contributing factor, as has been recently reported in human WAS cells41.
Since Th2 cytokines (IL-5 and IL-13) have been identified in the LP of patients with ulcerative colitis42, the colitis associated with WASP deficiency may offer unique insight into the pathogenesis of this form of human IBD. Unlike the other well-characterized spontaneous Th2 model of IBD (i.e., TCRα KO mice), WKO mice have near-normal levels of TCRα+β+ T cells in the periphery. In addition, WKO mice share the common feature of elevated IL-13 expression with the oxazolone-induced colitis model and human UC, in which NK-T cells have been demonstrated to be the source of IL-13 and possibly the initiating colitogenic cell population15, 42. Studies are underway to investigate whether NK-T cells play a pathogenic role in the immune dysregulation associated with WASP deficiency in our model.
Finally, colitis in WKO mice, unlike other animal models of IBD, offers the advantage of being the only model to have a human correlate where patients with the same genetic defect can also present with intestinal inflammations, Treg dysfunction, and clinical immunological sequelae associated with a Th2 phenotype (e.g., eczema and elevated IgE)29. Our data have implications to those investigators focusing on the pathogenesis of the WAS and the development of treatment strategies for this immunodeficiency that may utilize improvement of colitis as a measure of clinical efficacy43.
Supplementary Material
Acknowledgments
Grant support: This work was supported by grants from the NIH to S.B.S. (HL59561 and AI50950), A.K.B (DK47677), CN (DK55678), A.M. (DK64351), and to E.M. (DK64289 and DK74454); grants from the German Research Council (DFG), the Fritz-Thyssen-Foundation, and the Henkel-Foundation to C.K; postdoctoral support from the SICPA foundation and Lausanne University Hospital (M.H.M.); an AGA Student Research Fellowship Award and predoctoral and postdoctoral support from an NIH Training Grant (T32DK007191) to D. N.; CAPES to V. C.-A.; and grants from Broad Medical Foundation to E.M and A.M.
Non-standard abbreviations
- WAS
Wiskott-Aldrich Syndrome
- WASP
Wiskott-Aldrich Syndrome Protein
- KO
knockout
- WT
wildtype
- WKO
WASP KO
- WRDKO
WASP/RAG-2 double KO
- LP
lamina propria
- MLN
mesenteric lymph node
- IFN-γ
interferon-γ
- Ab
antibody
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is no conflict of interest to disclose.
References
- 1.Sartor RB. Innate immunity in the pathogenesis and therapy of IBD. J Gastroenterol. 2003;38(Suppl 15):43–7. [PubMed] [Google Scholar]
- 2.Korzenik JR. Past and current theories of etiology of IBD: toothpaste, worms, and refrigerators. J Clin Gastroenterol. 2005;39:S59–65. doi: 10.1097/01.mcg.0000155553.28348.fc. [DOI] [PubMed] [Google Scholar]
- 3.Strober W, Fuss IJ, Blumberg RS. The immunology of mucosal models of inflammation. Annu Rev Immunol. 2002;20:495–549. doi: 10.1146/annurev.immunol.20.100301.064816. [DOI] [PubMed] [Google Scholar]
- 4.Mizoguchi A, Mizoguchi E, Bhan AK. Immune networks in animal models of inflammatory bowel disease. Inflamm Bowel Dis. 2003;9:246–59. doi: 10.1097/00054725-200307000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Elson CO, Cong Y, McCracken VJ, Dimmitt RA, Lorenz RG, Weaver CT. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol Rev. 2005;206:260–76. doi: 10.1111/j.0105-2896.2005.00291.x. [DOI] [PubMed] [Google Scholar]
- 6.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ, Sher A. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–94. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–8. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 9.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–83. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–18. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–62. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 12.Neurath MF, Fuss I, Kelsall BL, Stuber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281–90. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson NJ, Hudak SA, Lesley RE, Menon S, Leach MW, Rennick DM. IL-12, but not IFN-gamma, plays a major role in sustaining the chronic phase of colitis in IL-10-deficient mice. J Immunol. 1998;161:3143–9. [PubMed] [Google Scholar]
- 14.Mizoguchi A, Mizoguchi E, Chiba C, Spiekermann GM, Tonegawa S, Nagler-Anderson C, Bhan AK. Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med. 1996;183:847–56. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–38. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 16.Dohi T, Fujihashi K, Rennert PD, Iwatani K, Kiyono H,, McGhee JR. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J Exp Med. 1999;189:1169–80. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habtezion A, Toivola DM, Butcher EC, Omary MB. Keratin-8-deficient mice develop chronic spontaneous Th2 colitis amenable to antibiotic treatment. J Cell Sci. 2005;118:1971–80. doi: 10.1242/jcs.02316. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida M, Shirai Y, Watanabe T, Yamori M, Iwakura Y, Chiba T, Kita T, Wakatsuki Y. Differential localization of colitogenic Th1 and Th2 cells monospecific to a microflora-associated antigen in mice. Gastroenterology. 2002;123:1949–61. doi: 10.1053/gast.2002.37049. [DOI] [PubMed] [Google Scholar]
- 19.Iqbal N, Oliver JR, Wagner FH, Lazenby AS, Elson CO, Weaver CT. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J Exp Med. 2002;195:71–84. doi: 10.1084/jem.2001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanai T, Kawamura T, Dohi T, Makita S, Nemoto Y, Totsuka T, Watanabe M. TH1/TH2-mediated colitis induced by adoptive transfer of CD4+CD45RBhigh T lymphocytes into nude mice. Inflamm Bowel Dis. 2006;12:89–99. doi: 10.1097/01.MIB.0000197237.21387.mL. [DOI] [PubMed] [Google Scholar]
- 21.Bamias G, Martin C, Mishina M, Ross WG, Rivera-Nieves J, Marini M, Cominelli F. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128:654–66. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 22.Iijima H, Takahashi I, Kishi D, Kim JK, Kawano S, Hori M, Kiyono H. Alteration of interleukin 4 production results in the inhibition of T helper type 2 cell-dominated inflammatory bowel disease in T cell receptor alpha chain-deficient mice. J Exp Med. 1999;190:607–15. doi: 10.1084/jem.190.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–39. doi: 10.1084/jem.188.10.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snapper SB, Rosen FS, Mizoguchi E, Cohen P, Khan W, Liu C-H, Hagemann TL, Kwan S-P, Ferrini R, Davidson L, Bhan AK, Alt FW. Wiskott-Aldrich syndrome protein-deficient mice reveal a role for WASP in T but not B cell activation. Immunity. 1998;9:81–90. doi: 10.1016/s1074-7613(00)80590-7. [DOI] [PubMed] [Google Scholar]
- 25.Ochs HD, Thrasher AJ. The Wiskott-Aldrich syndrome. J Allergy Clin Immunol. 2006;117:725–38. doi: 10.1016/j.jaci.2006.02.005. quiz 739. [DOI] [PubMed] [Google Scholar]
- 26.Rosen FS, Cooper MD, Wedgwood RJ. The primary immunodeficiencies. N Engl J Med. 1995;333:431–40. doi: 10.1056/NEJM199508173330707. [DOI] [PubMed] [Google Scholar]
- 27.Sullivan KE. Recent advances in our understanding of Wiskott-Aldrich syndrome. Curr Opin Hematol. 1999;6:8–14. doi: 10.1097/00062752-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh KH, Chang MH, Lee CY, Wang CY. Wiskott-Aldrich syndrome and inflammatory bowel disease. Ann Allergy. 1988;60:429–31. [PubMed] [Google Scholar]
- 29.Dupuis-Girod S, Medioni J, Haddad E, Quartier P, Cavazzana-Calvo M, Le Deist F, de Saint Basile G, Delaunay J, Schwarz K, Casanova JL, Blanche S, Fischer A. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 2003;111:e622–7. doi: 10.1542/peds.111.5.e622. [DOI] [PubMed] [Google Scholar]
- 30.Folwaczny C, Ruelfs C, Walther J, Konig A, Emmerich B. Ulcerative colitis in a patient with Wiskott-Aldrich syndrome. Endoscopy. 2002;34:840–1. doi: 10.1055/s-2002-34272. [DOI] [PubMed] [Google Scholar]
- 31.Schurman SH, Candotti F. Autoimmunity in Wiskott-Aldrich syndrome. Curr Opin Rheumatol. 2003;15:446–53. doi: 10.1097/00002281-200307000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Shehabeldin A, da Cruz LA, Butler J, Somani AK, McGavin M, Kozieradzki I, dos Santos AO, Nagy A, Grinstein S, Penninger JM, Siminovitch KA. Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott-Aldrich syndrome protein-deficient lymphocytes. J Exp Med. 1999;190:1329–42. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maillard MH, Cotta-de-Almeida V, Takeshima F, Nguyen DD, Michetti P, Nagler C, Bhan AK, Snapper SB. The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. J Exp Med. 2007;204:381–91. doi: 10.1084/jem.20061338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humblet-Baron S, Sather B, Anover S, Becker-Herman S, Kasprowicz DJ, Khim S, Nguyen T, Hudkins-Loya K, Alpers CE, Ziegler SF, Ochs H, Torgerson T, Campbell DJ, Rawlings DJ. Wiskott-Aldrich syndrome protein is required for regulatory T cell homeostasis. J Clin Invest. 2007;117:407–18. doi: 10.1172/JCI29539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marangoni F, Trifari S, Scaramuzza S, Panaroni C, Martino S, Notarangelo LD, Baz Z, Metin A, Cattaneo F, Villa A, Aiuti A, Battaglia M, Roncarolo MG, Dupre L. WASP regulates suppressor activity of human and murine CD4(+)CD25(+)FOXP3(+) natural regulatory T cells. J Exp Med. 2007;204:369–80. doi: 10.1084/jem.20061334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adriani M, Aoki J, Horai R, Thornton AM, Konno A, Kirby M, Anderson SM, Siegel RM, Candotti F, Schwartzberg PL. Impaired in vitro regulatory T cell function associated with Wiskott-Aldrich syndrome. Clin Immunol. 2007 doi: 10.1016/j.clim.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snapper SB, Meelu P, Nguyen D, Stockton BM, Bozza P, Alt FW, Rosen FS, von Andrian UH, Klein C. WASP deficiency leads to global defects of directed leukocyte migration in vitro and in vivo. J Leukoc Biol. 2005 doi: 10.1189/jlb.0804444. [DOI] [PubMed] [Google Scholar]
- 38.de Noronha S, Hardy S, Sinclair J, Blundell MP, Strid J, Schulz O, Zwirner J, Jones GE, Katz DR, Kinnon C, Thrasher AJ. Impaired dendritic-cell homing in vivo in the absence of Wiskott-Aldrich syndrome protein. Blood. 2005;105:1590–7. doi: 10.1182/blood-2004-06-2332. [DOI] [PubMed] [Google Scholar]
- 39.Fuss IJ, Marth T, Neurath MF, Pearlstein GR, Jain A, Strober W. Anti-interleukin 12 treatment regulates apoptosis of Th1 T cells in experimental colitis in mice. Gastroenterology. 1999;117:1078–88. doi: 10.1016/s0016-5085(99)70392-6. [DOI] [PubMed] [Google Scholar]
- 40.Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat Immunol. 2002;3:643–51. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 41.Trifari S, Sitia G, Aiuti A, Scaramuzza S, Marangoni F, Guidotti LG, Martino S, Saracco P, Notarangelo LD, Roncarolo MG, Dupre L. Defective Th1 Cytokine Gene Transcription in CD4+ and CD8+ T Cells from Wiskott-Aldrich Syndrome Patients. J Immunol. 2006;177:7451–61. doi: 10.4049/jimmunol.177.10.7451. [DOI] [PubMed] [Google Scholar]
- 42.Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, Fiocchi C, Strober W. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–70. [PubMed] [Google Scholar]
- 43.Klein C, Nguyen D, Liu CH, Mizoguchi A, Bhan AK, Miki H, Takenawa T, Rosen FS, Alt FW, Mulligan RC, Snapper SB. Gene therapy for Wiskott-Aldrich syndrome: rescue of T-cell signaling and amelioration of colitis upon transplantation of retrovirally transduced hematopoietic stem cells in mice. Blood. 2003;101:2159–66. doi: 10.1182/blood-2002-05-1423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.