Abstract
Small-molecule inhibitors of tau fibrillization are under investigation as tools for interrogating the tau aggregation pathway and as potential therapeutic agents for Alzheimer’s disease. Established inhibitors include thiacarbocyanine dyes, which can inhibit recombinant tau fibrillization in the presence of anionic surfactant aggregation inducers. In an effort to increase inhibitory potency, a cyclic bis-thiacarbocyanine molecule containing two thiacarbocyanine moieties was synthesized and characterized with respect to tau fibrillization inhibitory activity by electron microscopy and ligand aggregation state by absorbance spectroscopy. Results showed that the inhibitory activity of the bis-thiacarbocyanine was qualitatively similar to a monomeric cyanine dye, but was more potent with 50% inhibition achieved at ~80 nM concentration. At all concentrations tested in aqueous solution, the bis-thiacarbocyanine collapsed to form a closed clamshell structure. However, the presence of tau protein selectively stabilized the open conformation. These results suggest that the inhibitory activity of bis-thiacarbocyanine results from multivalency, and reveal a route to more potent tau aggregation inhibitors.
Keywords: Alzheimer’s disease, tau, neurofibrillary tangle, cyanine dye, aggregation
Introduction
Alzheimer’s disease (AD) is characterized in part by misfolding and self-association of the microtubule-associated protein tau within neuritic lesions [1]. Because protein aggregation reactions have been implicated in neuronal misfunction and cytotoxicity [2], small-molecule inhibitors of tau aggregation are being investigated as potential therapeutic agents for AD and other neurodegenerative diseases [3].
To facilitate the search for fibrillization antagonists, normally soluble recombinant tau preparations are typically treated with aggregation-inducing agents so that tau filaments form over experimentally tractable time periods from low bulk tau concentrations [4]. These inducers, which include anionic surfactant micelles, promote heterogeneous nucleation (i.e., nucleation owing to the presence of foreign particles) on their surfaces from which tau filaments then extend in an elongation reaction [5-7]. Tau fibrillization antagonists identified by in vitro screens involving surfactant inducers include thiacarbocyanine dyes such as N744 [8-10]. Cyanines are highly prone to self-association reactions that form dimers and higher order aggregates, leading to shifts in absorbance spectra relative to dye monomer [11]. Blue (hypsochromic) and red (bathochromic) shifted transitions are termed H-bands and J-bands, respectively. Although both classes of aggregate are composed of parallel dye molecules stacked plane-to-plane, they differ in the angle of slippage between successive molecular planes [9]. The degree of dye aggregation appears to modulate tau aggregation antagonist activity [10].
The utility of tau aggregation inhibitors will depend in part on potency. One strategy for maximizing potency is to present two or more binding moieties within a single multivalent ligand. For example, bivalent forms of acridine-based ligands inhibit prion misfolding in cellular models with up to an order of magnitude more potency than acridine monomers [12]. Multivalent ligands can act by increasing the local concentration of an active moiety. After an initial recruitment phase, improved potency results from binding avidity, which is the sum of the binding affinities of all multimeric interactions [13]. However, multivalent ligands containing rigid heterocycles can also fold into unique structures. For example, bis-thiacarbocyanines collapse in aqueous solution to form closed, clamshell structures resembling H-dimers [14]. The reaction is intramolecular, and so population of the closed structure is independent of ligand concentration. Because H-dimers have been implicated in the action of cyanine-mediated inhibition of tau aggregation [10], formation of closed clamshell ligands may have especially potent activity. Together these data predict that multivalent forms of thiacarbocyanines could have potent tau aggregation inhibitor activity, and may represent a novel route to more efficacious inhibitors.
Here we test this hypothesis using a cyclic bis-thiacarbocyanine that approximates a multivalent form of N744. Results show that the bis-thiazcarbocyanine inhibits the aggregation of full-length tau protein with >4-fold greater potency than the monomer N744. Absorbance spectroscopy measurements show that although the closed conformation predominates in aqueous solution, the presence of tau protein selectively stabilizes the fully open conformation. These data suggest that the improved potency observed with the bis-thiacarbocyanine results from ligand multivalency and not from ordered aggregate formation.
Materials and methods
Reagents
Recombinant full-length His6-htau40 [15] was prepared as described previously [16]. DMSO, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), isopropanol, methanol, and NaCl were from Fisher Scientific (Waltham, MA). Mixed histones (type II-A from calf thymus), dithiothreitol, pyridine, triethyl orthoformate, 1,5-dibromopentane, and 2-methylbenzothiazole were from Sigma-Aldrich (St. Louis, MO). Stock solutions of ODS (Research Plus, Manasquan, NJ) were prepared in 1:1 isopropanol/ddH2O and stored at room temperature. Glutaraldehyde, uranyl acetate, and 300 mesh carbon-coated copper grids were from Electron Microscopy Sciences (Ft. Washington, PA). Cyanine dye N744 [8-10] was custom synthesized by deCODE Genetics (Lemont, IL).
Chemical synthesis
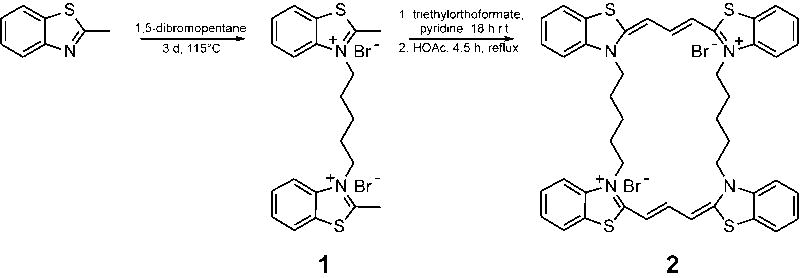
The bis-quaternary salt [19050-29-4]) (1) and cyclic N,N’-alkylene bis-thiacarbocyanine (2) were prepared as summarized in Fig. 1 [17, 18]. Synthesis of 1 has been explicitly described in the patent literature [19] and was repeated in a similar manner. 2-methylbenzothiazole (4.67 ml, 36.7 mmol) and 1,5-dibromopentane (2.5 ml, 18.4 mmol) were mixed in a sealed tube reaction vessel, cooled in dry ice/acetone, evacuated on a vacuum pump and heated in an oil bath at 115°C for three days. After cooling, the purple-pink solid was pulverized in a mortar and slurried with acetone, the pink solid collected in a filter, and dried. Yield was 7.72 g (80% yield). The salt was recrystallized from ethanol yielding 5.21 g (53.7%) of 1. Melting points (M.P.) were obtained on an Electrothermal melting point apparatus and were uncorrected. M.P. 250 - 260°C (literature 260°C) [19].
Fig. 1.
Synthetic scheme for bis-thiacarbocyanine 2.
To prepare N,N’ alkylene bis-thiacarbocyanine 2, compound 1 (495 mg, 0.937 mmol) was dissolved in 4 ml of pyridine and triethylorthoformate (5 ml, 30.1 mmol) and stirred overnight at room temperature. Glacial acetic acid (0.69 ml 12.1 mmol) was added and refluxed for 4.5 hrs. Solvents were removed in vacuo to leave 467 mg of very dark material. Thin layer chromatography on silica gel G plates (H2O: propanol: acetic acid 3:1:20 μl) [20] showed disappearance of starting material 1. Recrystallization from ethanol gave a 15.8% yield of 2. M.P. 220-229°C.
LC/MS
Samples were fractionated on a Vydac C18 MS reverse phase column (5 μm, 1.0 × 250 mm) and developed with a linear acetonitrile gradient (0% to 100%) operated at 50 μl/min. Eluted fractions were introduced (~20 μl/min) to a mass spectrometer (Micromass LC-Tof™ II, Wythenshawe, UK) equipped with an orthogonal electrospray source (Z-spray) operated in positive ion mode and calibrated (m/z 100 – 2000) with sodium iodide. Optimal electrospray ionization conditions were: capillary voltage 3000 V, source temperature 110°C and a cone voltage of 55 V. The electrospray gas was nitrogen. Q1 was set to optimally pass ions from m/z 100 – 2000 and all ions transmitted into the pusher region of the time-of-flight analyzer were scanned (m/z 100-1000) with a 1 s integration time. Data were acquired in continuum mode during the LC run.
Fibrillization assays
Tau was incubated (37°C for 18 h) without agitation in assembly buffer (10 mM HEPES, pH 7.5, 100 mM NaCl, 5 mM dithiothreitol) in the presence or absence of fibrillization inducer ODS and cyanine inhibitors. Control reactions were normalized for DMSO vehicle (2% (v/v) final concentration). Reactions were stopped with glutaraldehyde, stained with uranyl acetate, and subjected to electron microscopy as described previously [21].
Spectroscopy
Cyanine structure was assessed using absorbance spectroscopy [10]. Compound 2 was analyzed (Varian Cary 50 Bio) over the wavelength range 400 - 650 nm in water, methanol, or assembly buffer containing ODS inducer and either 4 μM htau40 or 0.6 μM mixed histones [6].
Results
Synthesis and inhibitory activity
The target cyclic N,N’-alkylene bis-thiacarbocyanine 2 was prepared as shown in Fig. 1 using the two-step synthetic route of Wilson [17, 18]. The molecule contains two cyanine moieties connected by two flexible pentameric linkers (Fig. 1). This structure was chosen for analysis because it is a multivalent ligand with strong spectral characteristics that is capable of intramolecular folding [14]. LC/MS analysis of 2 returned the expected mass (m/z 377 [M2+]), and indicated that it composed ~95% of the preparation by weight.
To determine whether the bis-thiacarbocyanine had increased anti tau-fibrillization potency relative to monomeric cyanine antagonist N744, htau40 was incubated in the presence of ODS inducer and increasing concentrations of 2 and under near-physiological conditions of pH, ionic strength, bulk tau concentration (4 μM), and reducing environment. Under these conditions, N744 activity was biphasic, with inhibition predominating at submicromolar concentrations (IC50~380 nM) followed by a loss of inhibition at higher concentrations (Fig. 2; [10]). Compound 2 also modulated tau fibrillization in a biphasic concentration dependent manner, but was a more potent inhibitor of htau40 fibrillization (IC50 ~80 nM) than N744 under the same reaction conditions (Fig. 2). It also lost potency at higher concentration, retracing the IC50 at ~600 nM (Fig. 2). These data show that bis-cyanine 2 can qualitatively reproduce the behavior of monomeric cyanine N744, but with greater potency.
Fig. 2.
Bis-thiacarbocyanine 2 is a potent inhibitor of tau fibrillization. Tau (4 μM) was incubated without agitation in assembly buffer containing ODS inducer (50 μM) and various concentrations of compound 2 (●) or N744 (○). Filaments were then stained with uranyl acetate and viewed by electron microscopy. Each data point represents total filament length expressed as the normalized percentage of filament length measured in DMSO vehicle alone (triplicate determinations ± SD), whereas the solid lines are drawn to aid visualization. Compound 2 was >4-fold more potent than N744 under these conditions.
Structure
Compound 2 can adopt multiple conformations and aggregation states [14]. In monomeric form, it populates either open or closed conformations (Fig. 3). The closed conformation, which is stabilized by the stacking of cyanine chromophores, predominates in polar solvents such as water but is destabilized in lower-polarity solvents such as methanol. In contrast, the open conformation does not support intramolecular interaction among cyanine chromophores (Fig. 3). Because of the intramolecular electronic coupling effects that accompany the closed conformation, its absorbance maximum is blue shifted relative to the open conformation and easily detected spectroscopically.
Fig. 3.
Model for the effects of solvent polarity and ligand concentration on the conformation and aggregation state of 2. At low concentrations, 2 is a monomer, with open and closed conformations populated depending on solvent polarity. In water, a polar solvent, the closed conformation predominates, whereas the open conformation predominates in less-polar solvents such as methanol. High ligand concentrations support aggregation. See text for details.
Cyanines including 2 also aggregate in solution from both open and closed conformations to form supramolecular structures [22]. Aggregation propensity is modulated by ligand concentration and solvent polarity (Fig. 3). Shifts in absorbance spectra accompany aggregation state [14]. To characterize 2 quaternary structure in solution, its absorbance spectrum was measured as a function of concentration and normalized on the basis of extinction coefficient. In water, the absorbance spectrum was characterized by three maxima at ~470, ~520, and ~560 nm (Fig. 4A). These correspond to H-aggregate, open monomer, and closed monomer, respectively [14]. Consistent with these assignments, increases in 2 concentration in water led to increased amounts of H-aggregate at the expense of open and closed monomers (Fig. 4A). The relative abundance of closed and open conformation strongly favored the former at all concentrations, indicating that closed monomer was stable in aqueous solution. A different absorbance pattern was observed when 2 concentration was held constant and solvent polarity was varied using methanol/water mixtures. Under these conditions, increasing concentrations of methanol stabilized the open monomer conformation at the expense of both aggregated and closed-monomer forms (Fig. 4B). These data show that distinguishing spectral characteristics of 2 isoforms can be used to assess its folded conformation in solution.
Fig. 4.
Effects of solvent polarity and ligand concentration on the conformation and aggregation state of bis-thiacarbocyanine 2. Absorbance spectra of 2 (A) at varying concentrations in water and (B) at constant concentration (50 μM) in methanol (40-100%) were plotted as (A) the extinction coefficient (ε) or (B) absorbance versus wavelength. Positions of absorbance bands corresponding to open monomer (O), closed monomer (C), and H-aggregate (H) species are indicated at the top of panel A. In aqueous solution, increasing ligand concentrations promote H-aggregate formation at the expense of both open and closed forms of monomer. In contrast, increasing concentrations of methanol solvent increases open monomer at the expense of closed monomer and H-aggregated species.
Compound 2 (0.4 μM) was incubated for 1 h (○, □) or 22 h (●, ■) in assembly buffer containing ODS (50 μM) and either (C) mixed histones or (D) htau40. Resultant absorbance measurements were plotted as function of wavelength. Positions of absorbance bands corresponding to open (O), closed (C), and H-aggregate (H) species are indicated at the top of panel C. Dotted lines in each panel depict the absorbance of 2 in assembly buffer alone. The presence of tau protein selectively stabilized 2 open conformation. See text for details.
Inhibitory conformation
To identify the source of bis-thiacarbocyanine inhibitory activity, 2 was incubated under various conditions and subjected to absorbance spectroscopy. Assembly buffer alone broadened absorbance peaks and strongly stimulated H-aggregate formation, potentially owing to its high ionic strength relative to water (Fig. 4C). Nonetheless, monomeric species still favored the closed conformation under these conditions (Fig. 4C). Spectra were then estimated under aggregation promoting conditions (i.e., in the presence of assembly buffer, tau, and ODS). Because anionic surfactant micelles alone influence the aggregation state of thiacarbocyanine dyes [14, 22, 23], 2 absorbance was first analyzed in reactions where tau was replaced with mixed histones, a protein preparation that induces ODS micellization but that does not fibrillize under standard assay conditions [24]. Histone concentrations were chosen on the basis of Corrin-Harkins plots [6] to yield the same surfactant critical micelle concentration as that resulting from the presence of tau protein [24]. Thus, the histone-containing reaction served to control for non-specific, micelle-associated changes in 2 absorbance. Results showed that at inhibitory concentrations of 2 (400 nM), ODS micelles greatly depressed H-aggregate formation and further broadened absorbance peaks (Fig. 4C). Nonetheless, closed monomer still predominated over open monomer after 1 h incubation and remained predominant up to 22 h incubation (Fig. 4C).
Substitution of tau protein for histone modified this pattern. H-aggregate formation was depressed as in the presence of histone, but the ratio of closed and open monomer conformations shifted (Fig. 4D). By 1 h incubation, the amounts of open monomer conformation exceeded those seen in the presence of histone, and by 22 h, the open conformation was clearly the predominant species present. These data indicate that open monomer conformation is selectively stabilized under tau aggregation conditions, and that it represents the predominant species at inhibitory ligand concentrations.
Discussion
These data show that increases in the potency of cyanine-based tau fibrillization inhibitors can be achieved through the creation of multivalent ligands. Qualitatively, the concentration effect relationship for the bis-cyanine examined here, compound 2, closely resembled cyanine monomer N744. Both compounds showed biphasic behavior, with inhibitory activity lost at high ligand concentions potentially owing to H-aggregate formation [10]. As a result, both compounds were inhibitory over a limited concentration range. Separation of aggregation propensity from inhibitory activity would increase the useful concentration range of these compounds.
N744 has been suggested to inhibit tau filament formation as an H-dimer [10]. Although 2 can fold to form closed monomers, which resemble putative N744 H-dimers, inhibitory activity was associated with the open rather than the closed conformation. Thus, the activity of 2 is more likely mediated by multivalency than by a folded conformation. May et al. predicted that a multivalent strategy might be generally applicable to protein fibrillization reactions owing to a role for oligomerization in the early stages of aggregation [12]. In this model, multivalent ligands that bind protein oligomers prevent their incorporation into nascent filaments. For example, 2 could bind at the interface of the β-sheet domains of one pair of tau proteins with one cyanine group, and this may pre-position the second group to disrupt incoming tau monomers that would otherwise extend the fibril. However, small-molecule inhibitors also have been proposed to act by sequestering proteins into small aggregates that are not part of the fibrillization pathway [25, 26]. In this model, multivalent ligands may be more efficient in recruiting proteins to assembly incompetent complexes that deplete protein substrate below the critical concentration needed to maintain filament formation. The observed migration of 2 from the closed to the open conformation in the presence of tau protein is consistent with either of these models.
The discovery that 2 is a potent, multivalent tau fibrillization inhibitor has several implications for the design of similar molecules in the future. The extended aromatic nature of cyanine compounds may be needed for activity, but certain characteristics of 2 could probably be altered to generate analogs with equal or greater potency. First, the linker that tethers cyanine moieties together could be optimized in terms of length, flexibility, and hydrophobicity so as to maximize presentation of binding surfaces, increase compound solubility, and minimize H-aggregate formation. It may not be necessary to have two linkers as in 2, and bis-cyanines with a single linker have been described [14, 27]. Second, the cyanine moiety could be optimized including the size and composition of the heterocyclic rings, and substituents connected to the rings. The structure of 2 may provide a useful template for the design of future inhibitors of tau aggregation.
Acknowledgments
We thank Ohio State University colleagues Mihaela Necula, Lauren Crissman, and Ranjan Batra for tau protein production and EM analysis, Erich Grotewold, Plant Biotechnology Center, for access to the Varian scanning spectrophotometer, Robert W. Curley, College of Pharmacy, for advice on chemical synthesis, and Kari Green-Church, the Ohio State University Mass Spectrometry and Proteomics Facility, for LC/MS analysis. This study was supported by grants from the National Institutes of Health (AG14452) and the Alzheimer’s Association (to J.K.).
Abbreviations
- AD
Alzheimer disease
- DMSO
dimethyl sulfoxide
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- LC
liquid chromatography
- MS
mass spectroscopy
- ODS
octadecyl sulfate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buee L, Bussiere T, Buee-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 2.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 3.Kuret J. Detection and Reduction of Neurofibrillary Lesions. In: Smith HJ, Sewell RDE, Simons C, editors. Protein Folding Diseases: Enzyme Inhibitors and Other Agents as Prospective Therapies. CRC Press, Taylor & Francis Books; Boca Raton, FL: 2007. pp. 287–324. [Google Scholar]
- 4.Kuret J, Congdon EE, Li G, Yin H, Yu X, Zhong Q. Evaluating triggers and enhancers of tau fibrillization. Microsc Res Tech. 2005;67:141–155. doi: 10.1002/jemt.20187. [DOI] [PubMed] [Google Scholar]
- 5.Chirita CN, Congdon EE, Yin H, Kuret J. Triggers of full-length tau aggregation: a role for partially folded intermediates. Biochemistry. 2005;44:5862–5872. doi: 10.1021/bi0500123. [DOI] [PubMed] [Google Scholar]
- 6.Chirita CN, Kuret J. Evidence for an intermediate in tau filament formation. Biochemistry. 2004;43:1704–1714. doi: 10.1021/bi036034b. [DOI] [PubMed] [Google Scholar]
- 7.Chirita CN, Necula M, Kuret J. Anionic micelles and vesicles induce tau fibrillization in vitro. J Biol Chem. 2003;278:25644–25650. doi: 10.1074/jbc.M301663200. [DOI] [PubMed] [Google Scholar]
- 8.Chirita CN, Necula M, Kuret J. Ligand-dependent inhibition and reversal of tau filament formation. Biochemistry. 2004;43:2879–2887. doi: 10.1021/bi036094h. [DOI] [PubMed] [Google Scholar]
- 9.Necula M, Chirita CN, Kuret J. Cyanine dye N744 inhibits tau fibrillization by blocking filament extension: implications for the treatment of tauopathic neurodegenerative diseases. Biochemistry. 2005;44:10227–10237. doi: 10.1021/bi050387o. [DOI] [PubMed] [Google Scholar]
- 10.Congdon EE, Necula M, Blackstone RD, Kuret J. Potency of a tau fibrillization inhibitor is influenced by its aggregation state. Arch Biochem Biophys. 2007;465:127–135. doi: 10.1016/j.abb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra A, Behera RK, Behera PK, Mishra BK, Behera GB. Cyanines during the 1990s: A review. Chem Rev. 2000;100:1973–2011. doi: 10.1021/cr990402t. [DOI] [PubMed] [Google Scholar]
- 12.May BC, Fafarman AT, Hong SB, Rogers M, Deady LW, Prusiner SB, Cohen FE. Potent inhibition of scrapie prion replication in cultured cells by bis-acridines. Proc Natl Acad Sci USA. 2003;100:3416–3421. doi: 10.1073/pnas.2627988100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handl HL, Vagner J, Han H, Mash E, Hruby VJ, Gillies RJ. Hitting multiple targets with multimeric ligands. Expert Opin Ther Targets. 2004;8:565–586. doi: 10.1517/14728222.8.6.565. [DOI] [PubMed] [Google Scholar]
- 14.Herz AH. Dye-Dye interactions of cyanines in solution and at silver bromide surfaces. Photogr Sci Eng. 1974;18:323–335. [Google Scholar]
- 15.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- 16.Carmel G, Mager EM, Binder LI, Kuret J. The structural basis of monoclonal antibody Alz50’s selectivity for Alzheimer’s disease pathology. J Biol Chem. 1996;271:32789–32795. doi: 10.1074/jbc.271.51.32789. [DOI] [PubMed] [Google Scholar]
- 17.Wilson CD. Polymeric cyanine dyestuffs. 2,425,772. US Patent. 1947
- 18.Wilson CD. Photographic emulsions. 2,393,351. US Patent. 1946
- 19.Heseltine DW, Brooks DA. Mordants for bleachable filter layers. 3,438,779. US Patent. 1969
- 20.Patnaik LN, Pattanaik BN, Mohanty M, Satapathy A. Thin-Layer Chromatographic Behavior of Some Styryl Cyanine Dyes Derived from Pyridine. J Chromatogr. 1989;481:331–342. [Google Scholar]
- 21.Necula M, Kuret J. Electron microscopy as a quantitative assay for studying tau fibrillization. Analytical Biochemistry. 2004;329:238–246. doi: 10.1016/j.ab.2004.02.023. [DOI] [PubMed] [Google Scholar]
- 22.West W, Lovell SP, Cooper W. Electronic spectra of cyanine dyes at low temperature. I. Monomeric and aggregate absorption spectra. Photogr Sci Eng. 1970;14:52–62. [Google Scholar]
- 23.Chibisov AK, Prokhorenko VI, Gorner H. Effects of surfactants on the aggregation behaviour of thiacarbocyanine dyes. Chem Phys. 1999;250:47–60. [Google Scholar]
- 24.Necula M, Kuret J. A static laser light scattering assay for surfactant-induced tau fibrillization. Anal Biochem. 2004;333:205–215. doi: 10.1016/j.ab.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 25.Masuda M, Suzuki N, Taniguchi S, Oikawa T, Nonaka T, Iwatsubo T, Hisanaga S, Goedert M, Hasegawa M. Small molecule inhibitors of alpha-synuclein filament assembly. Biochemistry. 2006;45:6085–6094. doi: 10.1021/bi0600749. [DOI] [PubMed] [Google Scholar]
- 26.Necula M, Kayed R, Milton S, Glabe CG. Small molecule inhibitors of aggregation indicate that amyloid beta oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282:10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- 27.Herz AH. Aggregation of sensitizing dyes in solution and their adsorption on to silver halides. Adv Colloid Interface Sci. 1977;8:237–298. [Google Scholar]