Abstract
The significance of multiple growth factors acting on individual neurons in the central nervous system is presently unclear. Cultured hippocampal neurons were used in the present study to compare the neurotrophic actions of fibroblast growth factor-2 (FGF-2) with the better characterized growth factors, insulin-like growth factor (IGF)-1 and brain-derived neurotrophic factor (BDNF). Additionally, cultures were utilized to identify possible interactions between FGF-2 and the other growth factors. Activation of the ERK and Akt pro-survival pathways, as well as neuronal survival itself, were studied. The maximal magnitude of Akt activation stimulated by FGF-2 was found to be similar to that stimulated by IGF-1 and BDNF. In contrast, IGF-1 was less effective at inducing ERK activation than were BDNF and FGF-2. All three agents were found to promote survival of neurons cultured under serum-free, low-insulin conditions, with FGF-2 surprisingly being significantly more effective than the other two peptides. Co-treatment with maximal concentrations of either IGF-1 or BDNF enhanced FGF-2-stimulated Akt and ERK activation. However, no enhancement of survival beyond that stimulated by FGF-2 was observed with co-treatment. These findings suggest that FGF-2 may play an important role in promoting the survival of hippocampal neurons. Additionally, an interesting dissociation was identified between the positive interaction of FGF-2 with both IGF-1 and BDNF in activating Akt and ERK, and the lack of enhancement of FGF-2-induced neuroprotection.
Keywords: hippocampus, IGF-1, BDNF, FGF-2, Akt, ERK
1. Introduction
Chronic treatment with antidepressants has been demonstrated to induce changes in the hippocampus consistent with neuroprotection. It has been hypothesized that these changes may be mediated by antidepressant-induced increases in growth factor expression. In fact, chronic antidepressant treatment has been shown to increase the level of brain-derived neurotrophic factor (BDNF) message (Duman et al., 1997) and IGF-1 protein expression (Khawaja et. al., 2004) in the hippocampus. IGF-1 has been shown, in vitro, to have neurotrophic and neuroprotective properties in hippocampal cultures (Nitta et. al., 2004; Zheng and Quirion, 2004; Johnson-Farley et al., 2006). Similarly, BDNF has been shown to inhibit, apoptosis in hippocampal neurons (Zheng and Quirion, 2004; Johnson-Farley et al, 2006).
Expression of FGF-2, a less well characterized growth factor, has also been reported to be increased by antidepressants in cortex and hippocampus (Mallei et al., 2002; Maragnolli et al., 2004). The peptide has been demonstrated to have a role in stimulating hippocampal neurogenesis (Palmer et al., 1999) and, and in some model systems, has been reported to have neurotrophic actions. For example FGF-2 promotes survival of cultured cerebellar granule cells (Bonthius et al., 2003) as well cultured embryonic chick ciliary ganglion neurons and cultured spinal chord neurons (Unsicker et al., 1987). It has been additionally reported to enhance survival of hippocampal neurons in culture (Walicke et al., 1986), and to confer protection from glutamate-induced cell death (Mattson et al., 1995; Lenhard et al., 2002). In vivo studies also suggest a neuroprotective role for FGF-2. In fact, the peptide has been reported to increase the survival of rat embryonic hippocampal CA3 donor cells grafted into adult hippocampi (Zaman and Shetty, 2003a; Zaman and Shetty, 2003b; Rao et al., 2005).
Our current study was designed to directly compare the neurotrophic actions of IGF-1, BDNF, and FGF-2 and to identify potential interactions. Differences in activity can provide insight into the specific roles of each growth factor. In addition to examining enhancement of cell survival, we have compared the coupling of receptors for these growth factors to activation of Akt (protein kinase B) and the Extracellular-regulated kinase (ERK) mitogen-activated protein (MAP) kinases. These kinases are thought to be key components in mediating growth factor-induced neuroprotection (Tamatani et al., 1998; Brunet et al., 1999; Hetman et al., 1999; Matsuzaki et al., 1999; Yamaguchi et al., 2001; Zheng and Quirion, 2004). We found significant differences in the neuroprotective efficacy of IGF-1, FGF-2, and BDNF and the pattern of receptor coupling to ERK and Akt. These may help explain differences in function and may provide insight into the role of these growth factors in mediating antidepressant-induced changes in the hippocampus.
2. Results
FGF-2 stimulates activation of Akt and ERK in hippocampal neurons
Treatment with FGF-2 was found to stimulate an approximate 4-fold maximal increase in the level of activated (phosphorylated) Akt, and a 12-fold maximal increase in the level of activated (phosphorylated) ERK. Maximal activation of both kinases was observed at 100 ng/ml FGF-2 (figure 1). Activation of both ERK and Akt occurred within 2 minutes of FGF-2 treatment, and was greatest at 5-10 minutes. At 30 minutes, the level of phosphorylated ERK and Akt had both decreased, but remained above basal levels (figure 2). The pattern of phosphorylation of p44 ERK1 and p42 ERK2 were similar.
Figure 1. FGF-2 stimulates activation of Akt and ERK in hippocampal neurons.
Hippocampal neurons were incubated for 5 minutes with the specified concentrations of FGF-2, and lysed. Supernatant was analyzed by immunoblotting with antibody to A) phospho-Akt (p-Akt) and total Akt or B) phospho-ERK (p-ERK) and total ERK. (Bands for both ERK1 (p44) and ERK2 (p42) are visible.) Net intensity of bands was calculated as described in the Methods section, and results are expressed as the means ± S.E.M. of 3 experiments, performed in duplicate. Experimental groups were compared by ANOVA followed by Dunnett’s multiple comparison post tests. **, p<0.01 vs. control. Representative immunoblots are shown.
Figure 2. FGF-2 rapidly stimulates activation of Akt and ERK.
Hippocampal neurons were incubated for the specified periods of time with 100 ng/ml FGF-2, and lysed. Supernatant was analyzed by immunoblotting with antibody to A) phospho-Akt (p-Akt) and total Akt or B) phospho-ERK (p-ERK) and total ERK. Results are expressed as the means ± S.E.M. of the net intensities of bands obtained from 3 experiments, performed in duplicate. Experimental groups were compared by ANOVA followed by Dunnett’s multiple Comparison post tests. **, p<0.01 vs. control. Representative immunoblots are shown.
FGF-2 stimulates neuronal survival
In order to study the neurotrophic actions of FGF-2 we used a model system in which neurons were cultured after the first 24 hours in vitro under low-insulin conditions (100 ng/ml). Cytosine arabinoside was included to prevent proliferation of non-neuronal cells. Cell viability was assessed 3 days after media change using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in which the yellow tetrazolium base compound is reduced by viable cells to a blue formazan product. Many neurons survived the 3 days of low-insulin culture conditions. However, a single treatment with FGF-2, at the beginning of the 72 hour period, significantly enhanced survival. Interestingly, the peptide was much more potent at enhancing survival than it was at stimulating activation of ERK and Akt (figure 3A). Maximal survival was obtained by treatment with 100 ng/ml FGF-2, but concentrations as low as 5 ng/ml FGF-2 induced neuroprotection. Significantly, nearly all cultured cells continued to stain positive for the MAP-2 neuronal marker at the end of 3 day period of FGF-2 treatment (not shown). This is consistent with enhancement of neuronal survival and not proliferation of contaminating, non-neuronal cells. Surprisingly, treatment with 100 ng/ml IGF or 20 ng/ml BDNF, concentrations that we have previously found to maximally activate ERK and Akt (Johnson-Farley et al., 2006), stimulated only 17% and 12% increases in neuronal survival (figure 3B).
Figure 3. FGF-2 more effectively enhances survival than BDNF and IGF-1.
After the first 24 hours of routine culture, neurons were cultured for the subsequent 72 hours under low-insulin conditions in the presence of the specified concentrations of FGF-2 and/or 100 ng/ml IGF-1 or 20 ng/ml BDNF. Cell viability was assessed on day 4 of culture by MTT assays in which the yellow tetrazolium base compound is reduced by viable cells to a blue formazan product, detected at a wavelength of 570 nm (O.D.570). Results were normalized in each experiment by dividing the mean absorbance of treated cultures by the mean absorbance of nontreated cultures to obtain fold-control values, and are expressed as the means ± S.E.M. of 3 experiments, performed in duplicate. A) Experimental groups were compared by ANOVA followed by Dunnett’s multiple comparison post tests. ***, p<0.001 vs. control. B, C) Experimental groups were compared by ANOVA followed by Newman-Keuls Multiple Comparison post tests. *, 0.5; **, p<0.01; ***, p<0.001 vs. FGF-2 treatment.
FGF-2 is more similar to BDNF than IGF-1 in stimulating activation of Akt and ERK
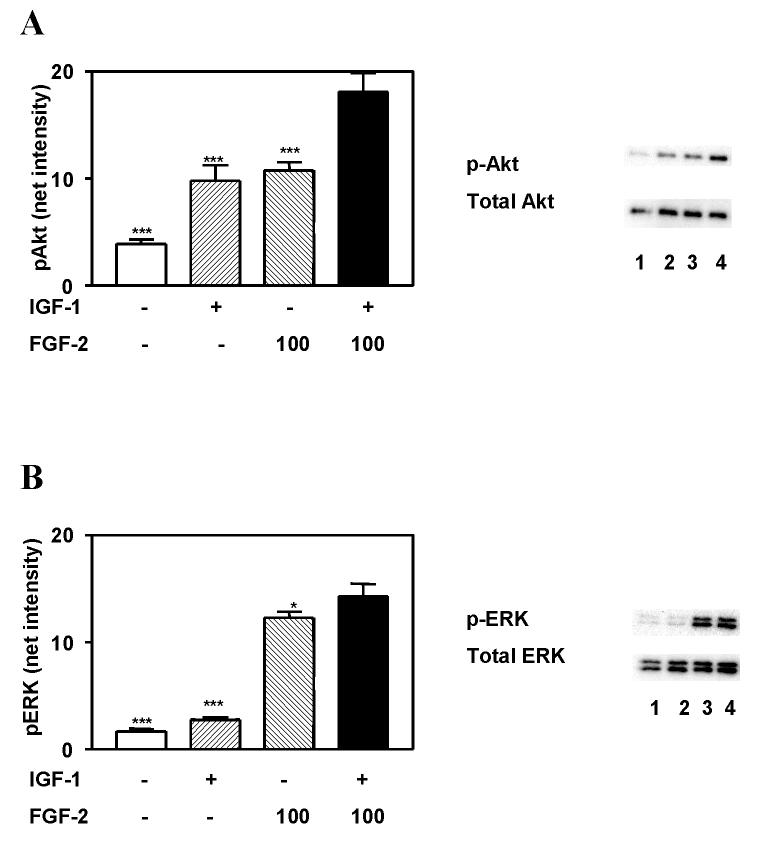
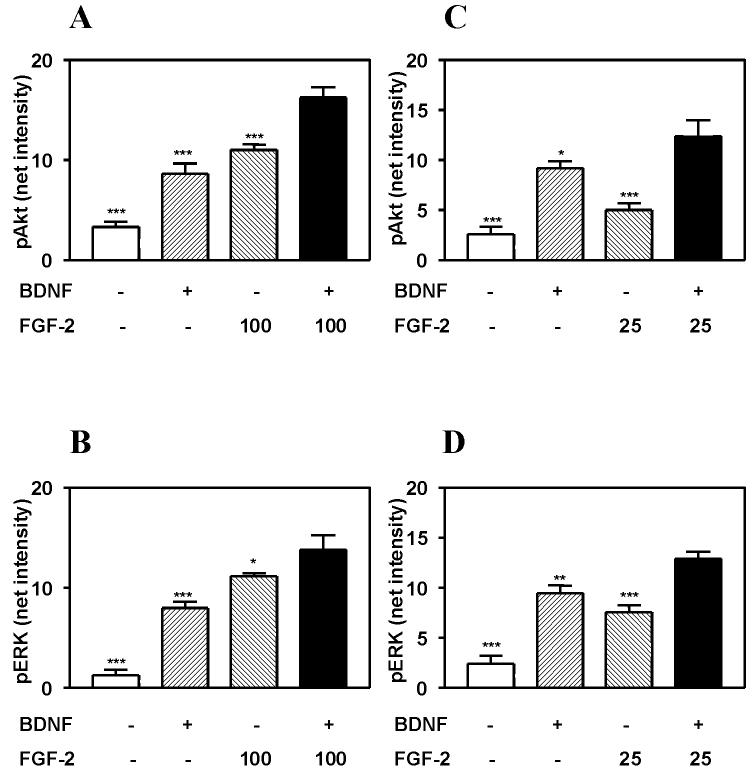
Akt and ERK have been shown to play major roles in mediating the neuroprotective actions of growth factors. We therefore sought to determine whether differences in the pattern of activation of these 2 kinases could account for the increased efficacy of FGF-2 vs. IGF-1 and BDNF in inducing neuroprotection. We have previously reported that maximal activation of ERK and Akt by IGF-1 and BDNF is stimulated by 100 ng/ml and 20 ng/ml concentrations, respectively (Johnson-Farley et al., 2006). The magnitude of IGF-1- and FGF-2- stimulated activation of Akt were found to be comparable (figure 4). However, the activation of ERK stimulated by IGF-1 was minimal compared to that stimulated by FGF-2. In contrast, the magnitude of both ERK and Akt activation stimulated by BDNF were relatively comparable to that stimulated by 100 ng/ml FGF-2 (figures 5A and 5B). Activation of Akt by the two peptides was found to not be statistically different. However, a small, but statistically significant difference, in the activation of ERK (p<0.01) was found with BDNF being somewhat less effective.
Figure 4. IGF-1 and FGF-2 cumulatively activate Akt and ERK.
Hippocampal neurons were incubated for 5 minutes with 100 ng/ml IGF-1 and/or 100 ng/ml FGF-2, and lysed. Supernatant was analyzed by immunoblotting with antibody to A) phospho-Akt (p-Akt) and total Akt or B) phospho-ERK (p-ERK) and total ERK. Results are expressed as the means ± S.E.M. of the net intensities of bands obtained from 6 experiments, performed in duplicate. Experimental groups were compared by ANOVA followed by Newman-Keuls Multiple Comparison post tests. *, p<0.05; ***, p<0.001 vs. FGF-2 plus IGF-1 co-treatment. Representative immunoblots are shown.
Figure 5. Co-treatment with BDNF stimulates greater activation of Akt and ERK than does FGF-2, alone.
Hippocampal neurons were incubated for 5 minutes with 20 ng/ml BDNF and/or either A, B) 100 ng/ml FGF-2 or C, D) 25 ng/ml FGF-2 and lysed. Supernatant was analyzed by immunoblotting with antibody to A, C) phospho-Akt (p-Akt) or B, D) phospho-ERK (p-ERK). Results are expressed as the means ± S.E.M. of the net intensities of bands obtained from 3 experiments, performed in duplicate. Experimental groups were compared by ANOVA followed by Newman-Keuls Multiple Comparison post tests. *, p<0.05; **, p<0.01; ***, p<0.001 vs. FGF-2 plus BDNF co-treatment.
Effect of co-treatment on cellular signaling and neuronal survival
We next looked at the interaction of FGF-2 with IGF-1 and BDNF. Simultaneous treatment of neurons with IGF-1 and FGF-2 stimulated a larger magnitude of Akt activation than stimulated by treatment with either peptide alone (figure 4). Activation appeared to be approximately additive. Similarly, maximal concentrations of IGF-1 and FGF-2 stimulated what appeared to be an additive increase in ERK activation, though nearly all of the ERK activation stimulated by co-treatment could be attributed to FGF-2.
Co-treatment with BDNF and FGF-2 also induced larger magnitudes of ERK and Akt activation than that stimulated by either growth factor alone (figures 5A and 5B). However, activation of ERK was clearly less than additive. Co-treatment with BDNF induced a statistically significant enhancement of FGF-2-stimulated activation, but the magnitude of difference was relatively small. Since this could reflect saturation of the activation pathways for ERK, we also studied co-treatment of BDNF with a submaximal dose of FGF-2 (25 ng/ml) (figures 5C and 5D). Activation of ERK was also not additive under these conditions.
We next sought to examine whether, at a cellular level, the activation of Akt and ERK stimulated by FGF-2 was similar to that stimulated by BDNF. Cells were stained with antibody directed at the activated form of either Akt or ERK. Untreated (control) neurons exhibited a range of baseline Akt activity (figure 6). However, treatment with either 20 ng/ml BDNF or 100 ng/ml FGF-2 resulted in clear activation, with many, though not all, neurons exhibiting brighter staining for phosphorylated Akt. At 5 minutes of treatment, activated Akt was localized primarily in the cell body and large processes. Immunostaining for activated ERK was similar, but not identical that for Akt. Consistent with our immunoblot studies (figures 1, 2, and 5), there was a bigger difference in the magnitude of ERK activation, than Akt activation, in individual FGF-2- and BDNF-treated neurons relative to untreated neurons. Most, but not all, neurons responded to BDNF and FGF-2 (figure 7). At 5 minutes of treatment, activated ERK was observed in the cell body and larger processes.
Figure 6. The pattern of activation of Akt by FGF-2 and BDNF are similar at the cellular level.
Hippocampal neurons were incubated for 5 min with 100 ng/ml FGF-2 or 20 ng/ml BDNF before fixation and permeabilization. Activation of Akt was analyzed by immunostaining with antibody to phospho-Akt (p-Akt). The nuclear dye DAPI was used to visualize all nuclei. A) Magnification was 20x. B) p-Akt and DAPI images at 60x magnification were overlaid.
Figure 7. The pattern of activation of ERK by FGF-2 and BDNF are similar at the cellular level.
Hippocampal neurons were incubated for 5 min with 100 ng/ml FGF-2 or 20 ng/ml BDNF before fixation and permeabilization. Activation of ERK was analyzed by immunostaining with antibody to phospho-ERK (p-ERK). The nuclear dye DAPI was used to visualize all nuclei. A) Magnification was 20x. B) p-ERK and DAPI images at 60x magnification were overlaid.
We next looked at the effect on neuronal survival of simultaneous treatment of FGF-2 with another growth factor. Interestingly, the greater activation of ERK and Akt induced by co-treatment with 100 ng/ml FGF-2 and either IGF-1 or BDNF, did not result in greater neuronal survival than was achieved with FGF-2, alone (figure 3B). It could be postulated that the lack of augmentation could have been the result of FGF-2, itself, inducing sufficient activation of Akt and ERK to promote maximal neuronal survival. We therefore examined the effect of co-treatment with IGF-1 or BDNF on the actions of 25 ng/ml FGF-2. This concentration induced submaximal activation of Akt and ERK (figure 1). Co-treatment with either IGF-1 or BDNF was found to induce more neuroprotection than did treatment with 25 ng/ml FGF-2, alone (figure 3C). However, although the differences between co-treatment and FGF-2, alone, were statistically significant, they were small.
These findings suggested that Akt and ERK, if relevant to the neuroprotective actions of FGF-2, were not solely responsible. In order to directly determine the roles of Akt and ERK in mediating the neurotrophic actions of FGF-2, cultures were pretreated with LY 294002, a PI3K inhibitor, or PD 98059, a MAP kinase kinase (MEK) inhibitor prior to FGF-2. 25 μM LY 294002 inhibited basal levels of Akt activity and completely inhibited FGF-2 stimulated activation of Akt (figure 8A). Cell survival was also reduced by LY 294002 relative to vehicle treated controls. Inclusion of FGF-2 in LY 294002-treated cultures, did increase cell survival but not to the extent seen in cultures treated with FGF-2 in the absence of the PI3K inhibitor (figure 8C). In contrast, pretreatment with the MAP kinase kinase (MEK) inhibitor, PD98059, inhibited activation of ERK, but did not significantly inhibit FGF-2-stimulated survival. However, a small inhibition in basal levels of survival were observed.
Figure 8. Effect of inhibition of Akt and ERK on FGF-2-induced neuronal survival.
A,C) Cultures were pretreated for 30 minutes with either 25 μM LY 294002, 100 μM PD 98059, or vehicle (0.2% dimethyl sulfoxide) prior to treatment with 100 ng/ml FGF-2 for 5 minutes. Supernatant was analyzed by immunoblotting with antibody to A) phospho-Akt (p-Akt) or C) phospho-ERK (p-ERK). Results are expressed as the means ± S.E.M. of the net intensities of bands obtained from 3 experiments, performed in duplicate. B) After the first 24 hours of routine culture, neurons were cultured for the subsequent 72 hours under low-insulin conditions in the presence or absence of 100 ng/ml FGF-2, after pretreatment for 30 minutes with either 25 μM LY 294002, 100 μM PD 98059, or vehicle (0.2% dimethyl sulfoxide). Cell viability was assessed on day 4 of culture by MTT assays in which the yellow tetrazolium base compound is reduced by viable cells to a blue formazan product, detected at a wavelength of 570 nm (O.D.570). Experimental groups were compared by ANOVA followed by Newman-Keuls Multiple Comparison post tests. *, p<0.05; ***, p<0.001 vs. same treatment in the absence of relevant inhibitor.
3. Discussion
It has been previously reported that FGF-2 protects cultured hippocampal neurons from glutamate-induced cell death (Lenhard et al., 2002). Our finding that FGF-2 confers protection under serum-free, low insulin conditions supports the hypothesis that FGF-2 plays a role in promoting survival of hippocampal neurons. It has been proposed that compounds with neurotrophic actions, such as FGF-2, may be useful clinically. One interesting aspect of this is their potential role in promoting graft survival in the treatment of brain injuries. In fact, pretreatment of dissociated embryonic hippocampal CA3 cells with FGF-2 prior to transplantation into lesioned adult rat hippocampus has been reported to increase graft survival (Zaman and Shetty 2003a). A separate study by Rao et al. (2005) reported enhanced neuronal integration of fetal hippocampal CA3 cells into injured CA3 regions of adult hippocampi after pretreatment with a combination of FGF-2 and BDNF. Additionally, treatment was found to suppress aberrant mossy fiber sprouting into the dentate supragranular layer, which is thought to be epileptogenic. The efficacy of treatment with the individual peptides was not compared. However, in a separate study, treatment with a combination of either BDNF + neurotrophin 3 (NT-3) + a caspase inhibitor was compared to treatment with a combination of FGF-2 + caspase inhibitor. Both treatments were found to markedly increase CA3 graft survival relative to no treatment. However, the combination of FGF-2 + caspase inhibitor appeared to stimulate more cell proliferation within the graft than did the other treatment combination (Hattiangady et al., 2006). There is conflicting evidence as to whether BDNF, itself, may enhance donor survival. Continuous intracerebral infusion of BDNF has been reported to enhance survival of fetal dopaminergic neurons transplanted into striatum (Yurek et al., 1996). However, Sauer et al. (1993) reported that although BDNF stimulated functional graft effects, it did not increase grafted dopaminergic neuron survival.
Surprisingly, our studies, in which FGF-2 was directly compared to IGF-1 and BDNF, demonstrated that FGF-2 was a more effective neuroprotective agent than the two better characterized peptides. The smaller degree of neuroprotection was not the result of a smaller percentage of the cell population expressing receptors for IGF-1 and BDNF. Greater than 90% of the cells in our cultures express IGF-1 receptors, TrkB receptors, and stain positive for the neuronal marker microtubule-associated protein (MAP)-2 (Johnson-Farley et al., 2006). Moreover, our immunocytochemistry studies of ERK and Akt activity demonstrated a similar percentage of cells responding to BDNF as to FGF-2. FGF-2, therefore, apparently induces stronger neurotrophic actions on individual neurons.
Interestingly, cell survival did not correlate with the magnitude of activation of Akt and ERK. This was somewhat surprising in that the two kinases are thought to play key roles in mediating growth factor-induced neuroprotection (Tamatani et al., 1998; Brunet et al., 1999; Hetman et al., 1999; Matsuzaki et al., 1999; Yamaguchi et al., 2001; Zheng and Quirion, 2004). In our studies, BDNF, IGF-1, and FGF-2 were found to be all approximately equally effective at activating Akt. Additionally, BDNF was almost as effective as FGF-2 at activating ERK. In contrast, IGF-1 and BDNF were both approximately equally effective at inducing neuroprotection, with both being much less effective than FGF-2. Moreover, further increasing Akt by combining FGF-2 with either IGF-1 or BDNF led to no further enhancement of neuroprotection. This is in contrast to our previous findings that IGF-1 and BDNF cumulatively enhance cell survival, in addition to cumulatively activating Akt (Johnson-Farley et al., 2006).
We cannot exclude a role for Akt in promoting cell survival. In fact, pretreatment with the PI3K inhibitor, LY 294002, decreased neuronal survival in both control and FGF-2 treated cultures. FGF-2 did increase cell survival in LY 294002-treated cultures, but not to the extent seen in cultures treated with FGF-2 in the absence of the PI3K inhibitor. It could be therefore be hypothesized that a minimal level of Akt activity is required for both basal levels of neuronal survival and maximal FGF-2-stimulated survival. In contrast, inhibition of ERK activity with PD 98059 did not attenuate the neuroprotective actions of FGF-2. Interestingly, FGF-2-dependent stimulation of synaptogenesis in cultured hippocampal neurons has been reported to be attenuated by ERK inhibitors (Li et al., 2002).
An additional pathway, beyond ERK and Akt would therefore likely appear to be required to mediate FGF-2’s neurotrophic effects. FGF-2 was found in our studies to be more potent at stimulating neuroprotection than at activating Akt. It would therefore be expected that FGF-2 would be similarly more potent at activating this unidentified pathway than at activating Akt. A possible relevant second messenger is nitric oxide, which has been reported to mediate FGF-2-induced protection of cerebellar neurons (Bonthius et al., 2003). However, in those studies, IGF-1-induced protection was also found to be mediated by nitric oxide. Alternatively, it is possible that FGF-2 induced upregulation of another growth factor that was responsible for the observed neuroprotection. In that regard, Lenhard et al. (2002), reported a role for glial-cell-line derived neurotrophic factor (GDNF) in mediating some actions of FGF-2. An additional potential mechanism is a direct nuclear effect of FGF-2. It has been reported that the peptide is internalized in neurons and can directly bind to chromatin in the nucleus (Walicke et al., 1991). It has been reported that cultured hippocampal neurons express types 1-3 of FGF receptors (Li et al., 2002) on the membrane of cell bodies, dendrites, and growth cones. Activation of ERK was reported to occur in the same, diffuse pattern. We similarly found that 5 minutes of treatment with either FGF-2 or BDNF resulted in activation of ERK within the cell bodies and larger neuronal processes.
Although much work has been done characterizing individual growth factors, very little is currently known about interactions between growth factors. Yet, a number of medications and drugs have been shown to increase the expression of multiple growth factors. Antidepressants for example, have been shown to increase the expression of IGF-1, BDNF, and FGF-2 (Duman et al., 1997; Mallei et al., 2002; Khawaja et. al., 2004; Maragoli et al., 2004). In our studies, we determined that the activation of Akt and ERK stimulated by IGF-1 and FGF-2 were approximately cumulative. In contrast, neurotrophin 3 (NT-3) has been reported to inhibit FGF-2-induced Akt activation as well as Akt-induced neuronal proliferation (Jin et al., 2005). We similarly found that the activation of ERK stimulated by co-treatment with FGF-2 and BDNF, which shares similarities to NT-3 (Soppet et al., 1991), was less than additive. It is possible that receptors for these 2 peptides activate pathways that exhibit negative interactions. Identifying positive and negative interactions between neurotrophins is critical to understanding the complex neuroplasticity mediated by the simultaneous presence of multiple growth factors.
4. Experimental Procedures
Materials
Recombinant human insulin-like growth factor-1 was purchased from Sigma (St. Louis, MO). Recombinant human brain-derived neurotrophic factor (BDNF) was purchased from Alomone Labs (Jerusalem, Israel), and recombinant human fibroblast growth factor-2 was purchased from Fisher Scientific (Pittsburgh, PA).
Cell Culture
Hippocampal neuronal cultures were prepared as previously described (Johnson-Farley et al., 2006). Hippocampi were isolated from embryonic day 18 (E18) Sprague-Dawley rats obtained from Charles River Laboratories (Raleigh, NC) and 106 cells were plated per poly(D-lysine) coated 35 mm Petri dish. Cells were maintained under serum-free conditions in Neurobasal media (Invitrogen, Carlsbad, CA) supplemented with 0.5 mM GlutaMax-1 (Invitrogen), 25 μg/ml insulin, 100 μg/ml transferrin, 60 μM putrescine, 20 nM progesterone, 30 nM selenium, 6 mg/ml glucose, 1 mg/ml bovine serum albumin-Fraction V, fatty acid free (Calbiochem, (San Diego, CA), and 7.5 units penicillin-7.5 μg streptomycin/ml at 37°C (95% air, 5% CO2). 0.6 μM cytosine arabinoside was additionally included at the time of plating to prevent proliferation of non-neuronal cells. Media was changed the day prior to second-messenger studies to low-insulin media (100 ng/ml) to prevent insulin from acting as an agonist at IGF-1 receptors (Rosenfeld and Hintz, 1980; Steele-Perkins et. al., 1988). This media contained no cytosine arabinoside, no antibiotics and contained a reduced concentration of albumin (0.1 mg/ml). Cultures were comprised primarily of neurons, with greater than 90% of cells expressing IGF-1 receptors, TrkB receptors, and staining positive for the neuronal marker microtubule-associated protein (MAP)-2 (Johnson-Farley et al., 2006).
Immunoblots
Mouse monoclonal anti-phospho-ERK1/ERK2 (Thr202/Tyr204), rabbit polyclonal anti-phospho-Akt (Ser 473), and rabbit polyclonal anti-total Akt were obtained from Cell Signaling (Beverly, MA). Rabbit polyclonal anti-total ERK1/ERK2 and horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Cells remained in culture for 5-7 days prior to use, with the media aspirated the day prior to use and replaced with culture media containing no antibiotics, no cytosine arabinoside, and reduced concentrations of insulin (100 ng/ml) and albumin (100 μg/ml). (No differences in relative responses to growth factors were seen in cultures used within this time period). Cells were treated with the addition of reagents directly to the culture media. BDNF was routinely used at 20 ng/ml and IGF-1 at 100 ng/ml. We have previously reported that maximum activation of ERK and Akt is elicited at these concentrations (Johnson-Farley et al., 2006). The concentration of FGF-2 varied in individual experiments and is specified in the figure legends. Cells were then washed with phosphate-buffered saline, and routinely lysed with a 26-gauge needle in 25 mM HEPES (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM β-glycerol phosphate, 50 mM NaF, 5 mM EDTA, 1 mM sodium orthovanadate, 250 μM 4-(2-aminoethyl)-benzene-sulfonylfluoride hydrochloride, 0.1% aprotinin, and 10 μg/ml leupeptin. After 30 minutes on ice, the lysate was centrifuged at 10,000 × g for 10 minutes at 4°C. Supernatant proteins were separated on 10% resolving gels (Cambrex, Rockland, Maine) and transferred to 0.45 μm Immobolin-P polyvinylidene difluoride membranes (Millipore Corporation, Bedford, MA). Bound antibodies were visualized using Enhanced Luminol Chemiluminescence Reagent (PerkinElmer Life Sciences, Boston, MA) and direct exposure to a Kodak Image Station 440CF with a cooled, full-frame-capture CCD camera (Kodak). Net intensity of bands was calculated directly from stored images using Kodak Digital Science 1D Image Analysis Software (version 3.5) on defined regions of interest. For analysis of pERK, both pERK1 and pERK2 bands were included in the regions of interest.
Cell viability
Media was aspirated 24 hours after the initiation of culture (24 hours in vitro), and replaced with media containing reduced concentrations of insulin (100 ng/ml) and albumin (100 μg/ml) and no antibiotics. 0.6 μM cytosine arabinoside was included in this low-insulin media to prevent induction of non-neuronal proliferation. Cultures were treated with the indicated growth factors one hour after media change. Cell viability was assessed 3 days later using the 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay in which the yellow tetrazolium base compound is reduced by viable cells to a blue formazan product. Cells were incubated at 37°C in humidified air with 5% CO2 for 40 minutes with 0.5 mg MTT/ml to allow reduction of MTT. The MTT containing media were subsequently discarded and cells containing formazan product dissolved in 0.9 ml dimethyl sulfoxide (DMSO). Absorbance at wavelength 570nm (O.D. 570) was measured using a spectrophotometer.
Statistics
Results are expressed as the means ± S.E.M. of three or more experiments, performed in duplicate. Experimental groups were compared by ANOVA followed by the indicated post test.
Immunofluorescence
Cells were plated, as described above, in poly(D-lysine)-coated 35-mm Petri dishes. Cultures were treated, washed with phosphate-buffered saline (PBS), and fixed in methanol for 6 min at -20°C. Cultures were incubated for 30 min at room temperature (22°C) in PBS-blocking buffer containing 10% goat serum (Vector Laboratories, Burlingame, CA). Cultures were then incubated at room temperature (22°C) for 2 h with (1:50) rabbit polyclonal anti-phospho-(Ser473)-Akt (Santa Cruz Biotechnology) or mouse monoclonal anti-phospho-(Thr202/Tyr204)-ERK1/ERK2 (Cell Signaling) in PBS containing 1.5% goat serum. Cultures were washed with PBS and incubated for 45 min at room temperature (22°C), with (1:1000) Alexa Fluor 488-conjugated goat anti-rabbit IgG or Alexa Fluor 568-conjugated goat anti-mouse IgG obtained from Invitrogen in PBS containing 1.5% goat serum. Cultures were then washed and coverslipped with Ultra Cruz Mounting Media (Santa Cruz Biotechnology) containing DAPI to visualize nuclei. Stained cultures were viewed under fluorescence illumination, and images were digitally captured using MetaVue 6.1 image analysis software (Molecular Devices, Sunnyvale, CA).
Acknowledgements
These studies were supported by NIMH grant MH60100 to D.S.C.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- FGF-2
fibroblast growth factor-2
- IGF-1
insulin-like growth factor-1
- PI3K
phosphatidylinositol 3-kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bonthius DJ, Karacay B, De D, Pantazis NJ. FGF-2, NGF, and IGF-1, but not BDNF, utilize a nitric oxide pathway to signal neurotrophic and neuroprotective effects against alcohol toxicity in cerebellar granule cell cultures. Dev. Brain. Res. 2003;140:15–28. doi: 10.1016/s0165-3806(02)00549-7. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch. Gen. Psych. 1997;54:597–605. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- Hattiangadya B, Raoa MS, Zamana V, Shetty AK. Incorporation of embryonic CA3 cell grafts into the adult hippocampus at 4-months after injury: Effects of combined neurotrophic supplementation and caspase inhibition. Neurosci. 2006;139:1369–1383. doi: 10.1016/j.neuroscience.2006.01.058. [DOI] [PubMed] [Google Scholar]
- Hetman M, Kanning K, Cavanaugh JE, Xia Z. Neuroprotection by Brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol 3-kinase. J. Biol. Chem. 1999;274:22569–22580. doi: 10.1074/jbc.274.32.22569. [DOI] [PubMed] [Google Scholar]
- Jin L, Hu X, Feng L. NT3 inhibits FGF2-induced neuronal progenitor cell proliferation via the PI3K/GSK3 pathway. J. Neurochem. 2005;93:1251–1261. doi: 10.1111/j.1471-4159.2005.03118.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Farley NN, Travkina T, Cowen DS. Cumulative activation of neuroprotective Akt pathway by Brain-Derived Neurotrophic Factor and Insulin-Like Growth Factor-1 in cultured hippocampal neurons. J. Pharmacol. Exp. Ther. 2006;316:1062–1069. doi: 10.1124/jpet.105.094433. [DOI] [PubMed] [Google Scholar]
- Khawaja X, Xu J, Liang J-J, Barrett JE. Proteomic Analysis of Protein Changes Developing in Rat Hippocampus After Chronic Antidepressant Treatment: Implications for Depressive Disorders and Future Therapies. J. Neurosci. Res. 2004;75:451–460. doi: 10.1002/jnr.10869. [DOI] [PubMed] [Google Scholar]
- Lenhard T, Schober A, Suter-Crazzolara C, Unsicker K. Fibroblast growth factor-2 requires glial-cell-line derived neurotrophic factor for exerting its neuroprotective actions on glutamate-lesioned hippocampal neurons. Mol. and Cell. Neurosci. 2002;20:181–197. doi: 10.1006/mcne.2002.1134. [DOI] [PubMed] [Google Scholar]
- Li A-J, Suzuki S, Suzuki M, Mizukoshi E, Imamura T. Fibroblast growth factor-2 increases functional excitatory synapses on hippocampal neurons. Eur. J. Neurosci. 2002;16:1313–1324. doi: 10.1046/j.1460-9568.2002.02193.x. [DOI] [PubMed] [Google Scholar]
- Mallei A, Shi B, Mocchetti I. Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol. Pharmacol. 2002;61:1017–1024. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- Maragnolli ME, Fumagalli F, Genarelli M, Racagni G, Riva MA. Fluoxetine and olanzapine have synergistic effects in the modulation of fibroblast growth factor 2 expression within the rat brain. Biol. Psych. 2004;55:1095–1102. doi: 10.1016/j.biopsych.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Mitsuda N, Namikawa K, Kiyama H, Miyake S, Tohyama M. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J. Neurochem. 1999;73:2037–2046. [PubMed] [Google Scholar]
- Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular Ca2+ concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J. Neurochem. 1995;65:1740–1751. doi: 10.1046/j.1471-4159.1995.65041740.x. [DOI] [PubMed] [Google Scholar]
- Nitta A, Zheng W, Quirion R. Insulin-Like Growth Factor 1 Prevents Neuronal Cell Death Induced by Corticosterone Through Activation of the PI3K/Akt Pathway. J. Neurosci. Res. 2004;76:98–103. doi: 10.1002/jnr.20057. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J. Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK. Fetal hippocampal CA3 cell grafts enriched with FGF-2 and BDNF exhibit robust long-term survival and integration and suppress aberrant mossy fiber sprouting in the injured middle-aged hippocampus. Neurobiol. Disease. 2006;21:276–290. doi: 10.1016/j.nbd.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Rosenfeld RG, Hintz RL. Characterization of a Specific Receptor for Somatomedin C (SM-C) on Cultured Human Lymphocytes: Evidence that SM-C Modulates Homologous Receptor Concentration. Endocrinol. 1980;107:1841–1848. doi: 10.1210/endo-107-6-1841. [DOI] [PubMed] [Google Scholar]
- Sauer H, Fischer W, Nikkhah G, Wiegand SJ, Brundin P, Lindsay RM, Bjorklund A. Brain-derived neurotrophic factor enhances function rather than survival of intrastriatal dopamine cell-rich grafts. Brain Res. 1993;626:37–44. doi: 10.1016/0006-8993(93)90560-a. [DOI] [PubMed] [Google Scholar]
- Soppet DS, Escandon E, Maragos J, Middlemas DS, Reid SW, Blair J, Burton LE, Stanton BR, Kaplan DR, Hunter T, Nikolics K, Parada LF. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991;65:895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- Steele-Perkins G, Turner J, Edman JC, Hari J, Pierce SB, Stover C, Rutter WJ, Roth RA. Expression and Characterization of a Functional Human Insulin-like Growth Factor I Receptor. J. Biol. Chem. 1988;263:11488–11492. [PubMed] [Google Scholar]
- Tamatani M, Ogawa S, Niitsu Y, Tohyama M. Involvement of Bcl-2 family and caspase-3-like protease in NO-mediated neuronal apoptosis. J. Neurochem. 1998;71:1588–1596. doi: 10.1046/j.1471-4159.1998.71041588.x. [DOI] [PubMed] [Google Scholar]
- Unsicker K, Reichert-Preibsch H, Schmidt R, Pettmann B, Labourdette G, Sensenbrenner M. Astroglial and fibroblast growth factors have neurotrophic functions for cultured peripheral and central nervous system neurons. Proc. Natl. Acad. Sci. USA. 1987;84:5459–5463. doi: 10.1073/pnas.84.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walicke PA, Baird A. Internalization and processing of basic fibroblast growth factor by neurons and astrocytes. J. Neurosci. 1991;11:2249–2258. doi: 10.1523/JNEUROSCI.11-07-02249.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walicke P, Cowan WM, Ueno N, Baird A, Guillemin R. Fibroblast growth factor promotes survival of dissociated hippocampal neurons and enhances neurite extension. Proc. Natl. Acad. Sci. USA. 1986;83:3012–3016. doi: 10.1073/pnas.83.9.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Tamatani M, Matsuzaki H, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Akt activation protects hippocampal neurons from apoptosis by inhibiting transcriptional activity of p53. J. Biol. Chem. 2001;276:5256–2564. doi: 10.1074/jbc.M008552200. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Lu W, Hipkens S, Wiegand SJ. BDNF enhances the functional reinnervation of the striatum by grafted fetal dopamine neurons. Exp. Neurol. 1996;137:105–118. doi: 10.1006/exnr.1996.0011. [DOI] [PubMed] [Google Scholar]
- Zaman V, Shetty AK. Pretreatment of donor cells with FGF-2 enhances survival of fetal hippocampal CA3 cell transplants in the chronically lesioned young adult hippocampus. Exper. Neuro. 2003a;183:11–24. doi: 10.1016/s0014-4886(03)00167-5. [DOI] [PubMed] [Google Scholar]
- Zaman V, Shetty AK. Fetal hippocampal CA3 cell grafts enriched with fibroblast growth factor-2 exhibit enhanced neuronal integration into the lesioned aging rat hippocampus in a kainate model of temporal lobe epilepsy. Hippocampus. 2003b;13:618–632. doi: 10.1002/hipo.10091. [DOI] [PubMed] [Google Scholar]
- Zheng W, Quirion R. Comparative Signaling Pathways of Insulin-Like Growth Factor-1 and Brain-Derived Neurotrophic Factor in Hippocampal Neurons and the Role of PI3 Kinase Pathway in Cell Survival. J. Neurochem. 2004;89:844–852. doi: 10.1111/j.1471-4159.2004.02350.x. [DOI] [PubMed] [Google Scholar]