Abstract
The study investigated the time course of the effects of acoustic and electric stimulation on the electrically evoked compound action potential (ECAP). Adult guinea pigs were used in acute experimental sessions. Bursts of acoustic noise and high-rate (5000 pulses/s) electric pulse trains were used as maskers. Biphasic electric pulses were used as probes. ECAPs were recorded from the auditory nerve trunk.
Simultaneous masking of the ECAP with acoustic noise featured an onset effect and a decrease in the amount of masking to a steady state. It was characterized by a two-component exponential function. The amount of masking increased with masker level and decreased with probe level. Post-stimulatory ECAP recovery often featured a non-monotonic time course, described by a three-component exponent. Electric maskers produced similar post-stimulatory effects in hearing and acutely deafened subjects.
Acoustic stimulation affects the ECAP in a level- and time-dependent manner. Simultaneous masking follows a time course comparable to that of adaptation to an acoustic stimulus. Refractoriness, spontaneous activity, and adaptation are suggested to play a role in ECAP recovery. Post-stimulatory changes in synchrony, possibly due to recovery of spontaneous activity and an additional hair-cell independent mechanism, are hypothesized to contribute to the observed non-monotonicity of recovery.
Keywords: Adaptation, Cochlear implant, Deafening, Electric-acoustic stimulation, Guinea pig, Recovery
1. Introduction
1.1. Background
Hearing loss is a common problem in the modern world. Electrical stimulation of the auditory nerve by a cochlear implant (CI) is the method of choice for treatment of severe and profound sensorineural hearing loss (NIH Consensus Statement, 1995). Recent advances in CI design, stimulation paradigms, and surgical techniques have led to changes in CI candidacy criteria, specifically, lowering the degree of hearing loss used as an indication for implantation. Consequently, patients with some degree of residual acoustic hearing are receiving CIs (NIH Consensus Statement, 1995; Cohen, 2004; Copeland & Pillsbury, 2004). The functionality of the remaining hair cells may be preserved in patients with residual hearing following implantation (Kiefer et al., 2002; Skarżyński et al., 2003; Gstoettner et al., 2006). This can be accomplished with a “soft” surgical approach (i.e., as atraumatic to the cochlea as possible) and the use of modified electrode arrays, inserted into the cochlea to a limited depth.
Clinical studies indicate that it is possible to take advantage of residual acoustic hearing in these cases for better speech comprehension — especially in noisy environments — and improved aesthetic quality of perceived sounds (von Ilberg et al., 1999; Gantz & Turner, 2004; Gstoettner et al., 2006; Turner et al., 2004; Kiefer et al., 2005; Gantz et al., 2006; Skarżyński et al., 2006). This can be achieved by employing combined electric and acoustic stimulation (EAS) of the auditory periphery with a CI and a hearing aid.
Damage to hair cells associated with sensorineural hearing loss is usually not uniform throughout the cochlea. Hair cells in the base of the cochlea are more susceptible to damage than those in the apex (Hinojosa et al., 1995, 2001; Sha et al., 2001). In a typical clinical case of EAS, a certain spatial segregation between areas of electric and acoustic stimulation within the cochlea may be expected (Wilson et al., 2003). It may be presumed that the CI, inserted into the basal turn of the cochlea, electrically excites mainly the higher-frequency region, which is devoid of hair cells. On the other hand, the apical (low frequency) portion of the implanted cochlea may have functional hair cells and thus be sensitive to acoustic stimulation provided by the hearing aid.
Nevertheless, populations of auditory nerve fibers (ANFs) receiving the two types of stimulation may overlap in the implanted cochleae (Kiefer et al., 2005). Therefore, the influence of one type of stimulus on responses to the other may be expected in the auditory periphery. Clinical studies on patients receiving EAS suggest that overlapping of the frequency ranges represented by acoustic and electric stimulation may be either advantageous or detrimental for auditory perception (Wilson et al., 2002; Kiefer et al., 2005). Acoustic simulations of EAS in normal-hearing listeners predict better speech recognition when there is no overlapping between the acoustic and simulated “electric” frequency representation maps, and when the gap between them is minimized (Dorman et al., 2005). In order to take advantage of acoustic-electric interactions that may occur in the auditory nerve, or to minimize their possible antagonistic effects on auditory perception, it is important to characterize them and to understand the underlying mechanisms.
1.2. Previous relevant research
Previous work in our laboratory addressed the effects of hair cells on auditory nerve responses to electric stimuli. A study by Hu et al. (2003) described electrically evoked compound action potentials (ECAPs) of the auditory nerve in guinea pigs temporarily deafened with furosemide. It was found that the presence of functional hair cells could affect the ECAP in several ways. Following furosemide treatment, ECAP growth functions exhibited greater saturation response amplitudes and steeper slopes compared to those recorded prior to the treatment. Stimulation with electric pulse trains produced smaller ECAP amplitude alternation and less adaptation, as demonstrated by greater adapted and refractory response amplitudes in ears with intact hair cell function. Miller et al. (2006) reported higher thresholds and greater dynamic ranges of single-fiber responses from acoustically sensitive cats compared to chemically deafened subjects. These observations led to a hypothesis that, relative to responses from deaf ears, the presence of hair cells had a desynchronizing effect on the electrically evoked response of the auditory nerve due to spontaneous neurotransmitter release.
Studies in our laboratory have also addressed effects of simultaneous acoustic stimulation on the ECAP. Acoustic stimulation has been demonstrated to produce a masking effect on the auditory nerve responses to single electric pulses (Miller et al., 2000). Specifically, the ECAPs exhibited a decrease in amplitude in the presence of wideband acoustic noise. Spatial properties of the observed interference of acoustic and electric stimuli were addressed by manipulating the spectral content of the acoustic noise masker (Abbas et al., 2001). It was found that for high-pass cut-off frequencies of an acoustic masker below 3 – 4 kHz, the amount of masking was independent of the cut-off frequency. As the electric probe stimulus was delivered to the basal portion of the cochlea, it was inferred that interaction between acoustic and electric stimuli was mainly occurring in the ANFs that innervated the base of the cochlea.
Masking of electrically evoked responses was also observed at the single-fiber level. The effects of an acoustic masker on single-unit response properties (threshold, jitter, and relative spread) were addressed by Abbas et al. (2003) and Miller et al. (2003). All three measures exhibited a positive correlation with the intensity level of the acoustic masker, thus indicating a general desynchronizing effect of acoustically driven activity on single-unit responses.
To investigate temporal properties of acoustic-electric interactions, effects of acoustic stimulation on auditory nerve responses to electric pulse trains presented at 250 pulses per second (pps) were studied using the ECAP and single-fiber measures (Nourski et al., 2005a; Miller et al., 2004). We observed decreased ECAP amplitudes as well as increased single-fiber thresholds and reduced firing synchrony in response to electric pulse trains during — as well as after — stimulation with broadband acoustic noise. Our findings suggested that acoustically driven activity had a desynchronizing simultaneous effect on the response of the ANFs (thereby reducing their contribution to the ECAP) and produced a cumulative post-stimulatory effect that reduced neural responses following the cessation of the acoustic stimulus. Part of this post-stimulatory effect was hypothesized to be due to suppression of ANF spontaneous activity by prior acoustic stimulation (Kiang et al., 1965).
1.3. Aims and hypotheses
The major goal of the present study was to provide a detailed description of the temporal properties of acoustic-electric interactions at the level of the auditory nerve. Responsiveness of the auditory nerve can decrease over the course of repeated electric stimulation (Haenggeli et al., 1998; Matsuoka et al., 2000). Our previous study of masking of the ECAP with acoustic noise demonstrated that the magnitude of the masking effect decreased with the probe pulse stimulation rate (Nourski et al., 2005a). This suggested that there was an interaction between adaptation to electric stimulation and acoustic masking of the electrically evoked response. To investigate the time course of acoustic-electric interactions while avoiding adaptation induced by electric stimulation, ideally, a single electric probe pulse would be presented at different times relative to the acoustic stimulus. However, such a paradigm would be prohibitively slow for obtaining a reasonable temporal resolution. An electric pulse rate of 5 pps was expected to produce no significant adaptation effects (Haenggeli et al., 1998; Matsuoka et al., 2000), so we chose such a low rate pulse train as the probe stimulus in the present study. As responses to each individual electric pulse within such a train are expected to be independent from preceding pulses, these stimuli were referred to as single pulses throughout the manuscript.
The first aim was to characterize the simultaneous effects of acoustic stimulation on the auditory nerve responses to electric stimuli. Acoustically evoked activity is expected to put the ANFs in a state of refractoriness and, consequently, increase excitation thresholds and desynchronize the population response to simultaneously presented electric stimuli. As the magnitude of the ECAP depends on firing rate as well as across-fiber firing synchrony, it was hypothesized that the simultaneous effect of acoustic stimulation would decrease ECAP amplitudes in response to pulsatile electric stimuli. Such a masking effect was hypothesized to be dependent on the amount of acoustically driven activity. Thus, the simultaneous masking effect should increase with level of the acoustic stimulus, and decrease over time due to peripheral auditory adaptation to the acoustic stimulus.
The second aim of this study was to assess post-stimulatory effects of acoustic stimulation on electrically evoked responses in the auditory nerve. Acoustic stimulation may be expected to put the ANFs in adapted states and thus decrease the ANF excitability to subsequent electric stimuli. Adaptation to the acoustic stimulus is also expected to produce a decrease in spontaneous activity, which could be expected to result in an enhancement of the auditory nerve gross-potential response to electric stimuli. As neural adaptation and decrease in spontaneous activity will have opposite effects on the electrically evoked population response of the auditory nerve, they may produce a complex temporal response. We hypothesized that acoustic stimulation would affect the auditory nerve responses to electric stimuli presented after the cessation of the acoustic stimulus, and the magnitude of the post-stimulatory effects would increase with the duration of the acoustic stimulus.
The third aim of the present study was to characterize the recovery of electrically evoked responses in the auditory nerve following electric stimulation, and compare it with recovery following acoustic stimulation. Such a comparison was based on a qualitative description of the shapes of recovery functions, and on a quantitative description of the time constants of recovery. Hair cell activity can affect electrically evoked responses of the auditory nerve (Moxon, 1971; Hu et al., 2003) and thus may play a role in ECAP recovery from electric masking. Specifically, a high-rate electric masker stimulus might depolarize inner hair cells which, in turn, would release the neurotransmitter. This could result in a depletion of the neurotransmitter store in the inner hair cells over the duration of the electric masker stimulus and a transient decrease in spontaneous activity in the auditory nerve after the masker offset. From that perspective, it may be hypothesized that recovery of spontaneous activity might contribute to ECAP amplitude recovery following high-rate electric stimulation. To address this possibility, ECAP forward masking was assessed before and after using chemical treatments to impair functionality of the hair cells. After such treatments, temporal properties of auditory nerve responses would presumably be dominated by mechanisms in the neuronal membranes rather than by a combination of hair cell and membrane mechanisms.
The two chemicals used for deafening were furosemide, a loop diuretic, and neomycin, an aminoglycoside antibiotic, each of which has specific advantages. Furosemide is applied systemically (i.v.) rather than locally, and therefore its administration does not risk direct mechanical trauma to the cochlea. As the effect of furosemide on hearing is reversible, it allows for A-B-A type comparisons, which are useful for assessing the experimental stability and attributing the effects to hair-cell functionality (Hu et al., 2003). Furosemide indirectly impedes hair cell function by inhibiting the function of the stria vascularis (Pike & Bosher, 1980; Ruggero & Rich, 1991) and reducing the endocochlear potential. Administration of furosemide has been demonstrated to correlate with decreases in spontaneous activity and threshold elevations in ANFs (Sewell, 1984a, 1984b; Searchfield et al., 2004). A limitation of using furosemide is a relatively brief period (on the order of minutes) of complete hearing loss following its administration (Hu et al., 2003). Consequently, only a limited amount of data can be collected in the deafened condition.
Aminoglycoside antibiotics such as neomycin are commonly used to produce deaf animal models for CI studies (Leake-Jones et al., 1982; Miller et al., 1998). Treatment with neomycin causes irreversible damage to hair cells (Anniko & Møller, 1978), thereby not subjecting the experimenter to the time limitations inherent with furosemide use. A disadvantage of using neomycin in an acute experimental setting is that intracochlear administration of the drug requires direct replacement of cochlear fluids and thus implies possible mechanical trauma to the cochlear structures, which could affect electric current paths and complicate before-and-after treatment comparisons. In addition, acute intracochlear administration of neomycin can damage the auditory nerve (Leake-Jones et al., 1982).
Portions of this study have appeared previously in abstract form (Nourski et al., 2005b, 2005c, 2006).
2. Methods
2.1. General approach
Nineteen adult albino guinea pigs (weight range 450 – 820 g), free from middle ear infection, were used in acute experimental sessions. In all experiments, evoked activity in the auditory nerve was assessed by measuring ECAP amplitudes produced in response to electric pulses. These electric pulses were referred to as probe stimuli. The masker stimuli used in the present study were bursts of broadband acoustic noise and high-rate electric pulse trains. They were presented simultaneously with, and prior to, presentation of the probe stimuli, to assess simultaneous and forward masking of the ECAP, respectively. As forward-masking functions reflect a recovery of the response to the probe stimulus following stimulation with the masker stimulus, the terms “forward masking of the ECAP” and “ECAP recovery” were used interchangeably throughout the manuscript.
2.2. Surgical preparation
Initial anesthesia was accomplished with a combination of ketamine hydrochloride (40 mg/kg), xylazine hydrochloride (7.5 mg/kg), and acepromazine maleate (0.5 mg/kg), administered intramuscularly. To reduce mucosal secretion, a single dose of atropine sulphate (0.05 mg/kg) was given subcutaneously at the beginning of the experimental session. After anesthesia was induced, the right external jugular vein was surgically exposed, and a catheter was inserted to provide a route for hydration with lactated Ringer's solution. Then the animal was tracheotomized and connected to a ventilator (Harvard Apparatus model 665) with an O2 supply (tidal volume 5 ml, respiration rate 50–55 cycles/min). Partial pressure of CO2 in the expired air and respiration rate were monitored with a capnometer (BCI model 9004). Peak CO2 partial pressure was maintained at 25–35 mm Hg. Core temperature, heart rate, and blood oxygen saturation were monitored with a vital signs monitor (PaceTech model 4000B). Core temperature was maintained at 38.5 ± 0.5°C with a heating pad and drapes. The level of anesthesia was assessed every 30 minutes using a paw-pinch reflex, and maintenance anesthesia (ketamine 20 mg/kg, xylazine 3.75 mg/kg, acepromazine 0.375 mg/kg) was given as needed.
The animal's head was immobilized with a custom-made head holder. After a subcutaneous injection of lidocaine hydrochloride (1%) with epinephrine (0.001%), an incision above and posterior to the left auricle was made. The skin flap and underlying muscles were retracted to expose the bulla. The cartilage of the left ear canal was cut to facilitate coupling with a speculum and earphone for acoustic stimulation. Next, a defect in the lateral wall of the bulla was made with a cutting burr to expose the cochlea. A cochleostomy was performed using a 30 gauge needle. It provided access to the scala tympani of the basal cochlear turn to allow for intracochlear electric stimulation. The left parietal bone superior to the external auditory meatus was thinned with a cutting burr and removed with a rongeur to expose the flocculus of the cerebellum. The flocculus was then retracted medially with two cotton balls to provide access to the auditory nerve trunk for the recording of evoked potentials.
Four subjects were temporarily deafened with 1% furosemide. Following the protocol of Hu et al. (2003), furosemide was delivered intravenously through a catheter in the right external jugular vein over a one-minute interval, at a dose of 80–100 mg/kg. The effectiveness of the deafening procedures was confirmed by measuring acoustically evoked compound action potentials (ACAPs) in response to click stimuli, following the approach of Hu et al. (2003). An absence of ACAP in response to the highest-level acoustic clicks (105 dB SPL) following drug administration, which corresponded to a shift of acoustic sensitivity of at least 60 dB, was required for including a subject in this experiment. The temporarily deafened condition was defined by absence of ACAP in response to 105 dB acoustic clicks both immediately prior to and immediately after data collection.
Three subjects were acutely deafened with 10% neomycin sulfate. Following the approach of Miller et al. (1998), perilymph was gently aspirated with a microsyringe via the round window and replaced, over a period of several minutes, with small boli (∼10 μl) of neomycin until a volume of 50 μl was delivered. The same criterion (zero-amplitude ACAP in response to 105 dB SPL acoustic clicks) was used for inclusion of a subject in this experiment.
Stimulation and recording were performed in a double-walled sound booth. After the experimental session, the animal was euthanized by an overdose of pentobarbital sodium. Surgical and experimental protocols were approved by The University of Iowa Animal Care and Use Committee, and were conducted according to the animal use standards of the National Institutes of Health.
2.3. Stimulus presentation
Acoustic and electric stimuli presented in experimental sessions were generated by a 16-bit digital-to-analog converter at a sampling rate of 100,000 samples/s. The converter was controlled by custom software written using LABVIEW® programming environment. Acoustic clicks were produced by driving an earphone (Beyerdynamic model DT-48) with biphasic pulses (100 μs/phase, alternating polarity) via an attenuator (Hewlett-Packard model 350C), and a custom-designed impedance-matching transformer. The clicks were presented at a rate of 33 per second. Bursts of broadband (20 Hz–20 kHz) acoustic noise were produced by a noise generator (Grason-Stadler model 455C) and gated by an electronic switch (Wilsonics model BSIT) using a rise/fall time of 1 ms. The gated output of the generator was fed to the attenuator, the transformer, and the earphone coupled to an otoscopic speculum. The sound pressure level (SPL) in the ear canal in individual subjects was estimated using a 1/4-inch condenser microphone (Radio Shack model 33-3013) coupled to the speculum. The highest noise level was approximately 105 dB SPL (overall level).
For electric stimulation of the auditory nerve, an electrode made from Teflon-insulated Pt/Ir (90/10%) wire (a 1 mm length stripped of its insulation) was used. The electrode was inserted through the cochleostomy into the scala tympani, to a depth of about 1 mm. A return needle electrode was placed in the left forepaw to facilitate monopolar intracochlear electric stimulation.
Biphasic (40 μs/phase, cathodic polarity-first) rectangular electric pulses were delivered to an optically isolated, capacitatively coupled current source. The output of the current source was monitored with an oscilloscope through an optically isolated amplifier.
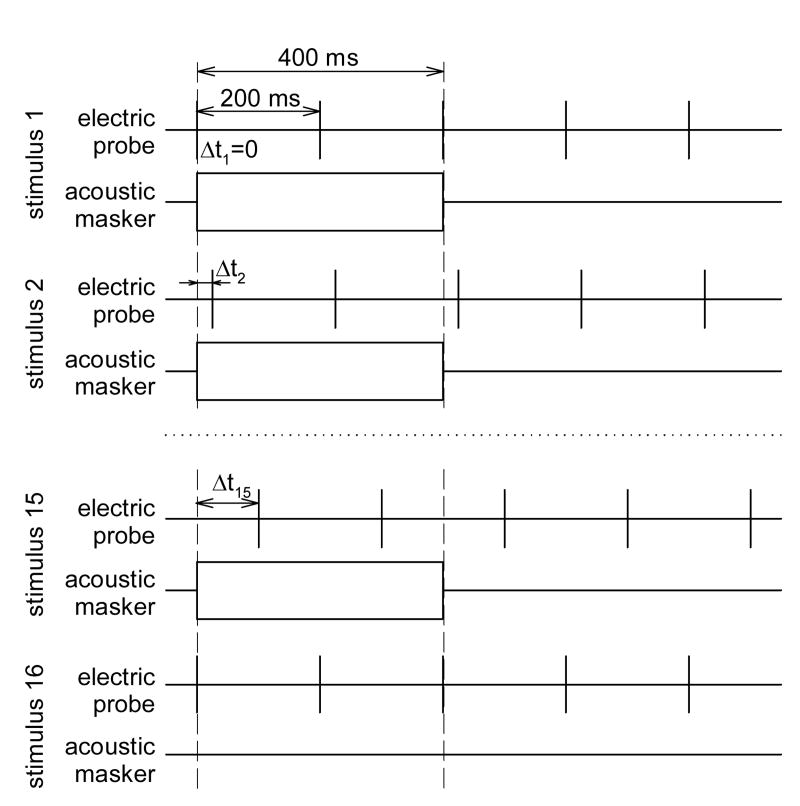
Fig. 1 presents the stimulus paradigm used to evaluate the time course of simultaneous and post-stimulatory effects of acoustic noise on the ECAP evoked by single electric pulses. Electric probe pulses were presented at a rate of 5 pps. A series of combined “acoustic masker and electric probe” stimuli was presented with the electric pulses delayed relative to the onset of acoustic noise. Within each such series, this delay, Δt, was increased from 0 to 150 ms in approximately logarithmic steps (Δt=0, 1, 2, 3, 4, 5, 7, 10, 15, 20, 35, 50, 70, 100, and 150 ms). Each series of fifteen combined electric and acoustic stimuli was followed by an electric probe-only control (stimulus 16 in Fig. 1). Responses to probe pulses within each stimulus (1 through 16) were averaged independently.
Fig. 1.
Schematic of the stimulus presentation paradigm used to study the effects of acoustic noise on auditory nerve responses evoked by electric pulses. Rectangles indicate bursts of acoustic noise. Short solid vertical lines indicate electric pulses, long dashed vertical lines indicate the onset and the offset of the masker stimulus. Δt, probe onset delay time.
The duration of the acoustic noise burst was 400 ms in most experiments. The silent interval between bursts of noise was set to 1200 ms to avoid cumulative across-stimulus effects of acoustic noise on the auditory nerve responses.
Forward masking of the ECAP with high-rate electric stimulation was studied using a similar stimulus paradigm. An electric pulse train, presented at a rate of 5000 pps with a 400 ms duration, was used as the masker. Electric probe pulses were presented following the offset of the masker train at rate of 5 pps. A series of fifteen combined masker and probe stimuli was presented with the masker-probe interval increasing from 0 to 150 ms using the same steps as in the acoustic masking experiment described above. The silent interval between masker pulse trains was set to 1200–1600 ms to minimize auditory fatigue effects. Each series of fifteen masker and probe combinations was followed by a probe-only control.
2.4. Response recording
Evoked potentials were recorded from the auditory nerve in a bipolar mode, using a pair of Pt/Ir (90/10%) ball electrodes with insulated shanks. The positive input electrode was placed about 1 mm superior to the nerve trunk, and the negative-input reference electrode was positioned 2 mm superior to the positive input. The space around the recording electrodes was filled with saline. A ground needle electrode was placed in neck muscle.
The recorded potentials were amplified with a relatively low gain of 20 dB to avoid saturation of the amplifier (DL Instruments model 1201) by the electric stimulus artifact. The recordings were low-pass filtered (6 pole Butterworth, 30 kHz cutoff, 120 dB/decade slope) and digitized by a 16-bit analog-to-digital converter at a rate of 50,000 samples/s for subsequent analysis. Acoustic sensitivity was assessed by measuring the ACAPs in response to click stimuli and determining a threshold response. ECAP growth functions were obtained by presenting single biphasic electric pulses (40 μs/phase) at different levels. ACAP thresholds and ECAP growth functions were obtained repeatedly throughout the course of each experiment, to monitor the stability of the animal preparation.
2.5. Data analysis
Click-evoked ACAP thresholds were determined using a visual criterion. The data presented in this study were obtained from acoustically sensitive animals that exhibited acoustic threshold shifts of 15 dB or less throughout the duration of the experimental sessions.
ECAP amplitudes, analyzed using custom-designed software written in the MATLAB® Version 6.1 programming environment, were measured from the first negative peak, N1, to the following positive peak, P2 (Miller et al., 1998). The latencies of the N1 peak were approximately 0.4–0.5 ms. This indicated that the measured responses were generated by direct depolarization of ANFs rather than through a hair cell related mechanism (β or δ responses) (Moxon, 1971; van den Honert & Stypulkowski, 1984).
Responses were collected at several stimulus levels to construct ECAP growth functions. To facilitate inter-subject comparison, stimulus levels for electric probe stimuli were chosen based on growth functions normalized to their saturation (maximum) amplitude. In most experiments, electric probe pulses were presented at current levels that produced a response corresponding to 40, 60, 80, and 95% of saturation response amplitude. Electric masker pulses were presented at levels corresponding to 5, 20, 40, and 60% of saturation response amplitude.
To provide a relative measure of the effect of the masker stimulus on the response to the probe, response amplitudes obtained in the combined masker and probe condition were normalized to those of the probe-only condition and plotted for each probe pulse as a function of time. Changes in ECAP amplitude during and after presentation of the masker stimuli (simultaneous and forward-masking functions) were described by fitting exponential functions to normalized ECAP amplitude data (see Results for a description of the models). Model parameters were fit using the Marquardt-Levenberg least-squared error algorithm with SigmaPlot version 7.101 software.
3. Results
3.1. General features
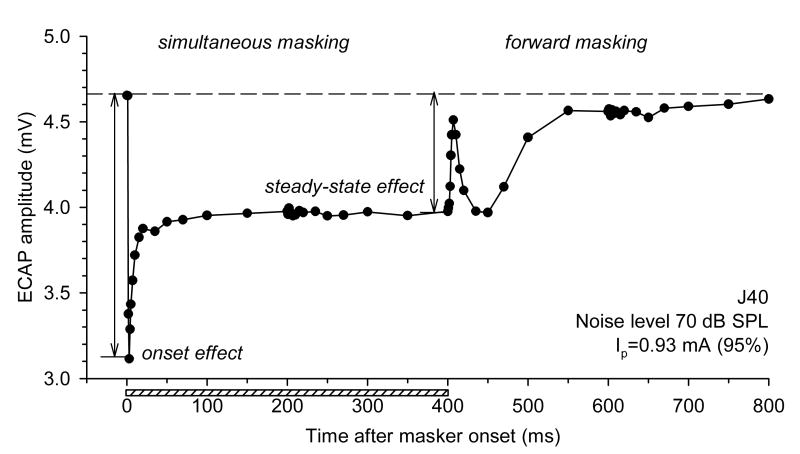
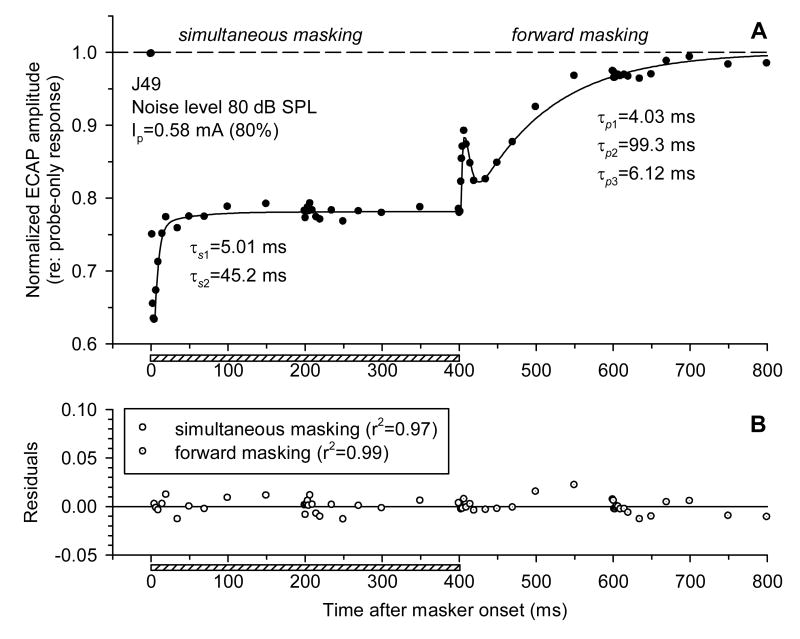
Fig. 2 demonstrates an example of changes in the ECAP in response to single electric pulses presented with different delays relative to stimulation with broadband acoustic noise. When electric probe pulses were presented simultaneously with acoustic noise (simultaneous masking), ECAP amplitudes in response to them decreased compared to the probe-only condition (indicated by a dashed line in Fig. 2). The maximum decrease of ECAP amplitude was observed shortly after the onset of the noise (“onset effect” in Fig. 2). ECAP amplitudes to pulses presented with greater delays relative to the masker onset gradually increased to an asymptote (“steady-state effect” in Fig. 2). The transition from the onset of the masking effect to the steady state followed, approximately, an exponential time course.
Fig. 2.
Simultaneous and post-stimulatory effects of acoustic noise on the ECAP. ECAP amplitudes in response to probe electric pulses are plotted as functions of time after the masker onset. The dashed line corresponds to the probe-only (control) response. The horizontal bar indicates the presentation time of the acoustic masker. Acoustic masker level 70 dB SPL, electric probe level 0.93 mA (corresponding to 95% of the maximum single-pulse ECAP amplitude).
ECAP amplitudes to pulses presented after the offset of the acoustic stimulus often exhibited non-monotonic changes over time. The ECAPs underwent a rapid phase of recovery within several milliseconds after the masker offset. This was followed by a transient decrease in response amplitudes and then further recovery to amplitudes corresponding to the unmasked control condition. This pattern was observed in all subjects (but not all stimulus conditions), in which ECAP recovery from masking was studied (n=13).
3.2. Level effects
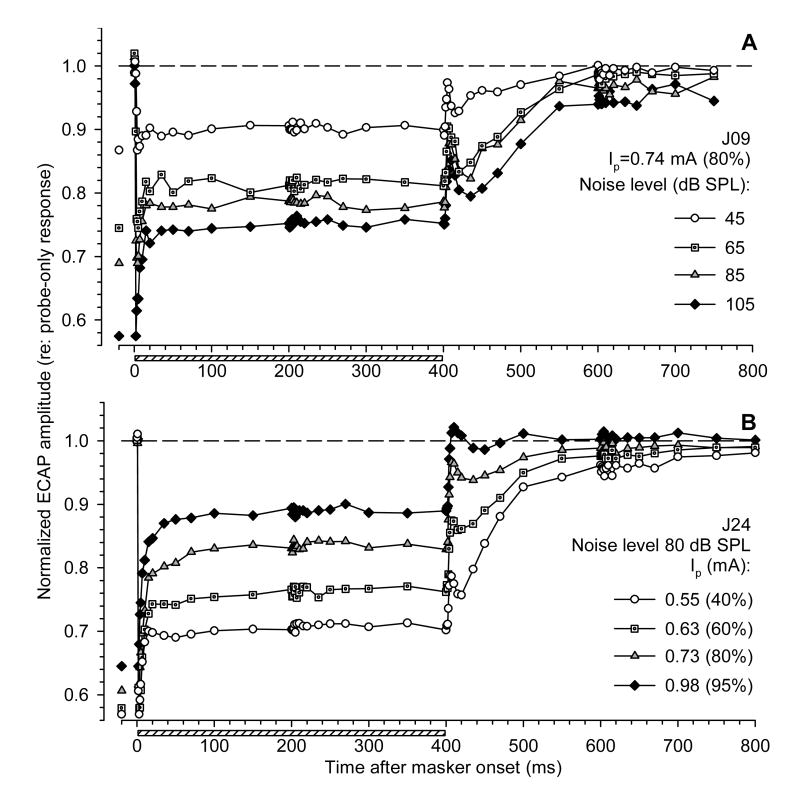
Fig. 3 presents examples of acoustic-electric interactions across stimulus levels. The effects of changing masker and probe levels are shown in Fig. 3A and 3B, respectively. In Fig. 3, and in following figures that demonstrate simultaneous and forward-masking functions, ECAP amplitudes in response to probe pulses are presented in normalized form (relative to probe-only responses). To clearly indicate the onset masking effect of noise, minimum normalized ECAP amplitudes within 5 ms following noise onset are re-plotted in Fig. 3 on the left side of the graphs.
Fig. 3.
Effects of stimulus intensity on simultaneous and forward masking of the ECAP with acoustic noise. ECAP amplitudes in response to probe electric pulses are normalized to probe-only responses, and plotted as functions of time after masker onset. A: Fixed electric probe level (0.74 mA); data for four levels of acoustic masker are shown. B: Fixed acoustic masker level (80 dB SPL); data for four levels of electric probe are shown. Horizontal bars indicate presentation time of the acoustic masker. Minimum normalized ECAP amplitudes within 5 ms following noise onset (i.e., the onset effect of the acoustic masker) are re-plotted on the left for clarity.
Fig. 3A demonstrates an example of the effects of acoustic noise presented at four different levels on responses to fixed-level electric probe pulses. Higher masker levels produced greater decreases in the normalized response to the probe both at onset and in steady-state, corresponding to a greater masking effect. The post-stimulatory effects of noise were also greater at higher masker levels. These trends were consistently observed in all subjects for which the acoustic noise was presented at several levels (n=5).
The effects of a fixed-level acoustic masker on ECAPs evoked by different level electric probes are illustrated in Fig. 3B. Greater post-stimulatory effects of noise were observed in responses to probe stimuli presented at lower intensities. This finding was consistent across subjects, for which the effects of probe stimulus level were examined (n=12). A fixed-level masker produced comparable onset effects on responses across probe levels, but smaller steady-state effects as probe level was increased.
3.3. Onset of the masking effect
The examples of masking functions presented above (Fig. 2, 3) demonstrated that onset of the masking effect of acoustic noise on the ECAP was relatively abrupt, but not instantaneous. Given the transient nature of some speech signals, the timing of the onset effect of acoustic stimulation on the ECAP may be particularly relevant to the acoustic-electric interactions that could occur in CI users with significant residual acoustic hearing. Furthermore, a precise description of the timing of the onset effect may be important for an accurate mathematical description of the time course of masking.
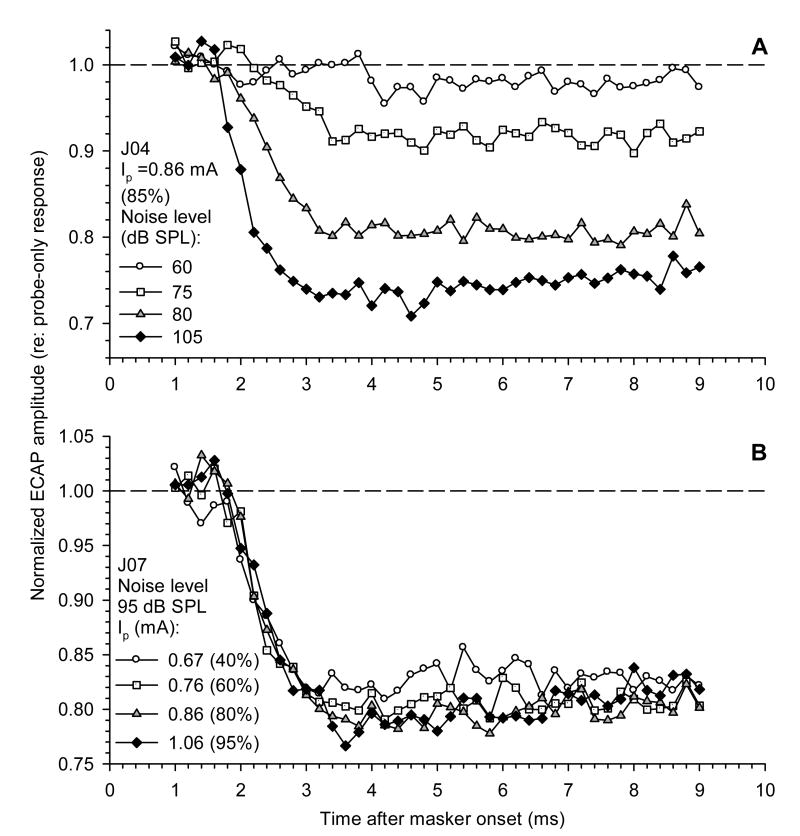
To investigate this effect in greater detail, relatively brief (10 ms) acoustic noise bursts were used as masker stimuli. Single electric probe pulses were systematically presented at different delays relative to the noise burst. To achieve good temporal resolution, the delay between the onset of the masker and the presentation of the probe was increased from 1 to 9 ms in 0.2 ms steps.
Examples of masking functions obtained from two subjects, using this approach, are shown in Fig. 4. In Fig. 4A, the masking functions for four different noise levels are presented under a fixed probe level condition. In Fig. 4B, the effects of a fixed-level masker on responses to probe stimuli of four levels are shown. Plots of normalized ECAP amplitudes in response to pulses demonstrate that the masking effect of acoustic stimulation is not instantaneous. The maximum effect on ECAP amplitude (i.e., the minimum amplitude compared to the control) had a latency of 3–4 ms following onset of acoustic noise. Figure 4A illustrates that the latency, as well as the relative magnitude of the onset effect, were dependent on acoustic masker level, with the maximum decrement occurring earlier at higher masker levels. In contrast, masking functions obtained for different probe levels using a fixed-level masker exhibited very similar latency and shape (Fig. 4B).
Fig. 4.
Onset of the masking effect of acoustic noise on the ECAP. ECAP amplitudes in response to probe electric pulses are normalized to probe-only responses and plotted as functions of time after masker onset. A: Fixed electric probe level (0.86 mA); data for four acoustic masker levels are shown. B: Fixed masker level (95 dB SPL); data for four probe levels are shown. Masker duration 10 ms.
The latency of the masking effect of acoustic noise illustrated in Fig. 4 may be the result of several factors. These include delays associated with propagation of the acoustic stimulus and the synaptic delay. In addition, the acoustic noise stimuli were gated with a 1 ms rise/fall time, and thus the envelope of the acoustic stimulus might also contribute to the latency of the masking effect.
3.4. Duration effects
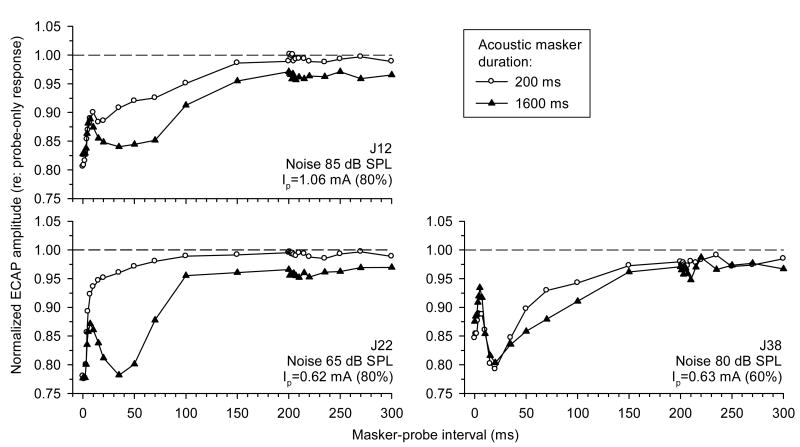
A remarkable feature of forward masking of the ECAP with acoustic noise was that it often featured a non-monotonic time course. Our previous results, obtained using 250 pps pulse-train probe stimuli, demonstrated that the post-stimulatory effects of acoustic noise on the ECAP were duration-dependent (Nourski et al., 2005a). Fig. 5 explores these duration effects, using a single probe pulse paradigm to compare recovery of the ECAP from masking with relatively short and long duration acoustic noise maskers (200 and 1600 ms, respectively). Note that in this figure, normalized ECAP amplitudes are plotted as functions of masker-probe interval (i.e., the time after masker offset), thus focusing on the post-stimulatory recovery of the ECAP. Fig. 5 demonstrates that the greater masker duration produced a slower post-stimulatory recovery of the ECAP. The recovery was incomplete at 300 ms following the offset of the 1600 ms masker in all three subjects. In addition, the greater masker duration produced a more pronounced non-monotonic time course of the forward-masking functions (particularly, in subjects J12 and J22). These trends are consistent with the electric pulse-train data presented previously (Nourski et al., 2005a).
Fig. 5.
Effects of noise duration on recovery of the ECAP from masking with acoustic noise. ECAP recovery functions following stimulation with 200 ms and 1600 ms bursts of acoustic noise are shown with open circles and filled triangles, respectively. ECAP amplitudes in response to probe electric pulses are normalized to probe-only responses, and are plotted as functions of masker-probe interval. Data from three subjects (J12, J22, and J38) are shown.
3.5. Forward masking of the ECAP by high-rate electric stimulation in hearing and deafened subjects
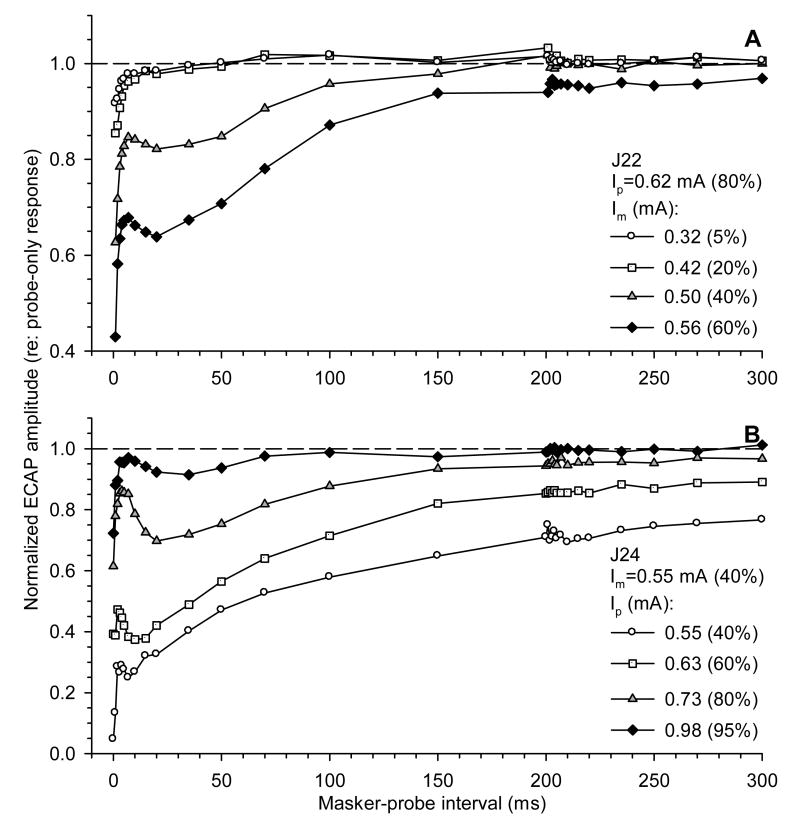
To study ECAP electric forward masking, we first addressed the effects of stimulus level on ECAP recovery following high-rate pulse-train stimulation. Fig. 6 shows examples of recovery functions from two subjects. ECAP amplitudes in response to probe pulses are plotted as functions of masker-probe interval. In Fig. 6A, the probe level was fixed and the masker level was varied. ECAP recovery functions could assume different shapes. Recovery from lower-level maskers (0.32 and 0.42 mA) was relatively fast and monotonic. Higher masker levels (0.50 and 0.56 mA) produced a non-monotonic recovery pattern. In these cases, a fast recovery phase was followed by a plateau or a depression in ECAP amplitude and then a slower phase of recovery.
Fig. 6.
Effects of stimulus intensity on forward masking of the ECAP with 5000 pps electric pulse trains. ECAP amplitudes in response to probe electric pulses are normalized to probe-only responses and plotted as functions of masker-probe interval. A: Fixed electric probe level (0.62 mA); data for four masker levels are shown. B: Fixed electric masker level (0.55 mA); data for four probe levels are shown.
Fig. 6B demonstrates the effect of a constant-level masker on responses to probe pulses presented at different levels. A fixed-level masker produced greater masking on lower-level probe stimuli. Non-monotonic patterns of recovery from masking were evident across probe stimulus levels.
The examples presented in Fig. 6 demonstrate that ECAP recovery from high-rate electric stimulation depends upon level. Comparison between panels A and B suggests that an increase in the masker level relative to the probe could produce greater masking and a slower ECAP recovery. To further investigate this trend, the effect of the high-rate electric masker was measured in data from eight subjects as the normalized ECAP amplitude to the probe pulse presented immediately after the masker offset (a masker-probe interval of 0 ms). It was found that masker stimuli presented at levels lower than half the current level of the probe had a relatively small (<10% decrease compared to unmasked control) effect on the response to the probe pulse presented immediately after the masker offset. In contrast, the masker presented at a level equal to or greater than that of the probe produced a large (>90% decrease) masking effect on the response to the probe (data not shown).
Next, we addressed the question of whether the non-monotonic pattern of ECAP amplitude recovery from high-rate electric stimulation reflects the recovery of spontaneous activity. For this purpose, ECAP recovery from masking was measured before and after using chemical treatments (furosemide and neomycin) that impair functionality of the hair cells.
Immediately after furosemide injection, ACAP amplitudes in response to high level (105 dB SPL) test clicks decreased to zero and, after a relatively short period of time (several minutes), underwent a gradual recovery. ECAP amplitudes in response to single electric pulses exhibited a considerable increase immediately after furosemide treatment, and then decreased to the normal baseline level.
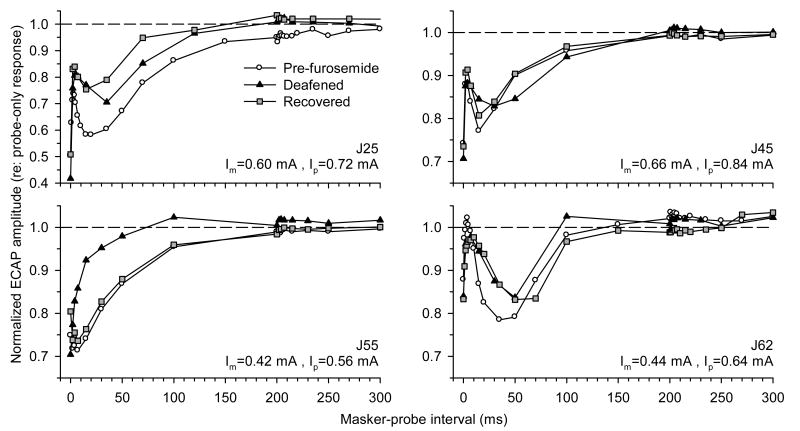
ECAP forward-masking functions obtained from four subjects before furosemide injection, immediately after the treatment, and following recovery of acoustic sensitivity (as assessed by the ACAP), are shown in Fig. 7. In three subjects (J25, J45, and J62) out of four, the non-monotonic pattern of ECAP recovery was observed in both the hearing and the deafened conditions.
Fig. 7.
Forward masking of the ECAP with 5000 pps electric pulse trains at different times relative to furosemide treatment. ECAP amplitudes in response to probe electric pulses are normalized to probe-only responses, and are plotted as functions of masker-probe interval. Forward-masking functions were obtained before (○), immediately after (▲), and following recovery from (■) furosemide administration. Data from four subjects (J25, J45, J55, and J62) are shown.
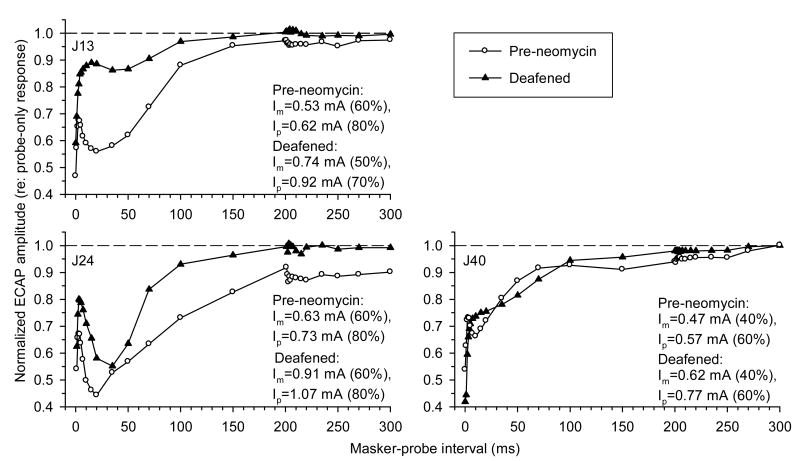
Fig. 8 presents comparisons of ECAP forward-masking functions obtained in three subjects before and after deafening with neomycin. Following administration of neomycin, ACAP amplitudes in response to 105 dB SPL test clicks decreased to zero and exhibited no recovery during the rest of the experimental sessions. In two subjects (J13 and J24) out of three, the non-monotonic time course of ECAP recovery was observed in hearing as well as deafened conditions.
Fig. 8.
Forward masking of the ECAP with 5000 pps electric pulse trains before and after neomycin treatment. ECAP amplitudes in response to probe electric pulses are normalized to probe-only responses and plotted as functions of masker-probe interval. Forward-masking functions were obtained before (○) and following (▲) neomycin administration. Data from three subjects (J13, J24, and J40) are shown.
These data demonstrate that non-monotonic recovery of the ECAP cannot be attributed solely to the recovery of spontaneous activity of hair cells, as the chemical treatments are expected to abolish or greatly reduce that activity.
3.6. Phenomenological models
To provide a quantitative description of the time course of simultaneous and forward masking, we performed a nonlinear regression analysis of the simultaneous and forward-masking functions. Simultaneous masking of the ECAP with acoustic noise appeared to follow an exponential time course (see Fig. 2, 3). We attempted to describe it by fitting an exponential function to the data. Single exponential equations with three free variables (a time constant, an asymptote to account for steady-state masking effect, and a time offset to account for the latency of the masking effect) did not completely provide good fits to the data. Specifically, in many cases, a single exponent could not adequately model the initial “rapid” phase of simultaneous masking (a relatively sharp increase in ECAP amplitude within several milliseconds following the onset effect). Therefore, a second exponential component was added to the equation. The following model was used to describe the time course of simultaneous masking of the ECAP with acoustic noise:
| (1) |
where
A = normalized ECAP amplitude; t = time after masker onset; t0 = latency of the onset effect; As1, As2 = magnitude coefficients (As1 ≥ 0, As2 ≥ 0); τs1, τs2 = rapid and short-term time constant, respectively (τs1 < τs2); Ass = steady-state masking.
ECAP forward-masking functions often had a non-monotonic shape (see Fig. 2, 3). A non-monotonic pattern of recovery was observed in 62 acoustic forward-masking functions out of a total of 100, obtained from thirteen subjects. To account for this, we used two exponential components to describe the recovery of the ECAP, and introduced a third component with an opposite sign. The model for the description of forward masking of the ECAP therefore included two recovery components and one enhancement component:
| (2) |
where
A = normalized ECAP amplitude; t = masker-probe interval (time after masker offset); t0 = latency; Ap1, Ap2, Ap3 = magnitude coefficients (Ap1 ≥ 0, Ap2 ≥ 0, Ap3 ≥ 0); τp1, τp2, = rapid and short-term recovery time constant, respectively (τp1 < τp2); τp3 = enhancement time constant.
Fig. 9 demonstrates an example of regression analyses of simultaneous and forward-masking functions using the equations presented above. In Fig. 9A, normalized ECAP amplitudes (filled circles) are plotted along with the regression curves (solid lines). Fig. 9B presents the residuals of curve fitting for both simultaneous and forward-masking functions. A relatively flat distribution of the residuals, as well as high coefficients of determination (r2), demonstrate that the chosen mathematical models provided a good description of the time course of the acoustic-electric interactions. For cases of monotonic recovery functions, the magnitude of the non-contributing enhancement component (A3) was set to zero, and the model (Eq. 2) was accordingly reduced to a two-component exponential decay function.
Fig. 9.
Regression analysis of the time course of simultaneous and forward masking. A: ECAP amplitudes in response to probe electric pulses normalized to probe-only responses and plotted against time after masker onset. Solid lines represent regression curves fitted to the data (symbols). B: Residuals of least-squares method curve fitting are plotted as a function of time after masker onset (see text for description of regression analysis). Horizontal bars indicate the presentation time of the acoustic masker.
High-rate electric pulse trains produced post-stimulatory effects on the ECAP that appeared to follow a time course similar to the effects of an acoustic noise masker. We therefore sought to determine whether the time course of forward masking could be described by the same mathematical model for both types of masker stimuli (acoustic and electric). Based on the acoustic forward-masking data presented above, a quantitative description of electric forward-masking functions was performed using a three-component exponential decay function with two recovery components and one enhancement component (see Eq. 2).
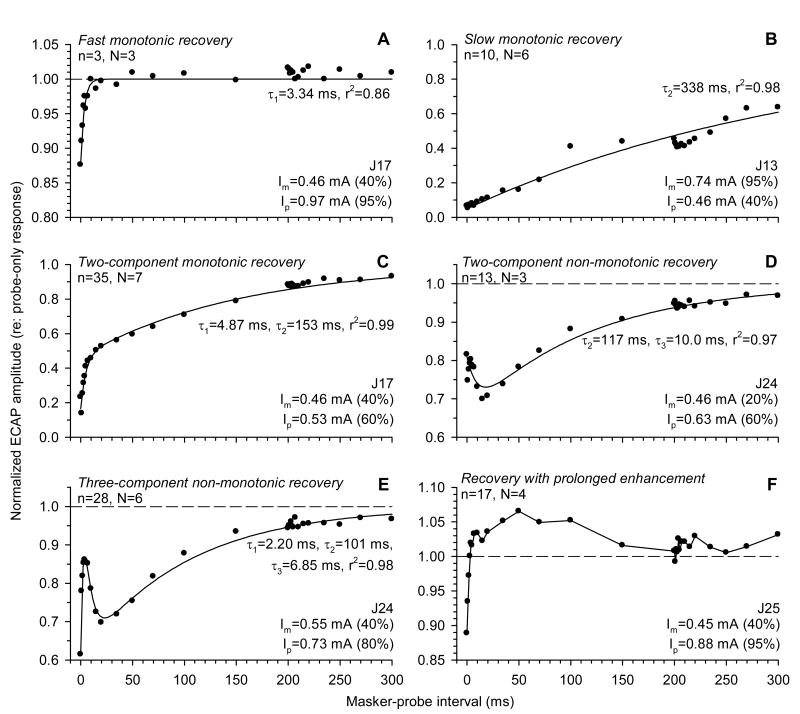
Fig. 10 presents examples of curve fits obtained using this model. The recovery functions, which could assume different shapes, were categorized according to the model components. The proposed three-component model was found to be adequate for the description of the majority of recovery functions (Fig. 10A-E). In some cases, one or two components of the model could be set to zero and still provide good fits. This includes one- and two-component monotonic recovery functions (Fig. 10A-C) and non-monotonic forward masking functions that did not have a rapid recovery phase (Fig. 10D). In other cases, all three components were evident (Fig. 10E).
Fig. 10.
Examples of ECAP recovery functions. ECAP amplitudes in response to probe electric pulses are normalized to probe-only responses and plotted as functions of masker-probe interval. Masker and probe intensities (Im and Ip, respectively) are indicated in each panel. Regression curves fitted to the data (symbols) in panels A-E are represented by solid lines. For each shape, n represents the number of individual functions (out of 106), and N corresponds to the number of subjects (out of 9) in which the particular shape was observed.
In four of nine subjects, ECAP electric forward-masking functions featured a prolonged period of enhancement after offset of the masker. An example of such a recovery function is shown in Fig. 10F. After masker offset, ECAP amplitudes underwent a fast increase to values exceeding the control (unmasked) response amplitudes and remained elevated for about 100 ms. As this phenomenon occurred on a longer time scale compared to the range of values of the enhancement time constant τp3 (see below), we did not use the three-component recovery model to try to fit such forward-masking functions.
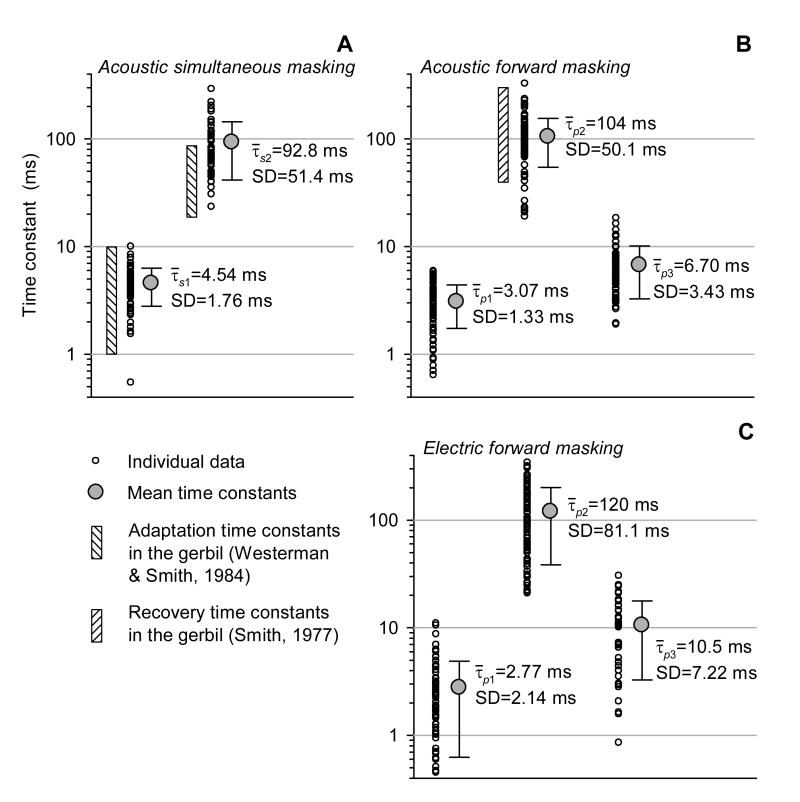
Fig. 11 presents a summary of time constants that described simultaneous (A) and forward (B) masking of the ECAP with 400 ms noise bursts, and forward masking with high-rate electric pulse trains (C). The time constants of acoustic and electric masking were obtained by a regression analysis of data from twelve and nine subjects, respectively. Fig. 11 also demonstrates the ranges of time constants that described single-fiber response adaptation to and recovery from acoustic stimulation, obtained in the gerbil by Westerman and Smith (1984) and Smith (1977), respectively.
Fig. 11.
Summary of time constants that described the time course of simultaneous (A) and forward (B, C) masking of the ECAP. Regression analysis of acoustic (A, B) and electric (C) masking was performed on data obtained from twelve and nine subjects, respectively. Small open circles represent time constants obtained from individual masking functions. Large filled circles indicate mean values of the time constants. Vertical shaded bars correspond to the ranges of adaptation and recovery time constants, obtained in the gerbil by Westerman and Smith (1984) and Smith (1977), respectively.
The two time constants that described simultaneous masking of the ECAP with noise in the present study (4.54±1.76 and 92.8±51.4 ms) were comparable to the ranges of time constants of rapid- and short-term single-fiber response adaptation reported by Westerman & Smith (1984) (1–10 and 20–89 ms, respectively). The short-term recovery time constant obtained by the regression analysis of our ECAP forward-masking data (τp2=104±51.4 ms) was consistent with the range of single-fiber recovery time constants (40–310 ms) reported by Smith (1977). The time constants that described simultaneous and forward masking of the ECAP by acoustic noise did not exhibit any systematic dependence on the masker stimulus level, as correlation coefficients (r2) between the values of the time constants and masker levels were smaller than 0.10. Although the group data demonstrate that the rapid recovery time constant (τp1=3.07±1.33 ms) and the enhancement time constant (τp3=6.70±3.43 ms) were similar, in each individual case τp3 had a greater value than τp1.
For electric masker stimuli, the mean values of time constants that described rapid, short-term recovery and enhancement components of the forward-masking functions, were τp1=2.77±2.14 ms, τp2=120±81.1 ms, and τp3=10.5±7.22 ms, respectively (see Fig. 11C). Comparison with regression analysis of acoustic forward masking shown in Fig. 11B indicates that recovery of the ECAP is very similar for the two modes of stimulation.
We also examined the dependence of time-constant estimates of electric forward masking on masker-to-probe level ratios (Im/Ip). While τp1 and τp3 did not exhibit any systematic change with stimulation level, the values of τp2 were found to be significantly greater at higher masker-to-probe ratios (r2=0.73), corresponding to a slower recovery. That is, the short-term recovery time constant (τp2) increased with a relative increase in the masker level. Non-monotonic recovery functions, for which the enhancement time constant τ3 was defined, were only obtained at Im/Ip≤1.0.
4. Discussion
4.1. Summary of results
The results presented in this study demonstrate that, in hearing subjects, responses to acoustic and electric stimuli could functionally interact at the level of the auditory nerve. Acoustic stimulation (masker) produced a decrease in ECAP amplitude in response to electric stimuli (probe) presented simultaneously, as well as after the cessation of the acoustic stimulus. The simultaneous masking effect of acoustic noise on the ECAP exhibited a maximum at the masker onset and then gradually decreased to a steady state. The time course of simultaneous masking could be described by a double decaying exponential function. The effects of acoustic stimulation on the ECAP were level and duration dependent. In general, greater simultaneous and post-stimulatory effects were observed at greater masker levels and durations. Fixed-level maskers had greater simultaneous and post-stimulatory effects on lower-level probe stimuli. Post-stimulatory effects of acoustic noise on the ECAP often followed a non-monotonic time course. The time course of ECAP forward masking in such cases could be described by a three-component exponential function with two recovery and one enhancement component.
ECAP recovery from masking with high-rate electric pulse trains was found to be similar (in terms of the shapes of the recovery functions and the time constants that described them) to ECAP recovery from masking with acoustic noise. Furthermore, a non-monotonic time course of ECAP recovery was observed in both hearing subjects and in subjects acutely deafened with furosemide or neomycin.
4.2. Comparison with other relevant ECAP and single-fiber studies
For a better understanding of mechanisms that contribute to the observed peripheral acoustic-electric interactions, it is important to consider our findings within the context of other relevant gross potential and single-unit studies of the auditory periphery. Miller et al. (2000) and Abbas et al. (2001) demonstrated that continuous presentation of broadband acoustic noise could produce a reduction in ECAP amplitudes in response to electric pulses. Miller et al. (2000) found that this simultaneous masking effect was larger for lower-intensity probe pulses. Our results for the steady-state effect of noise on the ECAP (see Fig. 3B) agree with that observation. One explanation for this level effect is that a relatively low intensity probe stimulus is less likely to overcome the increase in excitation threshold in ANFs due to a state of relative refractoriness following acoustically evoked spikes.
Abbas et al. (2001) demonstrated that relatively long bursts of acoustic noise (on the order of a minute) could produce decrements in ECAP amplitudes in response to electric pulses presented following the offset of the noise stimulus, with ECAP recovery time on the order of one minute. The present study did not address such long-term effects, instead focusing on temporal interactions spanning hundreds of milliseconds. We showed an effect of noise duration on post-stimulatory ECAP recovery for noise durations of 200 and 1600 ms (see Fig. 5). The data of Abbas et al. (2001) suggest that greater noise durations might cause a slower recovery of the ECAP.
Killian (1994) and Killian et al. (1994) addressed forward masking of the ECAP in guinea pigs using 100–300 ms sinusoidal electric maskers. Using 5 ms 15 kHz electric sinusoids and 20 μs/phase biphasic electric pulses, they reported trends similar to the findings of our study. Specifically, they observed greater ECAP masking and slower recovery at higher masker and lower probe levels (cf. Fig.6). They also reported occasional non-monotonic recovery functions that had a comparable time course to those presented in this manuscript. The recovery characteristics of the ECAP reported by Killian (1994) were very similar in cochleae with and without functional hair cells (cf. Fig. 7, 8). In addition, he observed enhancement of the ECAP following stimulation with relatively low frequency (50–400 Hz) sinusoidal electric maskers. While the origin of this phenomenon is unknown, it may be related to the prolonged post-masking enhancement of the ECAP observed in some of our experiments (see Fig. 10).
ECAP measures of acoustic-electric interactions presented in this manuscript as well as in our earlier study (Nourski et al., 2005a) were paralleled by single-fiber studies of Abbas et al. (2003, 2004) and Miller et al. (2004, 2005a, 2005b). These studies, performed on acute cat preparations, investigated how wideband acoustic noise modified ANF responses to 250 pps electric pulse trains. Despite inherent differences in the methods of measurement, the results of the aforementioned single-fiber studies are by and large consistent with the ECAP data presented here. Specifically, an acoustic stimulus produced an increase in spike rate, which was greatest immediately following the noise onset, and which was followed by a gradual asymptote to an approximately steady state (Abbas et al., 2003). Such a time course is consistent with that of simultaneous masking of the ECAP (see Fig. 2, 3), and with expected changes in acoustically evoked activity due to rapid and short-term adaptation (Kiang et al., 1965; Westerman & Smith, 1984). Measures of ANF firing synchrony to electric probe pulses demonstrated that noise stimulation also produced a simultaneous desynchronizing effect, as evidenced by increased jitter and decreased vector strength (Abbas et al., 2003).
Post-stimulatory effects of acoustic stimulation on ANF responses to electric pulses included a decrease in spike rate and an increase in firing synchrony (Abbas et al., 2004). Changes in these response properties were found to be related to acoustically driven activity (firing rates) during noise stimulation (Miller et al., 2004, 2005b). It is expected that post-stimulatory changes in spike counts and synchrony would have opposite effects on ECAP amplitude. There is some evidence that these properties might recover at different rates following noise onset (Miller et al., 2005a). This difference could account for the non-monotonic shape of ECAP forward-masking functions (see Fig. 2, 3).
Miller et al. (2005b) observed synchrony enhancements in single unit responses to probe electric pulses presented after stimulation with a broadband acoustic masker. They noted that such post-stimulatory synchrony enhancements could be observed in fibers with little to no spontaneous activity. This finding is inconsistent with the hypothesis that attributes post-stimulatory synchrony changes to cessation and recovery of inner hair cell spontaneous activity. It is, however, consistent with the result of the present ECAP study, where non-monotonic ECAP recovery could be observed in cochleae with the function of hair cells compromised by ototoxic treatment (see Fig. 7, 8). While the mechanisms that might account for these phenomena are unknown, they are likely to be localized to the neuronal membrane of the ANFs.
The single-fiber studies of Abbas et al. (2004) and Miller et al. (2004, 2005b) demonstrated that simultaneous, as well as post-stimulatory, changes in ANF firing rate and synchrony in response to electric probe pulses were more pronounced at higher acoustic stimulus levels and longer durations. This is consistent with the level- and duration-dependent trends observed in simultaneous and forward masking of the ECAP (see Fig. 3, 5).
4.3. Interpretation of the temporal aspects of acoustic-electric interactions in the auditory nerve
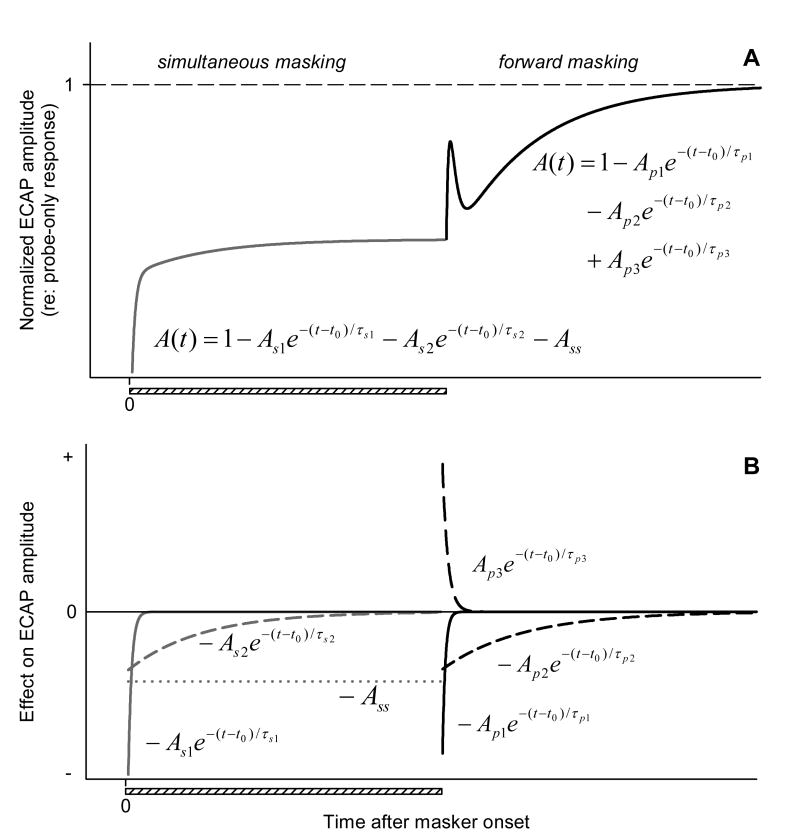
As noted above, we described the time course of simultaneous and forward masking of the ECAP using exponential equation-based phenomenological models (see Eq. 1, 2). This is illustrated as schematic curves in Fig. 12A. Fig. 12B examines how individual components of these curves would affect ECAP amplitude over time. With the proposed phenomenological models in mind, and considering the single-fiber data discussed above, changes in the ECAP amplitude during and after stimulation with acoustic noise may be interpreted as follows.
Fig. 12.
Phenomenological description of the time course of simultaneous and forward masking of the ECAP with acoustic noise. A: Schematic curve fits that described simultaneous and forward masking. B: Effects of individual components of the exponential equations shown in “A” on the ECAP amplitude over time.
Presentation of acoustic noise produces a net increase in the ANF firing rate (acoustically driven activity). Such an increase in activity does not enhance the amplitude of the ECAP, as the timing of the fibers' response to noise is not related to the timing of the probe pulse used to elicit the ECAP. Each fiber's discharge to the acoustic stimulus is followed by a refractory period during which the fiber's responsiveness to the probe is decreased. The refractoriness of ANFs due to acoustically evoked spikes contributes to the decrease of the ECAP, as fewer fibers discharge in response to the probe electric pulse once it is presented. In addition, those ANFs that are in a state of relative refractoriness, but do respond to the probe, may be expected to respond with reduced synchrony (Rubinstein et al., 1999) and decreased amplitude of individual spikes (Miller et al., 2001). These changes on a single-fiber level would also negatively affect the amplitude of the ECAP.
The time course of simultaneous masking is consistent with the expected changes in the desynchronizing effect of noise on electrically evoked activity over time. Peri-stimulatory changes in rate and synchrony associated with acoustic stimulation are somewhat attenuated over time due to a decrease of neurotransmitter release from acoustically stimulated hair cells that constitutes synaptic adaptation (Westerman & Smith, 1984). The amount of masking of the electrically evoked population response (the ECAP) decreases accordingly. The two exponential components of the simultaneous masking function (described by time constants τs1 and τs2; see Fig. 12) are therefore likely to represent rapid- and short-term components of auditory nerve response adaptation to the acoustic masker. The constant term (Ass) then corresponds to the amount of masking produced by the acoustic stimulus when the auditory periphery is in the adapted state, i.e., during steady-state acoustically driven activity.
The time course of forward masking may be approximated as a combination of three exponential processes. When the noise is turned off, there is a decrease in the rate of ANF firing to probe pulses, presumably as a result of neural adaptation to the noise stimulation. This results in a decrease of ECAP amplitude in response to the probe presented shortly after the noise onset (until the fibers fully recover from adaptation). The two exponential components that provide for ECAP increase over time (described by time constants τp1 and τp2; see Fig. 12) may reflect increases in driven rate associated with rapid- and short-term phases of recovery from ANF membrane adaptation.
The third exponential component of the forward-masking function, associated with time constant τp3, is indicative of a process that acts to decrease the ECAP amplitude over time, following the masker offset. One mechanism that could produce such an effect is transient depression of spontaneous activity, followed by the recovery of spontaneous release of neurotransmitter by hair cells after the offset of the noise burst (Kiang et al., 1965; Harris & Dallos, 1979). As a result, those ANFs that do fire in response to the electric probe stimulus shortly after the acoustic masker stimulus offset may do so with a relatively greater synchrony, therefore increasing the magnitude of the population response (ECAP) immediately after the masker offset. Thus, a post-stimulatory increase in firing synchrony that contributes to an enhancement of the ECAP amplitude, coupled with the opposite effect of membrane adaptation on firing rate, may determine the non-monotonic poststimulatory recovery of the ECAP. As rate and synchrony undergo recovery over time, their effects on the ECAP are diminished, and the ECAP amplitude eventually recovers as well.
The same explanation may be applied to the non-monotonicity of ECAP recovery from electric maskers in hearing subjects (see Fig. 6), as a high-rate electric masker may directly depolarize inner hair cells and cause them to release the neurotransmitter (van den Honert & Stypulkowski, 1984). From that perspective, synaptic adaptation and a subsequent transient decrease in auditory nerve spontaneous activity may be expected following the offset of the electric masker.
The acute effects of furosemide or neomycin on spontaneous activity within the auditory nerve were not directly evaluated in the present study, as single-unit activity was not assessed. While some across-subject variability of the ototoxic treatments is inevitable, we used a rather strict criterion for inclusion of subjects in the experiments (a minimum of 60 dB acoustic sensitivity threshold shift). The chemical treatments may be expected to produce a significant reduction in spontaneous activity of the ANFs, as demonstrated by Sewell (1984a, 1984b) and Searchfield et al. (2004) for acute administration of furosemide. While Shepherd and Javel (1997) noted that spontaneous activity might still be present in ANFs following acute aminoglycoside deafening, they suggested that such spontaneous activity had a non-synaptic origin. Litvak et al. (2001) reported spontaneous activity in 13 out of 106 single units studied in acutely deafened preparations. In the single-fiber studies of Miller et al. (2001) and Litvak et al. (2003), none of the ANFs from which recordings were made (n=37 and n=138, respectively) exhibited spontaneous activity following acute aminoglycoside deafening. This suggests that while spontaneous activity in aminoglicosyde-deafened ears cannot be ruled out, it is likely to be diminished following acute aminoglycoside administration.
Non-monotonic electric forward masking functions obtained in subjects acutely deafened with furosemide or neomycin (see Fig. 7, 8) suggest that post-stimulatory changes in hair-cell spontaneous activity cannot fully explain the non-monotonic pattern of ECAP recovery. While consistent with the general hypothesis of post-masking changes in ANF firing synchrony, this implies an additional mechanism, independent of the synaptic activity of hair cells, which affects temporal response properties of the ANFs during recovery from masking and contributes to its non-monotonic time course.
4.4. Clinical relevance and future directions
The acoustic-electric interactions that were observed in the present experimental study could potentially occur in CI patients with residual acoustic hearing. It should be noted that most of the data presented in this study were collected using relatively high-level electric probe stimuli. The probe pulses elicited ECAPs with amplitudes corresponding to at least 40% of saturation amplitude, i.e., well above ECAP thresholds. In CI users, ECAP thresholds are consistently higher than behavioral thresholds, and are often comparable to maximum comfortable stimulation levels (Brown et al., 1998, 2000; Eisen & Franck, 2004). This difference may limit the potential clinical relevance of the present study.
Another important difference between the experimental animal model used in this study and human EAS patients is the expected degree of overlapping of acoustically and electrically stimulated ANF populations within the cochlea. For our animal model of combined electric-acoustic stimulation, normal-hearing subjects were used. The surgical procedures were designed to preserve acoustic hearing as much as possible, so that a relatively large population of ANFs would be responsive to both stimulus modalities. In the case of human EAS patients, on the other hand, there is likely to be a spatial segregation of fibers within the auditory nerve that are responsive to acoustic and electric stimuli (Wilson et al., 2003). Consequently, such interactions might be limited, particularly in CI patients implanted with short electrode arrays (6 or 10 mm insertion depth). Such a shallow electrode insertion may result in stimulus segregation at the level of the auditory nerve. Indeed, in a study by Abbas et al. (2005), no physiological evidence was found for peripheral acoustic-electric interactions — or even electrophonic responses — in EAS patients implanted with short (10 mm insertion depth) electrode arrays.
There is a growing body of evidence that EAS is more beneficial for CI patients with residual hearing than either electric or acoustic stimulation alone (von Ilberg et al., 1999; Turner et al., 2004; Kiefer et al., 2005). This indicates that even if the stimuli delivered to the ear by the CI and the HA are conveyed by the auditory nerve independently, integration of information provided by the two modes of stimulation does take place in the central auditory system.
Peripheral electric-acoustic interactions are more likely to take place in EAS patients with CIs implanted to a greater insertion depth (around 20 mm). Moreover, it has been suggested that residual low-frequency hearing may be preserved with even deeper electrode insertion (James et al., 2005). Also, it is likely that as speech perception abilities in CI users continue to improve with newer devices and speech processing strategies, the acceptable levels of residual hearing in EAS candidates may expand further as well (Copeland & Pillsbury, 2004). Thus, understanding the physiological phenomena associated with ANF responses to simultaneous acoustic and electric stimulation is an important issue for EAS patients with greater levels of residual hearing and deeper electrode array insertion. It may be helpful for improving the processing of both electric and acoustic stimuli delivered to such patients by CIs and HAs, respectively.
The present work provides a basis for further investigation of neural mechanisms of adaptation and recovery and their contribution to acoustic-electric interactions in the auditory periphery. We hypothesized that continuous electric stimulation could affect temporal response properties of the ANFs by a mechanism independent of the synaptic activity of hair cells (see Fig. 7, 8). A single-fiber study by Miller et al. (2005b) has provided support for this hypothesis. It would be of special interest to address temporal properties of single unit responses (assessed as jitter and vector strength) to transient stimuli (electric pulses) following stimulation with electric adapters in hearing and deafened ears. This approach may help to determine the degree to which peripheral acoustic-electric interactions are specific to the two modes of stimulation and thus aid in the interpretation of the similarities and differences between ECAP recovery following acoustic and electric maskers, presented in our previous work (Nourski et al., 2005a) and this study.
Future research using single-fiber measures can also be directed at establishing a relationship between the spatial pattern of excitation within the cochlea and acoustic-electric interactions. For this purpose, it is important to perform a systematic evaluation of the characteristic frequencies of the ANFs which exhibit responses to combined acoustic and electric stimuli. Information on the responding fibers' characteristic frequencies may be useful in the assessment of their origin within the cochlea and, consequently, allow to estimate their position relative to the intracochlear stimulus electrode.
Acknowledgments
This work was supported by NIH contract N01-DC-2-1005.
Abbreviations
- ACAP
acoustically evoked compound action potential
- ANF
auditory nerve fiber
- CI
cochlear implant
- EAS
electric-acoustic stimulation
- ECAP
electrically evoked compound action potential
- pps
pulses per second
- SPL
sound pressure level
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas PJ, Miller CA, Rubinstein JT, Robinson BK. Seventh Quarterly Progress Report, Neural Prosthesis Program contract N01-DC-9-2106 NIH. 2001. Effects of remaining hair cells on cochlear implant function. [Google Scholar]
- Abbas PJ, Miller CA, Robinson BK, Jeng FC, Nourski KV. Fourth Quarterly Progress Report, Neural Prosthesis Program contract N01-DC-2-1005 NIH. 2003. Effects of remaining hair cells on cochlear implant function. [Google Scholar]
- Abbas PJ, Miller CA, Robinson BK, Jeng FC, Noh H, Nourski KV. Sixth Quarterly Progress Report, Neural Prosthesis Program contract N01-DC-2-1005 NIH. 2004. Effects of remaining hair cells on cochlear implant function. [Google Scholar]
- Abbas PJ, Etler C, Nourski K, Brown C, Miller C. Responses to electrical stimulation in individuals with residual hair cell function. Fourth International Symposium and Workshops: Objective Measures in Cochlear Implants; June 1–4, 2005; Hannover, Germany. 2005. [Google Scholar]
- Anniko M, Møller AR. A physiological and morphological study of the cochlea of the rat following treatment with atoxyl and neomycin. Acta Otolaryngol. 1978;86(34):201–211. doi: 10.3109/00016487809124737. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Abbas PJ, Gantz BJ. Preliminary experience with neural response telemetry in the Nucleus CI24M cochlear implant. Am J Otol. 1998;19:320–327. [PubMed] [Google Scholar]
- Brown CJ, Hughes ML, Luk B, Abbas PJ, Wolaver A, Gervais J. The relationship between EAP and EABR thresholds and levels used to program the Nucleus 24 speech processor: Data from adults. Ear Hear. 2000;21(2):151–163. doi: 10.1097/00003446-200004000-00009. [DOI] [PubMed] [Google Scholar]
- Cohen N. Cochlear implant candidacy and surgical considerations. Audiol Neurootol. 2004;9:197–202. doi: 10.1159/000078389. [DOI] [PubMed] [Google Scholar]
- Copeland BJ, Pillsbury HC., III Cochlear implantation for the treatment of deafness. Annu Rev Med. 2004;55:157–167. doi: 10.1146/annurev.med.55.091902.105251. [DOI] [PubMed] [Google Scholar]
- Dorman MF, Spahr AJ, Loizou PC, Dana CJ, Schmidt JS. Acoustic simulations of combined electric and acoustic hearing (EAS) Ear Hear. 2005;26(4):371–380. doi: 10.1097/00003446-200508000-00001. [DOI] [PubMed] [Google Scholar]
- Eisen MD, Franck KH. Electrically evoked compound action potential amplitude growth functions and HiResolution programming levels in pediatric CII implant subjects. Ear Hear. 2004;25(6):528–538. doi: 10.1097/00003446-200412000-00002. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C. Combining acoustic and electrical speech processing: Iowa/Nucleus hybrid implant. Acta Otolaryngol. 2004;124(4):344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE. Acoustic plus electric speech processing: Preliminary results of a multicenter clinical trial of the Iowa/Nucleus hybrid implant. Audiol Neurootol. 2006;11 1:63–68. doi: 10.1159/000095616. [DOI] [PubMed] [Google Scholar]
- Gstoettner WK, Helbig S, Maier N, Kiefer J, Radeloff A, Adunka OF. Ipsilateral electric acoustic stimulation of the auditory system: Results of long-term hearing preservation. Audiol Neurootol. 2006;11 1:49–56. doi: 10.1159/000095614. [DOI] [PubMed] [Google Scholar]
- Haenggeli A, Zhang JS, Vischer MW, Pelizzone M, Rouiller EM. Electrically evoked compound action potential (ECAP) of the cochlear nerve in response to pulsatile electrical stimulation of the cochlea in the rat: Effects of stimulation at high rates. Audiology. 1998;37:353–371. doi: 10.3109/00206099809072989. [DOI] [PubMed] [Google Scholar]
- Harris DM, Dallos P. Forward masking of auditory nerve fiber responses. J Neurophysiol. 1979;42(4):1083–1105. doi: 10.1152/jn.1979.42.4.1083. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, Riggs LC, Strauss M, Matz GJ. Temporal bone histopathology of cisplatin ototoxicity. Am J Otol. 1995;16(6):731–740. [PubMed] [Google Scholar]
- Hinojosa R, Nelson EG, Lerner SA, Redleaf MI, Schramm DR. Aminoglycoside ototoxicity: A human temporal bone study. Laryngoscope. 2001;111(10):1797–1805. doi: 10.1097/00005537-200110000-00025. [DOI] [PubMed] [Google Scholar]
- van den Honert C, Stypulkowski PH. Physiological properties of the electrically stimulated auditory nerv. II. Single fiber recordings. Hear Res. 1984;14:225–243. doi: 10.1016/0378-5955(84)90052-2. [DOI] [PubMed] [Google Scholar]
- Hu N, Abbas PJ, Miller CA, Robinson BK, Nourski KV, Jeng FC, Abkes BA, Nichols JM. Auditory response to intracochlear electric stimuli following furosemide treatment. Hear Res. 2003;185:77–89. doi: 10.1016/s0378-5955(03)00261-2. [DOI] [PubMed] [Google Scholar]
- von Ilberg C, Kiefer J, Tillein J, Pfenningdorff T, Hartmann R, Sturzebecher E, Klinke R. Electric-acoustic stimulation of the auditory system. New technology for severe hearing loss. ORL J Otorhinolaryngol Relat Spec. 1999;61(6):334–340. doi: 10.1159/000027695. [DOI] [PubMed] [Google Scholar]
- James C, Albegger K, Battmer R, Burdo S, Deggouj N, Deguine O, Dillier N, Gersdorff M, Laszig R, Lenarz T, Rodriguez MM, Mondain M, Offeciers E, Macias AR, Ramsden R, Sterkers O, von Wallenberg E, Weber B, Fraysse B. Preservation of residual hearing with cochlear implantation: How and why. Acta Otolaryngol. 2005;125(5):481–491. doi: 10.1080/00016480510026197. [DOI] [PubMed] [Google Scholar]
- Kiang NYS, Wanatabe T, Thomas EC, Clark LF. Research Monograph No 35. MIT Press; Cambridge, Massachusetts: 1965. Discharge patterns of single fibers in the cat's auditory nerve. [Google Scholar]
- Kiefer J, Tillein J, von Ilberg C, Pfennigdorf T, Stürzebecher E, Klinke R, Gstöttner W. Fundamental aspects and first results of the clinical application of combined electric and acoustic stimulation of the auditory system. In: Kubo T, Takahashi Y, Iwaki T, editors. Cochlear Implants: An Update. Karger Publications; The Hague, The Netherlands: 2002. pp. 569–576. [Google Scholar]
- Kiefer J, Pok M, Adunka O, Sturzebecher E, Baumgartner W, Schmidt M, Tillein J, Ye Q, Gstoettner W. Combined electric and acoustic stimulation of the auditory system: Results of a clinical study. Audiol Neurotol. 2005;10:134–144. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- Killian MJP. PhD dissertation. University of Utrecht; Utrecht, The Netherlands: 1994. Excitability of the electrically stimulated auditory nerve. [Google Scholar]
- Killian MJP, Klis SFL, Smoorenburg GF. Adaptation in the compound action potential response of the guinea pig VIIIth nerve to electric stimulation. Hear Res. 1994;81:66–82. doi: 10.1016/0378-5955(94)90154-6. [DOI] [PubMed] [Google Scholar]
- Leake-Jones PA, Vivion MC, O'Reilly BF, Merzenich MM. Deaf animal models for studies of a multichannel cochlear prosthesis. Hear Res. 1982;8:225–246. doi: 10.1016/0378-5955(82)90076-4. [DOI] [PubMed] [Google Scholar]
- Litvak L, Delgutte B, Eddington D. Auditory nerve fiber responses to electric stimulation: Modulated and unmodulated pulse trains. J Acoust Soc Am. 2001;110(1):368–379. doi: 10.1121/1.1375140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak LM, Smith ZM, Delgutte B, Eddington D. Desynchronization of electrically evoked auditory-nerve activity by high-frequency pulse trains of long duration. J Acoust Soc Am. 2003;114(4):2066–2078. doi: 10.1121/1.1612492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka AJ, Abbas PJ, Rubinstein JT, Miller CA. The neuronal response to electrical constant-amplitude pulse train stimulation: Evoked compound action potential recordings. Hear Res. 2000;149:115–128. doi: 10.1016/s0378-5955(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Rubinstein JT, Robinson BK, Matsuoka AJ, Woodworth G. Electrically evoked compound action potentials of guinea pig and cat: responses to monopolar, monophasic stimulation. Hear Res. 1998;119:142–154. doi: 10.1016/s0378-5955(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Rubinstein JT, Runge-Samuelson C, Robinson BK. Fifth Quarterly Progress Report, Neural Prosthesis Program contract N01-DC-9-2106 NIH. 2000. Effects of remaining hair cells on cochlear implant function. [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK. Response properties of the refractory auditory nerve fiber. J Assoc Res Otolaryngol. 2001;2(3):216–232. doi: 10.1007/s101620010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Abbas PJ, Hu N, Robinson BK. Second Quarterly Progress Report Neural Prosthesis Program contract N01-DC-2-1005 NIH. 2003. Effects of remaining hair cells on cochlear implant function. [Google Scholar]
- Miller CA, Abbas PJ, Noh H, Robinson BK, Nourski KV. Ninth Quarterly Progress Report, Neural Prosthesis Program contract N01-DC-2-1005 NIH. 2004. Effects of remaining hair cells on cochlear implant function. [Google Scholar]
- Miller C, Noh H, Abbas P, Robinson B, Nourski K, Jeng FC. Effects of combined acoustic and electric stimuli: Single auditory nerve fiber responses. Assoc Res Otolaryngol; 2005 MidWinter Meeting; February 19–24, 2005; New Orleans, LA, USA. 2005a. p. 357. [Google Scholar]
- Miller CA, Robinson BK, Abbas PJ, Nourski KV, Jeng FC. Twelfth Quarterly Progress Report, Neural Prosthesis Program contract N01-DC-2-1005 NIH. 2005b. Effects of remaining hair cells on cochlear implant function. [Google Scholar]
- Miller CA, Abbas PJ, Robinson BK, Nourski KV, Zhang F, Jeng FC. Electrical excitation of the acoustically sensitive auditory nerve: Single-fiber responses to electric pulse trains. J Assoc Res Otolaryngol. 2006;7:195–210. doi: 10.1007/s10162-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moxon EC. PhD dissertation. Massachusetts Institute of Technology; Boston, Massachusetts, USA: 1971. Neural and mechanical responses to electric stimulation of the cat's inner ear. [Google Scholar]
- NIH Consensus Statement. Cochlear implants in adults and children. NIH Consensus Statement. 1995;13(2):1–30. [PubMed] [Google Scholar]
- Nourski KV, Abbas PJ, Miller CA, Robinson BK, Jeng FC. Effects of acoustic noise on the auditory nerve compound action potentials evoked by electric pulse trains. Hear Res. 2005a;202:141–153. doi: 10.1016/j.heares.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Nourski KV, Abbas PJ, Miller CA, Robinson BK, Noh H. Acoustic-electric interactions in the auditory nerve: Masking of the electrically-evoked compound action potential by acoustic noise. 2005 Conference on Implantable Auditory Prostheses; July 30–August 4, 2005; Pacific Grove, California, USA. 2005b. p. 102. [Google Scholar]
- Nourski K, Abbas P, Noh H, Miller C, Robinson B. Acoustic and electric interactions in the auditory nerve: Simultaneous and forward masking of the electrically-evoked compound action potential. Assoc Res Otolaryngol; 2005 MidWinter Meeting; February 19–24, 2005; New Orleans, Louisiana, USA. 2005c. Assoc. Res. Otolaryngol. Abs.: 28. [Google Scholar]
- Nourski K, Abbas P, Noh H, Miller C, Robinson B. Forward masking of the electrically evoked compound action potential in animals with residual hair cell function. Assoc Res Otolaryngol; 2006 MidWinter Meeting; February 5 – 9, 2006; Baltimore, Maryland, USA. 2006. Assoc. Res. Otolaryngol. Abs.: 934. [Google Scholar]
- Pike DA, Bosher SK. The time course of strial changes produced by intravenous furosemide. Hear Res. 1980;3:79–89. doi: 10.1016/0378-5955(80)90009-x. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Wilson BS, Finley CC, Abbas PJ. Pseudospontaneous activity: Stochastic independence of auditory nerve fibers with electrical stimulation. Hear Res. 1999;127:108–118. doi: 10.1016/s0378-5955(98)00185-3. [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC. Furosemide alters Organ of Corti mechanics: Evidence for feedback of outer hair cells upon the basilar membrane. J Neurosci. 1991;11(4):1057–1067. doi: 10.1523/JNEUROSCI.11-04-01057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searchfield GD, Muñoz DJB, Thorne PR. Ensemble spontaneous activity in the guineapig cochlear nerve. Hear Res. 2004;192:23–35. doi: 10.1016/j.heares.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Sewell WF. The effects of furosemide on the endocochlear potential and auditory-nerve fiber tuning curves in cats. Hear Res. 1984a;14:305–314. doi: 10.1016/0378-5955(84)90057-1. [DOI] [PubMed] [Google Scholar]
- Sewell WF. The relation between the endocochlear potential and spontaneous activity in auditory nerve fibres of the cat. J Physiol. 1984b;347:685–696. doi: 10.1113/jphysiol.1984.sp015090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001;155:1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Javel E. Electrical stimulation of the auditory nerve. I. Correlation of physiological responses with cochlear status. Hear Res. 1997;108:112–144. doi: 10.1016/s0378-5955(97)00046-4. [DOI] [PubMed] [Google Scholar]
- Skarżyński H, Lorens A, Piotrowska A. A new method of partial deafness treatment. Med Sci Monit. 2003;9(4):CS26–CS30. [PubMed] [Google Scholar]
- Skarżyński H, Lorens A, Piotrowska A, Anderson I. Partial deafness cochlear implantation provides benefit to a new population of individuals with hearing loss. Acta Otolaryngol. 2006;126(9):934–940. doi: 10.1080/00016480600606632. [DOI] [PubMed] [Google Scholar]
- Smith RL. Short-term adaptation in single auditory nerve fibers: Some poststimulatory effects. J Neurophysiol. 1977;40(5):1098–1111. doi: 10.1152/jn.1977.40.5.1098. [DOI] [PubMed] [Google Scholar]
- Turner CW, Gantz BJ, Vidal C, Behrens A, Henry BA. Speech recognition in noise for cochlear implant listeners: Benefits of residual acoustic hearing. J Acoust Soc Am. 2004;115(4):1729–1735. doi: 10.1121/1.1687425. [DOI] [PubMed] [Google Scholar]
- Westerman LA, Smith RL. Rapid and short-term adaptation in auditory nerve responses. Hear Res. 1984;15:249–260. doi: 10.1016/0378-5955(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Wilson B, Wolford R, Lawson D, Schatzer R. Third Quarterly Progress Report, Neural Prosthesis Program contract N01-DC-2-1002 NIH. 2002. Speech processors for auditory prostheses. [Google Scholar]
- Wilson BS, Lawson DT, Müller JM, Tyler RS, Kiefer J. Cochlear implants: Some likely next steps. Annu Rev Biomed Eng. 2003;5:207–249. doi: 10.1146/annurev.bioeng.5.040202.121645. [DOI] [PubMed] [Google Scholar]