Cardiovascular disease remains the leading cause of death in the United States and Europe based on American Heart Association and British Heart Foundation data.1,2 Hyperlipidemia is one of the major risk factors and its treatment has come to the forefront of primary and subspecialty care as a preventative strategy against cardiovascular morbidity and mortality.1 The newest Adult Treatment Panel guidelines of the National Cholesterol Education Program (ATP III) identifies the optimal LDL lower than previous reports and recommends even more aggressive management in patients with a coronary heart disease risk equivalent.3 Despite their widespread use, clinically significant hepatic injury caused by lipid-lowering medications remains rare.4-6 The anti-hyperlipidemic drug with the highest potential for hepatic injury is the sustained-release formulation of niacin4. HMG CoA reductase inhibitors, otherwise known as statins, very rarely cause clinically significant liver injury, although asymptomatic elevation in aminotransferases is common.4 The notion that ezetimibe may have less risk of hepatotoxicity has recently been challenged and it may not be a “safe alternative” to statins in patients with pre-existing liver disease.7
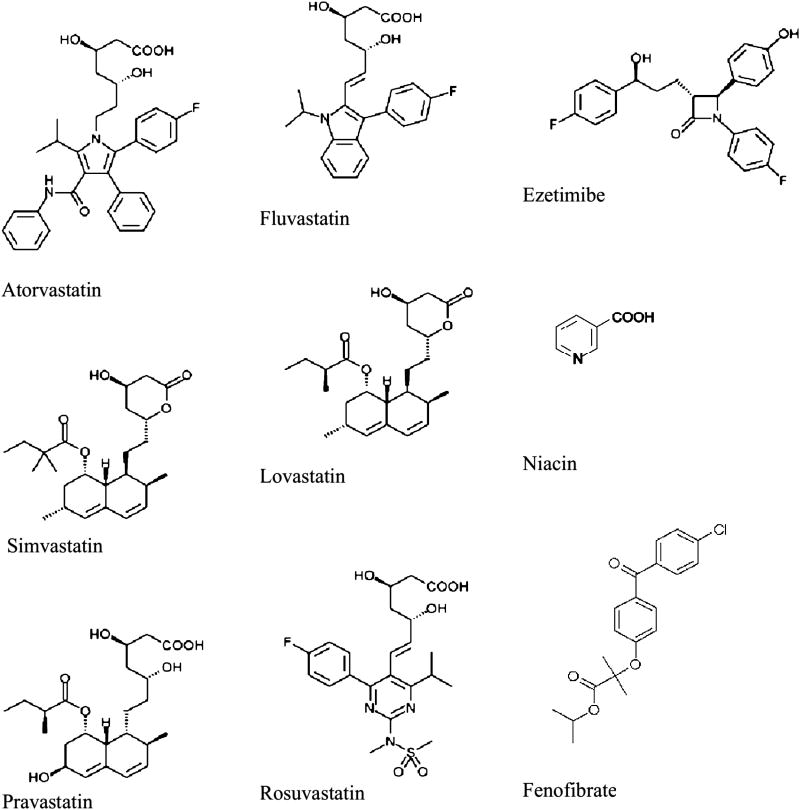
The pattern of liver injury from anti-hyperlipidemics is typically hepatocellular or mixed in nature with rare instances of pure cholestatic picture.4,5 The proposed mechanisms of hepatotoxicity are varied depending on the drug or drug class, and include effects on the cytochrome P450 system, impairment of bile acid transport proteins, immune-mediated inflammatory response to the medication or its metabolites, immune-mediated apoptosis by tumor necrosis factor, and oxidative stress due to intracellular damage.6 Figure 1 provides the chemical structures of commonly prescribed lipid-lowering medications with known hepatotoxicity, and Table 1 provides a concise overview of patterns of hepatotoxicity and recovery, as well as hepatotoxicity potential.
Figure 1. Chemical structures of commonly prescribed lipid-lowering medications with known hepatotoxicity.
Chemical structure of commonly available lipid lowering agents (Statins, Fibrates, Ezetimibe, and niacin)
Table 1.
Hepatotoxicity of various anti-hyperlipidemic agents
| Lipid-lowering agent or class | Mechanism of action | Typical pattern of biochemical derangement | Typical histologic appearance of injury | Avg. length of use prior to injury | Avg. recovery time | Hepatotoxicity potential |
|---|---|---|---|---|---|---|
| Statins | inhibits HMG CoA reductase (involved in cholesterol synthesis) | hepatocellular (although cholestatic and mixed injury are seen as well) | few cases have shown inflammation of portal tracts with mild piecemeal necrosis and mild, focal periportal fibrosis | often within first several months | 2-3 months (but usually within 10 weeks) | Serious liver injury is very rare |
| Niacin [especially SR formulation] | decreases hepatic TG esterification & LDL/VLDL production | mostly hepatocellular but may be mixed injury | generally varying patterns of necrosis but may also include centrilobular cholestasis or steatosis | 1 week-48 months | 1-2 months | common with SR formulation but rare with IR or ER |
| Fibrates [especially fenofibrate] | inhibits hepatic TG synthesis; decreases hepatic FFA extraction (gemfibrozil) | hepatocellular injury (especially with gemfibrozil) but mixed pattern of injury seen with fenofibrate | fenofibrate can cause ductopenia, chronic hepatits, and fibrosis (especially in combination with statin medications) | φ | φ | Serious liver injury is very rare |
| Ezetimibe | inhibits absorption of cholesterol at small intestine | rare cholestatic hepatitis or acute autoimmune hepatitis | n/a | φ | φ | very low, but seen in higher frequency than previously thought |
inadequate patient data to reliably quantify.
HMG CoA reductase inhibitors (Statins)
Statins competitively inhibit 3-hydroxy-3-methylglutarylcoenzyme A (HMG-CoA) reductase, an enzyme necessary for cholesterol biosysnthesis; they also act in several ways to decrease the level of low density lipoprotein (LDL) and increase the stability of atherosclerotic plaques.8,9 These medications are very effective is reducing cardiovascular mortality and are widely prescribed.8 More than 145 million prescriptions were written for statins in the U.S. in 2005 and more than 10,000 prescriptions were written for lovastatin, the first generic statin available in the United States (40% increase from the year before).10 The use of these drugs is likely to increase, given their effectiveness and relative safety, especially if any of them were to become available over-the-counter.
Although liver toxicity has been a concern since their initial introduction, several clinical trials have shown that statins are safe to use for the prevention of coronary disease and death, even in the setting of chronic liver deasease.11 Irreversible liver damage leading to death or liver transplantation appears to be extremely uncommon with statins.11 In fact, the incidence of liver enzyme elevations in the statin-treated population has not been consistently different than in placebo-treated patients.9,11-14 Concern surrounding the safety of this class of medications significantly increased in 2001 after the withdrawal of cerivastatin from the market due to the high incidence of rhabdomyolysis in patients taking the drug.15 Since that time, new drug applications for statin medications have been closely scrutinized.15 Rosuvastatin required a greater number of patients in the initial studies as well as more extensive post-marketing data.15 However, this was mostly due to concern over myotoxicity and renal toxicity rather than hepatotoxicity.
Initial studies of statins performed on animals revealed that very high doses of statins may cause hepatotoxicity, but typical therapeutic doses of the drug were not associated with significant liver injury.16,17 High doses of lovastatin caused significant hepatocellular necrosis in rabbits.16 This pattern of injury was also seen in a guinea pig model exposed to high doses of simvastatin.17 However, hepatocellular necrosis from statins is exceptionally rare in humans. Asymptomatic elevations in aminotransferases are common from statins but they do not necessarily indicate hepatic damage, especially when seen in the setting of normal bilirubin levels. The degree of elevation in transaminases does not, however, reflect the amount, or even the presence, of liver injury18. The increase in transaminases is a dose-related phenomenon, with higher doses leading to greater frequency of aminotransferase elevations.18
Atorvastatin
Atorvastatin-related hepatotoxicity has been associated with a mixed pattern of liver injury typically occurring several months after the initiation of the medication.19-22 There has also been a recent case report of underlying autoimmune hepatitis apparently revealed by atorvastatin.23 After broad experience with this medication (hundreds of thousands of treated patients), significantly increased transaminase levels greater than 3 times the upper limit of normal were only seen in 0.7% of cases.19
One case report described hepatocellular and cholestatic injury that occurred 12 weeks after the initiation of drug therapy that resolved within 2 months after discontinuation of the drug.20 Liver histology in this case revealed portal inflammation with presence of eosinophils in addition to mild piecemeal necrosis. This case did not show any evidence of cross-reactivity across drug class as simvastatin was subsequently introduced without recurrence of liver injury. Few other case reports described acute cholestatic hepatitis attributed to the use of atorvastatin.19-22 These cases appear to be associated with advanced age or chronic illnesses. For example, cholestatic hepatitis has been reported in a young woman with systemic lupus erythematosus.19 Immediate improvement in liver function studies and jaundice was seen with cessation of therapy with their normalization within a month. Two case reports have described cholestatic injury in patients over the age of 70.21,22 Both cases exhibited rapid improvement with cessation of the drug and recurrence of hepatitis with re-initiation of therapy.
Lovastatin
Mixed hepatic injury in hepatocellular and cholestatic patterns has also been noted with the use of lovastatin.24 Elevations in hepatic enzyme levels are dose-related and resolve with discontinuation of therapy.13,24,25 Although mild elevations in hepatic enzymes (ALT <3 times the upper limit of normal) have been shown to occur in up to 5% of patients on higher doses of therapy (80mg per day), reversal of liver enzyme levels has occurred spontaneously even with continuation of the medication.13 As with other statin medications, clinically significant disease, in the rare cases when it occurs, is seen within several months of the initiation of the drug and resolves within 2 months after discontinuation.24,25 One reported case in which a liver biopsy was done revealed histologic findings of centrilobular necrosis and cholestasis with a mixed inflammatory infiltrate.24 Based on 232 cases of acute liver injury potentially associated with lovastatin, it was estimated the risk of fulminant liver failure attributable to lovastatin was 2 in one million patients against a background of one in a million in the general population.13,14
Simvastatin
Simvastatin hepatotoxicity is hypothesized to occur due to drug-drug interactions.26,27 There have been several case reports involving amiodarone; this is of potential importance because these medications are often used together in the same patient with known cardiovascular disease.26 There have also been case reports describing hepatotoxicity when simvastatin is used in conjunction with flutamide, troglitazone, and diltiazem.27-29 The addition of diltiazem likely caused a significant increase in serum simvastatin level due to the inhibition of CYP3A4 caused by diltiazem.27 Troglitazone (no longer commercially available) is an inducer of CYP3A4 and, thus, hepatotoxicity cannot be attributed to potentiation of simvastatin in this case.28 Additionally, troglitazone itself is known to be hepatotoxic. As with other statin medications, mixed injury is seen at least as often as hepatocellular injury.28,29 Recovery within weeks to a few months of discontinuation of the drug is the general rule although a much longer course of recovery is sometimes seen.26-29
Pravastatin, Rosuvastatin, and Fluvastatin
Pravastatin has been reported to cause acute intrahepatic cholestasis.30 In this case, liver toxicity occurred within 2 months after initiating the drug and it resolved within 2 months after its discontinuation.30 Although never studied rigorously, there is a perception that pravastatin is less hepatotoxic than other statins. This has been attributed to its non-CYP based metabolism and its hydrophilic nature.31, 32
Rosuvastatin is the newest approved drug in this class and extensive clinical experience is lacking, although it has been the most carefully scrutinized statin to date as noted above33. Rosuvastatin has been shown to trigger an autoimmune-type hepatitis similar to atorvastatin.33 A case report published in 2005 noted a mixed pattern of liver injury and delayed recovery (>6 months before the hepatic enzyme normalization).33 Fluvastatin, as opposed to many other statins, is metabolized by cytochrome P450 2C9 instead of CYP3A4.31 The incidence of elevations in hepatic biochemistries is similar to the class as a whole although initial studies revealed a lower rate of hepatic enzyme elevation in the control population.31
Clinically significant hepatotoxicity caused by statins remains extremely rare although, as a class, asymptomatic elevations in transaminases less than three times the upper limit of normal (ULN) are common.34,35 There is a < 3% incidence of ALT = 3 times ULN associated with the use of statins and a minor increase in incidence is seen related to dose (Table 2). 34,35 There have been rare reports of significantly higher levels of transaminases, however, even these cases were not associated with clinical or histological evidence of liver dysfunction.36 Statins have no signature biochemical or histologic pattern for liver injury in patients who have actually developed liver toxicity. Hepatocellular, cholestatic, and mixed patterns of liver injury have all been reported in the literature (Table 3).4,5,13,14,37 A few case reports have noted an autoimmune-type reaction attributed to, or unmasked by, the use of statins.23,38,39 The risk of fulminant liver failure remains extremely low; Merck's World-wide Adverse Event Database (WAES) estimated the risk of fulminant liver failure attributable to lovastatin was 2 in one million patients based on 232 cases of acute liver injury potentially associated with lovastatin.13,14 Of the few cases of actual hepatotoxicity from statins, liver biopsy data show chronic inflammation of the portal tracts with mild piecemeal necrosis and, on rare occasion, mild focal periportal fibrosis.13
Table 2.
Rates of aminotransferase elevation and drug discontinuation for available statins
| Statin | Number of prescriptions written in 2004 (in millions) | Incidence of AST or ALT level > 3 times ULN | Rate of discontinuation |
|---|---|---|---|
| atorvastatin | 62.5 | 0-0.7% | n/a |
| fluvastatin | 1.9 | 1.2% | 0.6% |
| lovastatin | 7.4 | 0.6% | 0.2% |
| pravastatin | 12.0 | 1.3% | 0.1% |
| rosuvastatin | 6.3 | 0% | 0% |
| simvastatin | 23.8 | 1.8% | 0.5% |
Table 3.
Types of liver injury associated with statin use
| Frequency | Comment | |
|---|---|---|
| Asymptomatic elevations in aminotransferases | 0.1-3% | dose dependent; class effect; clinically not significant |
| Clinically significant acute liver injury | very rare | may be seen in combination with other medications, e.g. ezetimibe |
| Fulminant hepatic failure | extremely rare (isolated case reports) | it was estimated that risk of fulminant liver failure is 2 in one million |
| Autoimmune hepatitis | case reports | statins may induce AIH in genetically susceptible individuals |
Asymptomatic elevation in transaminases associated with statin use should not be considered as ongoing liver disease or injury. Increased aminotransferases have been theorized to represent a pharmacodynamic effect of lipid lowering, although the mechanism of the elevation remains unknown.14 There has been some suggestion that the elevation in AST (and, to a lesser extent, ALT) levels may result from muscle cell damage.14 Clinically significant liver injury may result from idiosyncratic mechanisms or the immunoallergic mechanisms noted above.5,37 There does seem to be a class effect with some evidence of cross-toxicity.5 Similar to other classes of medications, the statins have been linked to various tumors in rodents models that have not been noted in humans.40,41 However, the Scandinavian Simvastatin Survival Study (4S) reported no difference in cancer incidence between those on simvastatin and the placebo groups over a 10-year follow-up period.42 Furthermore, a previously published meta-analysis of five large clinical trials demonstrated no association between statin use and the risk of cancer over a 5-year period.40
The current recommendation to monitor liver function tests before and during statin therapy is controversial due to the lack of evidence-based supporting data, with some experts in the field calling for its reexamination.8,16,43 The existing guidelines state that liver enzymes should be checked before starting statin therapy and periodically evaluated thereafter.8 The package inserts continue to state that liver function tests should be assessed before and 12 weeks after initiating therapy with statins and also after each dose escalation. However, the Statin Safety Task Force Meeting conducted by the National Lipid Association (2005) has argued against routine monitoring of liver biochemistries before starting or during treatment with statins (Table 4).9
Table 4.
Summary of Recommendations of the Liver Expert Panel to the National Lipid Association on Statin Safety
|
Reproduced from reference 11 with permission
Niacin
Niacin, or nicotinic acid, is used primarily to increase HDL through a mechanism that is not entirely clear. A reduction in LDL is also seen and apparently occurs due to an inhibition of hepatic VLDL and triglyceride secretion and thus a reduction in the substrate needed to make LDL particles.46 The sustained-released (SR) preparation is easily available over-the-counter and may be used by patients seeking a perceived naturopathic solution to their high cholesterol or by patients seeking a cheaper option to treat themselves. Patients may possibly be using potentially toxic doses without the prior knowledge of their physicians. It is this unsupervised use of the SR formulation that often leads to the dose-related toxicity of niacin and should be discouraged.4,47-49 The onset of hepatotoxicity generally appears anywhere from 1 week to 48 months after the initiation of the drug and usually subsides with discontinuation.50-52 Recovery is usually seen in 1-2 months.48,50 Fulminant hepatic failure has been reported but is very rare.51,53,54 Combination therapy with statins does not increase the incidence of adverse effects of either medication.55
The typical pattern of injury involves an elevation in aminotransferase levels although a mixed pattern of hepatocellular and cholestatic injury can be seen.48,56 Histologic findings most often consist of liver necrosis to various degrees and in varying patterns, however centrilobular cholestasis and steatosis simulating the radiographic appearance of hepatobiliary neoplasia has been seen. A history of nausea and vomiting as well as sign and symptoms of acute liver failure provide clinical clues to niacin toxicity.59,60 Other clinical indicators of niacin hepatotoxicity include a sharp reduction of serum lipid levels preceding the liver injury as well as a presentation similar to acute abdomen with a cholestatic pattern of liver disease in the setting of fatty infiltration of the liver.59,61
Almost any formulation of niacin can cause hepatotoxicity in doses that exceed 2-3 grams per day, but the sustained-release (SR) formulation is significantly more hepatotoxic.62,63 The immediate-release (IR) formulations of niacin in usual therapeutic doses almost never cause serious liver injury.64,65 The over-the-counter SR formulation of the drug is not FDA approved for the treatment of dyslipidemia but still available to patients for this purpose as a supplement or “neutraceutical”. Half of the patients that take niacin SR develop a symptomatic elevation in transaminases.49,64,66
The hepatotoxicity of niacin is related to drug metabolism by a high-affinity, low-capacity amidation pathway that leads to toxic nicotinomide and pyrimidine metabolites; thus, the slower released SR formulation can lead to higher levels of toxic metabolites (Table 5).64,65,67 The alternative metabolic pathway is a low-affinity, high-capacity conjugation pathway that leads to prostaglandin-mediated vasodilation and subsequent cutaneous flushing.64,65,67 The rapidly-released IR formulation overwhelms the higher affinity amidation pathway and the majority is metabolized using the high-capacity conjugation pathway, leading to a much lower rate of hepatotoxicity.67 Extended-release (ER) niacin has an intermediate rate of dissolution and can be associated with both flushing and hepatotoxicity64.
Table 5.
Pathways and potential of niacin toxicity by formulation
| Formulation | Primary metabolic pathway | Toxic metabolites produced or toxic process initiated | Toxicity potential | |
|---|---|---|---|---|
| hepatotoxicity | cutaneous flushing | |||
| immediate release (IR) | conjugation | prostaglandins | extremely low | high |
| extended release (ER) | conjugation > amidation | prostaglandins > nicotinomide & pyrimidine metabolites | low | low |
| sustained release (SR) | amidation | nicotinomide & pyrimidine metabolites | high | very low |
Fibrates
Several studies have linked clofibrate and gemfibrozil to hepatomegaly, hepatocyte apoptosis, and hepatocarcinogenesis in rodent models.68-70 This increased cancer risk has not, however, been seen in humans, nor has the hepatomegaly or apoptosis.71 There is a case report in the Spanish literature of gemfibrozil leading to cholestatic hepatitis and still other rare reports of hepatocellular injury.71-75 Fenofibrate may very rarely instigate an autoimmune hepatitis-type reaction with resultant ductopenia, chronic hepatitis, and fibrosis, especially when used in combination with statin medications.71,76
Ezetimibe
Ezetimibe lowers cholesterol by inhibiting its intestinal absorption at the brush border of the small intestine.77 This medication does not induce or inhibit enzyme systems in the liver but undergoes enterohepatic circulation and is exposed to the liver and bile.7 Therefore, although there is limited systemic exposure to ezetimibe, it is not solely limited to the lumen and is, in fact, a systemic drug. Much of the previous data about the hepatotoxicity of ezetimibe came from the manufacturer's pre-release clinical trials. Ezetimibe was shown to be generally well tolerated with an incidence of adverse events similar to placebo.78 Recent studies, however, have noted that ezetimibe may rarely cause hepatotoxicity in the form of severe cholestatic hepatitis and acute autoimmune hepatitis.7 The mechanism of toxicity may be related to the metabolism of the drug; it is rapidly absorbed and glucuronidated, yielding an active metabolite and there is significant enterohepatic recirculation.77 The frequency of increased transaminases was noted to be slightly higher in patients receiving ezetimibe administered with HMG-CoA reductase inhibitors than in patients treated with HMG-CoA reductase inhibitors alone but whether this will translate into a higher rate of toxicity remains to be seen.78-81
Bile acid-binding resins
Bile acid-binding resins indirectly lower cholesterol by up-regulating the conversion of cholesterol into bile acids in the hepatocytes.82 These agents bind bile acids in the intestine which causes a subsequent reduction in the enterohepatic recirculation of bile acids.82-84 Furthermore, the decrease in intrahepatic cholesterol causes an up-regulation of LDL receptor expression.82 Drugs in this class are mostly noted to be safe for the liver and cholestyramine has actually been noted to be hepatoprotective.82,85 There is a case report of hepatotoxicity from colestipol.83 The patient in this report developed asymptomatic elevation in his transaminases to ten times the upper limit of normal. The hepatocellular injury occurred three months after the initiation of the drug and resolved within four weeks of discontinuation of the medication. Re-challenge was not attempted in this case. The exact mechanism of the hepatotoxicity of bile acid-binding resins is unknown.85
CETP (cholesteryl ester transfer protein) inhibitors
Trials were underway to evaluate the first drug in this class, but were cut short due to an increase in mortality of patients taking a combination of the first drug in this class, torcetrapib, with atorvastatin.86-88 Torcetrapib binds to CETP and blocks the transfer of lipids and phospholipids from HDL to other lipoproteins.89 Since it has been established that low high-density lipoprotein (HDL) cholesterol is as powerful a predictor for coronary heart disease (CHD) as is elevated low-density lipoprotein (LDL) cholesterol, this medication was highly anticipated, especially as a complementary medication to the statins. Indeed, initial studies revealed that the combination of atorvastatin and torcetrapib yielded striking improvements in the cholesterol profiles of study subjects and combination therapy appeared safe and well tolerated in initial studies.86,87 Unfortunately, the phase 3 trial was suspended due to a significant increase in mortality in patients receiving the combination compared to those receiving atorvastatin alone.88,90 Increased rates of hypertension are suspected to be involved and had previously been reported in patients randomized to torcetrapib, although it is unknown whether this effect definitively led to the mortality findings.88,90 Little is known about the potential hepatotoxicity of this class of medications at this time.
Use of lipid lowering medications in patients with pre-existing liver disease
As current recommendations state that statins should not be used in patients with active liver disease, practicing physicians typically avoid statins in patients with elevated liver enzymes. However, this is problematic because hyperlipidemic patients have a significant prevalence of underlying nonalcoholic fatty liver disease. Patients with nonalcoholic fatty liver disease would tremendously benefit from statins due to their heightened risk of cardiovascular disease.43, 91
Several recent studies examined if patients with underlying liver disease are increased risk for statin hepatotoxicity.91-96 In one study, the incidence of statin hepatotoxicity over a 6-month period in 342 hyperlipidemic patients with elevated liver enzymes who received statins was compared to 1437 hyperlipidemic patients with normal aminotransferases who received statins (statin controls) and 2245 patients with elevated liver enzymes who did not receive statins (liver disease controls).92 Elevations in liver biochemistries during a 6-month follow-up were categorized into mild-moderate or severe based on predefined criteria. Compared to statin controls, patients with elevated liver enzymes had higher incidence of mild-moderate elevations (4.7% vs. 1.9%, p=0.002) but not severe elevations (0.6% vs. 0.2%, p=ns).92 However, patients with elevated liver enzymes who received statins did not show higher incidence of mild-moderate elevations (4.7% vs 6.4%, p=ns) or severe elevations (0.6% vs. 0.4%, p=ns) than the liver disease controls who did not receive statins.92 These data point out that some individuals with elevated baseline liver enzymes have increases in their liver biochemistries, whether or not they receive statins. As most patients in this study received either atorvastatin or simvastatin, in a subsequent study of similar design, the same group examined if patients with elevated liver enzymes were more likely to have hepatotoxicity from lovastatin (first generation statin) than those with normal liver enzymes.25 This study also showed that hyperlipidemic individuals with elevated baseline liver enzymes did not have higher frequency of lovastatin hepatotoxicity than those with normal liver enzymes, as defined biochemically.25 More recently, investigators from the Palo Alto Veterans Administration Medical Center have shown that patients with chronic hepatitis C are not at higher risk for statin hepatotoxicity in comparison to hyperlipidemic patients with no hepatitis C.93 Similarly, a recent report from Dallas showed that statin use was not associated with hepatic steatosis or ALT levels in patients participating in the Dallas Heart Study.94 A recent randomized controlled study showed that pravastatin given at 80 mg/day dose is safe in patients with compensated chronic liver disease.95
Emerging data suggest that statins are actually of benefit in patients with underlying liver disease.96 A retrospective cohort study conducted on adult members of the Northern California Kaiser Permanente Medical Care Program also suggested that lovastatin may be beneficial in patients with pre-existing liver disease.95 In this study, 13,492 patients with lovastatin exposure had better clinical outcomes with no evidence of increased hepatotoxicity when compared to 79,628 patients without lovastatin exposure.96
Based on all these data, it is reasonable to conclude that patients with chronic liver disease and compensated cirrhosis should not be precluded from receiving statins to treat their hyperlipidemia. Although not specifically studied, it is our opinion that that these observations can be cautiously extended to other lipid lowering agents (e.g., fibrates and niacin). The Statin Safety Task Force of the National Lipid Association recently recommended that chronic liver disease or compensated cirrhosis should not be considered as a contraindication for statins (Table 4).9
Conclusion
Cardiovascular disease is the leading cause of death is the Western World and, therefore, the effort to reduce cardiovascular risk factors, including hyperlipidemia, has led to the increased use of lipid lowering agents. However, hyperlipidemic patients often have underlying fatty liver disease and thus may have elevated and fluctuating liver biochemistries. Therefore, caution should be applied before attributing elevated liver tests to lipid lowering agents.
Although the statins are the most publicized lipid-lowering medications in the arena of drug-induced liver injury, their potential to cause clinically significant liver injury is quite minimal. Sustained-release niacin, in fact, is the only drug in this category that causes clinically significant hepatotoxicity. Ezetimibe has recently been viewed as a safe alternative to statin therapy in the setting of pre-existing liver disease; however, recent evidence calls this belief into question. There has been a widespread perception that statins are deleterious for patients with underlying chronic liver disease. However, several recent studies and expert opinion currently fully endorse statin use in patients with nonalcoholic fatty liver disease and other chronic liver disease if clinically indicated.
Acknowledgments
This work is in part supported by K24 069290 (to NC). Dr. Chalasani has consulting agreements with Abbott Pharmaceuticals, Merck Pharmaceuticals, Ortho-McNeil, Metabasis, and Advanced Life Sciences. He has received speaking honoraria from Roche Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sidharth S. Bhardwaj, Visiting Assistant Professor of Medicine, Division of Gastroenterology and Hepatology, Indiana University School of Medicine, Indianapolis, IN.
Naga Chalasani, Associate Professor of Medicine, Division of Gastroenterology and Hepatology, Indiana University School of Medicine, Indianapolis, IN.
References
- 1.American Heart Association. [Accessed January 1, 2007];Cardiovascular disease statistics. 2006 Available at: http://www.americanheart.org/presenter.jhtml?identifier=4478.
- 2.British Heart Foundation statistics website. [Accessed January 1, 2007];CVD mortality in Europe. 2005 March 31; Available at: http://www.heartstats.org/topic.asp?id=753.
- 3.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, et al. Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Journal of the American College of Cardiology. 2004;44(3):720–32. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Parra JL, Reddy KR. Hepatotoxicity of hypolipidemic drugs. Clinics in Liver Disease. 2003;7(2):415–33. doi: 10.1016/s1089-3261(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 5.Chitturi S, George J. Hepatotoxicity of commonly used drugs: nonsteroidal anti-inflammatory drugs, antihypertensives, antidiabetic agents, anticonvulsants, lipidlowering agents, psychotropic drugs. Seminars in Liver Disease. 2002;22(2):169–83. doi: 10.1055/s-2002-30102. [DOI] [PubMed] [Google Scholar]
- 6.Bertolami MC. Mechanisms of hepatotoxicity. [Accessed January 8, 2007];Arquivos Brasileiros de Cardiologia. 2005 85(5):25–27. [online] Available at: < http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0066-782X2005002400007&lng=en&nrm=iso>. [PubMed]
- 7.Stolk MF, Becx MC, Kuypers KC, Seldenrijk CA. Severe hepatic side effects of ezetimibe. Clinical Gastroenterology & Hepatology. 2006;4(7):908–11. doi: 10.1016/j.cgh.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Talbert RL. Safety issues with statin therapy. Journal of the American Pharmacists Association: JAPhA. 2006;46(4):479–88. doi: 10.1331/154434506778073637. [DOI] [PubMed] [Google Scholar]
- 9.Cohen DE, Anania FA, Chalasani N. National Lipid Association Statin Safety Task Force Liver Expert Panel. An assessment of statin safety by hepatologists. American Journal of Cardiology. 2006;97(8A):77C–81C. doi: 10.1016/j.amjcard.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 10. [Accessed December 5, 2006];Drug Topics: Pharmacy Facts and Figures. 2006 Available at: http://www.drugtopics.com/drugtopics/article/articleList.jsp?categoryId=7604.
- 11.Vuppalanchi R, Chalasani N. Statins for hyperlipidemia in patients with chronic liver disease: are they safe? Clinical Gastroenterology & Hepatology. 2006;4(7):838–9. doi: 10.1016/j.cgh.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transplantation. 2004;10(8):1018–23. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 13.Tolman KG. The liver and lovastatin. American Journal of Cardiology. 2002;89(12):1374–80. doi: 10.1016/s0002-9149(02)02355-x. [DOI] [PubMed] [Google Scholar]
- 14.Tolman KG. Defining patient risks from expanded preventive therapies. American Journal of Cardiology. 2000;85(12A):15E–9E. doi: 10.1016/s0002-9149(00)00946-2. [DOI] [PubMed] [Google Scholar]
- 15.Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. American Journal of Cardiology. 2006;97(8A):44C–51C. doi: 10.1016/j.amjcard.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald JS, Gerson RJ, Kornbrust DJ, Kloss MW, Prahalada S, Berry PH, et al. Preclinical evaluation of lovastatin. American Journal of Cardiology. 1998;62(15):16J–27J. doi: 10.1016/0002-9149(88)90003-3. [DOI] [PubMed] [Google Scholar]
- 17.Horsmans Y, Desager JP, Harvengt C. Biochemical changes and morphological alterations of the liver in guinea-pigs after administration of simvastatin (HMG CoA reductase-inhibitor) Pharmacology & Toxicology. 1990;67(4):336–9. doi: 10.1111/j.1600-0773.1990.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson TA. Statin safety: lessons from new drug applications for marketed statins. American Journal of Cardiology. 2006;97(suppl):44C–51C. doi: 10.1016/j.amjcard.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez-Alonso J, Osorio JM, Guierrez-Cabello F, de la Osa AL, Leon L, Garcia JDM. Atorvastatin-induced cholestatic hepatitis in a young woman with systemic lupus erythematosus. Archives of Internal Medicine. 1999;159(15):1811–2. doi: 10.1001/archinte.159.15.1811-a. [DOI] [PubMed] [Google Scholar]
- 20.Nakad A, Bataille L, Hamoir V, Sempoux C, Horsman Y. Atorvastatin-induced acute hepatitis with absence of cross-toxicity with simvastatin. Lancet. 1999;353(9166):1763–4. doi: 10.1016/S0140-6736(99)00569-3. [DOI] [PubMed] [Google Scholar]
- 21.de Castro ML, Hermo JA, Baz A, de Luaces C, Perez R, Clofent J. Acute cholestatic hepatitis after atorvastatin reintroduction. Gastroenterologia y Hepatologia. 2006;29(1):21–4. doi: 10.1157/13083248. [Spanish; Case Report] [DOI] [PubMed] [Google Scholar]
- 22.Gershovich OE, Lyman AE. Liver function test abnormalities and pruritis in a patient treated with atorvastatin: case report and review of the literature. Pharmacotherapy. 2004;24(1):150–4. doi: 10.1592/phco.24.1.150.34803. [DOI] [PubMed] [Google Scholar]
- 23.Pelli N, Setti M, Ceppa P, Toncini C, Indiveri F. Autoimmune hepatitis revealed by atorvastatin. European Journal of Gastroenterology & Hepatology. 2003;15(8):921–4. doi: 10.1097/00042737-200308000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Grimbert S, Pessayre D, Degott C, Benhamou JP. Acute hepatitis induced by HMG-CoA reductase inhibitor, lovastatin. Digestive Diseases and Sciences. 1994;39(9):2032–3. doi: 10.1007/BF02088142. [DOI] [PubMed] [Google Scholar]
- 25.Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. Am J Med Sci. 2005;329:62–65. doi: 10.1097/00000441-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Ricaurte B, Guirguis A, Taylor HC, Zabriskie D. Simvastatin-amiodarone interaction resulting in rhabdomyolysis, azotemia, and possible hepatotoxicity. Annals of Pharmacotherapy. 2006;40(4):753–7. doi: 10.1345/aph.1G462. [DOI] [PubMed] [Google Scholar]
- 27.Kanathur N, Mathai MG, Byrd RP, Jr, Fields CL, Roy TM. Simvastatin-diltiazem drug interaction resulting in rhabdomyolysis and hepatitis. Tennessee Medicine. 2001;94(9):339–41. [PubMed] [Google Scholar]
- 28.Caldwell SH, Hespenheide EE, von Borstel RW. Myositis, microvesicular hepatitis, and progression to cirrhosis from troglitazone added to simvastatin. Digestive Diseases and Sciences. 2001;46(2):376–8. doi: 10.1023/a:1005505827545. [DOI] [PubMed] [Google Scholar]
- 29.Ashar U, Desai D, Bhaduri A. Flutamide-induced hepatotoxicity with possible potentiation by simvastatin. Journal of the Association of Physicians of India. 2003;51:75–7. [PubMed] [Google Scholar]
- 30.Hartleb M, Rymarczyk G, Januszewski K. Acute cholestatic hepatitis associated with pravastatin. American Journal of Gastroenterology. 1999;94(5):1388–90. doi: 10.1111/j.1572-0241.1999.01091.x. [DOI] [PubMed] [Google Scholar]
- 31.de Denus S, Spinler SA, Miller K, Peterson AM. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24(5):584–91. doi: 10.1592/phco.24.6.584.34738. [DOI] [PubMed] [Google Scholar]
- 32.Kubota T, Fujisaki K, Itoh Y, Yano T, Sendo T, Oishi R. Apoptotic injury in cultured human hepatocytes induced by HMG-CoA reductase inhibitors. Biochemical Pharmacology. 2004;67(12):2175–86. doi: 10.1016/j.bcp.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 33.Wolters LMM, Van Buuren HR. Rosuvastatin-associated hepatitis with autoimmune features. European Journal of Gastroenterology and Hepatology. 2005;17(5):589–90. doi: 10.1097/00042737-200505000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C. American College of Cardiology. American Heart Association. National Heart, Lung and Blood Institute. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation. 2002;106(8):1024–8. doi: 10.1161/01.cir.0000032466.44170.44. [DOI] [PubMed] [Google Scholar]
- 35.Farmer JA, Torre-Amione G. Comparative tolerability of the HMG-CoA reductase inhibitors. Drug Safety. 2000;23(3):197–213. doi: 10.2165/00002018-200023030-00003. [DOI] [PubMed] [Google Scholar]
- 36.Pfeffer MA, Keech A, Sacks FM, Cobbe SM, Tonkin A, Byington RP, et al. Safety and tolerability of pravastatin in long-term clinical trials: prospective Pravastatin Pooling (PPP) Project. Circulation. 2002;105(20):2341–6. doi: 10.1161/01.cir.0000017634.00171.24. [DOI] [PubMed] [Google Scholar]
- 37.Perger L, Kohler M, Fattinger K, Flury R, Meier PJ, Pauli-Magnus C. Fatal liver failure with atorvastatin. Journal of Hepatology. 2003;39(6):1095–7. doi: 10.1016/s0168-8278(03)00464-1. [DOI] [PubMed] [Google Scholar]
- 38.Graziadei IW, Obermoser GE, Sepp NT, Erhart KH, Vogel W. Drug-induced lupus-like syndrome associated with severe autoimmune hepatitis. Lupus. 2003;12(5):409–12. doi: 10.1191/0961203303lu313cr. [DOI] [PubMed] [Google Scholar]
- 39.Siddiqui J, et al. Autoimmune hepatitis induced by statins. 2005. Abstract submitted at DDW. [Google Scholar]
- 40.Guallar E, Goodman SN. Statins and cancer: a case of meta-uncertainty. American Journal of Medicine. 2001;110(9):738–40. doi: 10.1016/s0002-9343(01)00758-6. [DOI] [PubMed] [Google Scholar]
- 41.Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA. 1996;275(1):55–60. [PubMed] [Google Scholar]
- 42.Strandberg TE, Pyorala K, Cook TJ, Wilhelmsen L, Faergeman O, Thorgeirsson G, et al. 4S Group. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) Lancet. 2004;364(9436):771–7. doi: 10.1016/S0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 43.Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41(4):690–5. doi: 10.1002/hep.20671. [DOI] [PubMed] [Google Scholar]
- 44.Marzoa-Rivas R, Crespo-Leiro MG, Paniagua-Marin MJ, Llinares-Garcia D, Muniz-Garcia J, Aldama-Lopez G, et al. Safety of statins when response is carefully monitored: a study of 336 heart recipients. Transplantation Proceedings. 2005;37(9):4071–3. doi: 10.1016/j.transproceed.2005.09.163. [DOI] [PubMed] [Google Scholar]
- 45.Patel DN, Pagani FD, Koelling TM, Dyke DB, Baliga RR, Cody RJ, et al. Safety and efficacy of atorvastatin in heart transplant recipients. Journal of Heart & Lung Transplantation. 2002;21(2):204–10. doi: 10.1016/s1053-2498(01)00369-2. [DOI] [PubMed] [Google Scholar]
- 46.McCormack PL, Keating GM. Prolonged-release nicotinic acid: a review of its use in the treatment of dyslipidaemia. Drugs. 2005;65(18):2719–40. doi: 10.2165/00003495-200565180-00014. [DOI] [PubMed] [Google Scholar]
- 47.Vogt A, Kassner U, Hostalek U, Steinhagen-Thiessen E NAUTILUS Study Group. Evaluation of the safety and tolerability of prolonged-release nicotinic acid in a usual care setting: the NAUTILUS study. Current Medical Research & Opinion. 2006;22(2):417–25. doi: 10.1185/030079906x89766. [DOI] [PubMed] [Google Scholar]
- 48.Dalton TA, Berry RS. Hepatotoxicity associated with sustained-release niacin. American Journal of Medicine. 1992;93(1):102–4. doi: 10.1016/0002-9343(92)90689-9. [DOI] [PubMed] [Google Scholar]
- 49.Etchason JA, Miller TD, Squires RW, Allison TG, Gau GT, Marttila JK, et al. Niacin-induced hepatitis: a potential side effect with low-dose time-release niacin. Mayo Clinic Proceedings. 1991;66(1):23–8. doi: 10.1016/s0025-6196(12)61171-9. [DOI] [PubMed] [Google Scholar]
- 50.Henkin Y, Johnson KC, Segrest JP. Rechallenge with crystalline niacin after drug-induced hepatitis from sustained-release niacin. JAMA. 1990;264(2):241–3. [PubMed] [Google Scholar]
- 51.Hodis HN. Acute hepatic failure associated with the use of low-dose sustained-release niacin. JAMA. 1990;264(2):181. doi: 10.1001/jama.1990.03450020033012. [DOI] [PubMed] [Google Scholar]
- 52.Reimund E, Ramos A. Niacin-induced hepatitis and thrombocytopenia after 10 years of niacin use. Journal of Clinical Gastroenterology. 1994;18(3):270–1. doi: 10.1097/00004836-199404000-00037. [DOI] [PubMed] [Google Scholar]
- 53.Mullin GE, Greenson JK, Mitchell MC. Fulminant hepatic failure after ingestion of sustained-release nicotinic acid. Annals of Internal Medicine. 1989;111(3):253–5. doi: 10.7326/0003-4819-111-3-253. [DOI] [PubMed] [Google Scholar]
- 54.Clementz GL, Holmes AW. Nicotinic acid-induced fulminant hepatic failure. Journal of Clinical Gastroenterology. 1987;9(5):582–4. doi: 10.1097/00004836-198710000-00022. [DOI] [PubMed] [Google Scholar]
- 55.Moon YS, Kashyap ML. Niacin extended-release/lovastatin: combination therapy for lipid disorders. Expert Opinion on Pharmacotherapy. 2002;3(12):1763–71. doi: 10.1517/14656566.3.12.1763. [DOI] [PubMed] [Google Scholar]
- 56.American Society of Health-System Pharmacists. ASHP Therapeutic Position Statement on the safe use of niacin in the management of dyslipidemias. American Journal of Health-System Pharmacy. 1997;54(24):2815–9. doi: 10.1093/ajhp/54.24.2815. [DOI] [PubMed] [Google Scholar]
- 57.Scheer MS, Perlmutter S, Ross W, Katz DS. Ultrasonographic findings in niacin-induced hepatitis. Journal of Ultrasound in Medicine. 1999;18(4):321–3. doi: 10.7863/jum.1999.18.4.321. [DOI] [PubMed] [Google Scholar]
- 58.Kristensen T, Olcott EW. Effects of niacin therapy that simulate neoplasia: hepatic steatosis with concurrent hepatic dysfunction. Journal of Computer Assisted Tomography. 1999;23(2):314–7. doi: 10.1097/00004728-199903000-00026. [DOI] [PubMed] [Google Scholar]
- 59.Coppola A, Brady PG, Nord HJ. Niacin-induced hepatotoxicity: unusual presentations. Southern Medical Journal. 1994;87(1):30–2. doi: 10.1097/00007611-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 60.Lavie CJ, Milani RV. Safety and side effects of sustained-release niacin. JAMA. 1994;272(7):513–4. doi: 10.1001/jama.1994.03520070031016. [DOI] [PubMed] [Google Scholar]
- 61.Gray DR, Morgan T, Chretien SD, Kashyap ML. Efficacy and safety of controlled-release niacin in dyslipoproteinemic veterans. Annals of Internal Medicine. 1994;121(4):252–8. doi: 10.7326/0003-4819-121-4-199408150-00003. [DOI] [PubMed] [Google Scholar]
- 62.Capuzzi DM, Guyton JR, Morgan JM, Goldberg AC, Kreisberg RA, Brusco OA, et al. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. American Journal of Cardiology. 1998;82(12A):74U–81U. doi: 10.1016/s0002-9149(98)00731-0. [DOI] [PubMed] [Google Scholar]
- 63.Rader JI, Calvert RJ, Hathcock JN. Hepatic toxicity of unmodified and timerelease preparations of niacin. American Journal of Medicine. 1992;92(1):77–81. doi: 10.1016/0002-9343(92)90018-7. [DOI] [PubMed] [Google Scholar]
- 64.Pieper JA. Overview of niacin formulations: differences in pharmacokinetics, efficacy, and safety. American Journal of Health-System Pharmacy. 2003;60(13) 2:S9–14. doi: 10.1093/ajhp/60.suppl_2.S9. [DOI] [PubMed] [Google Scholar]
- 65.McKenney J. Niacin for dyslipidemia: considerations in product selection. American Journal of Health-System Pharmacy. 2003;60(10):995–1005. doi: 10.1093/ajhp/60.10.995. [DOI] [PubMed] [Google Scholar]
- 66.Lawrence SP. Transient focal hepatic defects related to sustained-release niacin. Journal of Clinical Gastroenterology. 1993;16(3):234–6. doi: 10.1097/00004836-199304000-00015. [DOI] [PubMed] [Google Scholar]
- 67.McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. JAMA. 1994;271(9):672–7. [PubMed] [Google Scholar]
- 68.Chen H, Huang CY, Wilson MW, Lay LT, Robertson LW, Chow CK, et al. Effect of the peroxisome proliferators ciprofibrate and perfluorodecanoic acid on hepatic cell proliferation and toxicity in Sprague-Dawley rats. Carcinogenesis. 1994;15(12):2847–50. doi: 10.1093/carcin/15.12.2847. [DOI] [PubMed] [Google Scholar]
- 69.Cornwell PD, De Souza AT, Ulrich RG. Profiling of hepatic gene expression in rats treated with fibric acid analogs. Mutation Research. 2004;549(12):131–45. doi: 10.1016/j.mrfmmm.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 70.Dix KJ, Coleman DP, Jeffcoat AR. Comparative metabolism and disposition of gemfibrozil in male and female Sprague-Dawley rats and Syrian golden hamsters. Drug Metabolism & Disposition. 1999;27(1):138–46. [PubMed] [Google Scholar]
- 71.Alsheikh-Ali AA, Kuvin JT, Karas RH. Risk of adverse events with fibrates. American Journal of Cardiology. 2004;94(7):935–8. doi: 10.1016/j.amjcard.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 72.Bustamante Balen M, Plume Gimeno G, Bau Gonzalez I, Berenguer Lapuerta J. Acute hepatitis caused by gemfibrozil. Gastroenterologia y Hepatologia. 1998;21(8):419–20. [PubMed] [Google Scholar]
- 73.de Diego Lorenzo A, Catalina V, Garcia Sanchez A, Escudero M, Cos E, Clemente G. Cholestatic hepatitis caused by gemfibrozil. Revista Espanola de Enfermedades Digestivas. 2001;93(9):610–1. [PubMed] [Google Scholar]
- 74.Grubisic-Cabo F, Vrdoljak E. Drug-induced hepatitis in a patient with malignant melanoma treated with interferon alfa 2b adjuvantly who had been administered gemfibrozil in therapy. Medical Oncology. 2006;23(1):121–4. doi: 10.1385/MO:23:1:121. [DOI] [PubMed] [Google Scholar]
- 75.Ho CY, Kuo TH, Chen TS, Tsay SH, Chang FY, Lee SD. Fenofibrate-induced acute cholestatic hepatitis. Journal of the Chinese Medical Association: JCMA. 2004;67(5):245–7. [PubMed] [Google Scholar]
- 76.Athyros VG, Papageorgiou AA, Hatzikonstandinou HA, Didangelos TP, Carina MV, Kranitsas DF, et al. Safety and efficacy of long-term statin-fibrate combinations in patients with refractory familial combined hyperlipidemia. American Journal of Cardiology. 1997;80(5):608–13. doi: 10.1016/s0002-9149(97)00430-x. [DOI] [PubMed] [Google Scholar]
- 77.Landmesser U, Bahlmann F, Mueller M, Spiekermann S, Kirchhoff N, Schulz S, et al. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111(18):2356–63. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- 78.Cruz-Fernandez JM, Bedarida GV, Adgey J, Allen C, Johnson-Levonas AO, Massaad R. Efficacy and safety of ezetimibe co-administered with ongoing atorvastatin therapy in achieving low-density lipoprotein goal in patients with hypercholesterolemia and coronary heart disease. International Journal of Clinical Practice. 2005;59(6):619–27. doi: 10.1111/j.1368-5031.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 79.Goldman-Levine JD, Bohlman LG. Ezetimibe/Simvastatin (Vytorin) for hypercholesterolemia. American Family Physician. 2005;72(10):2081–2. [PubMed] [Google Scholar]
- 80.Reyderman L, Kosoglou T, Cutler DL, Maxwell S, Statkevich P. The effect of fluvastatin on the pharmacokinetics and pharmacodynamics of ezetimibe. Current Medical Research & Opinion. 2005;21(8):1171–9. doi: 10.1185/030079905X46386. [DOI] [PubMed] [Google Scholar]
- 81.van Heyningen C. Drug-induced acute autoimmune hepatitis during combination therapy with atorvastatin and ezetimibe. Annals of Clinical Biochemistry. 2005;42(Pt 5):402–4. doi: 10.1258/0004563054890105. [DOI] [PubMed] [Google Scholar]
- 82.Armani A, Toth PP. Colesevelam hydrochloride in the management of dyslipidemia. Expert Review of Cardiovascular Therapy. 2006;4(3):283–91. doi: 10.1586/14779072.4.3.283. [DOI] [PubMed] [Google Scholar]
- 83.Sirmans SM, Beck JK, Banh HL, Freeman DA. Colestipol-induced hepatotoxicity. Pharmacotherapy. 2001;21(4):513–6. doi: 10.1592/phco.21.5.513.34501. [DOI] [PubMed] [Google Scholar]
- 84.Hofmann AF. Detoxification of lithocholic acid, a toxic bile acid: relevance to drug hepatotoxicity. Drug Metabolism Reviews. 2004;36(34):703–22. doi: 10.1081/dmr-200033475. [DOI] [PubMed] [Google Scholar]
- 85.Graf D, Kohlmann C, Haselow K, Gehrmann T, Bode JG, Haussinger D. Bile acids inhibit interleukin-6 signaling via gp130 receptor-dependent and - independent pathways in rat liver. Hepatology. 2006;44(5):1206–17. doi: 10.1002/hep.21368. [DOI] [PubMed] [Google Scholar]
- 86.Thuren T, Longcore A, Powell C, et al. Torcetrapib combined with atorvastatin raises HDL-C, lowers LDL-C, and is well tolerated: results from a phase 2 doseranging clinical trial. Circulation. 2005;112 17:II–179. Abstract 942. [Google Scholar]
- 87.Davidson M, McKenney J, Revkin J, Charles Shear C. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor torcetrapib when administered with and without atorvastatin to subjects with a low level of high-density lipoprotein cholesterol. Program and abstracts from the American College of Cardiology Annual Scientific Sessions; March 6-9, 2005; Orlando, Florida. 2005. Abstract 802. [Google Scholar]
- 88.Daley J. Pfizer scraps drug trial after deaths. [Accessed December 4, 2006];The Independent. 2006 December 4; [serial online] Available at: http://news.independent.co.uk/business/news/article2037520.ece.
- 89.Brewer HB., Jr High-density lipoprotein: a new potential therapeutic target for the prevention of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2004;24:387–391. doi: 10.1161/01.ATV.0000121505.88326.d2. [DOI] [PubMed] [Google Scholar]
- 90. [Accessed December 4, 2006];FDA Statement: Pfizer Stops All Torcetrapib Clinical Trials in Interest of Patient Safety. 2006 Available at: http://www.fda.gov/bbs/topics/NEWS/2006/NEW01514.html.
- 91.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. American Journal of Gastroenterology. 2004;99(8):1497–502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 92.Chalasani N, Aljadhey H, Kesterson J, et al. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology. 2004;126:1287–1292. doi: 10.1053/j.gastro.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 93.Khorashadi S, Hasson NK, Cheung RC. Incidence of statin hepatotoxicity in patients with hepatitis C. Clinical Gastroenterology & Hepatology. 2006;4(7):902–7. doi: 10.1016/j.cgh.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 94.Browning JD. Statins and hepatic steatosis: perspectives from the Dallas Heart Study. Hepatology. 2006;44:466–471. doi: 10.1002/hep.21248. [DOI] [PubMed] [Google Scholar]
- 95.Lewis JH, Fusco MJ, Medoff JR, Mortensen ME, Zweig S. Safety and efficacy of pravastatin 80 mg/day in 320 hypercholesterolemic patients with compensated chronic liver disease (CLD): results of a prospective, randomized, double-blind, placebo-controlled study. Gastroenterology. 2006;130:A–65. doi: 10.1002/hep.21848. [DOI] [PubMed] [Google Scholar]
- 96.Avins AL, Manos MM, Levin TR, Ackerson LM, Zhao WK, Murphy RC, Watson DJ, Hwang PMT, Replogle AR, Levine JG. Lovastatin is not hepatotoxic to patients with pre-existing liver disease. Gastroenterology. 2006;130:A–595. [Google Scholar]