Abstract
The uniquely designed limbs of the African elephant, Loxodonta africana, support the weight of the largest terrestrial animal. Besides other morphological peculiarities, the feet are equipped with large subcutaneous cushions which play an important role in distributing forces during weight bearing and in storing or absorbing mechanical forces. Although the cushions have been discussed in the literature and captive elephants, in particular, are frequently affected by foot disorders, precise morphological data are sparse. The cushions in the feet of African elephants were examined by means of standard anatomical and histological techniques, computed tomography (CT) and magnetic resonance imaging (MRI). In both the forelimb and the hindlimb a 6th ray, the prepollex or prehallux, is present. These cartilaginous rods support the metacarpal or metatarsal compartment of the cushions. None of the rays touches the ground directly. The cushions consist of sheets or strands of fibrous connective tissue forming larger metacarpal/metatarsal and digital compartments and smaller chambers which were filled with adipose tissue. The compartments are situated between tarsal, metatarsal, metacarpal bones, proximal phalanges or other structures of the locomotor apparatus covering the bones palmarly/plantarly and the thick sole skin. Within the cushions, collagen, reticulin and elastic fibres are found. In the main parts, vascular supply is good and numerous nerves course within the entire cushion. Vater–Pacinian corpuscles are embedded within the collagenous tissue of the cushions and within the dermis. Meissner corpuscles are found in the dermal papillae of the foot skin. The micromorphology of elephant feet cushions resembles that of digital cushions in cattle or of the foot pads in humans but not that of digital cushions in horses. Besides their important mechanical properties, foot cushions in elephants seem to be very sensitive structures.
Keywords: adipose tissue, collagen, elastic fibres, foot pad, Meissner corpuscles, prehallux, prepollex, torus, Vater–Pacinian corpuscles
Introduction
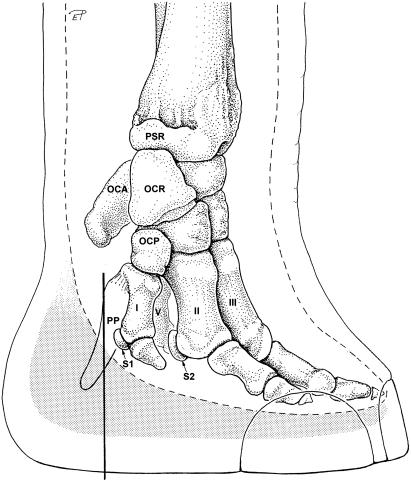
The limbs of elephants reveal many peculiarities both in structure and in kinematic patterns (Muybridge, 1899; Howell, 1944; Gambaryan, 1974; Hildebrand & Hurley, 1985; Hutchinson et al. 2003, in press; Weissengruber & Forstenpointner, 2004a; Weissengruber et al. 2006). All structures of the locomotor apparatus are integrated within a column-shaped, extended limb (Howell, 1944; Gambaryan, 1974). Although the larger forelimbs support about 60% of the body mass (Alexander et al. 1979), the hindlimbs are also well suited to weight bearing (Weissengruber & Forstenpointner, 2004a,b). Unlike in most mammals a 6th ray resembling a cartilaginous rod (Nauck, 1938; Smuts & Bezuidenhout, 1994) is present in the forelimb (the prepollex) as well as in the hindlimb (the prehallux) (Fig. 1: PP, Fig. 2: PH). The carpals/tarsals and metapodials are arranged and form an arch, similar to the human foot, and the toes are enclosed within a flexible sheath of skin (Weissengruber & Forstenpointner, 2004b; Weissengruber et al. 2006). None of the phalanges touches the ground directly (Virchow, 1910; Benz, 2005) – the distal phalanges are separated from the sole, firmly attached to the corium of the respective nail/hoof (Smuts & Bezuidenhout, 1993). Cushions occupy the spaces between carpal/tarsal, metapodial and digital bones or tendons, muscles (Eales, 1928; Weissengruber & Forstenpointner, 2004a) and ligaments, which cover the bones palmarly/plantarly, and the sole skin lies under all of these structures (Neuville, 1927; Benz, 2005) (Fig. 1).
Fig. 1.
Medial aspect of the distal part of the left forelimb in an African elephant. Black line: position of transverse section shown in Fig. 7, broken line: outline of soft tissues of the locomotor apparatus (ligaments, muscles, tendons), stippled: position of the foot cushion. PSR, processus styloideus radii; OCA, os carpi accessorium; OCR, os carpi radiale; OCP, os carpale primum; PP, prepollex; I, first metacarpal bone; II, second metacarpal bone; III, third metacarpal bone; V, fifth metacarpal bone; S1, promimal sesamoid bone of the 1st digit; S2, medial proximal sesamoid bone of the 2nd digit.
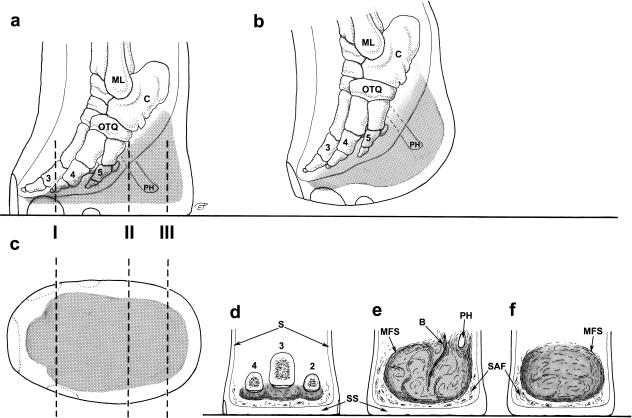
Fig. 2.
Distal part of the left hindlimb in an African elephant. (a) Lateral view, loaded; (b) lateral view, lifted; (c) distal view (sole); (d–f) plantar views, transverse sections in positions I, II and III (broken lines in a and c). Stippled: foot cushion (in a–c), digital compartments (in d) and metatarsal compartment (in e and f), black line (in a and b): outline of soft tissues of the locomotor apparatus (ligaments, muscles, tendons). ML, lateral malleolus (fibula); C, calcaneus; OTQ, os tarsale quartum; 2–5, 2nd−5th ray (proximal or middle phalanges); PH, prehallux; S, skin; SS, sole skin; MFS, main fibrous sheet of the metatarsal compartment; B, bundle of vessels, nerves and fibrous tissue; SAF, strips of adipose and fibrous tissue outside the metatarsal compartment of the foot cushion.
In the literature, the cushions in elephant feet have been frequently mentioned (Virchow, 1910; Neuville, 1935; Mariappa, 1955, 1986; Köther & Bürger, 1967; Fowler, 1980; Güßgen, 1988; Smuts & Bezuidenhout, 1994; Keet et al. 1997; Ramsay & Henry, 2001; Benz, 2005; Benz et al. 2005) and related to ‘noiseless stepping’ (Virchow, 1910), but detailed and concise anatomical data on their structure are still lacking. These cushions are roughly comparable with the foot/heel pads in humans (e.g. Tietze, 1921), the Pulvini digitales (digital cushions) in hoofed mammals (e.g. Neuville, 1935; Räber et al. 2004; Egerbacher et al. 2005) and the Tela subcutanea of the Tori metacarpei, metatarsei and digitales in domestic carnivores (Schaller, 1992; Liebich, 1999).
Foot problems are unfortunately common in captive elephants (Ruthe, 1961; Hittmair & Vielgrader, 2000; Gage, 2001; Benz et al. 2005), but they also occur in free-ranging animals (Keet et al. 1997). Yet diagnosis and treatment of disorders affecting deeper structures of the foot are difficult, owing to sparse morphological information on the foot cushions. Herein, to fill this important gap we investigate the structure of the palmar/plantar metapodial and digital cushions of the African elephant (Loxodonta africana Blumenbach 1797) and provide a detailed morphological description of how these unique structures are integrated with other foot structures.
Materials and methods
Animals, dissections and computed tomography
The manus and pedes of nine African elephants were examined after formalin fixation or deep freezing, using gross anatomical methods, standard histological techniques, computed tomography (CT) or magnetic resonance imaging (MRI) (see Table 1). Five juvenile elephants that had lived in the Krüger National Park (South Africa) were shot as part of the regular elephant culling programme during the 1990s. The dissections of these juveniles were carried out at the Department of Anatomy and Physiology, Faculty of Veterinary Science, University of Pretoria, Onderstepoort, South Africa. Two adult individuals (40 and 46 years) had been kept in the Tiergarten Schönbrunn Vienna. The younger individual of these was euthanized by zoo veterinarians because of severe disorders, and the other died of natural causes. Both were dissected at the Institute of Anatomy, University of Veterinary Medicine, Vienna. One juvenile individual (6.5 years old) had lived in Knowsley Safari Park (Knowsley, UK) and its pes was studied at The Royal Veterinary College (Hatfield, UK). The pes of an adult (26 years old) from Six Flags Marine World Theme Park (Vallejo, California, USA) was studied at the University of California-Davis (USA). CT was performed at both of these latter two locations using a Picker PQ5000 CT scanner (5 mm axial slice thickness, 140 kV, 200 mA, 512 × 512 pixels) or a GE HiSpeed CT scanner (helical CT scans, 5 mm thickness, 120 kV, 130 mA, 512 × 512 pixels), respectively. Furthermore, MRI scans of both feet were performed (GE Genesis Sigman MRI scanner: axial, sagittal, and coronal, 5 mm thickness, 10 mm spacing) to provide improved soft tissue data.
Table 1.
Specimens examined
| Number | Sex | Age | Origin | Used for |
|---|---|---|---|---|
| 5 | M | juvenile | Krüger National Park, RSA | dissection, histology |
| 1 | F | 40 years | Tiergarten Schönbrunn, Vienna, Austria | dissection, histology |
| 1 | F | 46 years | Tiergarten Schönbrunn, Vienna, Austria | dissection, histology |
| 1 | F | 6.5 years | Knowsley Safari Park, Knowsley, UK | dissection, CT, MRI |
| 1 | F | 26 years | Six Flags Marine World Theme Park, Vallejo, USA | CT, MRI |
Anatomical names follow NAV (2005).
Sample preparation for histological examination
One hindlimb of the 46-year-old individual was cut sagittally and an axial slice of the cushion was excised and fixed in buffered formalin according to the method given by Romeis (1989). Additionally, fixed slices of the hindfoot cushions of the second individual from the Tiergarten Schönbrunn Vienna and sections of fore- and hindlimbs of five juvenile individuals from the Krüger National Park were prepared.
Routine histology and specific staining methods
Tissue samples were taken at designated sites throughout the subcutaneous tissue, dehydrated in graded alcohols and embedded in Paraplast® (Vogel, Histo-Comp, Giessen, Germany) by means of a Tissue Tek VIP 2000 automatic embedding equipment (Miles Scientific Inc., Mishawaka, IN, USA). Serial sections were cut at 5-µm thickness. Sections were stained with haematoxylin and eosin (H&E), van Gieson's connective tissue stain for collagen fibres, Weigert's resorcin fuchsin for elastic fibres, alcian blue at pH 2.5 and pH 4.0 and safranin O for amorphous intercellular matrix, or Gomori's stain for reticulin fibres. All staining methods were performed according to Romeis (1989), except safranin O staining which was performed according to Lillie (1954).
Results
Macroscopic findings
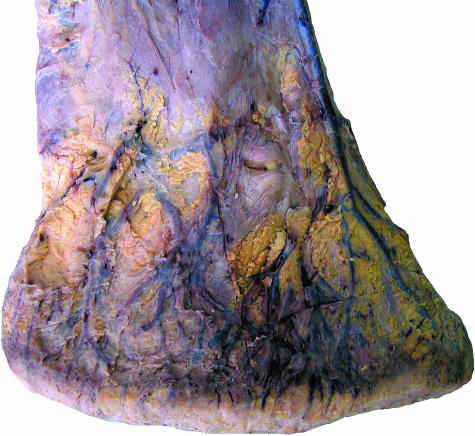
Between the skin and the deeper structures of the foot including the cushions, large veins embedded in adipose tissue are present (Fig. 3). Distally, these vessels form a network which is also visible proximal to the skin of the sole.
Fig. 3.
Superficial veins of the distal hindlimb of an African elephant, medial view.
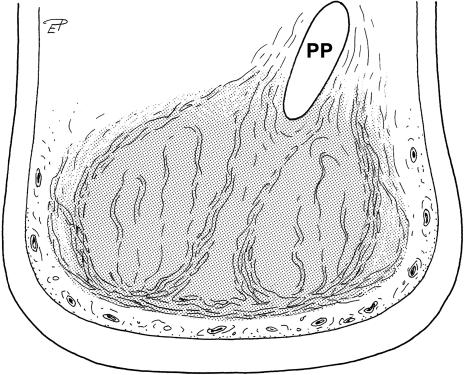
The skeletal elements of the foot and the long and short flexor muscle–tendon units are covered palmarly or plantarly by a thick layer of fibrous tissue. A transversely orientated ligamentous structure supporting the foot arch runs from the medial to the lateral ray of the foot. The cartilaginous prepollex, which resembles a slightly curved, elongated, blunt-ended cone, is attached to the Basis of the Os metacarpale primum (first metacarpal) (Fig. 1). It extends laterodistally towards the central part of the metacarpal cushion. The prehallux is a mediolaterally flattened cartilaginous rod and its distal end is widened (Fig. 2a,b). It is attached to the Ossa tarsale primum and metatarsale primum (first distal tarsal and first metatarsal) and extends on the medial and plantar sides of the foot towards the sole.
Many minor interindividual differences in size and shape of the different parts of the cushions and also of the subcutaneous tissue outside the cushions were present. The following statements describe either combinations of findings in different elephants or basic patterns found in all individuals.
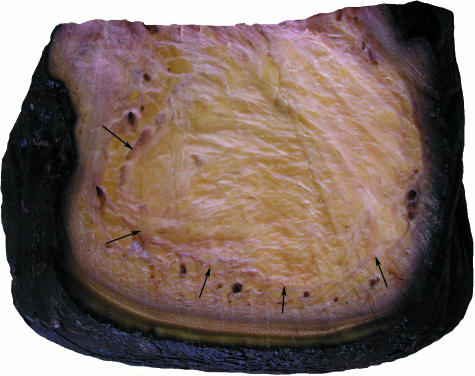
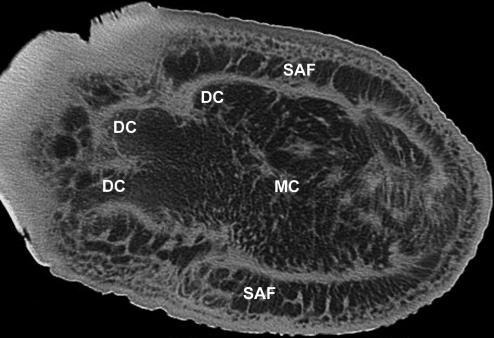
The cushions are complex structures of white or yellowish adipose tissue and fibrous connective tissue occupying the space between tarsal, metacarpal/metatarsal, digital bones or muscles, tendons, ligaments covering the bones palmarly/plantarly and the sole skin (Fig. 4). They resemble combinations of a Torus metacarpalis or metatarsalis, respectively, with Tori digitales. Therefore, we designate the different parts of the foot cushions as metacarpal/metatarsal or digital compartments. In the forelimb, the cushion (metacarpal compartment) ends proximally on a level with the proximal part of the prepollex (Fig. 1). The metatarsal compartment extends further proximally towards the calcaneus and, thus, the proximal part of this cushion may be designated as ‘tarsal’ (Fig. 2). Thick bundles of greyish fibrous tissue (see below) form a large metacarpal or metatarsal compartment and three more irregular digital compartments (Fig. 2d). In the forelimb and the hindlimb, the distal tendon of the M. flexor digitorum superficialis and the palmar/plantar fascia attach superficially (palmarly/plantarly) to the metacarpal/metatarsal compartments. Short toe flexors and other muscles of the Palma manus/Planta pedis lie dorsal of the foot cushions. The digital compartments are situated distal and palmar/plantar of the Phalanges proximales and mediae of the 2nd, 3rd and 4th digit and even – especially proximally – between the digital bones including the 1st and the 5th. The metapodial and digital compartments are not clearly separated from each other (Fig. 4). Both metapodial (Fig. 5) and digital compartments are filled with amorphous, white or yellowish adipose tissue and smaller strands of fibrous tissue. These smaller strands are even visible on CT scans (Fig. 6), forming chambers enveloping strands of adipose tissue. The large or ‘main’ fibrous sheets (see below) of the metapodial compartments seem continuous with those of the digital compartments and, thus, the strands of adipose tissue within the different compartments may also be continuous. The digital compartments are bordered and separated from each other by near-sagittally and horizontally orientated fibrous sheets (Fig. 2d) attached to the fascial sheets covering the toes and to the corium of the sole. Towards the tip of the toes the digital compartments become thinner and end irregularily on a level either with the distal part of the Phalanges proximales or with the Phalanges mediae. In forelimbs, they tend to be shorter. The small distal phalanges in both forelimbs and hindlimbs are not supported by digital compartments but by adipose and fibrous tissue lying outside the digital compartments (Fig. 6). Strands of fibrous and adipose tissue lie also between the digital compartments and medially and laterally on the foot (Figs 2 and 6).
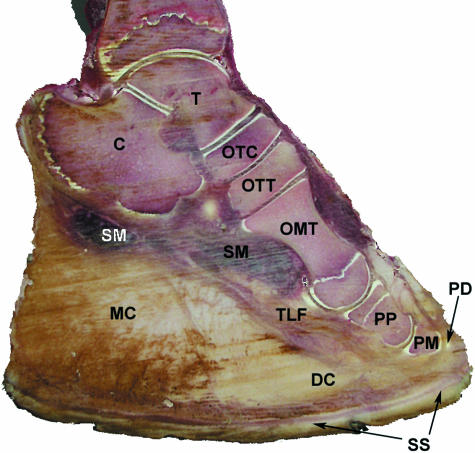
Fig. 4.
Sagittal section through the distal hindlimb of an African elephant. T, talus; C, calcaneus; OTC, os tarsi centrale; OTT, os tarsale tertium; OMT, os metatarsale tertium; PP, proximal phalanx; PM, medial phalanx; PD, distal phalanx; SM, short muscles of the planta pedis (Mm. interossei, adductores and abductores); TLF, tendon of long toe flexors (Mm. flexor digitorum medialis and lateralis); SS, sole skin; MC, metatarsal compartment of the foot cushion; DC, digital compartment.
Fig. 5.
Transverse section through the metatarsal compartment of the foot cushion and the surrounding skin in an African elephant. Arrows: main fibrous sheet.
Fig. 6.
CT image of the hindlimb of an African elephant, horizontal section. DC, digital compartments; MC, metatarsal compartment; SAF, strips of adipose and fibrous tissue outside the metatarsal compartment of the foot cushion.
A thick fibrous sheet (designated as the main fibrous sheet or capsule) is the peripheral boundary of the metacarpal/metatarsal compartment (Fig. 2e,f). These compartments are medially or laterally supported by the Ossa metacarpalia/metatarsalia prima, the proximal part of the prepollex, the entire prehallux, and the Ossa metacarpalia/metatarsalia quinta. The capsule is attached to the medial and lateral ray of the foot, the digital fascia of the 2nd, 3rd and 4th digits and to the deep palmar/plantar fascia covering the metacarpals/metatarsals palmarly/plantarly. The metacarpal compartment is roughly hemispherical. The metatarsal compartment, which extends further proximally compared with the corresponding compartment in the forelimb (see above), is more ovoid or pear-shaped. Fibrous strands within the metapodial compartments are rather straight or slightly S-shaped and run mainly in a proximodistal direction (Fig. 2e,f). Nevertheless, there are also radially orientated strands. Small blood vessels are visible within several strands. Two sagittally orientated fibrous sheets are situated in the central part of the metacarpal compartment (Fig. 7). These sheets converge towards the distal part of the prepollex (Fig. 7). Whereas in other parts of the cushion larger vessels are sparse or absent, one bundle consisting of larger vessels, nerves and fibrous tissue runs laterodistally into the central portion of the metacarpal or metatarsal compartment (Fig. 2e). Larger vessels course also on the outside of the main fibrous sheets of the metapodial compartments (Fig. 5), but the main vessels of the foot lie adjacent to the bones and thus outside the entire cushion. Proximal to the corium of the sole skin, strands of adipose and more delicate fibrous tissue are also found outside the metapodial compartments forming strips of cushion-like tissue along the medial or lateral side of the foot (Figs 2e,f and 6). These strips therefore fill the gap between the capsule of the metapodial compartments, the lateral or medial skin of the foot and the sole. The strips are usually wider on the lateral side of the foot and they are continuous with those lying outside of the digital compartments.
Fig. 7.
Transverse section of the distal part of the left forelimb of an African elephant (position shown in Fig. 1), caudal view. Stippled: metacarpal compartment of the foot cushion. PP, prepollex.
Microscopic findings
The sole of the elephant foot is covered by a thick keratinized squamous epithelium, the epidermis, which lies on a massive layer of dense connective tissue forming the dermis. The interface between epidermis and dermis contains many remarkably tall dermal papillae that interdigitate with epidermal pegs. The dermis represents a thick, reticulated layer of interwoven and very tightly packed bundles of collagen fibres with only few interspersed elastic fibres. Non-fibrillar connective tissue matrix between the collagen bundles is rarely detectable, showing neither alcianophilia nor staining with safranin O. The dermis is very rich in blood vessels and nerves. Numerous large arteries and veins lie within the connective tissue. The subcutaneous vascular network proximal to the sole skin and the superficial vessels surrounding the entire foot are visible even macroscopically (see above).
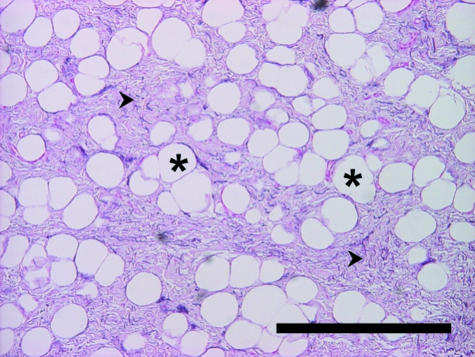
The foot cushions are formed by modified hypodermis. The subcutaneous lobules of adipose tissue are separated from each other by elastic strands (Fig. 8). Larger groups of fat lobules are enclosed by coarse bundles of collagen fibres, which thus form a supporting mesh-like network.
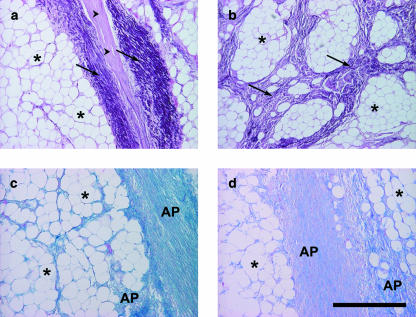
Fig. 8.
Tissue components in the foot cushion of the African elephant. Asterisk: adipocytes, arrowheads: collagen fibres, arrows: elastic fibres. AP, distinct alcianophilia of the non-fibrillar matrix at pH 2.5 and pH 4. Staining resorcin–fuchsin according to Weigert (a,b), alcian blue (pH 2.5) (c), alcian blue (pH 4.0) (d). Scale bar = 500 µm.
Numerous densely packed elastic fibres are arranged like schools of fish. They are restricted to loosely organized connective tissue as well as few intermingled thin collagen fibres. Reticulin fibres are detected between the elastin bundles. In contrast to the dermis, the non-fibrillar connective tissue matrix shows a relatively high cell density and alcianophilia at pH 2.5 and pH 4.0 (Fig. 8). In some areas it also stains slightly with safranin O.
Unilocular adipose tissue becomes prominent where the bundles of elastic fibres diverge. Fat cells, arranged in lobules, resemble compact islets within the framework of elastic bundles. The relative volume of adipose tissue appears equal in the cushions of juvenile and adult fore- and hindlimbs. Some adipocytes are also found as solitary cells. Scattered elastic and reticulin fibres are found between fat cells. Small amounts of loose connective tissue between and adjacent to adipocytes show alcianophilia, did not stain with safranin O and are well vascularized.
A differently organized type of adipose tissue is found within the dorsal part of the subcutaneous tissue between the digital bones and the sole skin, namely in the digital compartments (Fig. 9). In some specimens, this tissue is separated from the metapodial compartments by strands of dense connective tissue. In this area, the adipocytes do not cluster in lobules but almost every fat cell is surrounded solitarily by a thick layer of fibrillar matrix (Fig. 9). The fibrillar matrix is remarkably rich in collagen fibres building a scaffold for the adipose tissue whereas the number of elastic fibres is notably small. Fibrocytes and blood vessels are sparse. The non-fibrillar matrix of the digital cushions is rich in hyaluronan and is therefore alcianophilic at pH 4.0.
Fig. 9.
Tissue components of the digital compartments of the foot cushion of the African elephant. Asterisk: adipocytes, arrowheads: collagen fibres with few intermingled elastic fibres. Staining resorcin–fuchsin according to Weigert. Scale bar = 500 µm.
Whereas in other parts of the cushion larger vessels are rather sparse, one bundle consisting of large vessels, nerves and fibrous tissue runs into the central part of the metacarpal or metatarsal compartment (see above). Smaller vessels and nerves of variable size are evenly distributed throughout the loose connective tissue surrounding the fibrous septa or fat lobules, respectively. Furthermore, numerous capillaries are detectable within the adipose tissue.
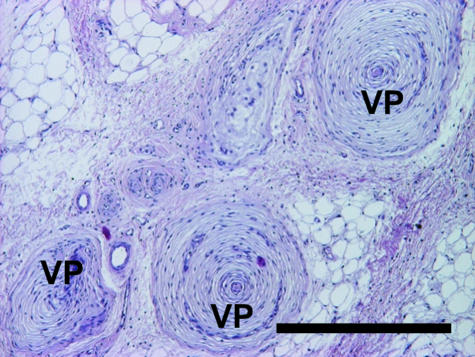
Vater–Pacinian corpuscles lie in the dermis close to the metacarpal/metatarsal compartments as well as in all compartments of the cushion, embedded within collagenous tissue (Fig. 10). Numerous corpuscles are found within the bundle of vessels and nerves which enter the metapodial compartments proximomedially and course toward the centre of the cushion. These specialized sensory receptors appear as solitary bodies as well as in groups of up to five corpuscles. In addition, other tactile corpuscles, namely Meissner corpuscles (Fig. 11), were found in dermal papillae, but not in metapodial or digital compartments.
Fig. 10.
Vater–Pacinian corpuscles (VP) embedded within collagenous tissue in the foot cushion. Staining Mayer's haematoxylin/eosin. Scale bar = 500 µm.
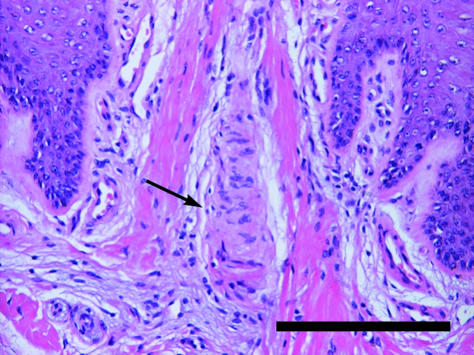
Fig. 11.
Meissner corpuscle in dermal papilla (arrow). Staining Mayer's haematoxylin/eosin. Scale bar = 200 µm.
Discussion
The hindfeet of African elephants are mediolaterally compressed (Fig. 2c) and the sole is smaller than the rounded sole surface in the forefeet. This pattern has been described in previous studies (Neuville, 1935; Smuts & Bezuidenhout, 1994; Ramsay & Henry, 2001). The larger circumference of the forelimb sole might be related to the assumption that also in graviportal elephants the majority of the body weight rests on the forelimbs, as occurs in cursorial, quadrupedal mammals.
Owing to the positions of the prepollex or the prehallux, respectively, and to the attachment of the cushion capsules to these flexible cartilages, it seems likely that they mainly serve to improve the stiffness and the joint (tarsus, carpus) stabilizing effect of the foot cushion as presumed by Ramsay & Henry (2001).
In our specimens, the long axes of the phalangeal bones of the manus form smaller angles with the horizontal (i.e. manus sole) than in the pes and they overlie a larger part of the sole surface. Because also the metacarpal compartment of the cushion is rather small compared with the size of the entire forefoot, it is likely that the toes of the forelimb are more involved in weight bearing than in the hindlimb. The more horizontal orientation of the manual phalanges also suggests that they incur relatively greater bending loads than in the pes. Considering the positions of the skeletal elements of the hindfoot, the major part of the body weight resting on the hindlimb is considered to pass through the metatarsal compartment. Nevertheless, the cushion also presumably helps to distribute the animal's weight over the entire sole (Ramsay & Henry, 2001). When loaded, the cushion is compressed and expands medially, laterally and palmarly/plantarly (Ramsay & Henry, 2001). In unloaded hindfeet of elephants the sole surface is convex (Smuts & Bezuidenhout, 1994) (Fig. 2b). This pattern was also found in our specimens and after removing the skin the rounded metatarsal compartment was clearly visible. By contrast, in the forelimb the surface of the sole as well as the distal surface of the metacarpal compartment remained flattened even when unloaded, which might be due to the different structure and shape of the metacarpal cushion. In both the forelimb and the hindlimb the expansion of the loaded cushion towards the skeletal elements of the feet except the cartilaginous prepollex/prehallux is extremely limited. It must be taken into account that slight movements of the digits, such as spreading within the skin-shoe, may be possible in the forelimb and hindlimb (Miall & Greenwood, 1878; Weissengruber & Forstenpointner, 2004a; G. E. Weissengruber, unpublished observations). Thus, weight distribution over certain parts of the cushion and the sole might be adjusted by means of altering the posture of the foot and the position of its skeletal elements. During load the digits are most probably dorsiflexed and spread (abducted or adducted, respectively).
As deformable foot cushions serve to absorb mechanical shock, store and return elastic strain energy, protect against local stress and keep pressures low (e.g. Ker, 1999; Miller-Young et al. 2002; König et al. 2003; Taylor et al. 2005), these structures should be organized accordingly and should be composed of appropriate tissues. Neuville (1927) mentioned that the cushions of elephants exhibit similarities to the feet of humans, camels and rhinoceroses. A macroscopic picture of a sagittal section of the foot in an Asian elephant shown in Neuville (1927) reveals a very similar pattern to what we have found in African elephants. Nevertheless, Asian elephants seem to have more connective tissue within the cushions than the African species (Benz, 2005). In domestic carnivores the cushions are composed of fat lobules, which co-operate with strands of dense connective tissue to disperse and absorb mechanical forces when being compressed (Alexander et al. 1986; Liebich, 1999). Digital cushions in horses mainly consist of interwoven and tightly packed bundles of collagen fibres in acidic mucinous matrix that is rich in hyaluronan but has only few interspersed elastic fibres (König et al. 2003; Egerbacher et al. 2005). Larger gaps are filled with myxoid tissue, and small areas of fibrocartilage are common in the stroma of the cushions (Egerbacher et al. 2005). In contrast to what is found in elephants and cattle, a large part of the horse's body weight is carried by the suspensory apparatus attached to the hoof walls and only a small load rests on the sole and heel segment including cushions (Räber et al. 2004). The suspensory apparatus is less well developed in cattle and absent in elephants. Thus, in elephants as well as in cattle (Räber et al. 2004), the cushions must support a considerably greater proportion of the body weight. Digital cushions in cattle comprise resilient loose connective tissue with varying amounts of associated soft fat enclosed in an envelope of collagenous connective tissue (Räber et al. 2004) and reveal therefore a similar structure as elephant cushions. Even the human foot pads (heel, ball) show a similar organization. They consist of columns of fat, which are confined in small chambers by fibrous connective tissue and reinforced with a mesh of elastic transverse and diagonal fibres (Blechschmidt, 1933; Bojsen-Møller & Flagstad, 1976; Kimani, 1984; Jahss et al. 1992; Miller-Young et al. 2002). Digital cushions in horses therefore exhibit a remarkably different composition and are less involved in weight bearing. Varying magnitudes, timings and distributions of mechanical loads that stress the cushions are likely to be responsible for the differences in tissue composition between the species.
Cushions in the feet of African elephants are highly specialized structures adapted to enable pain-free weight bearing and locomotion of the largest terrestrial animal. They compress and expand during the gait cycle, making elephant feet far more dynamic structures than might be assumed (Ramsay & Henry, 2001). The cushions are characterized by lobules of adipose tissue and by fibrous connective tissue arranged in main sheets and thinner septa. It seems logical that under high loads the chambered structure of the foot cushion in the African elephant guarantees protection for the foot bones by spreading the load over the whole palmar (plantar) surfaces of digital, metapodial and tarsal bones. Under compression, the volume of the liquid-filled chambers has to remain constant (Rome, 1998). Loading would result in sideways displacement of the adipose tissue, and the collagen septa would come under tension, limit the visco-elasticity of the adipose tissue and increase stiffness (Ker, 1999; Miller-Young et al. 2002; Tong et al. 2003).
The numerous strands of elastic fibrous connective tissue, which were also found in the cushions of Asian elephants (Neuville, 1935), presumably adds to the resilience of the cushions. Because only very few elastic fibres were found in the digital compartments, perhaps these parts of the foot do not undergo such severe deformations as the metacarpal/metatarsal compartments. The digital compartments might have higher stiffness than the metapodial compartments because their matrix is rich in collagen fibres which surround single adipocytes rather than form large clusters of cells. The strands of collagenous connective tissue seen in the histological samples of digital compartments are likely to confine the digital compartments as capsules and keep their shape more constant than the metapodial compartments. Compression and expansion of the loaded foot during the locomotion cycle (Keet et al. 1997; our personal observation) may therefore mainly be caused by expansion of the metapodial compartments.
The non-fibrillar matrix surrounding the elastic fibres contained hyaluronan and other proteoglycans, as indicated by alcianophilia at pH 4.0 and pH 2.5 (Fig. 8) and positive staining with safranin O, respectively. It was moderately well vascularized, as indicated by numerous small blood vessels and capillaries. Furthermore, the loosely organized connective tissue especially on the edges of the fat lobules stained for hyaluronan. Hyaluronan is a highly hydrated macromolecule that binds water and increases interstitial fluid viscosity (Lodish et al. 2004). It seems that in elephants adipose tissue and connective tissue rich in hyaluronan complement one another in contributing to the mechanical properties of the cushions, especially in determining the tissue stiffness. Other proteoglycans, indicated by positive staining with safranin O, might contribute to the compression strength of the cushions, as proteoglycans are known to confer gel-like properties on tissues (Lodish et al. 2004). In our specimens, cartilage was not discernible within the cushions, in contrast to the short description of Keet et al. (1997). Furthermore, myxoid tissue, the occurrence of which was described as a possible adaptation to compressive load in the digital cushion of horses (Egerbacher et al. 2005), was absent.
The observations of Benz (2005), who stated that there is much more connective tissue than adipose tissue in young elephants, could not be supported by our findings, which revealed a similar quota of adipose to connective tissue in juvenile and adult individuals. Furthermore, our findings stand in contrast to those in cattle (Räber et al. 2004). In the bovine digital cushion, it is presumed that the decreasing fat content in individuals of greater age is a reaction to increasing load and age (Räber et al. 2004). However, we acknowledge the limitations of our qualitative observations. A more thorough quantitative investigation of adipose tissue volume would be informative.
Vater–Pacinian corpuscles were found in the dermis and in the cushion itself (Fig. 10). These sensory receptor organs are responsive to pressure and especially to vibration (Cutts & Krause, 1983; Leem et al. 1993; Simonetti et al. 1998). As in other animals, including humans (Cauna & Mannan, 1958; Palmieri et al. 2003), in the African elephant the corpuscles are occasionally located in close proximity to blood vessels. In the distal limb parts of elephants [as well as of other animals (for example, see Leydig, 1854; Palmieri et al. 1980; Turnbull & Rasmusson, 1986; Bowker et al. 1993)], Vater–Pacinian corpuscles are likely to serve an important purpose in aiding the animals to negotiate the ground surface. Meissner corpuscles are found particularly in finger/toe pads and oral mucosae of humans, primates, certain rodents and marsupials (Ide, 1977; Tachibana et al. 1991; Halata & Baumann, 1999; Hoffmann et al. 2004). These rapidly adapting mechanoreceptors, which were found in the dermal papillae of the feet of our African elephants, are surprisingly absent in the presumably most sensitive organ of the elephant, the trunk (Hoffmann et al. 2004). Furthermore, these observations together with the detected dense innervation strengthen the hypothesis (Spinage, 1994) that the elephant foot is very sensitive and endowed with a fine sense of touch.
In conclusion, foot cushions in African elephants have a complex structure both macroscopically and microscopically. The cushions themselves form septate internal pads like the gel pads in modern running shoes, yet are more than just shock absorbers. They are part of an integrated system of tissues, including skeletal, cartilaginous, capsular, adipose, collagenous and elastic forms, contained within a tight integumentary sheath that also must influence their mechanical behaviour. The cushion anatomy is well matched to the demands of storing or absorbing mechanical forces when compressed, and distributing locomotor forces over large areas to keep foot tissue stresses within acceptable levels. In addition to the obvious mechanical functions, the cushions are important sensory structures. The high concentration of sensory receptors such as Vater–Pacinian corpuscles within the cushion and Meissner corpuscles in dermal papillae of the adjacent skin might rank an elephant's foot among the most sensitive parts of its body. Together, the mechanical and sensory functions of the feet enhance the ability of elephants effectively to move through and analyse their physical environment.
Acknowledgments
We greatly appreciate the assistance of Professor John T. Soley and Mr Leon de Villiers (Department of Anatomy and Physiology, Faculty of Veterinary Science, Onderstepoort, University of Pretoria, South Africa) and the co-operation provided by Dr Wolfgang Zenker from the Viennese Tiergarten Schönbrunn (Austria). We wish to thank Mag. Eva Polsterer (Vienna, Austria) for excellent assistance with graphics, and Sonja Dolezal, Magdalena Helmreich and Doris Rosenfellner (Institut für Histologie, Veterinärmedizinische Universität Wien, Austria) for strong technical support. Kim Luikart and Susan Stover at the University of California-Davis kindly assisted in arranging the CT and MRI scans done there. We appreciate discussions of this work with Charlotte Miller, Lei Ren and Caitlin O'Connell-Rodwell.
References
- Alexander R McN, Maloiy GMO, Hunter B, Jayes AS, Nturibi J. Mechanical stresses in fast locomotion of buffalo (Syncerus caffer) and elephant (Loxodonta africana) J Zool. 1979;189:135–144. [Google Scholar]
- Alexander R McN, Bennett MB, Ker RF. Mechanical properties and function of the paw pads of some animals. J Zool. 1986;209:405–419. [Google Scholar]
- Benz A. Inaugural Dissertation. Vetsuisse-Fakultät Universität Zürich: 2005. The elephant's hoof: macroscopic and microscopic morphology of defined locations under consideration of pathological changes. [Google Scholar]
- Benz A, Zenker W, Hildebrandt TB, Weissengruber G, Geyer H. About the macroscopic and microscopic morphology of elephants' hooves (Elephantidae) Verh Ber Erkrg Zootiere. 2005;42:167–170. [Google Scholar]
- Blechschmidt E. Die Architektur des Fersenpolsters. Gegenbaurs Morph Jb. 1933;73:20–68. [Google Scholar]
- Bojsen-Møller F, Flagstad KE. Plantar aponeurosis and internal architecture of the ball of the foot. J Anat. 1976;121:599–611. [PMC free article] [PubMed] [Google Scholar]
- Bowker RM, Brewer AM, Vex KB, et al. Sensory receptors in the equine foot. Am J Vet Res. 1993;54:1840–1844. [PubMed] [Google Scholar]
- Cauna N, Mannan G. The structure of human digital Pacinian corpuscles (Corpuscula lamellosa) and its functional significance. J Anat. 1958;92:1–20. [PMC free article] [PubMed] [Google Scholar]
- Cutts JH, Krause WJ. Structure of the paws in Didelphis virginiana. Anat Anz. 1983;154:329–335. [PubMed] [Google Scholar]
- Eales NB. The anatomy of a foetal African elephant, Elephas africanus (Loxodonta africana). Part II. The body muscles. Trans Roy Soc Edinburgh. 1928;55:609–642. [Google Scholar]
- Egerbacher M, Helmreich M, Probst A, König H, Böck P. Digital cushions in horses comprise coarse connective tissue, myxoid tissue, and cartilage but only little unilocular fat tissue. Anat Histol Embryol. 2005;34:112–116. doi: 10.1111/j.1439-0264.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- Fowler ME. Hoof, claw, and nail problems in nondomestic animals. J Am Med Vet Assoc. 1980;177:885–893. [PubMed] [Google Scholar]
- Gage LJ. Treatment of osteomyelitis in elephant feet. In: Csuti B, Sargent EL, Bechert US, editors. The Elephant's Foot. Ames, IA: Iowa State University Press; 2001. pp. 117–118. [Google Scholar]
- Gambaryan PP. How Mammals Run. New York: John Wiley & Sons; 1974. [Google Scholar]
- Güßgen B. Dissertation. Tierärztliche Hochschule Hannover: 1988. Vergleichende Zusammenstellung der Literaturbefunde über die Anatomie des Indischen und Afrikanischen Elefanten als Grundlage für tierärztliches Handeln. [Google Scholar]
- Halata Z, Baumann KI. Sensory nerve endings in the hard palate and papilla incisiva of the rhesus monkey. Anat Embryol. 1999;199:427–437. doi: 10.1007/s004290050241. [DOI] [PubMed] [Google Scholar]
- Hildebrand M, Hurley JP. Energy of the oscillating legs of a fast-moving cheetah, pronghorn, jackrabbit, and elephant. J Morph. 1985;184:23–31. doi: 10.1002/jmor.1051840103. [DOI] [PubMed] [Google Scholar]
- Hittmair KM, Vielgrader HD. Radiographic diagnosis of lameness in African elephants (Loxodonta africana) Vet Radiol Ultrasound. 2000;41:511–515. doi: 10.1111/j.1740-8261.2000.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann JN, Montag AG, Dominy NJ. Meissner corpuscles and the somatosensory acuity: the prehensile appendages of primates and elephants. Anat Rec Part A. 2004;281A:1138–1147. doi: 10.1002/ar.a.20119. [DOI] [PubMed] [Google Scholar]
- Howell AB. Speed in Animals: Their Specialization for Running and Leaping. Chicago: University of Chicago Press; 1944. [Google Scholar]
- Hutchinson JR, Famini D, Lair R, Kram R. Are fast-moving elephants really running? Nature. 2003;422:493–494. doi: 10.1038/422493a. [DOI] [PubMed] [Google Scholar]
- Hutchinson JR, Schwerda D, Famini DJ, Dale R, Fischer M, Kram R. The locomotor kinematics of Asian and African elephants: changes with speed and size. J Exp Biol. doi: 10.1242/jeb.02443. in press. [DOI] [PubMed] [Google Scholar]
- Ide C. Development of Meissner corpuscle of mouse toe pad. Anat Rec. 1977;188:49–67. doi: 10.1002/ar.1091880107. [DOI] [PubMed] [Google Scholar]
- Jahss MH, Michelson JD, Desai P, et al. Investigations into the fat pads of the sole of the foot: anatomy and histology. Foot Ankle. 1992;13:233–242. doi: 10.1177/107110079201300502. [DOI] [PubMed] [Google Scholar]
- Keet DF, Grobler DG, Raath JP, Gouws J, Carstens J, Nesbit JW. Ulcerative pododermatitis in free-ranging African elephant (Loxodonta africana) in the Kruger National Park. Onderstepoort J Vet Res. 1997;64:25–32. [PubMed] [Google Scholar]
- Ker RF. The design of soft collagenous load-bearing tissues. J Exp Biol. 1999;202:3315–3324. doi: 10.1242/jeb.202.23.3315. [DOI] [PubMed] [Google Scholar]
- Kimani JK. The structural and functional organization of the connective tissue in the human foot with reference to the histomorphology of the elastic fibre system. Acta Morph Neerl-Scand. 1984;22:313–323. [PubMed] [Google Scholar]
- König HE, Macher R, Polsterer-Heindl E, et al. Stoßbrechende Einrichtungen am Zehenendorgan des Pferdes. Vet Med Austria. 2003;90:267–273. [Google Scholar]
- Köther H, Bürger M. Verh Ber 9. Prag: International Symp Erkr Zootiere; 1967. Ein Beitrag zur Behandlung eines Fußleidens beim indischen Elefanten; pp. 249–251. [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Leydig F. Über die Vater-Pacinischen Körperchen der Taube. Z Wissenschaftliche Zool. 1854;5:75–86. [Google Scholar]
- Liebich H-G. Funktionelle Histologie der Haussäugetiere. 3. Stuttgart: Schattauer; 1999. [Google Scholar]
- Lillie RD. Histopathologic Technic and Practical Histochemistry. New York: Blakiston Division, McGraw–Hill; 1954. [Google Scholar]
- Lodish H, Berk A, Matsudaira P, et al. Molecular Cell Biology. 5. New York: W.H. Freeman; 2004. [Google Scholar]
- Mariappa D. The anatomy of the foetal Indian elephant. Part II. The muscles, nerves and blood vessels of the fore-limb. Indian Vet J. 1955;32:170–202. [Google Scholar]
- Mariappa D. Anatomy and Histology of the Indian Elephant. Oak Park, MI: Indira; 1986. [Google Scholar]
- Miall LC, Greenwood F. The anatomy of the Indian elephant. Part I. The muscles of the extremities. J Anat Physiol. 1878;12:261–287. [PMC free article] [PubMed] [Google Scholar]
- Miller-Young JE, Duncan NE, Baroud G. Material properties of the human calcaneal fat pad in compression: experiment and theory. J Biomech. 2002;35:1523–1531. doi: 10.1016/s0021-9290(02)00090-8. [DOI] [PubMed] [Google Scholar]
- Muybridge E. Animals in Motion. New York: Dover; 1899. [Google Scholar]
- Nauck ET. Extremitätenskelett der Tetrapoden. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der Vergleichenden Anatomie der Wirbeltiere, Fünfter Band. Berlin and Vienna: Urban & Schwarzenberg; 1938. pp. 71–248. [Google Scholar]
- NAV. Nomina Anatomica Veterinaria. 5. World Association of Veterinary Anatomists; 2005. http://www.wava-amav.org/Downloads/nav_2005.pdf. [Google Scholar]
- Neuville H. Note préliminaire sur l’organisation du pied des éléphants. Bull Mus Natl d'Histoire Naturelle. 1927;33:60–64. [Google Scholar]
- Neuville H. Sur quelques caractères anatomiques du pied des éléphants. Arch Mus Natl d'Histoire Naturelle, Paris, 6e Série. 1935;13:111–183. [Google Scholar]
- Palmieri G, Asole A, Panu R. Sul contingente nervoso sensitivo dei cuscinetti digitali del suino. Arch Ital Anat Embriol. 1980;85:211–219. [PubMed] [Google Scholar]
- Palmieri G, Sanna M, Minelli LB, et al. On the sensitive innervation of the ostrich's foot pads. Ital J Anat Embryol. 2003;108:25–37. [PubMed] [Google Scholar]
- Räber M, Lischer CJ, Geyer H, Ossent P. The bovine digital cushion – a descriptive anatomical study. Vet J. 2004;167:258–264. doi: 10.1016/S1090-0233(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Ramsay EC, Henry RW. Anatomy of the elephant foot. In: Csuti B, Sargent EL, Bechert US, editors. The Elephant's Foot. Ames, IA: Iowa State University Press; 2001. pp. 9–12. [Google Scholar]
- Rome K. Mechanical properties of the heel pad: current theory and review of the literature. Foot. 1998;8:179–185. [Google Scholar]
- Romeis B. Mikroskopische Technik. 17. München und Wien: Urban & Schwarzenberg; 1989. [Google Scholar]
- Ruthe H. Fußleiden der Elefanten. Wiss Z Humboldt-University Berlin, Math-Nat R. 1961;10:471–516. [Google Scholar]
- Schaller O. Illustrated Veterinary Anatomical Nomenclature. Stuttgart: Ferdinand Enke; 1992. [Google Scholar]
- Simonetti S, Dahl K, Krarup C. Different indentation velocities activate different populations of mechanoreceptors in humans. Muscle Nerve. 1998;21:858–868. doi: 10.1002/(sici)1097-4598(199807)21:7<858::aid-mus3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Smuts MMS, Bezuidenhout AJ. Osteology of the thoracic limb of the African elephant (Loxodonta africana) Onderstepoort J Vet Res. 1993;60:1–14. [PubMed] [Google Scholar]
- Smuts MMS, Bezuidenhout AJ. Osteology of the pelvic limb of the African elephant (Loxodonta africana) Onderstepoort J Vet Res. 1994;61:51–66. [PubMed] [Google Scholar]
- Spinage C. Elephants. London: Poyser; 1994. [Google Scholar]
- Tachibana T, Fujiwara N, Nawa T. Mechanoreceptors of the hard palate of the Mongolian gerbil include special junctions between epithelia and Meissner lamellar cells: a comparison with other rodents. Anat Rec. 1991;231:396–403. doi: 10.1002/ar.1092310313. [DOI] [PubMed] [Google Scholar]
- Taylor DD, Hood DM, Potter GD, Hogan HA, Honnas CM. Evaluation of displacement of the digital cushion in response to vertical loading in equine forelimbs. Am J Vet Res. 2005;66:623–629. doi: 10.2460/ajvr.2005.66.623. [DOI] [PubMed] [Google Scholar]
- Tietze A. Ueber den architektonischen Aufbau des Bindegewebes in der menschlichen Fußsohle. Bruns' Beiträge Zur Klinischen Chirurgie. 1921;123:493–506. [Google Scholar]
- Tong J, Lim CS, Goh OL. Technique to study the biomechanical properties of the human calcaneal heel pad. Foot. 2003;13:83–91. [Google Scholar]
- Turnbull BG, Rasmusson DD. Sensory innervation of the racoon forepaw: 1. Receptor types in glabrous and hairy skin and deep tissue. Somatosensory Res. 1986;4:43–62. doi: 10.3109/07367228609144597. [DOI] [PubMed] [Google Scholar]
- Virchow H. Hand und Fuss des Elefanten, nach Form zusammengesetzt. Sitzungsberichte der Gesellschaft der Naturforschenden Freunde Berlin. 1910. pp. 77–87.
- Weissengruber GE, Forstenpointner G. Musculature of the crus and pes of the African elephant (Loxodonta africana): insight into semiplantigrade limb architecture. Anat Embryol. 2004a;208:451–461. doi: 10.1007/s00429-004-0406-1. [DOI] [PubMed] [Google Scholar]
- Weissengruber GE, Forstenpointner G. Shock absorbers and more: design principles of the lower hindlimb in African elephants (Loxodonta africana) J Morph. 2004b;260:339. [Google Scholar]
- Weissengruber GE, Fuss FK, Egger G, Stanek G, Hittmair KM, Forstenpointner G. The elephant knee joint: morphological and biomechanical considerations. J Anat. 2006;208:59–72. doi: 10.1111/j.1469-7580.2006.00508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]