Abstract
The functional anatomy of the pelvic limb of the ostrich (Struthio camelus) was investigated in order to assess musculoskeletal specialization related to locomotor performance. The pelvic limbs of ten ostriches were dissected and detailed measurements of all muscle tendon units of the pelvic limb were made, including muscle mass, muscle length, fascicle length, pennation angle, tendon mass and tendon length. From these measurements other muscle properties such as muscle volume, physiological cross-sectional area (PCSA), tendon cross-sectional area, maximum isometric muscle force and tendon stress were derived, using standard relationships and published muscle data. Larger muscles tended to be located more proximally and had longer fascicle lengths and lower pennation angles. This led to an expected proximal to distal reduction in total muscle mass. An exception to this trend was the gastrocnemius muscle, which was found to have the largest volume and PCSA and also had the highest capacity for both force and power production. Generally high-power muscles were located more proximally in the limb, while some small distal muscles (tibialis cranialis and flexor perforatus digiti III), with short fibres, were found to have very high force generation capacities. The greatest proportion of pelvic muscle volume was for the hip extensors, while the highest capacity for force generation was observed in the extensors of the ankle, many of which were also in series with long tendons and thus were functionally suited to elastic energy storage.
Keywords: architecture, biomechanics, locomotion, muscle, ostrich, tendon
Introduction
The ostrich (Struthio camelus) is the largest living avian biped and it couples this size with ability for high-speed locomotion. Ostriches have features of cursorial animals that mean they are effective locomotors. They travel long distances and are also able to run fast to escape predation. Maximum running speed in this species has not yet been accurately measured. However, speeds of 12 and 17 m s−1 have been estimated from video footage during periods of sustained running (Alexander et al. 1979). This capacity for high-speed locomotion requires specific musculoskeletal design. For example, the ostrich has a reduced wing skeleton and musculature in the thoracic limb, and combines this with a compact body, small head and neck, and strong legs and feet. The ostrich also has long, slender, lightweight pelvic limb bones and well-developed pelvic limb muscles, with large muscles in the proximal limb and long tendons, coupled with a lever system to stretch these with extension of the digit, in the distal limb. Comparisons can be made with other species that exhibit high running speeds, and similar trends in limb design have been observed in other animals specialized for high-speed locomotion, such as the horse (Brown et al. 2003; Payne et al. 2005a) and the greyhound (Pasi & Carrier, 2003).
The locomotor musculature of the ostrich plays an important role in optimization of running performance. Muscles are required to do work on the system, by creating muscle force and hence generating moments about the joints, in order to move the limb segments and support the centre of mass. Muscle architecture is usually designed to achieve this in the most metabolically efficient way, ensuring sustainable efficient locomotion. Passive mechanisms, such as using the muscle tendon units (MTUs) as energy-storing springs, increase locomotor efficiency, reducing the requirement for muscle mass and hence resulting in a lightened limb, which requires less energy during the swing phase of a stride (Biewener, 1998). However, there is inevitably a limit beyond which greater elasticity is not desirable and a limb that is too passive may have reduced position control (Alexander, 2002).
The anatomy of the ostrich has been reported on in several different studies. In the 19th century these consisted of general description of the musculoskeletal system and internal organs (Langer, 1859; Haughton, 1865; MacAlister, 1865; Garrod & Darwin, 1872; Gadow, 1880). More recently, scientists have aimed to clarify the descriptive anatomy of the ostrich in order to follow modern nomenclatural rules (Mellet, 1985; Mellet, 1994; Pavaux & Lignereux, 1995; Fuss, 1996; Liswinaso, 1996; Gangl et al. 2004). These studies have primarily focused on the pelvic limb. In spite of the above, there is surprisingly little data available on muscle architecture or muscle function in this highly specialized species of bird. Some attempts have been made to quantify muscle mass or architecture (Alexander et al. 1979; Mellet, 1994; Hutchinson, 2004) but these studies are limited to selected muscle groups and do not present detailed information on muscle dimensions or other properties. In other athletic animals, such as the horse (Brown et al. 2003; Payne et al. 2005a,b), detailed musculoskeletal geometry and muscle architecture has been described enabling more accurate modelling of the limb. This study aims to provide detailed information on the architecture of the MTUs of the pelvic limb of the ostrich, and to compare these data with similar data for the pelvic limb of a quadrupedal runner, the horse.
Methods
Eleven pelvic limbs from ten juvenile African black ostriches (Struthio camelus var. domesticus), seven male and three female, were used in this study (Table 1). Ostriches ranged in age from 22 to 38 weeks (53–105 kg) and were estimated to be between 40 and 90% of expected adult mass (expected female adult mass is between 110 and 120 kg, and male adult mass is between 120 and 140 kg). All had been raised from chicks with identical feeding and regular exercise as a result of experimental protocols. Ostriches had been kept in a group in a large paddock to allow natural behaviour and free exercise. Limbs were dissected soon after the birds were killed and always within 24 h of death. During any interim period between death of the animal and dissection, birds were stored in a refrigerated room at 4 °C, ensuring effects of the onset of rigor mortis were small. A single pelvic limb was dissected in all but one of the ostriches (number 366, both legs were dissected in this bird).
Table 1.
Subjects
| Ostrich no. | Sex (M/F) | Age (weeks) | Mass (kg) | Height (m) | Leg (L/R) |
|---|---|---|---|---|---|
| 366 | M | 24 | 60 | 1.15 | R & L |
| 372 | M | 24 | 53 | 1.07 | L |
| 373 | M | 24 | 55 | 1.08 | R |
| 371 | M | 25 | 73 | 1.22 | R |
| 365 | M | 26 | 69 | 1.17 | R |
| 357 | M | 27 | 55 | 1.10 | R |
| 378 | F | 28 | 75 | 1.23 | R |
| 363 | F | 32 | 86 | 1.27 | R |
| 369 | M | 39 | 80 | 1.26 | R |
| 367 | F | 43 | 105 | 1.39 | R |
Each limb was skinned and superficio-lateral muscles of the proximal limb were removed first, followed by deep proximal and then deep distal muscles. Each muscle was identified and removed, including any tendons of origin or insertion. Muscle belly length (to the nearest millimetre) was measured as the length between the proximal ends of the most proximal and distal ends of the most distal fibres of the muscle, using a ruler. Any external tendon was then removed from the muscle and the slack length of the tendon (to the nearest millimetre) was measured with a tape measure. Tendon mass was then measured, to the nearest 0.1 g, using electronic scales (EKS®, UK). Fascicle length of the released muscle was measured by cutting the muscle to reveal the orientation of the fibres and bisecting the muscle belly longitudinally along the plane of the fibres, separating the muscle, and measuring the length of fascicles from at least ten random sites within the muscle belly, at different depths between the origin and insertion. Where no internal tendon was evident, successive incisions were carefully made until the plane of muscle fascicles was exposed, enabling measurements to be taken as described above. For several smaller muscles, fascicles could not be clearly defined and were therefore not measured. No fascicle measurements were made on muscles of the second limb of ostrich number 366. Pennation angle of the fibres was determined using a protractor to measure the angle between the fascicles and internal tendon (or aponeurosis where no tendon was present). For muscles with multiple bellies, fascicle lengths and pennation angles were measured individually for each muscle belly. Total pelvic muscle mass was calculated by adding the masses of all MTUs in the pelvic limb and multiplying by two, to account for both limbs.
Muscle volume was calculated by dividing muscle mass by muscle density (1.06 g cm−3; Mendez & Keys, 1960; Brown et al. 2003) and physiological cross-sectional area (PCSA) was then determined by dividing muscle volume by mean fascicle length. Architectural index [expressing the relative fibre lengths of muscles, which is proportional to the velocity of contraction (Sharir et al. 2006), with respect to series elastic element length and hence relating passive elasticity to active length change] was calculated by dividing fibre length by muscle length. Mean tendon cross-sectional area was estimated by calculating tendon volume [tendon mass/density (1.12 g cm−3; Ker et al. 1988)], and dividing this by tendon length. Muscle maximum isometric force, Fmax, was estimated by multiplying PCSA by maximum isometric stress of vertebrate skeletal muscle (0.3 MPa; Wells, 1965; Woledge et al. 1985; Zajac, 1989; Medler, 2002). Tendon stress at maximum isometric force was then calculated by dividing muscle Fmax by tendon cross-sectional area, and an estimate of strain (%) at this stress was determined by dividing this by Young's modulus for tendon (1.5 GPa; Bennett et al. 1986; Bennett & Stafford, 1988). Power output was estimated to be proportional to muscle volume (this assumes similar stress/length-specific velocity relationships for all muscles, which is a reasonable approximation if fibre type distribution is similar in all muscles). Such power estimates were used to group and compare muscles with relatively high force or relatively high power, and to allow comparison with trends previously described in the horse, rather than to establish quantifiable power outputs, as contraction velocities of the muscles measured were unknown.
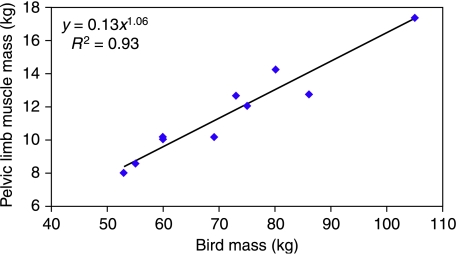
Due to age-related variation in size and mass of the ostriches, measured data were scaled to that of the largest ostrich (number 367), as this bird was almost at adult mass and height. Mass-related measurements were scaled in relation to body mass, as pelvic limb mass was found to scale, as expected, with body mass, M (∝ M1.06, Fig. 1). Therefore, in order to compare relative values, masses were divided by body mass (to give muscle mass per kg of body mass) and multiplied by the mass of the largest bird (105 kg). Length measurements were scaled in a similar way in relation to tibiotarsus length (∝M0.38, where M is body mass; N. C. Smith et al. in preparation).
Fig. 1.
Scaling of pelvic limb mass was found to be directly proportional to body mass, M1.06 (n = 10).
Results
Pelvic limb muscle of the ostrich
Total pelvic limb muscle mass [considering both limbs, and assuming symmetry (see below)] of the ostrich was found to account for 33.7 ± 2.1% (mean ± SD) of body mass, corresponding to a mean pelvic limb muscle mass of 16.97 ± 1.08 kg for a 105-kg ostrich. Symmetrical distribution of muscle mass between both pelvic limbs was confirmed in ostrich number 366 where masses of all muscles from both limbs were obtained, such that the difference in total mass of muscle between limbs was less than 0.2% of total muscle mass (left leg, M = 18.41 kg; right leg, M = 18.44 kg).
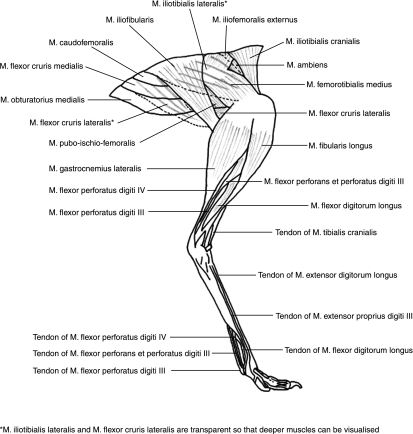
All 36 previously recognized muscles were identified in the pelvic limb of the ostrich, with origins and insertions generally agreeing with published data (Gangl et al. 2004), although some minor variations were observed between birds. Descriptions of individual muscles, and abbreviations used in the text below, are given in Table 2, with origins and insertions based both on observed and on published data (Mellet, 1985; Gangl et al. 2004). A diagram of the superficial muscles of the pelvic limb, shown on a lateral view of a limb in mid-stance posture, is shown in Fig. 2. Included in Table 2 are estimates of muscle action, based on anatomical positioning. Functions of the individual muscles of the pelvic limb of the ostrich have not, as far as we are aware, been previously published, and are therefore based on our personal observations. Detailed discussion of the specific actions of muscles and effective moment arms at the joints will be provided in a separate publication (N. C. Smith et al. in preparation).
Table 2.
Pelvic limb muscles of the ostrich
| Muscle | Abbreviation | Origin | Insertion | Action |
|---|---|---|---|---|
| M. iliotibialis cranialis | ITCr | Cranial aspect of ilium | Via aponeurosis on medial surface of proximal tibiotarsus | Hip flexion, knee extension |
| M. iliotrochantericus caudalis | ITrCa | Acetabular ilium | Femoral trochanter | Hip flexion, abduction, internal rotation |
| M. iliotrochantericus cranialis | ITrCr | Lateral preacetabular ilium | Femoral trochanter | Hip flexion, abduction, internal rotation |
| M. iliofemoralis externus | IFE | Dorsal acetabular ilium | Femoral trochanter | Hip flexion and abduction |
| M. iliotibialis lateralis | ITL | Postacetabular ilium | Via aponeurosis on lateral surface of knee | Hip extension and abduction |
| M. flexor cruris lateralis | FCL | Caudal ilium | Medial distal femur | Hip extension and adduction |
| M. flexor cruris medialis | FCM | Caudal ilium | Medial aspect of tendofascial sheet of stifle | Hip extension and adduction (knee flexion) |
| M. caudofemoralis | CFem | Postacetabular ilium and caudal vertebrae | Proximal caudal femur | Hip extension |
| M. ischiofemoralis | IsFem | Lateral ischium | Proximal caudal femur | Hip extension |
| M. pubo-ischio-femoralis | PIF | Ischium and cranial pubis | Caudal femur, medial to GastC | Hip extension, adduction |
| M. obturatorius lateralis | OL | Medial ischium and pubis | Medial femoral trochanter | Hip extension and adduction |
| M. obturatorius medialis | OM | Ventral ischium and lateral pubis | Lateral proximal femoral trochanter | Hip extension, adduction, rotation |
| M. pectineus | Pect | Lateral surface of pubis | Medial aspect of proximal tibiotarsus, along with ITCr and FTI | Hip adduction |
| M. iliofemoralis internus | IFI | Lateral preacetabular ilium, between ITrCa and ITrCr | Proximal medial aspect of femur | Hip adduction and stabilizer |
| M. iliofibularis | IFib | Postacetabluar ilium, ventral to ITL | Lateral proximal fibula | Knee flexion and hip extension |
| M. femorotibialis externus | FTE | Lateral surface of femur | Proximal tibiotarsus | Knee extension |
| M. femorotibialis medius | FTM | Lateral femoral trochanter | Proximal tiobiotarsus via tendofascial sheet of stifle | Knee extension |
| M. femorotibialis accessorius | FTA | Cranial femur | Proximal tiobiotarsus via tendofascial sheet of stifle | Knee extension |
| M. ambiens | Amb | Lateral surface of ilium, deep to ITCr | Fuses with tendon of origin of FPDIII | Knee extension and hip flexion |
| M. femorotibialis internus | FTI | Medial shaft of femur | Medial aspect of proximal tibiotarsus, along with Pect and ITCr | Knee adduction |
| M. tibialis cranialis | TCr | Lateral condyle of femur and medial tiobiotarsus | Distal dorsal tarsometatarsus | Ankle flexion |
| M. gastrocnemius – medialis | GastM | Medial tendofascial sheet of knee and proximal medial tibiotarsus | Plantar distal tarsometatarsus | Ankle extension and knee flexion |
| – caudalis | GastC | Caudal surface of femur Aponeurosis of ITL and | ||
| – lateralis | GastL | lateral femoral condyle | ||
| M. fibularis longus | FibL | Lateral tendofascial sheet of the knee and proximal fibula | Proximal tarsometatarsus | Ankle extension |
| M. fibularis brevis | FibB | Tendinous. Tibiotarsus, distal to fibula | Proximal lateral tarsometatarsus | Ligament function |
| M. flexor hallucis longus | FHL | Distal femur | Tendon of FDL | Ankle extension Knee extension |
| M. popliteus | Pop | Proximal caudal tibiotarsus | Proximal caudal tibiotarsus | Fibular pronation |
| M. flexor perforans et perforatus digiti III | FPetPIII | Proximal tibiotarsus and medial surface of tendon of FTE and tendon of origin of FPDIII | Middle IP joint of digit III | Digit III flexion (Ankle extension) |
| M. flexor perforatus digiti III | FPDIII | Via tendon on lateral condyle of femur, lateral collateral ligament and proximal caudal tibiotarsus | First phalanx and proximal IP joint of digit III | Digit III flexion (Ankle extension) |
| M. flexor perforatus digiti IV | FPDIV | On tendon of origin of lateral head of FPDIII | 1st phalanx and proximal and intermediate IP joints of digit IV | Digit IV flexion (Ankle extension) |
| M. flexor digitorum longus | FDL | Proximal caudal tibiotarsus | 4th phalanx of digit III and 5th phalanx of digit IV | Digits III and IV flexion (ankle extension) |
| M. extensor digitorum longus | EDL | Cranial proximal tibiotarsus | Proximal IP joint, 2nd and 3rd phalanges of digit III, proximal IP joint, 4th and 5th phalanges of digit IV. | Digits III and IV extension, ankle flexion |
| M. extensor proprius digiti III | EPDIII | Fascia of intertarsal joint | 4th phalanx of digit III | Digit III extension |
| M. extensor brevis digiti III | EBDIII | Dorsal distal tarsometatarsus | 1st phalanx of digit III | Digit III extension |
| M. extensor brevis digiti IV | EBDIV | Dorsal tarsometatarsus proximal to EBDIII | 1st phalanx of digit IV | Digit IV extension |
| M. abductor digiti IV | ADIV | Proximal plantar surface of tarsometatarsus | 1st phalanx of digit IV | Digit IV abduction |
| M. lumbricalis | Lumb | On distal tendon of FDL | MTP joints of digits III and IV | Accessory to FDL |
Origins and insertions based on observed and published data (Mellet, 1985; Gangl et al. 2004). Primary muscle actions are listed based on our anatomical observations.
Fig. 2.
Lateral view of the anatomy of the pelvic limb of the ostrich.
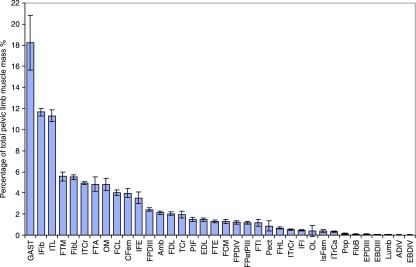
Mean values for muscle data, scaled as previously described, are provided in Table 3, and the percentage of total pelvic muscle mass (including tendons) of each MTU is shown in Fig. 3. The largest muscle in the pelvic limb was the ankle extensor gastrocnemius, which on average accounted for 18% of the total muscle of the pelvic limb. This muscle could be separated into three distinct heads, medial, lateral and caudal, decreasing in size in that order. The heads fused in the mid to distal region of the tibiotarsus and inserted onto a large, wide tendon that passed caudally over the ankle and accounted for some 20% of the total tendon mass in the limb. With the exception of gastrocnemius the usual trend was for larger muscles to be located more proximally in the limb, above the knee joint. Specifically, there was almost twice as much muscle mass located proximal to the knee than distal to it (65 and 35%, respectively). Of the proximal muscles, iliotibialis lateralis and iliofibularis both had a mean mass of approximately 2 kg (± 0.3 kg), each accounting for a further 11% of total pelvic limb muscle mass. The femorotibial group, consisting of femorotibialis medius, femorotibialis externus, femorotibialis internus and femorotibialis accessorius, also together accounted for a large proportion of total pelvic limb muscle mass (12.5%), with FTM and FTA being responsible for the bulk of this. After gastrocnemius, fibularis longus was the largest distal limb muscle (5.5% total muscle mass), providing the cranial contour of the shank. The digital flexors combined accounted for a further 7.4% and the tendons of the digital flexors accounted for most of the MTUs lying distal to the ankle joint. Although the small muscles originating below the ankle joint were identified and measured, they were found to account for less than 0.3% of total muscle mass, with a mean combined mass of 36 g (± 16 g).
Table 3.
Normalized mean muscle data
| Muscle | Muscle mass (g)* | Volume (cm3)* | Length (mm)* | Mean fascicle length (mm)† | PCSA (cm2)† | Pennation angle (°)† | Force (N)† |
|---|---|---|---|---|---|---|---|
| Iliotibialis cranialis | 872 (775–961) | 822 | 333 | 273 (237–309) | 30 | 0 | 900 |
| Iliotibialis lateralis | 1997 (1720–2235) | 1884 | 426 | 122 (83–186) | 158 | 37 | 4740 |
| Iliofibularis | 2067 (1866–2317) | 1950 | 367 | 257 (229–302) | 76 | 0 | 2280 |
| Ambiens | 367 (311–394) | 346 | 174 | 47 (24–71) | 80 | 41 | 2420 |
| Pectineus | 153 (27–338) | 145 | 191 | 53 (19–77) | 23 | 35 | 700 |
| Iliotrochantericus caudalis | 54 (39–77) | 51 | 124 | 45 (30–59) | 12 | 28 | 350 |
| Iliotrochantericus cranialis | 90 (79–100) | 85 | 150 | 63 (54–80) | 14 | 27 | 410 |
| Iliofemoralis internus | 75 (56–94) | 71 | 135 | 53 (42–64)‡ | 14‡ | 29‡ | 430‡ |
| Iliofemoralis externus | 619 (489–789) | 584 | 197 | 56 (41–67) | 107 | 37 | 3230 |
| Femorotibialis externus | 219 (190–253) | 207 | 227 | 49 (30–67) | 44 | 33 | 1330 |
| Femorotibialis medius | 944 (815–1082) | 891 | 221 | 98 (76–129) | 93 | 41 | 2800 |
| Femorotibialis internus | 207 (128–365) | 196 | 210 | 47 (16–63)§ | 49§ | 33§ | 1470§ |
| Femorotibialis accessorius | 755 (616–841) | 713 | 215 | 71 (37–97)‡ | 108‡ | 4‡ | 3230‡ |
| Flexor cruris lateralis | 714 (653–818) | 674 | 378 | 222 (117–295) | 33 | 0 | 980 |
| Flexor cruris medialis | 221 (152–263) | 208 | 217 | 34 (22–50) | 63 | 42 | 1880 |
| Caudofemoralis | 705 (590–818) | 665 | 297 | 127 (107–162) | 54 | 25 | 1630 |
| Ischiofemoralis | 74 (56–105)† | 69† | 174† | 29 (19–42) | 22 | 35 | 750 |
| Obturatorius lateralis | 82 (15–298)‡ | 77‡ | 134‡ | 56 (37–84)** | 15 | 17 | 450 |
| Obturatorius medialis | 846 (606–958) | 798 | 444 | 69 (56–87) | 117 | 32 | 3500 |
| Pubo-ischio-femoralis | 261 (215–314) | 247 | 288 | 73 (57–124) | 36 | 30 | 1080 |
| Tibialis cranialis | 312 (187–369) | 295 | 432 | 13 (7–20) | 255 | 36 | 7670 |
| Extensor digitorum longus | 218 (192–257) | 206 | 341 | 71 (36–92) | 31 | 30 | 930 |
| Fibularis longus | 940 (816–1029) | 887 | 347 | 63 (52–73) | 140 | 34 | 4200 |
| Fibularis brevis | – | – | – | – | – | – | – |
| Gastrocnemius – medial | 1553 (1143–1838) | 1465 | 410 | 107 (65–137) | 141 | 31 | 4240 |
| – lateral | 1051 (456–1400) | 992 | 424 | 92 (63–122) | 113 | 32 | 3380 |
| – caudal | 471 (268–570) | 445 | 313 | 100 (72–119) | 44 | 39 | 1330 |
| (Total) | (3076) (1867–3655) | (2902) | (298) | (8950) | |||
| Popliteus | 29 (15–38) | 27 | 82 | 29 (13–51)¶ | 1¶ | – | 330¶ |
| Flexor perforans et perforatus digiti III | 163 (131–196) | 154 | 248 | 25 (15–35) | 65 | 41 | 1960 |
| Flexor perforatus digiti III | 322 (257–361) | 304 | 399 | 15 (12–18) | 207 | 36 | 6210 |
| Flexor perforatus digiti IV | 169 (130–223) | 159 | 289 | 27 (18–35) | 62 | 37 | 1860 |
| Flexor hallucis longus | 104 (82–147) | 98 | 286 | 44 (35–64)‡ | 22‡ | 28‡ | 670‡ |
| Flexor digitorum longus | 289 (248–347) | 272 | 333 | 42 (26–54)‡ | 65‡ | 28‡ | 1960‡ |
| Extensor proprius digiti III | 7 (4–30)† | 6† | 112† | 27 (10–38)¶ | 2¶ | 15¶ | 60¶ |
| Extensor brevis digiti III | 7 (4–10)‡ | 6† | 102† | 40 (19–63)¶ | 1¶ | 25¶ | 40¶ |
| Extensor brevis digiti IV | 4 (2–7)‡ | 4‡ | 102‡ | – | – | – | – |
| Abductor digiti IV | 4 (2–8)† | 4‡ | 246† | – | – | – | – |
| Lumbricalis | 7 (5–11) | 6 | 62 | 46 (39–57)** | 1** | – | – |
| Total | 16 972 (15 527–18 725) |
In some muscles, fascicle measurements were not possible due to limits of size and/or poor fascicle definition.
n = 11, unless otherwise indicated
n = 10
n = 9
n = 8
n = 7
n = 6
Fig. 3.
Mean MTU mass (± SD) as a proportion of total pelvic limb muscle mass for all muscles of the pelvic limb of the ostrich (n = 11).
Pelvic limb tendons
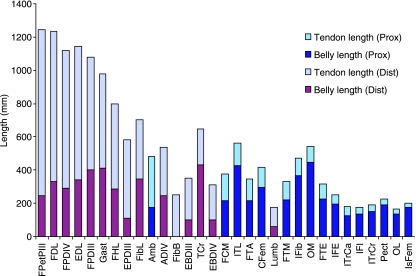
Of the total muscle mass of the pelvic limb, 4.6% was accounted for by free tendon. The proportional lengths of muscle belly and tendon lengths within MTUs, for those muscles where tendons are present, are shown in Fig. 4. Raw data on the mass and slack length of significant tendons (i.e. tendon mass is > 5 g) are given in Table 4. The digital flexor muscles had the longest tendons, averaging about 900 mm in length. These tendons were long and slender with cross-sectional areas of less than 1.5 cm2. The heaviest tendon in the distal limb was that of gastrocnemius (176 ± 28 g), while in the proximal limb it was the wide, flat tendon of insertion of FTM, FTE and FTA (170 ± 32 g), which covered the knee and contained the patella. Many of the proximal muscles had no discernible tendon and of those that did the tendons tended to be relatively short and light. The only proximal limb muscle with a tendon length exceeding muscle length was ambiens, where the long tendon passed through the tendinofascial sheet of the knee to insert onto the origin of flexor perforatus digiti III. Ambiens and FPDIII therefore provide a direct musculotendinous link between the digits and the ilium.
Fig. 4.
MTU length measurements, showing proportion of tendon length to muscle belly length for both proximal (Prox) and distal (Dist) pelvic limb muscles of the ostrich (n = 11).
Table 4.
Mean measured data for significant (mass > 5 g) tendons of the pelvic limb
| Muscle tendon unit | Mass (g) | Volume (cm3) | Length (mm) | CSA (cm2) | Stress (MPa) | Strain (%) |
|---|---|---|---|---|---|---|
| Gastrocnemius | 176 | 157 | 571 | 2.75 | 15.4 | 1.0 |
| Femorotibialis accessorius | 137 | 122 | 132 | 9.24 | 3.5 | 0.2 |
| Flexor perforatus digiti III | 108 | 96 | 680 | 1.48 | 42.0 | 2.8 |
| Flexor digitorum longus | 71 | 64 | 903 | 0.71 | 37.2 | 2.5 |
| Femorotibialis medius | 53 | 47 | 109 | 3.63 | 7.7 | 0.5 |
| Flexor perforans et perforatus digiti III | 49 | 44 | 996 | 0.44 | 44.3 | 3.0 |
| Flexor perforatus digiti IV | 48 | 43 | 830 | 0.53 | 34.8 | 2.3 |
| Extensor digitorum longus | 43 | 38 | 801 | 0.48 | 19.3 | 1.3 |
| Fibularis longus | 37 | 33 | 354 | 0.98 | 42.7 | 2.8 |
| Tibialis cranialis | 36 | 33 | 214 | 1.63 | 47.0 | 3.1 |
| Iliotibialis lateralis | 16 | 15 | 137 | 1.08 | 43.8 | 2.9 |
| Flexor hallucis longus | 16 | 14 | 512 | 0.32 | 21.0 | 1.4 |
| Femortibialis externus | 13 | 11 | 91 | 1.34 | 10.0 | 0.7 |
| Fibularis brevis | 12 | 11 | 250 | 0.52 | 0.0 | 0.0 |
| Ambiens | 12 | 11 | 309 | 0.34 | 70.5 | 4.7 |
| Flexor cruris medialis | 10 | 9 | 161 | 0.58 | 32.6 | 2.2 |
| Iliofemoralis externus | 10 | 9 | 55 | 1.86 | 17.4 | 1.2 |
| Obturatorius medius | 6 | 5 | 95 | 0.56 | 62.2 | 4.2 |
| Caudofemoralis | 5 | 5 | 117 | 0.48 | 33.9 | 2.3 |
| Iliofibularis | 5 | 4 | 106 | 0.43 | 53.5 | 3.6 |
Muscle architecture
For specific muscles scaled fibre length varied both between birds and within the muscle belly. Variations within the muscle were accounted for by multiple measurements at different locations in each muscle, and the range of scaled mean fascicle length between birds is shown in Table 3. The longest muscle fascicles were found in the more proximal limb muscles. Iliotibialis cranialis, iliofibularis and flexor cruris lateralis had the longest mean fascicle lengths (27, 25 and 22 cm, respectively), lying parallel to the tendon or aponeurosis. All other muscles showed some degree of pennation. Individual values of pennation angle varied from 0 to 55°, with the majority of distal limb muscles showing an angle greater than 30°. A highly pennate fascicular arrangement was also observed in several of the proximal muscles such as flexor cruris medialis (mean pennation angle of 42°), femorotibialis medius, femorotibialis accessorius and ambiens (all had a mean pennation angle of 41°). The calculation of architectural index (AI = fascicle length/muscle length) enabled comparison of relative fibre length between muscles. The highest AI values were those of the aforementioned parallel fibred ITC, IFib and FCL (0.8, 0.7 and 0.6, respectively) and proximal limb muscles tended to have higher index values than distal muscles. Distally the greatest AI was observed in the heads of gastrocnemius (AI values: caudal 0.32, medial 0.26, lateral 0.22), with the shortest relative fascicle lengths observed in the pennate digital flexors (AI values ranged from 0.04 to 0.12) and the ankle flexor, tibialis cranialis (AI of 0.03). Owing to relatively short fascicles, maximum PCSA was also observed in these distal muscles, being greatest in tibialis cranialis (255 cm2) and flexor perforatus digiti III (207 cm2) and also large in medial and lateral heads of gastrocnemius (141 and 113 cm2, respectively), while proximally, the greatest PCSA was observed in iliotibialis lateralis (158 cm2).
Functional distribution of muscle
Functional muscle volume was defined as the volume of muscle available at a single joint that could perform a particular movement, in this case flexion or extension. Distribution of functional muscle volume was determined in order to establish the maximum muscle volume, as a percentage of total pelvic limb muscle volume that could either flex or extend each joint (Table 5). Muscles that could act at more than one joint were included in the relevant functional group at each joint crossed, although there is potential for power transfer across multiple joints. The largest volumes for each joint were those of the extensors of the hip, the flexors of the knee, the extensors of the ankle and the flexors of the digit. The greatest volume contribution overall was that of hip extensor muscles (41.0%), with potential hip extensor volume being more than three times that of hip flexion (11.8%). The greatest difference at a specific joint was seen between ankle flexion (3.1%) and ankle extension (29.3%), with extensor muscle volume exceeding flexor volume by more than nine times.
Table 5.
Functional division of muscle volume of the pelvic limb
| Function | Volume percentage (mean ± SD) | Estimated total force (N) |
|---|---|---|
| Hip flexion | 11.8 ± 1.2 | 6 000 |
| Hip extension | 41.0 ± 4.3 | 15 000 |
| Knee flexion | 25.4 ± 3.0 | 8 300 |
| Knee extension | 20.4 ± 2.0 | 10 500 |
| Ankle flexion | 3.1 ± 0.4 | 6 700 |
| Ankle extension | 29.3 ± 4.8 | 20 700 |
| Digit flexion | 5.6 ± 0.7 | 9 800 |
| Digit extension | 1.4 ± 0.2 | 900 |
Isometric force-generating capacity is directly related to muscle PCSA. Differing architecture of muscles enabled some smaller volume muscles with short fibres to have a relatively large PCSA, as previously mentioned, and therefore relative force-generating capacity of muscle groups differed from muscle volumes. The combined heads of the gastrocnemius muscle can be seen to have the greatest force-generating capacity, with an estimate of 8950 N, while the relatively small distal muscles of tibialis cranialis, flexor perforatus digiti III and fibularis longus also show impressive Fmax estimates of 7670, 6210 and 4200 N, respectively. Proximally the largest potential force-generating capacities were those of iliotibialis lateralis (4740 N), obturatorius medialis (3500 N), femorotibialis accessorius (3230 N) and femorotibialis medius (2800 N). In terms of functional groups, hip extensors still had a greater force-generating capacity than hip flexors, but the ratio between them was less than that of their volume. Knee extensor force capacity (10500 N) exceeded that of knee flexors (8300 N) despite a lower muscle volume. Ankle extensors were found to have the highest force-generating capacity, exceeding 20 kN, due to the existence of many short fibred muscles, which resulted in high PCSA. Many of these muscles also act to flex the digits, and the large PCSAs of these muscles lead to a high force-generating capacity of almost 10 kN of the digital flexors despite their making a relatively small contribution to the total volume of pelvic limb muscle (5.6%).
Power output of the muscles was considered to be proportional to muscle volume and therefore, due to sheer size of the muscle, gastrocnemius was not only estimated to have the greatest potential for muscle force production, but also for power output. Other muscles potentially capable of generating high power included iliotibialis lateralis (also with high force-generating potential) and iliofibularis, which despite only a moderately high force capacity (2280 N) would be estimated to have one of the highest power outputs. Most of the high-power-generating muscles were located in the proximal limb, with some muscles with relatively low force-generating potential, such as iliotibialis cranialis (900 N), suggesting capacity for relatively high power. Distally, fibularis longus showed a high capacity for both force (4200 N) and relative power. Other distal muscles tended to have relatively low potential for power production, with the high force-generating tibialis cranialis and flexor perforatus digiti III estimated to be capable of only low power production. The remainder of the digital flexors also had low capacity for power production, coupled with moderately high estimated force capacities (of approximately 2000 N). The trends of potential for power generation of the flexors and extensors at the joints closely followed those of the muscle volume. Power of the hip extensors would be expected to be greatest, as a number of high-force, high-power muscles act to extend the hip. Ankle extensor power would also be expected to be high, largely due to the main ankle extensor, gastrocnemius. Total potential power of the flexors of the ankle and the flexors of the digit were low when compared with their force-generating capabilities.
Discussion
Muscle architecture of the pelvic limb of the ostrich was quantified, and functional division of muscle mass within the limb was established. Muscle mass of the pelvic limb was found to account for a large proportion of total body mass (33.7 ± 2.1%). This value for relative pelvic limb muscle mass is a great deal larger than that which has been observed in humans (17–20%; Janssen et al. 2000), suggesting that their musculoskeletal structure and pelvic limb musculature is much better adapted for running locomotion than our own, due to a greater proportion of total muscle being available for locomotion. As the pelvic limb muscle mass can be considered to be the total locomotor muscle mass of the ostrich, this relates more closely to the combined amount of total locomotor muscle that might be found in an athletic quadruped, such as the horse, when considering thoracic and pelvic musculature (∼20% in the equine pelvic limb alone; Payne et al. 2005b).
The distribution of muscle mass throughout the pelvic limb demonstrated a proximal-to-distal reduction, as expected. This relative reduction in distal limb mass is a consistent feature of animals adapted for high-speed locomotion (Brown et al. 2003; Pasi & Carrier, 2003; Payne et al. 2005a,b) and supports the observation that high-speed animals have lighter distal limbs (Hildebrand & Hurley, 1985; Steudel, 1990) in order to reduce the effects of rotational inertia in the swinging limb (Cavagna & Kanecko, 1977; Fedak et al. 1982). The largest proximal muscles (ITL and IFib) were also those with the highest capacity for power production, due to the direct relationship that exists between muscle volume and power, and therefore other substantial muscles (FTM, FTA, ITC, FCL, OM and CFem) of the proximal limb were also estimated to have high relative powers. Some of these powerful muscles also demonstrated a relatively large capacity for force production due to large PCSAs, despite having relatively long fascicle lengths (ITL, IFib). Conversely, other long parallel-fibred muscles such as ITC and FCL showed low capacities for force production. The muscle group with the greatest potential for power production was the hip extensors. This trend of high-power muscles, capable of doing large amounts of work, being situated in the proximal limb has also been observed in the pelvic limb of the horse (Payne et al. 2005a). However, powerful muscles were not exclusive to the proximal limb: the gastrocnemius muscle (originating on the distal femur and acting to flex the knee and extend the ankle) demonstrated the largest capacity for both force and power production in the pelvic limb, occupying the lateral, caudal and medial aspects of the tibiotarsus. The cranial aspect was occupied by another relatively high-force, high-power-generating muscle, fibularis longus. All other muscles in the distal limb were estimated to be low-power muscles, but short pennate fascicles lead to these relatively small muscles having high PCSA and hence capacity to generate large forces. This trend was not limited to the distal muscles, however, as short fascicled muscles were also evident in the proximal limb and small muscles such as ambiens and FCM also showed potential for relatively high force compared with power production.
Potential for power production was estimated, as described in the methods, based on the assumption that all of the muscles have the same force/velocity relationship and therefore muscle power is proportional to muscle volume. This estimate was used due to there currently being no data available on the maximum contraction velocity of ostrich muscle. Studies have, however, been carried out on the contraction properties and power production of avian muscle, with the majority considering power of flight muscles (pectoralis). In most of these studies, estimates have been made of shortening velocity derived from calculated power requirements (Biewener et al. 1998; Askew & Marsh, 2001), with the exception of chicken pectoralis contraction velocity, which has been measured directly (Reiser et al. 1996). However, flight muscle has a very different role to that of the pelvic limb and at extremes of performance, such as take off, power outputs and hence contraction velocities were seen to be very high compared with those measured in terrestrial mammals. Contraction velocity of pelvic limb avian muscle [lateral gastrocnemius (LG) and peroneus longus (PL), both with a mix of fast glycolytic (FG) and fast oxidative glycolytic fibres] was measured in vivo in the wild turkey (mass 4 kg; Nelson et al. 2004), and found to be 13.0 (LG) and 14.8 L s−1 (PL), yielding a more suitable comparison. Smaller mammals have been demonstrated to have higher contraction velocities than larger mammals (McMahon, 1975; Medler, 2002), which might be expected to hold true for avian muscle. Comparative studies between numerous species have attempted to determine the scaling relationships of muscle properties such as Vmax. One such study (which considered a body mass range that would encompass that of ostriches) suggested that Vmax in terrestrial animals scales to 15.9 × (Mass)−0.25 (Medler, 2002). This relationship would suggest maximum contraction velocities of 5.0 and 11.2 L s−1 for an ostrich (100 kg) and turkey (4 kg), respectively. Therefore, the contraction velocity of ostrich muscle for a 100-kg ostrich would be expected to be about half that of a 4-kg wild turkey. However, the estimate of turkey muscle Vmax is also less than the directly measured published value, suggesting these may be underestimates. The distribution of fibre types within a muscle would also have a large effect on maximum contraction velocity, and fast type II (FOG) muscle fibres do not necessarily dominate in the ostrich pelvic limb. Fibre typing in four pelvic limb muscles of the ostrich (Velotto & Crasto, 2004) revealed an even mix of FG, FOG and slow oxidative (SO) fibres in the gastrocnemius, and only FOG and SO fibres in fibularis longus and the two parts of tibialis cranialis. Therefore, in order to establish accurate measurements of muscle power output, muscle fibre typing and contraction velocities for each fibre type would be required.
Extensive tendons were observed in many of the distal limb muscles. Gastrocnemius inserted onto the tarsometatarsus via a thick, flat tendon that passed over the caudal aspect of the ankle joint. The digital flexor tendons lay deep to this tendon. Digital flexor tendons were all long and thin, with flexor perforans and perforatus digiti III being the longest (990 mm) with the smallest CSA (0.4 cm2), and flexor perforatus digiti III being the shortest (680 mm) with the largest CSA (1.5 cm2). The greater CSA of FPDIII enabled it to wrap around and encase the smaller tendons of FPetPIII and FPDIV. The tendon of FDL ran deep to this tendon bundle, with a length of 900 mm and CSA 0.7 cm2, receiving the tendon of FHL mid-tarsometarsus. Tendon stress was estimated from maximum force produced by the corresponding muscles, with the exception of FDL where the forces produced by both FDL and the received FHL were combined. The estimates of maximum force production in FDL, FPDIV and FPetPIII were all very similar (in the region of 2000 N) whereas FPDIII was found to have a force-generating capacity more than three times this value (6210 N) due to having the shortest fascicle length of all the digital flexors. The greater CSA of this tendon would limit peak stress in the tendon to 42 MPa at maximum isometric muscle force. Stresses in FPDIV and FDL were estimated as 35 and 37 MPa, respectively, while FPetPIII was estimated to undergo the greatest stress of the flexor tendons (44 MPa) due to it having the smallest CSA. It is reassuring to find that these estimates of tendon stress are very close to 40 Mpa, which is the mean stress as derived by Alexander et al. (1979) based on the estimated limb force of an ostrich running with a duty factor of 0.29. These estimates of tendon stress are also appropriate for significant elastic energy storage. Estimates of strain in these tendons show that all three tendons within the superficial bundle (FPDIII, FPDIV, FPetPDIII) have similar percentage strains at maximum isometric muscle force (2.3–2.9%) and that strain in the deep FDL is also similar (2.5%). Tendons in the proximal limb were generally much shorter and lighter, but some tendons were still estimated to experience large stresses and strains. The greatest of these was ambiens (stress 70.5 MPa, strain 4.7%), a high-force, low-power-producing muscle, with tendon length exceeding belly length. The large volume of elastic tendon in the limb is in-keeping with economical locomotion, as long tendons enable storage of elastic strain energy when stretched, which is then recovered on release, hence reducing mechanical work required and optimizing economy (Alexander, 1991). In addition, elasticity of the aponeurosis of the muscles, which could account for almost as much mass in the limb as tendon, can also contribute to this energy-saving mechanism (Ettema & Huijing, 1989). The mechanism of storing and returning energy in elastic tendons is well documented in the horse and studies have estimated that in a galloping horse, this enables muscular work to be halved (Dimery et al. 1986; Minetti et al. 1999). This reduction in the work of running would enable the ostrich to use less muscle mass and optimize metabolic energy expenditure, effectively enabling the high-speed sustained running that is commonly observed in ostriches.
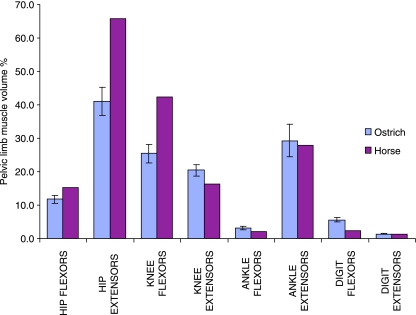
Similarities in the muscular design and structure of the ostrich pelvic limb and that of a fast running quadrupedal species (the horse) have been discussed. This particular comparison is useful as the ostrich is the fastest living biped and comparison with an athletically comparable species can be more useful than a less athletic biped when considering specializations for high-speed locomotion. Despite these similarities, there are obviously specific functional requirements for running bipedally or quadrupedally. By comparing the functional distribution of muscle in the pelvic limb of the ostrich and the horse some of these differences may be highlighted. A comparison of relative functional muscle volume for the pelvic limb of the ostrich and the horse (Payne et al. 2005a) is shown in Fig. 5.
Fig. 5.
Muscle functional distribution at each joint, shown for both the ostrich and the horse, as percentage of total pelvic limb muscle volume. Horse data were taken directly from Payne et al. (2005a).
Ostrich and horse functional muscle volumes tend towards extending the hip, flexing the knee, extending the ankle and flexing the digit. However, the proportions of total pelvic limb muscle vary in that the muscle volumes capable of extending the hip and flexing the knee are much reduced in the ostrich than in the horse (41% of pelvic limb muscle volume available for hip extension in the ostrich, compared with 66% in the horse, and 25% compared with 42% for knee flexion, respectively). The second largest functional volume in the ostrich accounts for ankle extension (29%), which is a volume similar to that seen in the horse (28%), and both of these are significantly greater than the volumes of muscle available for ankle flexion (3% in the ostrich, 2% in the horse). Although also only accounting for a small percentage of total volume, the digital flexor volume of the ostrich (5.5%) was found to be more than double that seen in the horse (2.4%).
The greater combined percentage of muscle volume able to act at all of the joints demonstrates more multijoint muscles in the horse than in the ostrich. In particular, the horse has a greater number (and mass) of muscles that can act to both extend the hip and flex the knee, resulting in the much larger volume of muscle available for each of these actions in the horse than in the ostrich. These large functional volumes observed in the horse are consistent with the muscle requirements for propelling the animal. This functional division, particularly hip extension, is important in terms of powering locomotion of the animal. In a quadruped forelimbs tend to have a greater role in weight support, while pelvic limbs dominate in power (Cavagna & Kanecko, 1977; Jayes & Alexander, 1978; Heglund et al. 1982; Full et al. 1991). This division of labour enables thoracic and pelvic limbs to develop their own specialized functional anatomy (Payne et al. 2005a). As the extensor muscles must actively shorten in order to accelerate the body, a greater volume of muscle is required for accelerating than decelerating, where eccentric contractions occur to absorb energy, which can generate a greater force at high velocity (Katz, 1939). Therefore, less muscle volume is required for deceleration than acceleration. A biped does not have the ability to optimize for either one or the other of these tasks, as the limb must function to both accelerate the body and support the body weight. This thoraco-pelvic variation in extensor muscle is seen in the horse (Payne et al. 2005a,b) and the propulsive requirements of the pelvic limb of the horse. Horses can accelerate by torque at the hip joint as the centre of mass is forward of the hip, providing stability, while in the ostrich the centre of mass is closer to the hip, making it harder to accelerate solely by hip extension without large pitching movements. During walking in the ostrich femoral excursions are minimal but extension at the hip does increase during high-speed locomotion, with the large hip extensors able to generate high powers. Ankle extensor muscle volume available is also large and similar in both species (29%). Extension of the ankle also plays a large part in propulsion, as extension of the ankle lengthens the leg and acts to propel the centre of mass up and forwards. The large relative volumes of muscle dedicated to this limb extension in the pelvic limbs of both the horse and the ostrich are functionally desirable to do work and hence achieve acceleration and high-speed locomotion.
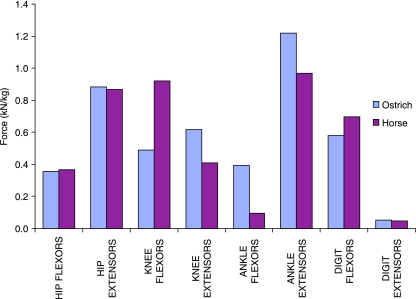
Maximum functional muscle force per kilogram of pelvic limb muscle is shown in Fig. 6 for both the ostrich and the horse. These data are presented per kilogram of pelvic limb muscle to enable a direct comparison between the proportions of muscle volume functionally available at each joint and the force-generating capacity as defined by the differing architecture of that muscle. The force-generating capacity of the hip flexors (horse, 0.36 kN kg−1; ostrich, 0.35 kN kg−1) and extensors (horse, 0.86 kN kg−1; ostrich, 0.88 kN kg−1) can be seen to be very similar in the two species, despite large differences in functional volume, due to the greater effective PCSA of many muscles of the ostrich. The difference between the ostrich and the horse is greater in the knee flexors, with almost double the potential force generation in the horse (0.92 kN kg−1) than the ostrich (0.49 kN kg−1). This capacity for knee flexion remains much greater in the horse than the ostrich, primarily because the major knee flexor of the ostrich, iliofibularis, has only moderate force capabilities but high power. The largest component of force production in the ostrich can be seen at the ankle joint, with both flexion (0.39 kN kg−1) and extension (1.22 kN kg−1) force capacity exceeding that of the horse (0.10 and 0.97 kN kg−1). With force generation of both the ankle flexors and the extensors greater in the ostrich than the horse and the greater force per kilogram acting in these muscles, more energy would be able to be stored in the tendons crossing the ankle and metatarsophalangeal joints. Of course, the effective moment arms of these muscles also play an essential role in defining applied forces and joint moments, and must be considered before definitive conclusions can be made.
Fig. 6.
Maximum functional force production per kilogram of pelvic limb muscle acting at each joint for the pelvic limb of both the ostrich and the horse.
The large volume and potential force generation of the ankle extensor muscles in the ostrich suggest that extension of the ankle may be more significant during locomotion and in acceleration of the centre of mass than hip extension, compared with the horse. This finding is consistent with the theory that while quadrupeds can to some degree separate propulsion and weight support with thoracic–pelvic specialization, hence using the hip extensor muscle to generate a torque at the hip, bipeds rely less on generation of torque at the hip to lift and propel the centre of mass (Usherwood & Wilson, 2005). Losses occur during locomotion so work must be done during a stride even at steady speeds (and more so in acceleration or incline locomotion). Animals also need to accelerate, run uphill and jump. In that situation net work on the centre of mass is required, over and above that needed to meet locomotor losses and it is likely that quadrupeds and bipeds use different combinations of leg extension and leg retraction to achieve this. In quadrupeds there is greater scope for localized muscles doing this work around hip extension than in bipeds, where less localized and dual role muscles are responsible for both anti-gravity and work on the system. Therefore, extension of the ankle is an alternative mechanism ideal for bipedal locomotion. The high force capacity of the ankle extensors and digit flexors also optimizes elastic performance of the limb, by further stretching of the tendons, and hence increasing the capacity for storage of elastic energy.
Trends in potential power production of muscles at each of the joints of the pelvic limb would be expected to be similar to those of muscle volume for both the ostrich and the horse. The power of the muscles of the horse pelvic limb exceeds that of the ostrich for muscles acting at proximal joints, while relative power would be expected to be slightly greater in the ostrich only for the ankle extensors and digit flexors. This demonstrates a difference in the pelvic muscle structure of the ostrich, optimized for maximizing force production and supporting the body weight (with a greater proportion of short-fibred, high-PCSA muscles ideal for achieving this) with reduced capacity for power output, and that of the horse with comparably less effective muscular force production, due to reduced weight support requirement, but high-power-producing propulsive muscle structure (with a greater proportion of work-producing long-fibred muscles). However, this comparison does not take account of possible differences in muscle moment arms and it must be considered that power estimates of both the ostrich and the horse are based on the assumptions previously discussed and that therefore muscle power may vary from and indeed exceed that indicated here.
Conclusions
The muscle architecture of the pelvic limb muscles of the ostrich was quantified, producing a detailed description of measured and derived muscle properties within the limb. Expected trends were observed in the proximal to distal reduction of muscle mass in the limb, mainly due to having larger muscles situated proximally. The exception to this was gastrocnemius, which not only had the greatest mass and largest tendon of the pelvic limb but was also found to have the greatest potential for both force generation and power output. High relative power production is directly related to volume and as such most high-power muscles were in the proximal limb, the majority of which acted to extend the hip. Some of the proximal muscles also had capacity to generate high force due to having relatively large PCSA as a result of their size. By contrast, some distal muscles such as tibialis cranialis and flexor perforatus digiti III were found to be strong despite their small size due to very short fascicles and large PCSA. Functional distribution of muscle volume of the ostrich showed the hip extensors to account for the greatest proportion of total muscle volume in the pelvic limb. The hip extensor, knee flexor, ankle extensor and digit flexor volumes all exceeded those of the opposing function. Potential power production of the muscles acting at the joints was directly related to the muscle volumes, but capacity for force production was dependent not only on muscle size but also effective PCSA. As such, the ankle extensors accounted for the greatest force capacity. These high-force ankle extensors were all in series with long tendons, and therefore potentially have high capacity for energy storage.
Acknowledgments
We would like to thank Erica Gummery from the Royal Veterinary College for providing the line drawing shown in Fig. 2. This work was funded by the BBSRC. N.C.S. was funded by a Royal Veterinary College studentship. A.M.W. holds a BBSRC research development fellowship and a Royal Society Wolfson Research Merit Award.
References
- Alexander RM, Maloiy GMO, Njau R, Jayes AS. Mechanics of running in the ostrich (Struthio camelus) J Zool Lond. 1979;187:169–178. [Google Scholar]
- Alexander RM. Energy-saving mechanisms in walking and running. J Exp Biol. 1991;160:55–69. doi: 10.1242/jeb.160.1.55. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Tendon elasticity and muscle function. Comp Biochem Physiol – Part A: Mol Integrative Physiol. 2002;133:1001–1011. doi: 10.1016/s1095-6433(02)00143-5. [DOI] [PubMed] [Google Scholar]
- Askew GN, Marsh RL. The mechanical power output of the pectoralis muscle of blue-breasted quail (Coturnix chinensis): the in vivo length cycle and its implications for muscle performance. J Exp Biol. 2001;204:3587–3600. doi: 10.1242/jeb.204.21.3587. [DOI] [PubMed] [Google Scholar]
- Bennett MB, Ker RF, Dimery NJ, Alexander RM. Mechanical properties of various mammalian tendons. J Zool Lond. 1986;209:537–548. [Google Scholar]
- Bennett MB, Stafford JA. Tensile properties of calcified and uncalcified avian tendons. J Zool Lond. 1988;214:343–351. [Google Scholar]
- Biewener AA. Muscle-tendon stresses and elastic energy storage during locomotion in the horse. Comp Biochem Physiol Part B: Biochem Mol Biol. 1998;120:73–87. doi: 10.1016/s0305-0491(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Corning WR, Tobalske BW. In vivo pectoralis muscle force-length behavior during level flight in pigeons (Columba livia) J Exp Biol. 1998;201:3293–3307. doi: 10.1242/jeb.201.24.3293. [DOI] [PubMed] [Google Scholar]
- Brown NAT, Pandy MG, Kawcak CE, McIlwraith CW. Force- and moment-generating capacities of muscles in the distal forelimb of the horse. J Anat. 2003;203:101–113. doi: 10.1046/j.1469-7580.2003.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna G, Kanecko M. Mechanical work and efficiency in level walking and running. J Physiol. 1977;268:467–481. doi: 10.1113/jphysiol.1977.sp011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimery NJ, Alexander RM, Ker RF. Elastic extension of the leg tendons in the locomotion of horses (Equus caballas) J Zool Lond. 1986;205:415–425. [Google Scholar]
- Ettema GJC, Huijing PA. Properties of the tendinous structures and series elastic component of EDL muscle-tendon complex of the rat. J Biomech. 1989;22:1209–1215. doi: 10.1016/0021-9290(89)90223-6. [DOI] [PubMed] [Google Scholar]
- Fedak M, Heglund N, Taylor C. Energetics and mechanics of terrestrial locomotion. II. Kinetic energy changes of the limbs and body as a function of speed and body size in birds and mammals. J Exp Biol. 1982;97:23–40. doi: 10.1242/jeb.97.1.23. [DOI] [PubMed] [Google Scholar]
- Full RJ, Blickhan R, Ting LH. Leg design in hexapedal runners. J Exp Biol. 1991;158:369–390. doi: 10.1242/jeb.158.1.369. [DOI] [PubMed] [Google Scholar]
- Fuss FK. Tibiofibular junction of the south african ostrich (Struthio camelus australis) J Morph. 1996;227:213–226. doi: 10.1002/(SICI)1097-4687(199602)227:2<213::AID-JMOR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Gadow H. Zur Vergleichenden Anatomie der Muskulatur des Beckens und der Hinteren Gliedmasse der Ratinen. Jena: Gustav Fischer; 1880. [Google Scholar]
- Gangl D, Weissengruber GE, Egerbacher M, Forstenpointner G. Anatomical description of the muscles of the pelvic limb in the ostrich (Struthio camelus) Anat Histol Embryol J Vet Med Series C. 2004;33:100–114. doi: 10.1111/j.1439-0264.2003.00522.x. [DOI] [PubMed] [Google Scholar]
- Garrod AH, Darwin F. Notes on an ostrich lately living in the Society's collection. Proc ZoolSoc Lond. 1872. pp. 356–363.
- Haughton S. Notes on animal mechanics. No III: On the muscular mechanism of the leg of the ostrich. Proc Roy Irish Acad. 1865;9:50–61. [Google Scholar]
- Heglund NC, Cavagna GA, Taylor CR. Energetics and mechanics of terrestrial locomotion. III. Energy changes of the centre of mass as a function of speed and body size in birds and mammals. J Exp Biol. 1982;97:41–56. doi: 10.1242/jeb.97.1.41. [DOI] [PubMed] [Google Scholar]
- Hildebrand M, Hurley JP. Energy of the oscillating legs of a fast-moving cheetah, pronghorn, jackrabbit, and elephant. J Morph. 1985;184:23–31. doi: 10.1002/jmor.1051840103. [DOI] [PubMed] [Google Scholar]
- Hutchinson JR. Biomechanical modeling and sensitivity analysis of bipedal running ability. I. Extant taxa. J Morphol. 2004;262:421–440. doi: 10.1002/jmor.10241. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Jayes AS, Alexander RM. Mechanics of locomotion of dogs (Canis familiaris) and sheep (Ovis aries) J Zool Lond. 1978;185:289–308. doi: 10.1111/j.1469-7998.1978.tb03334.x. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. J Physiol. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ker RF, Alexander RM, Bennet MB. Why are mammalian tendons so thick? J Zool Lond. 1988;216:309–324. [Google Scholar]
- Langer K. Uber die Fussgelenke der Vogel. Denkschriften Akademie Wissenschaften Wien. 1859;16:93–130. [Google Scholar]
- Liswinaso D. University of Glasgow: 1996. A morphological and diagnostic imaging study of the distal pelvic limb of the ostrich (Struthio camelus) MSc thesis. [Google Scholar]
- MacAlister A. On the anatomy of the ostrich (Struthio camelus) Proc Roy Irish Acad. 1865;9:1–24. [Google Scholar]
- McMahon TA. Using body size to understand the structural design of animals: quadrupedal locomotion. J Appl Phys. 1975;39:619–627. doi: 10.1152/jappl.1975.39.4.619. [DOI] [PubMed] [Google Scholar]
- Medler S. Comparative trends in shortening velocity and force production in skeletal muscles. Am J Physiol Regul Integr Comp Physiol. 2002;283:R368–R378. doi: 10.1152/ajpregu.00689.2001. [DOI] [PubMed] [Google Scholar]
- Mellet FD. University of Stelenbosch: 1985. The ostrich as a meat animal – anatomical and muscle characteristics. MSc Agric thesis. [Google Scholar]
- Mellet FD. A note on the musculature of the proximal part of the pelvic limb of the ostrich (Struthio camelus) J S Afr Vet Assoc. 1994;65:5–9. [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabol. 1960;9:184–188. [Google Scholar]
- Minetti AE, Ardig OLP, Reinach E, Saibene F. The relationship between mechanical work and energy expenditure of locomotion in horses. J Exp Biol. 1999;202:2329–2338. doi: 10.1242/jeb.202.17.2329. [DOI] [PubMed] [Google Scholar]
- Nelson FE, Gabaldon AM, Roberts TJ. Force-velocity properties of two avian hindlimb muscles. Comp Biochem Physiol – Part A: Mol Integrative Physiol. 2004;137:711–721. doi: 10.1016/j.cbpb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Pasi BM, Carrier DR. Functional trade-offs in the limb muscles of dogs selected for running vs. fighting. J Evol Biol. 2003;16:324–332. doi: 10.1046/j.1420-9101.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- Pavaux C, Lignereux Y. A myologic dissection of the leg and foot of the ostrich (Struthio camelus) Anat Histol Embryol J Vet Med Series C. 1995;24:127–131. doi: 10.1111/j.1439-0264.1995.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Payne RC, Hutchinson JR, Robilliard JJ, Smith NC, Wilson AM. Functional specialisation of pelvic limb anatomy in horses (Equus caballus) J Anat. 2005a;206:557–574. doi: 10.1111/j.1469-7580.2005.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RC, Veenman P, Wilson AM. The role of the extrinsic thoracic limb muscles in equine locomotion. J Anat. 2005b;206:193–204. doi: 10.1111/j.1469-7580.2005.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser PJ, Greaser ML, Moss RL. Contractile properties and protein isoforms of single fibres from the chicken pectoralis red strip muscle. J Physiol (Lond) 1996;493:553–562. doi: 10.1113/jphysiol.1996.sp021403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharir A, Milgram J, Shahar R. Structural and functional anatomy of the neck musculature of the dog (Canis familiaris) J Anat. 2006;208:331–351. doi: 10.1111/j.1469-7580.2006.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudel K. The work and energetic cost of locomotion. I. The effects of limb mass distribution in quadrupeds. J Exp Biol. 1990;154:273–285. doi: 10.1242/jeb.154.1.273. [DOI] [PubMed] [Google Scholar]
- Usherwood JR, Wilson AM. No force limit on greyhound sprint speed. Nature. 2005;438:753–754. doi: 10.1038/438753a. [DOI] [PubMed] [Google Scholar]
- Velotto S, Crasto A. Histochemical and morphometrical characterization and distribution of fibre types in four muscles of ostrich (Struthio camelus) Anat Histol Embryol J Vet Med Series C. 2004;33:251–256. doi: 10.1111/j.1439-0264.2004.00535.x. [DOI] [PubMed] [Google Scholar]
- Wells JB. Comparison of mechanical properties between slow and fast mammalian muscle. J Physiol. 1965;178:252–269. doi: 10.1113/jphysiol.1965.sp007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woledge RC, Curtin NA, Homsher E. Energetic aspects of muscle contraction. Monogr Physiol Soc. 1985;41:1–357. [PubMed] [Google Scholar]
- Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Engng. 1989;17:359–411. [PubMed] [Google Scholar]