Abstract
The aim of this study was to assess whether enzymatically isolated chondrons from normal adult articular cartilage could be used as a model for the onset of osteoarthritis, by comparison with mechanically extracted chondrons from osteoarthritic cartilage. Enzymatically isolated chondrons (EC) were cultured for 4 weeks in alginate beads and agarose gel constructs. Samples were collected at days 1 and 2, and weekly thereafter. Samples were immunolabelled for types II and VI collagen, keratan sulphate and fibronectin and imaged using confocal microscopy. Mechanically extracted chondrons (MC) were isolated, immunohistochemically stained for type VI collagen and examined by confocal microscopy. In culture, EC showed the following characteristics: swelling of the chondron capsule, cell division within the capsule and remodelling of the pericellular microenvironment. This was followed by chondrocyte migration through gaps in the chondron capsule. Four types of cell clusters formed over time in both alginate beads and agarose constructs. Cells within clusters exhibited quite distinct morphologies and also differed in their patterns of matrix deposition. These differences in behaviour may be due to the origin of the chondrocytes in the intact tissue. The behaviour of EC in culture paralleled the range of morphologies observed in MC, which presented as single and double chondrons and large chondron clusters. This preliminary study indicates that EC in culture share similar structural characteristics with MC isolated from osteoarthritic cartilage, confirming that some processes that occur in osteoarthritis, such as pericellular remodelling, take place in EC cultures. The study of EC in culture may therefore provide an additional tool to investigate the mechanisms operating during the initial stages of osteoarthritis. Further investigation of specific osteoarthritic phenotype markers will, however, be required in order to validate the value of this model.
Keywords: agarose gel, alginate bead, chondrocyte, chondron, confocal microscopy
Introduction
Adult articular cartilage is divisible into four distinct horizontal layers: the superficial, middle, deep and calcified cartilage layers. Each is characterized by specific proteoglycan concentrations, collagen fibre orientations, glycoprotein distributions and chondrocyte morphology. In addition, the chondrocytes in each layer are surrounded circumferentially by a 2- to 3-µm-thick pericellular microenvironment, which is rich in collagen types II, VI, IX and XI, hyaluronan, proteoglycans such as aggrecan, decorin and biglycan, and glycoproteins such as fibronectin, link protein and laminin. Integrins and other cell-surface receptors anchor the chondrocyte to this integrated macromolecular microenvironment and together they represent the chondron (Benninghoff, 1925; Szirmai, 1969), the primary structural (Poole et al. 1984, 1988b; Poole, 1997), functional (Knight et al. 2001; Hing et al. 2002) and metabolic (Winter et al. 1998; Handley et al. 2002) unit of adult articular cartilage. In the middle and deep layers, each chondron is further surrounded by a morphologically differentiated territorial matrix which is under the metabolic control of the chondrocyte (Winter et al. 1998; Handley et al. 2002), while a distinct interterritorial matrix is present between adjacent territories. Thus, articular chondrocyte heterogeneity relates not only to the position within the cartilage matrix, but also to the nature of the microenvironment which surrounds and interacts with each cell.
Investigations of articular cartilage physiology and metabolism in vitro generally employ cultured explants or enzymatically isolated chondrocytes. Cartilage explants have proved to be extremely practical for short-term studies of cartilage metabolism (Winter et al. 1998; Handley et al. 2002), but the use of isolated chondrocytes is still the preferred approach for longer term studies. Enzymatic isolation of chondrocytes usually involves processing the entire tissue depth with little regard for the heterogeneity of the resultant chondrocyte population. These chondrocytes become uniformly rounded upon extraction and are considered to be homogeneous. A limited number of studies have shown that chondrocytes from different matrix layers maintain their different phenotypes in culture with respect to cytoskeletal organization, proteoglycan metabolism and cluster formation (Zanetti et al. 1985; Aydelotte & Kuettner, 1988; Idowu et al. 2000). Superficial layer cells, in particular, behave differently from middle and deep layer cells, indicating that the composition of the tissue source should be carefully considered when studying isolated chondrocytes.
In order to maintain the isolated chondrocyte phenotype and discourage dedifferentiation in vitro, it is necessary to culture isolated chondrocytes in three-dimensional matrices. Early studies employed gels such as agarose by either embedding the cells within the gel (Aydelotte & Kuettner, 1988; Benya & Shaffer, 1982; Chang & Poole, 1996) or suspending them in medium over the gel (Archer et al. 1990). Although these agarose gels proved extremely well suited to investigations of cell growth, matrix production and biomechanical studies, biochemical analysis of synthesized macromolecules was complicated by the presence of the agarose. The introduction of alginate bead culture allowed more systematic approaches to biochemical studies, as calcium chelation can be used to depolymerize the gel, thereby allowing centrifugal separation of cell-associated and further-removed matrix fractions (Mok et al. 1994). Although isolated chondrocyte cultures are useful for investigating chondrocyte–pericellular matrix interactions in vitro, they lack the macromolecular complexity or structural integrity of intact chondrons mechanically extracted from normal adult articular cartilage (Poole et al. 1988b).
Mechanical disruption of normal adult cartilage by low-speed homogenization produces a heterogeneous mixture of single and double chondrons, chondron columns, small cartilage chips (< 500 µm3) and collagenous debris (Poole et al. 1988b). Studies using spontaneously osteoarthritic canine cartilage resulted in the isolation of clonal osteoarthritic clusters of varying size (Poole et al. 1997). Although fundamentally important in establishing the chondron as the primary functional unit of adult articular cartilage, the yield of single viable chondrons for comparative studies is low compared with conventionally isolated chondrocytes. Mechanically isolated chondrons (MC) are therefore considered ‘mini-cartilage explants’, with studies focusing on the analysis of individual chondrons in sufficient numbers to elucidate the molecular anatomy and physiology of the chondron (Poole, 1997). However, isolated chondron viability is difficult to maintain beyond 14 days (Knight et al. 2001; Hing et al. 2002), and the small yield, combined with the innate heterogeneity of the preparation, precludes their use for biochemical studies.
The development of a method to enzymatically isolate chondrocytes and their pericellular microenvironment from adult human articular cartilage has enabled further investigation of chondron structure and metabolism in vitro, as yields comparable with isolated ‘naked’ chondrocytes can be achieved (Lee et al. 1997). These ‘enzymatically isolated chondrons’ (EC) share similar morphological features with MC, but exhibit a substantial reduction in the size of the pericellular microenvironment (Knight et al. 2001; Hing et al. 2002). Type VI collagen is the principal molecule retained in the microenvironment, with reduced amounts of type II collagen and aggrecan, and complete loss of fibronectin. The viability of EC can be maintained over extended culture periods, thereby allowing more direct comparison with traditional chondrocyte cultures (Larson et al. 2002).
In this study of canine EC in culture, both agarose gel and alginate bead culture systems were employed to study the influence of the residual pericellular microenvironment on chondrocyte behaviour. These preparations were compared with MC and clonal chondrocyte clusters mechanically extracted from canine osteoarthritic cartilage by low-speed homogenization.
Materials and methods
Materials
Dulbecco's modified Eagle's medium (DMEM), fetal calf serum (FCS), Earle's balanced salt solution (EBSS), dispase and ascorbic acid were supplied by Life Technologies Inc. (New Zealand). Sodium alginate, HEPES, agarose (Type IX-A, ultra-low gelling temperature), streptomycin sulphate and penicillin were obtained from Sigma Chemical Co. (Australia). Collagenase (Type 2) was provided by Worthington Biochemical Corp. (USA). 5-Chloromethylfluorescein diacetate (CMFDA) and ethidium homodimer-1 were purchased from Molecular Probes Inc. (USA). The polyclonal antibody against type VI collagen was a gift from Dr S. Ayad (University of Manchester, UK). The monoclonal antibody 5D4 against keratan sulphate was purchased from Seikagaku Corp. (USA), and the polyclonal antibody against type II collagen was supplied by Rockland Laboratories Inc. (USA). The polyclonal antibody against fibronectin was purchased from Life Technologies Inc. Streptavidin-Texas Red was obtained from Amersham Pharmacia Biotech (UK). Vectashield was supplied by Vector Laboratories (USA).
EC extraction
Articular cartilage was resected in a sterile manner from the tibial plateaux of three adult dogs (2–4 years) that were euthanized under veterinary supervision at the Auckland City Pound. The samples were processed separately but in parallel. The tissue was diced and then digested with dispase and collagenase as previously described (Lee et al. 1997). The digested mixture was removed from the incubator, diluted with phosphate-buffered saline (PBS) and centrifuged for 10 min at 300 g. The supernatant was discarded and two additional washes were performed with DMEM. The EC suspension was diluted to the required volume with DMEM, divided into two portions and processed into either alginate beads or agarose gel at a density of 2 × 106 cells mL−1.
Preparation of alginate beads
For alginate bead culture, the EC suspension was diluted 1 : 10 with 1.25% sodium alginate in 20 mm HEPES buffer. Using a syringe equipped with a 21-gauge needle, the sodium alginate/EC mixture was extruded drop-wise into 0.1 m calcium chloride. The alginate beads were allowed to polymerize at room temperature for 10 min followed by two washes in 0.15 m sodium chloride and two washes in DMEM. The beads were then transferred to six-well plates that had been precoated with 3% agarose in EBSS.
Preparation of agarose gel constructs
For agarose culture, a 6% agarose solution in EBSS was prepared by autoclaving and then placed on a Heraeus Universal Turning Device in an incubator to equilibrate to 37 °C. The EC suspension was added to the 6% agarose at a ratio of 1 : 1 and rotated for 10 min at 37 °C. The resultant agarose/EC mixture was transferred into six-well plates to a depth of 5 mm and placed at 4 °C to gel for 20 min. Cylinders (5 mm in diameter) were cored out and placed into six-well plates that had been precoated with 3% agarose in EBSS.
Culture conditions
Cultures were maintained in DMEM supplemented with 10% fetal bovine serum, 50 µg mL−1 ascorbate, 100 U mL−1 penicillin and 100 µg mL−1 streptomycin, and the medium was changed every other day. The cultures were maintained for 4 weeks and examined daily using a Nikon Diaphot inverted microscope. Samples were collected on days 1 and 2 and weekly thereafter. Chondrocyte viability was determined on sample collection days using 25 µm CMFDA and 5 µm ethidium homodimer-1 (Poole et al. 1996). Samples were labelled for 2 h at 37 °C, washed three times in PBS, fixed in 4% paraformaldehyde in PBS for 30 min at room temperature, washed in PBS and then examined using a Nikon Diaphot fluorescence microscope. Samples for immunohistochemistry were labelled with CMFDA only and stored in PBS at 4 °C.
MC extraction
Samples of osteoarthritic articular cartilage were resected in a sterile manner from the tibial plateaux of five adult dogs (7–11 years) that were euthanized under veterinary supervision due to immobility of the stifle joint. Histology revealed the presence of osteophytes in all animals sampled and gross macroscopic evaluation confirmed central cartilage erosion and bone ebonation with some intact cartilage at the periphery of the joint. Samples of cartilage were resected and subjected to mechanical extraction procedures as previously described (Poole et al. 1988a,b). The osteoarthritic cartilage homogenate containing MC and clonal chondrocyte clusters was embedded in 4% agarose gel according to established procedures (Poole et al. 1997) and later processed for immunohistochemistry.
Immunohistochemistry and confocal laser scanning microscopy
For immunohistochemical studies, all samples were processed simultaneously. Agarose constructs were cut in half to improve antibody penetration. Alginate beads were treated in the same manner as agarose constructs throughout the procedure, except that calcium chloride (0.1 g L−1) was added to every buffer to maintain polymerization. Immunohistochemistry was performed as previously described (Poole et al. 1988a, 1991a). The primary antibodies included a polyclonal anti-type VI collagen antibody (Poole et al. 1988a), 5D4, a monoclonal antibody directed against keratan sulphate (Poole et al. 1991a), a polyclonal anti-fibronectin antibody and a polyclonal anti-type II collagen antibody. Biotinylated secondary antibodies and streptavidin-linked Texas Red were used for detection. Samples were mounted in Vectashield and examined using a Leica TCS 4D confocal laser scanning microscope (Leica, Germany).
Results
Morphology of canine EC
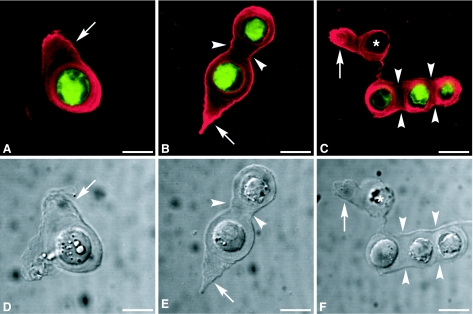
The morphologies of single and double chondrons, and chondron columns, immediately after enzymatic isolation from adult canine tibial cartilage, are shown in Fig. 1, with viable chondrocytes (Fig. 1A–C, green) forming intimate contacts with the residual pericellular microenvironment. Immunolabelling for type VI collagen (Fig. 1A–C, red) defined the extent of the pericellular microenvironment, which was also evident by differential interference contrast microscopy (Fig. 1D–F), confirming the presence of chondron ‘tails’ (Fig. 1A,B,D,E) and interconnecting segments between adjacent chondrons in columns of two or more (Fig. 1B,C,E,F). The majority of the EC preparation consisted of single chondrons, with only small numbers of double chondrons and few columns of three or more.
Fig. 1.
Confocal photomicrographs of single optical sections illustrating enzymatically isolated chondrons at day 0. (A–C) Viable chondrocytes labelled with CMFDA are shown in green with type VI collagen labelling shown in red. (D–F) Differential interference contrast images. Arrows point to the tail regions of the chondrons, and arrowheads indicate interconnecting segments. The asterisks (C,F) indicate that no viable chondrocyte is present. Scale bars = 10 µm.
Morphology and behaviour of EC in culture
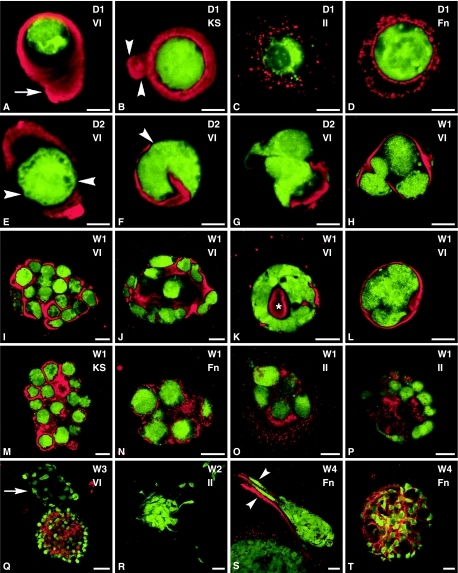
Images of immunohistochemical staining of EC in culture are presented in Fig. 2. Little difference was seen between the behaviour of EC in agarose constructs and alginate beads. After 1 day in culture, staining for both type VI collagen (Fig. 2A) and keratan sulphate (Fig. 2B) was localized to the pericellular microenvironment of the chondron. Chondrons labelled for keratan sulphate sometimes displayed an intense band immediately adjacent to the chondrocyte surface with a broader, less intense area surrounding it. The type II collagen distribution at day 1 was mostly limited to intense punctate labelling immediately around the chondrocyte (Fig. 2C), with a small proportion of chondrocytes showing long strands or remnants of type II collagen entangling the chondrocyte in a seemingly random arrangement. Staining for fibronectin was absent at day 1, but by day 2 a stippled pattern was present around the chondrocyte (Fig. 2D).
Fig. 2.
Confocal photomicrographs of single optical sections illustrating enzymatically isolated chondrons cultured in alginate beads (A,B,E,J,K,M) or agarose constructs (C,D,F–I,L,N–T). In all images, viable chondrocytes labelled with CMFDA are shown in green. Labelling for type II collagen (II), type VI collagen (VI), keratan sulphate (KS) or fibronectin (Fn), as indicated in the images, is shown in red. (A) Arrow points to tail region, (B) arrowheads indicate interconnecting segments, (E, F) arrowheads indicate gaps in the pericellular microenvironment, (K) asterisk indicates no viable cell is present, (Q) arrow points to cluster without type VI collagen labelling, (S) arrowheads point to migrating cells labelled for fibronectin. Labelling on images: D = day, W = week. Scale bars: A–G = 5 µm; H–P = 10 µm; Q–T = 20 µm.
EC remodelling and chondrocyte migration
By day 2, small gaps 1–2 µm in diameter were occasionally seen in the pericellular microenvironments of EC stained for type VI collagen (Fig. 2E,F). These gaps were only seen when the entire chondron was optically sectioned by confocal laser scanning microscopy and were not evident in day 0 or day 1 samples. Staining with CMFDA identified viable chondrocytes protruding through these small gaps in the pericellular microenvironment (Fig. 2F). Further evidence for this migration was found where cell processes, 2–5 µm in length, extended through these spaces into the surrounding matrix (Fig. 2G). In addition, chondrocytes that had migrated out of their microenvironment often seemed to use the vacated chondron ‘ghost’ as a focal substrate for continued proliferation (Fig. 2G).
EC cluster formation and classification
By the end of week 1, each viable chondrocyte remaining within a chondron had divided at least once inside the confines of the original microenvironment (Fig. 2G,H) and a variety of clusters had formed in both agarose constructs and alginate beads (Fig. 2H–P).
Classification of cultured EC clusters
Four types of clusters were identified based on their cell morphology and pattern of type VI collagen labelling, and two of them represented the majority of the observed clusters. One of these numerous cluster types was composed of rounded cells in either an oval or a round conformation (Fig. 2I). These clusters showed intense, but discrete, labelling for type VI collagen (Fig. 2I) and keratan sulphate (Fig. 2M) around each individual chondrocyte.
The other numerous cluster type had a spiral configuration (Fig. 2J) composed of concentric layers of cells that became very densely packed together as the culture progressed. There was diffuse labelling for type VI collagen, type II collagen and keratan sulphate between each chondrocyte in these clusters with more intense punctate labelling around the outermost cells.
Two further cluster types were identified in smaller numbers. One had a layer of flattened interleaved cells around the outside of the cluster with few viable cells in the centre (Fig. 2K). Intense labelling for type VI collagen was found in the central region of this cluster type where no viable cells were present, while no type VI collagen was evident between the cells in the cluster. Staining for keratan sulphate was also absent between individual cells (data not shown).
The final cluster type contained cells so tightly packed that their membrane boundaries became difficult to discern with CMFDA (Fig. 2L) and the cellular mass appeared amorphous. These cellular masses were enveloped by a thin band of extracellular matrix that included keratan sulphate and type VI collagen, confined the chondrocytes within and conferred a smooth external appearance to the cluster.
Although the classification of these cluster types was based on the pattern of type VI collagen staining, the chondrocytes also continued to synthesize other molecules, such as fibronectin (Fig. 2N) and type II collagen (Fig. 2O,P).
The apparent size of individual cells in the clusters also varied depending on the cluster type. For example, the cells which were very tightly packed appeared smaller than cells in more loosely packed clusters. In addition, in some cluster types, the size of individual cells was uncertain as the cell boundaries could not be clearly observed.
Chondrocyte migration out of EC clusters
From weeks 2 to 4, some cells migrated out of existing clusters (Fig. 2Q–S) and formed new clusters in the adjacent matrix. The clusters or groups that resulted from these migrating cells were dominated by elongated or irregularly shaped cells which did not sequester type VI collagen (Fig. 2Q), type II collagen (Fig. 2R) or aggrecan (data not shown). However, fibronectin was usually observed to line the cell's path (Fig. 2S) or as a continuous layer surrounding each cell (Fig. 2T) in a cluster.
Chondrocyte loss from EC agarose constructs and alginate beads
By the end of week 3, large outgrowths of cell clusters could be seen protruding from the surface of both agarose constructs and alginate beads (data not shown). Some of these clusters had fallen out of their supporting gel and were visible as large floating balls of cells in the surrounding medium. Others appeared to have settled and begun to proliferate on the surface of the agarose base. The floating clusters were removed by pipette suction when the medium was changed. At 4 weeks, however, large sheets of de-differentiated chondrocytes had spread over the entire surface of the basal agarose layer in both culture systems. Moreover, although the majority of chondrocytes remained viable over the culture period, dead cells became more numerous in the central regions of the very large dense clusters over time.
Type VI collagen distribution in MC isolated from osteoarthritic cartilage
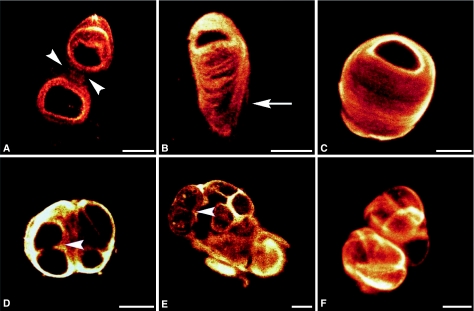
MC isolated from osteoarthritic cartilage and stained for type VI collagen are shown in Fig. 3. A variety of morphologies were observed, including single and double chondrons (Fig. 3A,B). The swollen tail region of single chondrons (Fig. 3B,C) was an indication of pericellular remodelling. In addition, chondrocyte division was identified as having occurred within the confines of the expanded pericellular microenvironment (Fig. 3D). A variety of chondrocyte clusters predominated in MC preparations from osteoarthritic cartilage, and two examples are illustrated (Fig. 3E,F). In one example, the chondrocytes in a cluster continued to proliferate upon an acellular mass of type VI collagen (Fig. 3E). In the other example, the cluster was entirely composed of closely packed chondrocytes, each enclosed by a thin veil of type VI collagen (Fig. 3F). Overall, the variety of chondrocyte clusters extracted reflected the heterogeneity of cluster patterns seen in histological sections of intact osteoarthritic cartilage.
Fig. 3.
Confocal photomicrographs showing extended focus images, from z series, of mechanically extracted chondrons from osteoarthritic cartilage labelled for type VI collagen. (A) Double chondron with arrowheads pointing to an interconnecting segment. (B) Single chondron with an arrow indicating the tail region of the chondron, which shows evidence of swelling. (C) Single chondron with a swollen microenvironment. (D–F) Chondron clusters with arrowheads indicating areas of pericellular remodelling. Scale bars = 10 µm.
Discussion
Osteoarthritic joints usually show a multiplicity of articular cartilage morphologies, from apparently normal cartilage at the periphery, through to degenerative matrix changes, cellular loss and cartilage erosion in the centre. These heterogeneous changes are often difficult to study in biopsy or necropsy tissue samples, and models are required to examine not only matrix catabolism, but also the changing role of the chondrocyte during division, re-differentiation and ultimately loss from the tissue. In the present study, methods to enzymatically extract chondrons from adult human articular cartilage were successfully modified and adapted for the extraction of chondrons from adult canine tibial cartilage. Investigation of the behaviour of these EC preparations in three-dimensional culture revealed chondron remodelling, cell division, migration and proliferation to form clusters with characteristic morphologies. These changes in chondron morphology and chondrocyte behaviour appeared to mimic those seen in heterogeneous samples of MC from osteoarthritic canine tibial cartilage. We believe that canine tibial EC in culture recapitulate the events associated with the initiation of osteoarthritis, namely remodelling of the chondron microenvironment, chondrocyte division and migration within the microenvironment, and cell proliferation to form clusters (Poole, 1997).
Extraction of EC from adult canine tibial cartilage
Enzymatic extraction of canine tibial cartilage produced EC with morphological features in common with MC (Poole et al. 1988b). Single EC predominated with each chondrocyte surrounded by a distinct microenvironment that frequently had a ‘tail’ trailing from one pole, as seen in MC. Double and, less frequently, triple EC columns were also identified, in which individual chondrocytes and their microenvironments were interconnected by continuous segments of the capsule (Poole et al. 1988b). The strong labelling for type VI collagen and keratan sulphate and absence of fibronectin were also consistent with previous studies (Lee et al. 1997, 2000). However, in our canine EC, limited type II collagen staining presented only as stippled fragments or short strands within the microenvironment, whereas previous research on human EC showed a more significant retention of type II collagen (Lee et al. 1997). Since the human cartilage used in that study was from both normal and osteoarthritic subjects (mean ages of 50 and 61 years, respectively), it is possible that the type II collagen present in the human EC samples was more strongly cross-linked (Bank et al. 1998; Bailey & Paul, 1999; Brama et al. 1999), thereby making it more resistant to enzymatic extraction than that in our canine cartilage (mean age 3 years). By contrast, types II, VI and IX collagen, laminin, fibronectin, hyaluronan, aggrecan and link protein were all retained within the pericellular microenvironment of canine MC (Poole et al. 1988a, 1991a, 1997). Therefore, although morphological features appear common between MC and EC, the marked differences in their molecular anatomy suggest that MC are likely to have greater structural and macromolecular integrity than EC.
It is clear that total removal of the interterritorial and territorial matrices, and loss of important components from the chondron microenvironment during the enzymatic digestion procedure must disrupt both cell–matrix and matrix–matrix interactions. The removal of matrix ligands, such as fibronectin and type II collagen, would lead to the interruption of outside-in signalling from the extracellular matrix. Integrins α1β1, α3β1 and α5β1 have previously been reported on the surface of freshly isolated bovine chondrocytes, and their levels of expression increased in culture from days 3 to 7 (Loeser et al. 1995). In the current study, fibronectin was detected on the cell surface and in the pericellular microenvironment within 2 days of extraction, indicating that some integrins were still expressed by EC. Fibronectin also co-localizes with type VI collagen in MC (Chang et al. 1997) and during the process of reassembly of the pericellular microenvironment in agarose-cultured chondrocytes (Chang & Poole, 1997). This suggests that fibronectin may play an important role not only in chondrocyte adhesion to the pericellular microenvironment, but also in restoration of the macromolecular integrity of the chondron.
Another important receptor affected by enzymatic chondron extraction is CD44, the cell-surface receptor for hyaluronan, which was partially lost during extraction of human EC, but returned after 1–3 days in culture (Lee et al. 1997). Although we did not specifically examine the distribution of CD44, the increased retention of keratan sulphate over time noted here and in previous studies (Knight et al. 2001; Hing et al. 2002) is thought to reflect up-regulation of CD44, as hyaluronan is responsible for facilitating the retention and anchorage of aggrecan.
A previous study investigated EC and concluded that the pericellular microenvironment is likely to have a functional role that is dependent on its biomechanical properties (Guilak et al. 1999).
We believe that the loss of key components from the extracellular matrix and pericellular matrix surrounding the chondrocyte in EC may provide a stimulus for the subsequent remodelling and events observed in our study due to changes in the biophysical conditions.
EC cultured in agarose constructs and alginate beads
Culture of canine EC in either hydrogel revealed remodelling of the pericellular microenvironment, initiation of chondrocyte division, cellular migration out of the pericellular capsule, and accelerated proliferation to form clusters with characteristic morphologies. It would therefore appear that, in order to reassemble a fully functional pericellular microenvironment, the residual chondron matrix must be remodelled and key components restored.
Within the first few days of EC culture, the chondrocyte divided within the confines of the pericellular capsule, suggesting that the chondrocyte must be capable of remodelling the microenvironment to provide sufficient space for division to occur. Although metalloproteinases are likely to be involved in this remodelling process (Loeser, 1994), serine proteases may also play a role, as intact type VI collagen comprises the major component of EC (Kielty et al. 1993; Everts et al. 1995, 1996; Myint et al. 1996). Phagocytosis of type VI collagen has been shown to occur in fibroblasts (Everts et al. 1995, 1996) and although it is likely that chondrocytes have the same capability, remodelling of type VI collagen has not yet been examined specifically in chondrons. A study of type VI collagen metabolism using osteoarthritic canine cartilage found small gaps in the chondron capsule and noted that some capsules appeared to be ‘breaking down’ (Arican et al. 1996). The presence of similar gaps in the type VI collagen-stained capsules of canine EC suggests that the chondrocyte may be focusing proteolytic enzymes at specific parts of the cell surface (Murphy & Gavrilovic, 1999), thinning the capsule and creating gaps through which it could subsequently divide and migrate.
In normal adult articular cartilage, chondrocytes seldom divide within their microenvironment and the turnover of type II collagen is very low (Buttle et al. 1995). During osteoarthritis, however, the degradation of heterotypic fibrillar collagens in the pericellular microenvironment represents an early event in the disease process (Poole et al. 1991b, 1997). This localized damage to the pericellular collagen network leads to a loss of pericellular proteoglycans, and together these factors facilitate chondrocyte division followed by continued proliferation to form clonal chondrocyte clusters as the disease progresses. The use of collagenase to disrupt the collagenous network of the extracellular matrix of bovine articular cartilage has been shown to cause an increase in chondrocyte proliferation (Lee et al. 1993). A separate study using various enzymes to disrupt the extracellular matrix of cartilage explants indicated that the collagen levels and organization of the matrix had more influence over the control of proteoglycan synthesis (Lee et al. 1994). We argue that the process of enzymatically isolating chondrons mimics this osteoarthritic pathology, and that the resulting damage to the pericellular microenvironment promotes pericellular remodelling, initiation of chondrocyte division and cellular proliferation to form large chondrocyte clusters with characteristic morphologies.
A feature of these early chondron remodelling and cell division events in EC was the tendency for one of the chondrocytes to migrate out of the chondron microenvironment by first extending elongated pseudopodia and cytoplasm through a gap in the capsule, then ‘dragging’ the remainder of the cell onto the surface of the capsule. These changes in chondrocyte morphology from rounded to elongated are directly related to cellular locomotion and are known to be induced by changes in the cytoskeletal assembly mediated by fibronectin, integrins, hyaluronan and hyaluronan receptors (McCarthy & Turley, 1993). Other studies of isolated chondrocytes cultured in agarose have also demonstrated a dramatic increase in cytoskeletal organization during the culture period that correlated with changes in the chondrocyte phenotype (Idowu et al. 2000). It is therefore possible that changes in the composition and integrity of the pericellular microenvironment during extraction of EC may directly result in cellular shape changes and the induction of locomotory behaviour. Initially, we found no evidence of the chondrocytes in EC migrating away from the capsule into the hydrogel. Rather, the cell appeared to remain anchored to the outside of the capsule and continued to proliferate, gradually enclosing the original capsule within the forming cluster.
At later time points, however, large numbers of fibroblastic cells often formed cellular tracts migrating away from dense chondrocyte clusters. The cells in these tracts did not sequester type II collagen or aggrecan, indicating that they had de-differentiated (Benya & Shaffer, 1982), but continued to synthesize and retain fibronectin, an essential requirement for adhesion and spreading (Enomoto et al. 1993; Enomoto-Iwamoto et al. 1997). Chondrocytes cultured in alginate beads have been observed to migrate along microtracks inside the bead (Aydelotte et al. 1998), and it is well established that chondrocytes migrate when cultured on plastic (Benya & Shaffer, 1982; Frenkel et al. 1996). Moreover, chondrocytes have been shown to migrate in vitro when cultured on fibronectin-coated membranes in Boyden chambers (Chang et al. 2003) and a separate study of chondrocytes from osteoarthritic cartilage (Reboul et al. 2001) revealed that hepatocyte growth factor, which can stimulate chondrocyte migration, may be involved in cartilage repair. In the current study, the migratory behaviour of chondrocytes in the latter half of the culture period resulted in large numbers of cells falling out of the agarose constructs and alginate beads, contaminating the acellular agarose support and confounding analysis of cell proliferation and synthesis.
Although little published comment has previously been made about cells that contaminate the media and exposed surfaces of agarose construct and alginate bead cultures, we found it to be a common problem that must affect the reliability of proliferation data achieved with cultures beyond 2–3 weeks.
Cluster morphology may reflect the cellular zonal origin
When EC were cultured in hydrogels for 2–4 weeks, they formed a variety of cluster morphologies that are thought to result from their differing zonal origins. In studies on isolated chondrocytes, it has been shown that phenotypic differences are maintained when chondrocytes from different zones are cultured in agarose (Aydelotte & Kuettner, 1988). Superficial layer chondrocytes cultured in agarose gel showed minimal staining with alcian blue, whereas deep layer chondrocytes formed clusters with rounded cells that showed intense alcian blue staining between each cell in the cluster. The pattern of alcian blue staining seen around the deep layer chondrocytes is consistent with that seen in the two principal cluster types in the current study, which makes sense given that the majority of the chondrons in the canine tibial cartilage samples used are from the middle and deep layers (Arokoski et al. 1999). Identification, in small numbers, of two further cluster types comprising flattened cells that lacked pericellular keratan sulphate and type VI collagen suggests that these clusters may be derived from superficial layer cells, which make up about 4% of the total tissue volume (Arokoski et al. 1999).
The external shape of the clusters may also indicate the origin of the cells in each cluster. In a study of isolated human articular cartilage chondrocytes cultured over agarose, clusters formed from superficial layer cells had smooth outlines and were either highly cellular or amorphous (Archer et al. 1990). Clusters formed from deep layer chondrocytes were termed lobulate. The majority of clusters formed from isolated EC in the current study also contained rounded cells separated by matrix, and appeared lobulate, whereas small numbers had flattened cells encircling the cluster and creating a smooth outline. As stated previously, these differences are consistent with the structural organization of the tissue (Arokoski et al. 1999), and future studies on cultured EC using markers such as superficial zone protein will enable further elucidation of the origin of the cells within these clonal chondron clusters.
MC from osteoarthritic cartilage provide evidence of chondron remodelling
The current study compared the behaviour of EC in culture with the structure of MC from osteoarthritic cartilage in order to assess any similarities and to determine whether EC cultures could be used as a model for the initiation of osteoarthritis. We have shown that a heterogeneous mixture of chondron morphologies can be mechanically extracted from spontaneously osteoarthritic canine tibial cartilage. They vary from normal chondron structures with tails through to complex clusters of chondrocytes showing clear evidence of pericellular type VI collagen remodelling. A comparative study of EC found that the frequency of enlarged chondrons was greater in osteoarthritic cartilage than in normal cartilage, primarily due to increased matrix deposition (Lee et al. 2000). Although studies on MC isolated from osteoarthritic cartilage have revealed substantial changes in the fibrillar collagen composition and proteoglycan concentration (Poole et al. 1991a, 1997), studies of isolated chondrocytes in agarose gel demonstrated increased pericellular type VI collagen deposition in the presence of interleukin-1, a key catabolic cytokine in the initiation and progression of osteoarthritis (Chang & Poole, 1996). In addition, a recent study found that the gene expression of fibroblast activation protein alpha was up-regulated in isolated chondrocytes and cartilage explants following treatment with interleukin-1 and oncostatin M, and was higher in osteoarthritic cartilage than in normal articular cartilage (Milner et al. 2006). Increased levels of type VI collagen protein and mRNA have also been found in the clusters present in osteoarthritic cartilage (Ronziere et al. 1990; Loeser, 1993; Arican et al. 1996), suggesting that increased synthesis and deposition of type VI collagen compensate for the loss of other pericellular macromolecules during osteoarthritis (Pullig et al. 1999). Moreover, integrin α1β1, a receptor for type VI collagen, was identified as the most frequently expressed β1 integrin in human osteoarthritic cartilage (Loeser et al. 1995, 2000; Loeser, 2000). Therefore, type VI collagen, which is resistant to the matrix metalloproteinases driving the osteoarthritic process (Kielty et al. 1993), appears to play a key role in the remodelling process, substituting for the fibrillar collagens by providing a resistant but adaptable pericellular coat for the rapidly dividing and synthesizing chondrocytes.
We previously compared the biophysical properties of isolated chondrocytes, EC and MC (Knight et al. 2001), and determined that the pericellular matrix of MC was more mechanically robust than that of EC immediately after extraction.
Other studies (Alexopoulos et al. 2003, 2005) investigated the biophysical properties of chondrons that were mechanically isolated from normal and osteoarthritic cartilage using a novel microaspiration technique, and found that the pericellular matrix stiffness of chondrons from osteoarthritic cartilage was significantly lower than that of chondrons from normal adult cartilage.
Another observed feature of MC isolated from osteoarthritic cartilage was evidence of cell division within a swollen pericellular microenvironment, namely migration of cells within the microenvironment and remodelling of its morphological characteristics, as the cells progressively formed the clonal clusters characteristic of osteoarthritic cartilage (Pfander et al. 2001). Cell-to-cell communication through gap junctions may play a critical role in the formation of these clusters (Donahue et al. 1995; Chi et al. 2004), and other studies have suggested that cell death may occur by apoptosis following a period of proliferation (Sandell & Aigner, 2001).
It has been shown that 75% of surface layer chondrocytes and 10% of deep layer chondrocytes in intact tissue express alpha smooth muscle actin (Kim & Spector, 2000). In a separate study, chondrocyte-like cells that strongly expressed alpha smooth muscle actin were found in samples obtained from human temporomandibular joint discs with severe pathological conditions (Leonardi et al. 2002). The latter authors suggested that cells with this ‘contractile phenotype’ located in these severely affected zones may play a role in repair.
Conclusions
We conclude that canine tibial EC cultured in three-dimensional hydrogels mimic processes associated with the initiation and progression of chondron remodelling, one of the early events in the osteoarthritic process. These events represent the previously proposed three phases of chondron remodelling, namely swelling and remodelling of the pericellular microenvironment, initiation of cell division and migration associated with the swollen microenvironment, and continued proliferation and modified synthesis to form clonal chondrocyte clusters. Further studies investigating the involvement of proteolytic enzymes and the expression of smooth muscle actin in the migrating chondrocytes are required to validate this EC model. However, we believe that EC in culture could provide a useful model for studying the chondron remodelling that occurs during the initiation and progression of osteoarthritis.
Acknowledgments
We thank the Health Research Council of New Zealand and the Arthritis Foundation of New Zealand who provided the funding for this research. Confocal microscopy was carried out in the Biomedical Imaging Research Unit at the University of Auckland.
References
- Alexopoulos LG, Haider MA, Vail TP, Guilak F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J Biomechan Engineering-Trans ASME. 2003;125:323–333. doi: 10.1115/1.1579047. [DOI] [PubMed] [Google Scholar]
- Alexopoulos LG, Williams GM, Upton ML, Setton LA, Guilak F. Osteoarthritic changes in the biphasic mechanical properties of the chondrocyte pericellular matrix in articular cartilage. J Biomechanics. 2005;38:509–517. doi: 10.1016/j.jbiomech.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Archer C, McDowell J, Bayliss M, Stephens M, Bentley G. Phenotypic modulation in sub-populations of human articular chondrocytes in vitro. J Cell Sci. 1990;97:361–371. doi: 10.1242/jcs.97.2.361. [DOI] [PubMed] [Google Scholar]
- Arican M, Carter SD, Bennett D, Ross G, Ayad S. Increased metabolism of collagen VI in canine osteoarthritis. J Comparative Pathol. 1996;114:249–256. doi: 10.1016/s0021-9975(96)80046-6. [DOI] [PubMed] [Google Scholar]
- Arokoski JPA, Hyttinen MM, Helminen HJ, Jurvelin JS. Biomechanical and structural characteristics of canine femoral and tibial cartilage. J Biomed Mater Res. 1999;48:99–107. doi: 10.1002/(sici)1097-4636(1999)48:2<99::aid-jbm1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connective Tissue Res. 1988;18:205–222. doi: 10.3109/03008208809016808. [DOI] [PubMed] [Google Scholar]
- Aydelotte MB, Thonar E, Mollenhauer J, Flechtenmacher J. Culture of chondrocytes in alginate gel – variations in conditions of gelation influence the structure of the alginate gel, and the arrangement and morphology of proliferating chondrocytes. In Vitro Cell Dev Biol Anim. 1998;34:123–130. doi: 10.1007/s11626-998-0094-x. [DOI] [PubMed] [Google Scholar]
- Bailey AJ, Paul RG. The mechanisms and consequences of the maturation and ageing of collagen. Proc Indian Acad Sci-Chem Sci. 1999;111:57–69. [Google Scholar]
- Bank RA, Bayliss MT, Lafeber F, Maroudas A, Tekoppele JM. Ageing and zonal variation in post-translational modification of collagen in normal human articular cartilage – the age-related increase in non-enzymatic glycation affects biomechanical properties of cartilage. Biochem J. 1998;330:345–351. doi: 10.1042/bj3300345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff A. Form und Bau der Gelenkknorpel in Ihren Bezeihungen zur Junktion. Zweiter Teil. Der aufbau des Gelenkknorpels in seinen Bezeihungen zur Funktion. Z Zellforch. 1925;2:783–862. [Google Scholar]
- Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- Brama PAJ, TeKoppele JM, Bank RA, van Weeren PR, Barneveld A. Influence of site and age on biochemical characteristics of the collagen network of equine articular cartilage. Am J Vet Res. 1999;60:341–345. [PubMed] [Google Scholar]
- Buttle DJ, Bramwell H, Hollander AP. Proteolytic mechanisms of cartilage breakdown: a target for arthritis therapy. J Clin Pathol: Mol Pathol. 1995;48:m167–m177. doi: 10.1136/mp.48.4.m167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Lauffenburger DA, Morales TI. Motile chondrocytes from newborn calf: migration properties and synthesis of collagen II. Osteoarthritis Cartilage. 2003;11:603–612. doi: 10.1016/s1063-4584(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Chang J, Poole CA. Sequestration of type VI collagen in the pericellular microenvironment of adult chondrocytes cultured in agarose. Osteoarthritis Cartilage. 1996;4:275–285. doi: 10.1016/s1063-4584(05)80105-0. [DOI] [PubMed] [Google Scholar]
- Chang J, Poole CA. Confocal analysis of the molecular heterogeneity in the pericellular microenvironment produced by adult canine chondrocytes cultured in agarose gel. Histochem J. 1997;29:515–528. doi: 10.1023/a:1026467724216. [DOI] [PubMed] [Google Scholar]
- Chang J, Nakajima H, Poole CA. Structural colocalisation of type VI collagen and fibronectin in agarose cultured chondrocytes and isolated chondrons extracted from adult canine tibial cartilage. J Anat. 1997;190:523–532. doi: 10.1046/j.1469-7580.1997.19040523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SS, Rattner JB, Matyas JR. Communication between paired chondrocytes in the superficial zone of articular cartilage. J Anat. 2004;205:363–370. doi: 10.1111/j.0021-8782.2004.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue HJ, Guilak F, Vander Molen MA, et al. Chondrocytes isolated from mature articular cartilage retain the capacity to form functional gap junctions. J Bone Miner Res. 1995;10:1359–1364. doi: 10.1002/jbmr.5650100913. [DOI] [PubMed] [Google Scholar]
- Enomoto M, Leboy PS, Menko AS, Boettiger D. Beta 1 integrins mediate chondrocyte interaction with type I collagen, type II collagen, and fibronectin. Exp Cell Res. 1993;205:276–285. doi: 10.1006/excr.1993.1087. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Iwamoto M, Nakashima K, et al. Involvement of alpha-5-beta-1 integrin in matrix interactions and proliferation of chondrocytes. J Bone Miner Res. 1997;12:1124–1132. doi: 10.1359/jbmr.1997.12.7.1124. [DOI] [PubMed] [Google Scholar]
- Everts V, Korper W, Niehof A, Jansen I, Beertsen W. Type VI collagen is phagocytosed by fibroblasts and digested in the lysosomal apparatus – involvement of collagenase, serine proteinases and lysosomal enzymes. Matrix Biol. 1995;14:665–676. doi: 10.1016/s0945-053x(05)80030-7. [DOI] [PubMed] [Google Scholar]
- Everts V, Vanderzee E, Creemers L, Beertsen W. Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. Histochem J. 1996;28:229–245. doi: 10.1007/BF02409011. [DOI] [PubMed] [Google Scholar]
- Frenkel SR, Clancy RM, Ricci JL, Dicesare PE, Rediske JJ, Abramson SB. Effects of nitric oxide on chondrocyte migration, adhesion, and cytoskeletal assembly. Arthritis Rheum. 1996;39:1905–1912. doi: 10.1002/art.1780391118. [DOI] [PubMed] [Google Scholar]
- Guilak F, Jones WR, Ting-Beall HP, Lee GM. The deformation behavior and mechanical properties of chondrocytes in articular cartilage. Osteoarthritis Cartilage. 1999;7:59–70. doi: 10.1053/joca.1998.0162. [DOI] [PubMed] [Google Scholar]
- Handley CJ, Winter GM, Ilic MZ, et al. Distribution of newly synthesized aggrecan in explant cultures of bovine cartilage treated with retinoic acid. Matrix Biol. 2002;21:579–592. doi: 10.1016/s0945-053x(02)00078-1. [DOI] [PubMed] [Google Scholar]
- Hing WA, Sherwin AF, Ross JM, Poole CA. The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge. Osteoarthritis Cartilage. 2002;10:297–307. doi: 10.1053/joca.2002.0517. [DOI] [PubMed] [Google Scholar]
- Idowu BD, Knight MM, Bader DL, Lee DA. Confocal analysis of cytoskeletal organisation within isolated chondrocyte sub-populations cultured in agarose. Histochem J. 2000;32:165–174. doi: 10.1023/a:1004095207330. [DOI] [PubMed] [Google Scholar]
- Kielty CM, Lees M, Shuttleworth CA, Woolley D. Catabolism of intact type VI collagen microfibrils: susceptibility to degradation by serine proteinases. Biochem Biophys Res Commun. 1993;191:1230–1236. doi: 10.1006/bbrc.1993.1349. [DOI] [PubMed] [Google Scholar]
- Kim AC, Spector M. Distribution of chondrocytes containing alpha-smooth muscle actin in human articular cartilage. J Orthopaedic Res. 2000;18:749–755. doi: 10.1002/jor.1100180511. [DOI] [PubMed] [Google Scholar]
- Knight MM, Ross JM, Sherwin AF, Lee DA, Bader DL, Poole CA. Chondrocyte deformation within mechanically and enzymatically extracted chondrons compressed in agarose. Biochim Biophys Acta-Gen Subjects. 2001;1526:141–146. doi: 10.1016/s0304-4165(01)00118-0. [DOI] [PubMed] [Google Scholar]
- Larson C, Kelley S, Blackwood A, Banes A, Lee G. Retention of the native chondrocyte pericellular matrix results in significantly improved matrix production. Matrix Biol. 2002;21:349–359. doi: 10.1016/s0945-053x(02)00026-4. [DOI] [PubMed] [Google Scholar]
- Lee DA, Bentley G, Archer CW. Proteoglycan depletion alone is not sufficient to stimulate proteoglycan synthesis in cultured bovine cartilage explants. Osteoarthritis Cartilage. 1994;2:175–185. doi: 10.1016/s1063-4584(05)80067-6. [DOI] [PubMed] [Google Scholar]
- Lee GM, Johnstone B, Jacobson K, Caterson B. The dynamic structure of the pericellular matrix on living cells. J Cell Biol. 1993;123:1899–1907. doi: 10.1083/jcb.123.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GM, Poole CA, Kelley SS, Chang J, Caterson B. Isolated chondrons – a viable alternative for studies of chondrocyte metabolism in vitro. Osteoarthritis Cartilage. 1997;5:261–274. doi: 10.1016/s1063-4584(97)80022-2. [DOI] [PubMed] [Google Scholar]
- Lee GM, Paul TA, Slabaugh M, Kelley SS. The incidence of enlarged chondrons in normal and osteoarthritic human cartilage and their relative matrix density. Osteoarthritis Cartilage. 2000;8:44–52. doi: 10.1053/joca.1999.0269. [DOI] [PubMed] [Google Scholar]
- Leonardi R, Villari L, Piacentini C, Bernasconi G, Travali S, Caltabiano C. Immunolocalization of vimentin and alpha-smooth muscle actin in dysfunctional human temporomandibular joint disc samples. J Oral Rehabil. 2002;29:282–286. doi: 10.1046/j.1365-2842.2002.00829.x. [DOI] [PubMed] [Google Scholar]
- Loeser RF. Integrin-mediated attachment of articular chondrocytes to extracellular matrix proteins. Arthritis Rheumatism. 1993;36:1103–1110. doi: 10.1002/art.1780360811. [DOI] [PubMed] [Google Scholar]
- Loeser RF. Modulation of integrin-mediated attachment of chondrocytes to extracellular matrix proteins by cations, retinoic acid, and transforming growth factor beta. Exp Cell Res. 1994;211:17–23. doi: 10.1006/excr.1994.1053. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Carlson CS, McGee MP. Expression of beta 1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Exp Cell Res. 1995;217:248–257. doi: 10.1006/excr.1995.1084. [DOI] [PubMed] [Google Scholar]
- Loeser RF. Chondrocyte integrin expression and function. Biorheology. 2000;37:109–116. [PubMed] [Google Scholar]
- Loeser RF, Sadiev S, Tan L, Goldring MB. Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for alpha 1 beta 1 and alpha 2 beta 1 integrins in mediating chondrocyte adhesion to types II and VI collagen. Osteoarthritis Cartilage. 2000;8:96–105. doi: 10.1053/joca.1999.0277. [DOI] [PubMed] [Google Scholar]
- McCarthy J, Turley EA. Effects of extracellular matrix components on cell locomotion. Crit Rev Oral Biol Med. 1993;4:619–637. doi: 10.1177/10454411930040050101. [DOI] [PubMed] [Google Scholar]
- Milner JM, Kevorkian L, Young DA, et al. Fibroblast activation protein alpha is expressed by chondrocytes following a pro-inflammatory stimulus and is elevated in osteoarthritis. Arthritis Res Ther. 2006;8:U254–U261. doi: 10.1186/ar1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok SS, Masuda K, Hauselmann HJ, Aydelotte MB, Thonar EJ. Aggrecan synthesized by mature bovine chondrocytes suspended in alginate. Identification of two distinct metabolic matrix pools. J Biol Chem. 1994;269:33021–33027. [PubMed] [Google Scholar]
- Murphy G, Gavrilovic J. Proteolysis and cell migration: creating a path? Curr Opin Cell Biol. 1999;11:614–621. doi: 10.1016/s0955-0674(99)00022-8. [DOI] [PubMed] [Google Scholar]
- Myint E, Brown DJ, Ljubimov AV, Kyaw M, Kenney MC. Cleavage of human corneal type VI collagen alpha-3 chain by matrix metalloproteinase-2. Cornea. 1996;15:490–496. [PubMed] [Google Scholar]
- Pfander D, Kortje D, Weseloh G, Swoboda B. Cell proliferation in osteoarthritic human cartilage. Z Orthopadie Ihre Grenzgebiete. 2001;139:375–381. doi: 10.1055/s-2001-17977. (in German) [DOI] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW. Morphological and functional interrelationships of articular cartilage matrices. J Anat. 1984;138:113–138. [PMC free article] [PubMed] [Google Scholar]
- Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage. I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci. 1988a;90:635–643. doi: 10.1242/jcs.90.4.635. [DOI] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW. Chondrons extracted from canine tibial cartilage: preliminary report on their isolation and structure. J Orthopaedic Res. 1988b;6:408–419. doi: 10.1002/jor.1100060312. [DOI] [PubMed] [Google Scholar]
- Poole CA, Glant TT, Schofield JR. Chondrons from articular cartilage. (IV). Immunolocalization of proteoglycan epitopes in isolated canine tibial chondrons. J Histochem Cytochem. 1991a;39:1175–1187. doi: 10.1177/39.9.1717545. [DOI] [PubMed] [Google Scholar]
- Poole CA, Matsuoka A, Schofield JR. Chondrons from articular cartilage. III. Morphologic changes in the cellular microenvironment of chondrons isolated from osteoarthritic cartilage. Arthritis Rheumatism. 1991b;34:22–35. doi: 10.1002/art.1780340105. [DOI] [PubMed] [Google Scholar]
- Poole CA, Brookes NH, Gilbert RT, et al. Detection of viable and non-viable cells in connective tissue explants using the fixable fluoroprobes 5-chloromethylfluorescein diacetate and ethidium homodimer-1. Connective Tissue Res. 1996;33:233–241. doi: 10.3109/03008209609028880. [DOI] [PubMed] [Google Scholar]
- Poole CA. Articular cartilage chondrons – form, function and failure. J Anat. 1997;191:1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CA, Gilbert RT, Herbage D, Hartmann DJ. Immunolocalization of type IX collagen in normal and spontaneously osteoarthritic canine tibial cartilage and isolated chondrons. Osteoarthritis Cartilage. 1997;5:191–204. doi: 10.1016/s1063-4584(97)80014-3. [DOI] [PubMed] [Google Scholar]
- Pullig O, Weseloh G, Swoboda B. Expression of type VI collagen in normal and osteoarthritic human cartilage. Osteoarthritis Cartilage. 1999;7:191–202. doi: 10.1053/joca.1998.0208. [DOI] [PubMed] [Google Scholar]
- Reboul P, Pelletier JP, Tardif G, et al. Hepatocyte growth factor induction of collagenase 3 production in human osteoarthritic cartilage – Involvement of the stress-activated protein kinase/c-Jun N-terminal kinase pathway and a sensitive p38 mitogen-activated protein kinase inhibitor cascade. Arthritis Rheumatism. 2001;44:73–84. doi: 10.1002/1529-0131(200101)44:1<73::AID-ANR11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Ronziere MC, Ricard-Blum S, Tiollier J, Hartmann DJ, Garrone R, Herbage D. Comparative analysis of collagens solubilized from human foetal, and normal and osteoarthritic adult articular cartilage, with emphasis on type VI collagen. Biochim Biophys Acta. 1990;1038:222–230. doi: 10.1016/0167-4838(90)90209-x. [DOI] [PubMed] [Google Scholar]
- Sandell LJ, Aigner T. Articular cartilage and changes in arthritis – An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szirmai F. Structure of cartilage. In: Engel A, Larsson T, editors. In Ageing of Connective and Skeletal Tissue. Stockholm: Nordiska Bokhandelns; 1969. pp. 163–184. [Google Scholar]
- Winter GM, Poole CA, Ilic MZ, Ross JM, Robinson HC, Handley CJ. Identification of distinct metabolic pools of aggrecan and their relationship to type VI collagen in the chondrons of mature bovine articular cartilage explants. Connective Tissue Res. 1998;37:277–293. doi: 10.3109/03008209809002445. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Ratcliffe A, Watt FM. Two subpopulations of differentiated chondrocytes identified with a monoclonal antibody to keratan sulfate. J Cell Biol. 1985;101:53–59. doi: 10.1083/jcb.101.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]