Abstract
Both the giant panda (Ailuropoda melanoleuca) and the red panda (Ailurus fulgens) possess a ‘false-thumb’, actually an enlarged radial sesamoid bone, which contributes to the gripping action of the hand. These species are not closely related, however, as one is an ursid and the other an ailurid, so the fact that they share this adaptation implies a remarkable convergence. We studied the functional anatomy of this structure in the red panda, comparing it with existing descriptions of the grasping mechanism in both pandas. Previous interpretations of the radial sesamoid in Ailurus as a rod-like structure without direct articulation to the wrist bones are inaccurate. There are various important differences between the red panda and the giant panda. In the former, the lesser development of the radial sesamoid, its connection with the flexor retinaculum, the presence of an insertion of the muscle abductor pollicis longus in the first metacarpal, which enhances its supinatory action, and the presence of a muscle flexor brevis digitorum manus point to thin-branch climbing features serving as an exaptation to the more recent role of the red panda hand in the manipulation of bamboo.
Keywords: functional anatomy, panda, radial, sesamoid
Introduction
The ‘false-thumb’ is a small bone, the radial sesamoid, that is present in many carnivores, which has enlarged in pandas, contributing to gripping actions, and compensating for the lack of prehensile abilities in a hand where the pollex is aligned with the other digits. Although the giant panda (Ailuropoda melanoleuca) and the red panda (Ailurus fulgens) are not closely related (Goldman et al. 1989; Ledje & Arnason, 1996; Flynn & Nedbal, 1998; Bininda-Emonds et al. 1999; Flynn et al. 2000, 2005) the fact that they share this trait, together with other anatomical features associated with their adaptation to herbivory, has encouraged the idea that both species might have a relatively recent common ancestor. Such perceptions have led to proposed classifications of the red panda as a member of the ursidae, reflected in standard reference books (Wozencraft, 1989a,b, 1993). Both extant species have Asiatic distributions, but whereas the fossil record of the giant panda group is restricted to Eurasia, with early members of the extant genus Ailuropoda (Pei, 1974) as well as the Late Miocene genera Ailurarctos and Agriarctos (Qiu & Qi, 1989), fossils of red panda relatives are known from sites both in Eurasia, including the genera Simocyon, Magerictis and Parailurus (Ginsburg et al. 1997, 2001; Wang, 1997; Sasagawa et al. 2003; Peigné et al. 2005), and in North America, including Parailurus and Pristinailurus (Tedford & Gustafson, 1977; Sasagawa et al. 2003; Wallace & Wang, 2004). In spite of this relatively rich record, the lack of postcranial elements has precluded any insight into the evolution of the false-thumb in the red panda group. However, the recent discovery that Simocyon, a Miocene red panda relative with a carnivorous diet, had a false-thumb (Salesa et al. 2006), has shown that this feature evolved in early members of the red panda lineage prior to the development of secondary herbivory, probably as an adaptation for thin-branch climbing, reinforcing the notion of its independent evolution in both pandas.
The anatomy of the giant panda was described by Davis (1964) in a landmark study, and more recently the gripping mechanism in this species has been re-studied, leading to a new functional interpretation of the action of the false-thumb (Endo et al. 1999a,b, 2001a). The radial sesamoid and related structures in the red panda have recently been the subject of gross dissection (Endo et al. 2001b), suggesting the presence of significant anatomical differences from the giant panda and the possibility of a very different functionality. Nonetheless, the precise relationships between the radial sesamoid and the whole carpus in anatomical articulation remain poorly understood, and a functional model that satisfactorily explains the action of the false-thumb in red pandas is still lacking. In this study we combined gross dissection with computed tomography (CT) scan imagery and detailed observation of skeletal morphology, providing for the first time an accurate indication of the anatomical relationships of the bones of the forearm and hand, allowing a more precise understanding of its gripping mechanism. Finally, we have integrated our morphological and functional observations into an evolutionary context, accounting for the fossil record to provide a scenario for the separate origins of the false-thumb in the two panda lineages.
Materials and methods
We dissected two specimens, one male and one female, of Ailurus fulgens at the Facultad de Medicina of the Universidad de Valladolid (Spain). The animals died in 2005/6 at the Zoological Garden of Madrid (Spain). They were kept refrigerated until just before dissection, so the muscles were still in excellent condition and all the structures and muscular attachments were clearly visible. We used radiographic and CT scan images taken on the intact, right-side forelimb of the male specimen as reference for the anatomical position of the forearm bones in articulation. It was scanned in a helicoidal CT scanner (Somatom, Siemens) with a 0.75-mm slice thickness and 0.5-mm gaps. For detailed osteological observations, we used the skeleton of another specimen (no. 2031) from the Museo Anatómico of the Universidad de Valladolid. We concentrated on those muscles related to the movements of the radial sesamoid, and to the grasping action of the manus.
Radial sesamoid and associated musculature in Ailurus fulgens
Radial sesamoid
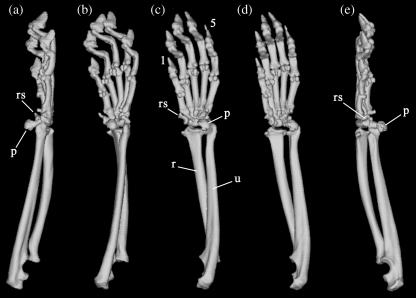
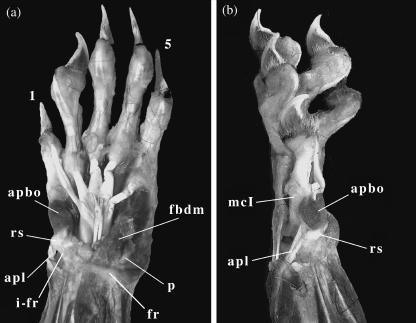
The radial sesamoid of Ailurus fulgens, although enlarged in relation to other carnivores, is a small bone (Fig. 1), measuring about 5.5 mm in maximum length in our specimen 2031. It is medio-distally orientated (Fig. 1) and has a clear synovial articulation with the surface of the posterior tubercle of the scapholunar. Its shape is subrectangular, elongated, and with a concave distal tip in which a cartilaginous cap is attached; the whole structure is connected to the flexor retinaculum (Fig. 2). A comparison of the CT scan images and the gross dissection photographs shows that the distal cartilaginous cap hides the actual bone from view during dissection unless cut off. This suggests that the observation of Endo et al. (2001b) regarding the lack of articulation of the radial sesamoid with any other bones of the wrist and hand may have been the result of a misinterpretation of the more superficial structures. The ‘ulnar end’ described by those authors can best be interpreted as a fibrous condensation of the ligamentous tissue of the flexor retinaculum, while their ‘radial part’, judging from the photographs, is more likely to correspond to the actual cartilaginous cap that makes up the distal third of the radial sesamoid.
Fig. 1.
Three-dimensional reconstructed CT images of the right forelimb of male Ailurus fulgens. (a) Lateral view; (b) latero-ventral view; (c) ventral view; (d) medio-ventral view; (e) medial view. 1, first digit; 5, fifth digit; p, pisiform; r, radius; rs, radial sesamoid; u, ulna.
Fig. 2.
Photographs of the dissected left forelimb of male Ailurus fulgens. (a) Ventral view; (b) medial view. 1, first digit; 5, fifth digit; apbo, muscles abductor pollicis brevis and opponens pollicis; apl, muscle abductor pollicis longus; fbdm, muscle flexor brevis digitorum manus; fr, flexor retinaculum; i-fr, insertion of flexor retinaculum on radial sesamoid; cI, first metacarpal; p, pisiform; rs, radial sesamoid (notice that instead of the actual bone, it is the distal cartilaginous cap that is visible in these images).
Abductor pollicis brevis and opponens pollicis muscles
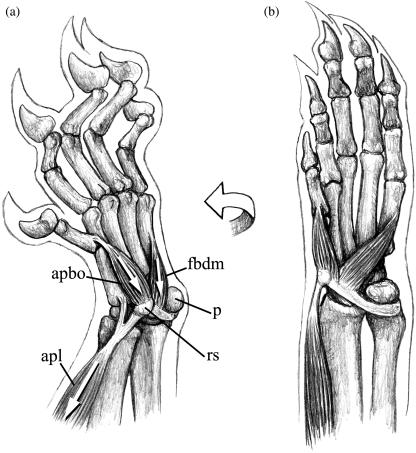
These muscles are thick and short, their fibres being so closely disposed that both are almost fused, as in the giant panda (Davis, 1964). They originate on the antero-medial face of the radial sesamoid and on the adjacent portion of the flexor retinaculum (a section of the palmar fascia, which defines the carpal groove) and insert on the proximal end of the first phalanx of the thumb, the attachment of the opponens pollicis being placed just below the insertion of the abductor pollicis brevis (Figs 2 and 3), as in Ailuropoda melanoleuca (Davis, 1964). Despite their thickness, their attachment areas on the first phalanx of the pollex and radial sesamoid are small. Although Davis (1964) interpreted these muscles as adductors of the radial sesamoid in Ailuropoda melanoleuca, our observations in Ailurus fulgens point to their action as flexors of pollex and the palm (Fig. 4).
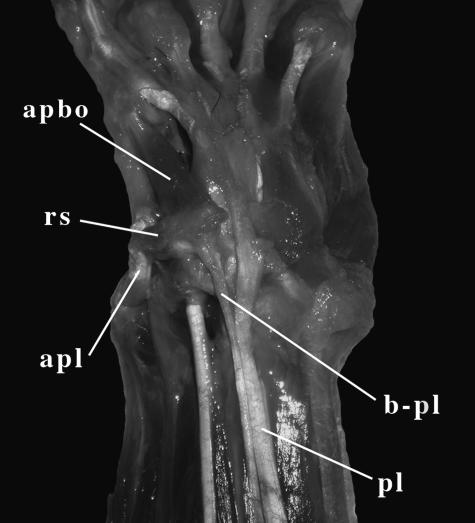
Fig. 3.
Ventral view of the left wrist of female Ailurus fulgens. apbo, muscles abductor pollicis brevis and oppones pollicis; apl, muscle abductor pollicis longus; b-pl, branch of muscle palmaris longus inserted on radial sesamoid; pl, muscle palmaris longus; rs, radial sesamoid.
Fig. 4.
Simplified drawing of the bones and selected muscles of the left hand of Ailurus fulgens. Small arrows show the direction of pull of muscular fibres during contraction. Curved arrow shows the direction of rotation of the hand due to the supinatory action of the contracting muscle abductor pollicis longus. (a) Medio-ventral view, extended articulation. (b) Ventral view, partly flexed articulation. Note that even in slight flexion, digit 1 overlaps digit 2, whilst digit 5 overlaps digit 4, showing initial ‘closure of the palm’. apbo, muscles abductor pollicis brevis and oppones pollicis; apl, muscle abductor pollicis longus; fbdm, muscle flexor brevis digitorum manus; p, pisiform; rs, radial sesamoid.
Abductor pollicis longus muscle
This muscle, mostly developed on the anterior face of the forearm, attaches in a small area, on the postero-lateral face of the radial sesamoid, and marginally on the base of the first metacarpal, through a narrow, long tendon that crosses a notch below the muscle brachioradialis, and broadening distally (Figs 2 and 3). Its contraction contributes to the abduction and flexion of thumb over the palm and, as observed during our dissection, pulling its fibres leads to supination of the hand. Davis (1964) noticed that in Ailuropoda melanoleuca this muscle does not attach on the first metacarpal, although the tendon ‘is divisible throughout its length into two elements, and the more lateral of these shows a tendency to subdivide further’ (Davis, 1964, p. 179).
Flexor brevis digitorum manus muscle
This muscle is absent in the giant panda and ursids in general (Davis, 1964) but is present in Ailurus fulgens (Fig. 2). In this species, this muscle develops as a wide, thin sheet, originating on the flexor retinaculum and inserting on the proximal epiphysis of the fifth metacarpal. Its contraction produces a medial rotation of the fifth metacarpal, thus providing a closing motion of the palm, which contributes to grasping actions. Although not directly attached to the radial sesamoid, this muscle can contribute to stabilization of that bone against the pull of abductor muscles, by way of the connection with the flexor retinaculum, described above.
Palmaris longus muscle
Its main tendon passes beneath the flexor retinaculum and inserts onto the palmar fascia. In Ailurus fulgens this muscle sends a branch that extends in a radial direction, inserting on the radial sesamoid (Fig. 3). Its contraction adducts the latter, or stabilizes it against the pull of the abductors.
Functional anatomy of the radial sesamoid in pandas
Use of the false-thumb
Both extant panda species use the false-thumb in food manipulation, holding stalks of bamboo and separating the leaves (Davis, 1964; Chorn & Hoffmann, 1978; Roberts & Gittleman, 1984). Detailed observations of this type of behaviour in the red panda indicate that ‘food items are grasped in a single forepaw and brought to the mouth while sitting, standing or occasionally lying on the back’ (Roberts & Gittleman, 1984, p. 5). The digits and wrist bones are used with remarkable dexterity, leading to the interpretation of the radial sesamoid as an opposable ‘sixth digit’ (Davis, 1964; Gould, 1980). Recently, this mechanism has been restudied in the giant panda (Endo et al. 1999a,b, 2001), producing a new interpretation of the gripping action as a ‘double pincer-like apparatus’. In this scenario, the radial sesamoid is not seen as an independent digit, but as part of a mechanism by which the whole manus flexes around two carpals (the scapholunar and unciform) enabling it to hold objects between the digits and the radial sesamoid and pisiform bones (Endo et al. 2001a). This is achieved by the rotation of the scapholunar on the distal surface of the radius, and of the proximal articulations of the fourth and fifth metacarpals on the distal surface of the unciform. The suggestion by Endo et al. (1999a, p. 299) that the muscles abductor pollicis brevis and opponens pollicis would act as ‘a cushion for objects grasped between the radial sesamoid and the first metacarpal’ seems to derive from observations on dead specimens where muscles are inert, whereas in live animals the contraction implies a shortening of the fibres, not a folding. In the living giant panda, it is the palmar pads that serve as cushions, and the muscles, contracting deep below, act to guide the motions of the hand.
Differences between the two pandas
The radial sesamoid in Ailuropoda melanoleuca is relatively large, laterally compressed and with a distal end that hooks medially (Davis, 1964), whereas in Ailurus fulgens it is proportionally smaller, not compressed and with a concave distal end. These differences have significant functional implications: the smaller size in Ailurus fulgens implies a lesser rotation arc, and the smaller protrusion of the bone suggests its reduced grasping capacity. The similar morphology of the radial sesamoid between Ailurus and Simocyon, including a concave distal tip, suggests the presence in the fossil taxon of a cartilaginous cap.
A recent gross anatomical study of the hand in Ailurus fulgens (Endo et al. 2001b) has led to the proposal of a radically different gripping mechanism in this species, including the action of the radial sesamoid as a supporting ridge, controlled by the motions of the flexor retinaculum, palmar aponeurosis, flexor and abductor muscles. But, as commented above, the purported differences in the structure of the radial sesamoid (including the lack of articulation with the carpus, a medio-lateral orientation and a rod-like morphology) apparently stem from a misinterpretation of superficial structures, and the functional model deriving from those observations is open to question.
Davis (1964) pointed to the absence of a muscle flexor brevis digitorum manus in the giant panda, while acknowledging its presence in all procyonids; as this author considered Ailurus fulgens to be a procyonid (Davis, 1964, p. 322), the statement would be inclusive of the red panda. Our study supports that difference, and also confirms the presence of an insertion of the tendon of the muscle abductor pollicis longus on the first metacarpal, and shows a previously unreported insertion of the muscle palmaris longus on the radial sesamoid.
The double insertion of the abductor pollicis longus in Ailurus fulgens (on the first metacarpal and on the radial sesamoid) enhances the supinatory action of this muscle in contrast to Ailuropoda melanoleuca, where the flexor action predominates. Davis (1964) listed the abduction of the radial sesamoid as the only action of this muscle, but he may have overemphasized the independent motions of that bone, in view of interpretations discussed above, which view it not as a digit, but as part of a grasping mechanism involving the wrist and hand (Endo et al. 1999a,b, 2001).
The presence in Ailurus fulgens of the muscle flexor brevis digitorum manus (equivalent to the opponens minimi digiti in human anatomy) provides an enhanced ability to flex the palm, bringing the fifth digit towards the thumb, an action reinforced by the connection of the radial sesamoid with the flexor retinaculum. Such an action resembles the ‘converging grasp’ of the manus described for the arboreal procyonid kinkajou, Potos flavus (McClearn, 1992). This muscle is reported in all procyonids (Davis, 1964) and in Ailurus fulgens, currently considered to belong in the family Ailuridae (Peigné et al. 2005), and in a more or less vestigial state in many other carnivores (Barone, 2000, p. 819), suggesting that this character is a plesiomorphy for the Carnivora. It is an example of the contrahentes muscles that, according to Haines (1955), augment the adduction force for squeezing a support between the marginal digits creating a ‘clasping’ or ‘opposable’ hand. The connection of the palmaris longus with the radial sesamoid in Ailurus fulgens would contribute to this adduction of the palm.
Function of manus in climbing carnivores
Although the hypertrophy of the radial sesamoid is known only in the pandas (Davis, 1964; Chorn & Hoffmann, 1978; Roberts & Gittleman, 1984) and in the fossil ailurid Simocyon batalleri (Salesa et al. 2006), a moderate development of that bone has been observed in other small climbing carnivores, associated with the presence of a large and laterally orientated pisiform (Taylor, 1974, 1989). These features are related with radial and ulnar deviation necessary for increased rotary movements in the wrist during arboreal locomotion, and they are notorious in climbing species such as the African palm civet (Nandinia binotata) and genets (genus Genetta). By contrast, more terrestrial species, including mongooses of the genera Ichneumia and Bdeogale, display small or absent radial sesamoids, and their pisiforms are posteriorly orientated (Taylor, 1974, 1989).
On the other hand, the kinkajou (Potos flavus) has been observed to grasp thin branches with a grip termed ‘pseudo-opposition’ (Napier & Napier, 1967) in which the digits converge toward the centre of the palm (McClearn, 1992). Such an action partly compensates for the lack of a true opposable thumb like that of primates, and the well-developed muscle flexor brevis digitorum manus of Ailurus fulgens is at least indicative of a similar function (Fig. 4).
Our observation of the supinatory action of the muscle abductor pollicis longus (Fig. 4) is coherent with this model, as supination of the hand is an essential part of forelimb motions during thin-branch climbing, and also for bringing food items to the mouth. The contribution of this muscle to supination is recognized in human anatomy (Khale et al. 1992).
Thus, the morphological features of the hand of Ailurus fulgens fit in the model of small to medium-sized climbing carnivores with more or less omnivorous diets, which need to combine an efficient locomotion along thin branches with the ability to manipulate small food items. Field observations confirm the proficiency of Ailurus fulgens as a small-branch climber thanks to the flexibility of its limb joints (see Roberts & Gittleman, 1984, and references therein).
Evolutionary considerations
The widespread presence of adaptations for the radial deviation of the hand and for the grasping action of the digits in small extant climbing carnivores and in the puma-sized fossil ailurid Simocyon batalleri (Peigné et al. 2005; Salesa et al. 2006) strongly suggests that the morphological features of the manus in Ailurus fulgens were originally selected for their advantages for arboreal locomotion. Later, when Ailurus and other members of the Ailuridae evolved a more vegetarian diet, this set of adaptations worked as exaptations for the new role of the hand in the manipulation of bamboo stalks. Nonetheless, the anatomy of the hand and the morphology of the radial sesamoid in particular remain conservative in Ailurus fulgens, not showing any significant change from the assumedly primitive pattern observed in S. batalleri.
By contrast, the anatomy of the hand of the giant panda reflects its ursid affinities, showing features inherited from large, terrestrial ancestors. The typical ursid hand consists of five subparallel digits lacking grasping abilities, owing to their adaptations for ambulatory terrestrial locomotion and digging (Gambaryan, 1974). Ursids have lost the insertion of the abductor pollicis longus on the first metacarpal, favouring flexion over abduction of the hand, and they also lost the muscle flexor brevis digitorum manus. In spite of this distinctively terrestrial design of the manus some ursids display sizeable radial sesamoids, already showing the pattern of muscle insertions of the giant panda, as observed by Davis (1964). But the development of an hypertrophied radial sesamoid in an essentially ursid hand in the giant panda reflects a very different evolutionary scenario to that of the ailurids, suggesting that the need to manipulate food items was the main adaptive pressure leading to the selection of this highly specialized trait in Ailuropoda melanoleuca. This is an interesting example of how a significant convergence in form is not necessarily the result of a selection pressure due to similar circumstances. In fact, the fossil record suggests that, during most of the evolutionary history of the two panda lineages, their particular forearm and manus adaptations served quite different purposes, i.e. food manipulation and arboreal locomotion.
Acknowledgments
We would like to thank F. Cano from the department of Radiology of the Hospital Clínico Universitario de Valladolid (Spain) for carrying out the radiography and CT scanning of the forelimb of Ailurus fulgens; we also thank the Comunidad Autónoma de Madrid (Dirección General de Patrimonio Histórico) for continuous funding and research permissions. This study is part of research project CGL2005-03900/BTE (Dirección General de Investigación-MCYT). The National Geographic Society provided additional support (Grant 6964-01).
References
- Barone R. Anatomie comparée des mammifères domestiques. Arthrologie et Myologie editions Vigot; 2000. Tome 2. [Google Scholar]
- Bininda-Emonds OR, Gittleman JL, Purvis A. Building large trees by combining phylogenetic information: a complete phylogeny of the extant Carnivora (Mammalia) Biol Rev. 1999;74:143–175. doi: 10.1017/s0006323199005307. [DOI] [PubMed] [Google Scholar]
- Chorn J, Hoffmann RS. Ailuropoda melanoleuca. Mamm Species. 1978;110:1–6. [Google Scholar]
- Davis DD. The giant panda: a morphological study of evolutionary mechanisms. Fieldiana: Zool Mem. 1964;3:1–339. [Google Scholar]
- Endo H, Hayashi Y, Yamagiwa D, et al. CT examination of the manipulation system in the giant panda (Ailuropoda melanoleuca) J Anat. 1999a;195:295–300. doi: 10.1046/j.1469-7580.1999.19520295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Yamagiwa D, Hayashi Y, Koie H, Yamaya Y, Kimura J. Role of the giant panda's ‘pseudo-thumb’. Nature. 1999b;397:309–310. doi: 10.1038/16830. [DOI] [PubMed] [Google Scholar]
- Endo H, Sasaki M, Hayashi Y, Koie H, Yamaya Y, Kimura J. Carpal bone movements in griping action of the giant panda (Ailuropoda melanoleuca) J Anat. 2001a;198:243–246. doi: 10.1046/j.1469-7580.2001.19820243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Sasaki M, Kogiku H, Yamamoto M, Arishima K. Radial sesamoid bone as a part of the manipulation system in the lesser panda (Ailurus fulgens) Ann Anat. 2001b;183:181–184. doi: 10.1016/S0940-9602(01)80045-5. [DOI] [PubMed] [Google Scholar]
- Flynn JJ, Nedbal MA. Phylogeny of the Carnivora (Mammalia): congruence vs incompatibility among multiple data sets. Mol Phylogenet Evol. 1998;9:414–426. doi: 10.1006/mpev.1998.0504. [DOI] [PubMed] [Google Scholar]
- Flynn JJ, Nedbal MA, Dragoo JW, Honeycutt RL. Whence the red panda? Mol Phylogenet Evol. 2000;17:190–199. doi: 10.1006/mpev.2000.0819. [DOI] [PubMed] [Google Scholar]
- Flynn JJ, Finarelli JA, Zehr S, Hsu J, Nedbal MA. Molecular phylogeny of the Carnivora (Mammalia): assessing the impact of increased sampling on resolving enigmatic relationships. Sist Biol. 2005;54:317–337. doi: 10.1080/10635150590923326. [DOI] [PubMed] [Google Scholar]
- Gambaryan PP. How Mammals RunAnatomical Adaptations. New York: Wiley; 1974. [Google Scholar]
- Ginsburg L, Morales J, Soria D, Herráez E. Découverte d'une forme ancestrale du Petit Panda dans le Miocène moyen de Madrid (Espagne) CR Acad Sci Paris. 1997;325:447–451. [Google Scholar]
- Ginsburg L, Maridet O, Mein P. Un Ailurinae (Mammalia, Carnivora, Ailuridae) dans le Miocène moyen de Four (Isère, France) Geodiversitas. 2001;23:81–85. [Google Scholar]
- Goldman D, Rathna Giri P, O'Brien SJ. Molecular genetic-distance estimates among the Ursidae as indicated by one- and two-dimensional protein electrophoresis. Evolution. 1989;43:282–295. doi: 10.1111/j.1558-5646.1989.tb04228.x. [DOI] [PubMed] [Google Scholar]
- Gould SJ. The Panda's Thumb. New York: Norton; 1980. [Google Scholar]
- Haines RW. The anatomy of the hand of certain insectivores. Proc Zool Soc Lond. 1955;125:761–777. [Google Scholar]
- Khale W, Leonhardt H, Platzer W. Color Atlas and Textbook of Human Anatomy, Locomotor System. Vol. 1. New York: Georg Thieme; 1992. [Google Scholar]
- Ledje C, Arnason U. Phylogenetic relationships within caniform carnivores based on analyses of the mitochondrial 12S rRNA gene. J Mol Evol. 1996;42:135–144. doi: 10.1007/BF02202112. [DOI] [PubMed] [Google Scholar]
- McClearn D. Locomotion, posture, and feeding behavior of kinkajous, coatis and raccons. J Mammal. 1992;73:245–261. [Google Scholar]
- Napier JR, Napier PH. A Handbook of Living Primates. New York: Academic Press; 1967. [Google Scholar]
- Pei WC. A brief evolutionary history of the giant panda. Acta Zool Sin. 1974;20:188–190. [Google Scholar]
- Peigné S, Salesa MJ, Antón M, Morales J. Ailurid carnivoran mammal Simocyon from the late Miocene of Spain and the systematics of the genus. Acta Palaeontol Pol. 2005;50:219–238. [Google Scholar]
- Qiu Z, Qi G. Ailuropod found from the Late Miocene deposits in Lufeng, Yunnan. Vertebrata Palasiatica. 1989;27:153–169. [Google Scholar]
- Roberts MS, Gittleman JL. Ailurus fulgens. Mamm Species. 1984;222:1–8. [Google Scholar]
- Salesa MJ, Antón M, Peigné S, Morales J. Evidence of a false-thumb in a fossil carnivore clarifies the evolution of pandas. Proc Natl Acad Sci USA. 2006;103:379–382. doi: 10.1073/pnas.0504899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasagawa I, Takahashi K, Sakumoto T, Nagamori H, Yabe H, Kobayashi I. Discovery of the extinct red panda Parailurus (Mammalia, Carnivora) in Japan. J Vert Paleontol. 2003;23:895–900. [Google Scholar]
- Taylor ME. The functional anatomy of the forelimb of some African Viverridae (Carnivora) J Morph. 1974;143:307–336. doi: 10.1002/jmor.1051430305. [DOI] [PubMed] [Google Scholar]
- Taylor ME. Locomotor adaptations by carnivores. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Vol. 1. New York: Cornell University Press; 1989. pp. 382–409. [Google Scholar]
- Tedford RH, Gustafson EP. First North American record of the extinct panda Parailurus. Nature. 1977;265:621–623. [Google Scholar]
- Wallace SC, Wang X. Two new carnivores from an unusual late Tertiary forest biota in eastern North America. Nature. 2004;431:556–559. doi: 10.1038/nature02819. [DOI] [PubMed] [Google Scholar]
- Wang X. New cranial material of Simocyon from China and its implications for phylogenetic relationship to the red panda (Ailurus) J Vert Paleontol. 1997;17:184–198. [Google Scholar]
- Wozencraft WC. The phylogeny of the recent Carnivora. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Vol. 1. New York: Cornell University Press; 1989a. pp. 495–535. [Google Scholar]
- Wozencraft WC. Appendix: classification of the recent Carnivora. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Vol. 1. New York: Cornell University Press; 1989b. pp. 569–593. [Google Scholar]
- Wozencraft WC. Order Carnivora. In: Wilson DE, Reeder MD, editors. Mammal Species of the World. Washington, DC: Smithsonian Institution Press; 1993. pp. 279–348. [Google Scholar]