Abstract
IRBIT is an IP3R [IP3 (inositol 1,4,5-trisphosphate) receptor]-binding protein that competes with IP3 for binding to the IP3R. Phosphorylation of IRBIT is essential for the interaction with the IP3R. The unique N-terminal region of IRBIT, residues 1–104 for mouse IRBIT, contains a PEST (Pro-Glu-Ser-Thr) domain with many putative phosphorylation sites. In the present study, we have identified a well-conserved PP1 (protein phosphatase-1)-binding site preceeding this PEST domain which enabled the binding of PP1 to IRBIT both in vitro and in vivo. IRBIT emerged as a mediator of its own dephosphorylation by associated PP1 and, hence, as a novel substrate specifier for PP1. Moreover, IRBIT-associated PP1 specifically dephosphorylated Ser68 of IRBIT. Phosphorylation of Ser68 was required for subsequent phosphorylation of Ser71 and Ser74, but the latter two sites were not targeted by PP1. We found that phosphorylation of Ser71 and Ser74 were sufficient to enable inhibition of IP3 binding to the IP3R by IRBIT. Finally, we have shown that mutational inactivation of the docking site for PP1 on IRBIT increased the affinity of IRBIT for the IP3R. This pinpoints PP1 as a key player in the regulation of IP3R-controlled Ca2+ signals.
Keywords: calcium; casein kinase 1; inositol 1,4,5-trisphosphate receptor (IP3R); phosphorylation; protein phosphatase-1 (PP1); signalling
Abbreviations: AHCY, S-adenosylhomocysteine hydrolase; AKAP9, A-kinase anchoring protein 9; AMPK, AMP-activated kinase; CaMK, Ca2+/calmodulin-dependent protein kinase; CK1, casein kinase 1; DTT, dithiothreitol; GFP, green fluorescent protein; EGFP, enhanced GFP; GST, glutathione transferase; HRP, horseradish peroxidase; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; IPTG, isopropyl β-D-thiogalactoside; IRBIT, IP3R-binding protein released by IP3; MAPK, mitogen-activated protein kinase; mIRBIT, mouse IRBIT; MK-2, MAPK-activated protein kinase 2; NIPP1, nuclear inhibitor of PP1; PKC, protein kinase C; PKD, protein kinase D; PP1, protein phosphatase-1; PP2, protein phosphatase-2; TBS, Tris-buffered saline
INTRODUCTION
IP3R [IP3 (inositol 1,4,5-trisphosphate) receptor] is a key component for intracellular Ca2+ signalling [1]. The complex regulation of Ca2+ signals has been partly attributed to the diversity of IP3R isoform expression and their subcellular distribution, assembly of heterotetrameric IP3R complexes, proteolytic degradation of IP3R and regulation of IP3R activity by various cellular regulators. The last includes Ca2+ itself as well as a broad diversity of interacting proteins, which for the IP3-binding domain of IP3R include calmodulin and related Ca2+-binding proteins, Homer, Gβγ, the PKC (protein kinase C), anchoring protein RACK1 (receptor for activated C kinase 1) and IRBIT (IP3R-binding protein released by IP3) [2,3]. In addition, IP3R contains a large number of potential and/or documented phosphorylation sites. Among the kinases that target IP3R are the serine/threonine protein kinases PKA (protein kinase A), PKB (protein kinase B), PKC, PKG (protein kinase G) and the MAPK (mitogen-activated protein kinase) ERK (extracellular-signal-regulated kinase), the tyrosine kinases Fyn and Lyn, Rho kinase and the cyclin-dependent kinase Cdk1, whereas the phosphatases involved in this process include PP1 (protein phosphatase-1), PP2A (protein phosphatase-2A) and PP2B (protein phosphatase-2B; also termed PP3 or calcineurin), as reviewed in [4,5] and shown recently [6].
IRBIT interacts directly with the ligand-binding domain of the IP3R [7]. This interaction reduces the affinity of IP3R for IP3 and suppresses IP3-induced Ca2+ release [7]. We have shown previously that a PEST (Pro-Glu-Ser-Thr) region (residues 65–92 in the mouse sequence) in the N-terminal domain of IRBIT is essential for the interaction with the ligand-binding domain of the IP3R [7]. The PEST region contains many in silico-predicted phosphorylation sites [8–10], but the relevance of these sites for the interaction between IRBIT and IP3R remains to be elucidated. Collins et al. [11] have shown that IRBIT residues Thr82, Ser84 and Ser85 are sites phosphorylated in vivo. However, Ando et al. [10] have demonstrated recently that mutation of Ser85 to alanine results in a dramatic increase of the in vivo phosphorylation of IRBIT, whereas mutation of two other residues, Ser68 and Ser71, to alanine results in a strong decrease (see Figure 2F in [10]). These workers suggested that Ser68 acts as an initiator site for a phosphorylation cascade involving CaMKs (Ca2+/calmodulin-dependent protein kinases) and CK1 (casein kinase 1) [10]. Taken together, these findings are indicative of a very complex regulation of the phosphorylation status of IRBIT.
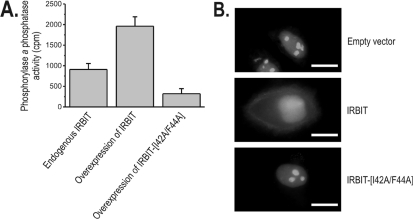
Figure 2. IRBIT and PP1 interact in vivo.
(A) COS-1 cells were co-transfected with pEGFP-PP1α and either the pTRACER-IRBIT constructs [for overexpression of untagged IRBIT or IRBIT(I42A/F44A)] or the empty vector (for endogenous IRBIT). IRBIT was immunoprecipitated from the cell lysates, and the trypsin-releasable phosphorylase a phosphatase activity of PP1 was measured in the immunoprecipitates. The PP1 activities of the immunoprecipitates in the absence of IRBIT antibodies were subtracted, and results are expressed as means±S.D. of the resulting specific PP1 activity (n=3). (B) HeLa cells were transiently co-transfected with pEGFP-PP1γ1 and pEXPR-IBA103-IRBIT (wild-type or I42A/F44A mutant) or empty vector pEXPR-IBA103 as a control. After 24 h, cells were analysed for GFP fluorescence. Scale bar, 20 μm.
Given the importance of phosphorylation for the function of IRBIT in suppressing IP3R activity [7,10], in the present study, we searched for a protein phosphatase involved in the dephosphorylation and, hence, inactivation of IRBIT. We found that IRBIT contains a specific well-conserved binding site for PP1. Using site-directed mutagenesis and competition experiments, we have shown that this docking site is a key player in the binding of PP1 both in vitro and in vivo. Moreover, IRBIT was found to be a substrate specifier for PP1 and to target the activity of PP1 towards IRBIT itself. Analysis of the phosphorylation profile of IRBIT using in vitro phosphorylation experiments and MS revealed that phosphorylation of Ser68 promoted subsequent phosphorylation of Ser71 and Ser74. We have demonstrated that PP1 specifically dephosphorylated Ser68, but not Ser71 or Ser74. Hereby, PP1 can specifically inactivate the initiation step of a phosphorylation cascade that is involved in the inhibition of IP3 binding to the IP3R by IRBIT. We also found that phosphorylation of Ser71 and Ser74 is sufficient for this inhibition. Finally, we have shown that mutational inactivation of the docking site for PP1 on IRBIT increased the affinity of IRBIT for the IP3R. These results suggest a novel mechanism by which PP1 can affect intracellular Ca2+ signalling.
MATERIALS AND METHODS
Materials
BSA and IPTG (isopropyl β-D-thiogalactoside) were obtained from Sigma. GSTrap FF columns, pGEX6p2, glutathione Sepharose 4B, secondary antibodies coupled to HRP (horseradish peroxidase), ECL® Plus and HyperFilm were from GE Healthcare. The vector pEXPR-IBA103 was from IBA GmbH. Sypro® Orange, NuPAGE® gels, Mops buffer, pTRACER/CMV-Bsd and all cell culture media and supplements were from Invitrogen. Slide-A-Lyzer dialysis cassettes were from Pierce Biotechnology. Yeast extract was from Remel, and peptone was from ICN. PKD (protein kinase D) [12], CK1 [13] and the catalytic subunit of PP1 [14] were purified as described previously. KRX Escherichia coli cells and the QuikChange™ site-directed mutagenesis kit were from Stratagene.
Preparation of IRBIT mutants and GST (glutathione transferase) fusion proteins
We used the pEXPR-IBA103-IRBIT construct described previously [7] as a template for subcloning residues 1–530 and 1–104 into the bacterial pGEX6p2 vector. KRX E. coli cells were transformed with the vectors. Colonies were grown overnight in 50 ml of DYT medium [16 g/l peptone, 10 g/l yeast extract and 5 g/l NaCl (pH 7.1)] at 37 °C. DYT medium (350 ml) was added to this pre-culture, and bacteria were grown further at 28 °C until the D600 was 1.5. Protein expression was induced by adding 0.1 mM IPTG to the bacterial culture, which was grown further at 14 °C for another 8 h. Bacterial cells were harvested and lysed by sonication. Lysates were cleared via centrifugation (10000 g for 10 min) at 4 °C and filtered through a 0.22 μm pore-size filter. GST fusion proteins were purified using GSTrap FF columns coupled to the ÄKTA FPLC system (GE Healthcare). All fusion proteins were dialysed against TBS [Tris-buffered saline; 10 mM Tris/HCl (pH 7.5) and 100 mM NaCl], using a Slide-A-Lyzer dialysis cassette with a cut-off of 10 kDa, and stored at −80 °C. Protein quantification was done using BSA as a standard.
The site-directed mutations of the PP1 docking site were performed using the QuikChange™ site-directed mutagenesis kit. The I42A/F44A mutant was made using 5′-CAAGAAGCAAGCCCAGGCTGCTGATGACATGC-3′ and 5′-GTCATCAGCAGCCTGGGCTTGCTTCTTGGG-3′ as the primers, in which the mutations are underlined. The K40R/Q41R/I42V/Q43R mutant was constructed using 5′-CTCCCAAGAGGCGAGTCCGGTTTGCTGATGAC-3′ and 5′-ATCAGCAAACCGGACTCGCCTCTTGGGAGC-3′. Mutagenesis was performed on the pEXPR-IBA103-IRBIT construct. The wild-type and I42A/F44A mutant were transferred via PCR to pTRACER/CMV-Bsd, resulting in pTRACER-IRBIT and pTRACER-IRBIT(I42A/F44A) respectively, allowing eukaryotic overexpression of untagged IRBIT. The N-terminal 104 amino acids containing the PP1-docking-site mutations were transferred via PCR into the pGEX6p2 vector for the purification of GST–IRBIT1–104(I42A/F44A) and GST–IRBIT1–104(K40R/Q41R/I42V/Q43R) respectively. The site-directed mutations of Ser68 to asparagine, glumatate and alanine were performed using the QuikChange™ site-directed mutagenesis kit on the pGEX6p2-IRBIT(1–104) vector, enabling purification of GST–IRBIT1–104(S68D), GST–IRBIT1–104(S68E) and GST–IRBIT1–104(S68A) respectively. All constructs were sequenced on a Genetic Analyser 3100 using Big Dye Terminator V1.1 technology (Applied Biosystems).
In vitro phosphorylation and dephosphorylation of IRBIT
Purified GST–IRBIT1–104 (or the appropriate mutants) was added to a reaction mixture containing 50 mM glycylglycine (pH 7.5), 50 μg/ml BSA and 20 μg/ml PKD and/or 30 μg/ml CK1. The phosphorylation reaction was started by addition of an Mg2+/[γ-32P]ATP mixture giving a final concentration of 2 mM MgCl2 and 0.1 mM ATP (with 0.1 μCi/μl [γ-32P]ATP) in the assay. After incubation for 1 h at 30 °C, the reaction was stopped by addition of 10 mM EDTA and incubation on ice. For subsequent dephosphorylation, 60 nM PP1 was added to the reaction mixture, and the mixture was incubated for 10 min at 30 °C (unless stated otherwise). Samples were separated on a 10% (w/v) Bis-Tris NuPAGE® gel. After staining with Coomassie Blue, radioactivity was detected using phosphoscreens and a STORM 840 PhosphoImager (GE Healthcare). Quantification was done using TotalLab software (Non-linear Dynamics).
Pull-down assay between GST–IRBIT and PP1
Purified GST–IRBIT fusion proteins (650 pmol) were immobilized on glutathione–agarose beads and incubated with the catalytic subunit of PP1. After washing, the trypsin-released phosphorylase a phosphatase activity was measured as described previously [15,16].
Pull-down assay between GST–IP3R11–604 and IRBIT
Bacterial fusion protein GST–IP3R11–604 was purified from E. coli as described for GST–IRBIT1–104. IRBIT or IRBIT mutants were transfected as pEXPR-IBA103 constructs in COS-1 cells using GeneJuice (EMD Biosciences). At 2 days after transfection, cells were collected and lysed in RIPA buffer [25 mM Hepes (pH 7.5), 0.3 mM NaCl, 1.5 mM MgCl2, 0.5 mM DTT (dithiothreitol), 20 mM β-glycerophosphate, 10% (v/v) glycerol, 1 mM Na3VO4 and 1% (v/v) Triton X-100] as described previously [7]. The lysate was added to immobilized GST–IP3R11–604, the incubate was washed and bound proteins were subsequently eluted with glutathione. IRBIT present in the eluates was detected using an HRP-conjugated Strep-Tactin monoclonal antibody after SDS/PAGE and blotting, as described previously [7]. Presence of the GST fusion proteins in the eluates was confirmed by Ponceau staining of the immunoblot (results not shown).
Production of an anti-IRBIT antibody
A New Zealand white rabbit was immunized with purified GST–IRBIT1–104 by subcutaneous injection with complete Freund's adjuvant and subsequent injection of the purified GST–IRBIT1–104 alone at 14-day intervals. The whole procedure was performed by Sigma–Genosys. To confirm the specificity of the antibody, COS-1 cells were used that were transfected with pTRACER-IRBIT to enable overexpression of wild-type untagged IRBIT. The whole-cell lysates obtained were analysed by immunoblotting with the anti-IRBIT antibody. As shown in Supplementary Figure S1(A) (at http://www.BiochemJ.org/bj/407/bj4070303add.htm), the anti-IRBIT antibody recognized a protein with a size of approx. 60 kDa in untransfected COS-1 cells, and this size corresponded to that of endogenous IRBIT. Overexpression of IRBIT resulted in an increased signal of the approx. 60 kDa fragment, confirming that the anti-IRBIT antibody recognized IRBIT. The antibody was validated in immunoprecipitation experiments on endogenous IRBIT present in COS-1 cells. As shown in Supplementary Figure S1(B), the anti-IRBIT antibody enabled immunoprecipitation of IRBIT.
Immunoprecipitations from COS-1 cells
COS-1 cells were used that were co-transfected with the pEGFP-PP1α vector [17] and the pTRACER-IRBIT constructs using GeneJuice (EMD Biosciences), according to the manufacturer's protocol [13]. Transfection with the empty pTRACER vector was used for experiments with endogenous IRBIT. After 2 days, cells were collected and lysed in lysis buffer [50 mM Tris/HCl (pH 7.5), 0.3 M NaCl, 1% (v/v) Triton X-100, 0.5 mM DTT, 0.5 mM PMSF, 0.5 mM benzamidine and 5 μM leupeptin]. The lysate was cleared by centrifugation (10 min at 3800 g at 4 °C) and incubated for 1 h with Protein A–TSK-Sepharose™ (Affiland) to remove non-specific interactions. The Protein A–TSK-Sepharose™ was subsequently removed via centrifugation (2 min at 1500 g at 4 °C). The anti-IRBIT antibody designed against the unique N-terminal region of IRBIT was added for 1 h at 10 °C, followed by incubation with Protein A–TSK-Sepharose™ for another 1 h. For control experiments, buffer was added instead of the anti-IRBIT antibody. Following centrifugation (20 s at 1000 g), the pellet was washed once with TBS supplemented with 0.1 M LiCl and three times with TBS supplemented with 0.1% Nonidet P40. Next, the pellet was washed once with, and resuspended in, 20 mM Tris/HCl (pH 7.5) supplemented with 1 mM DTT and 0.1 mg/ml BSA. The trypsin-released phosphorylase a phosphatase activity in the precipitates was measured as described previously [15,16]. The background PP1 activity of control experiments without the anti-IRBIT antibody was subtracted from the total PP1 activity in the IRBIT immunoprecipitates. The resulting values represent the specific PP1 activity present in the IRBIT immunoprecipitates.
Subcellular localization using fluorescence microscopy
HeLa cells were transiently co-transfected with pEGFP-PP1γ1 [17] and pEXPR-IBA103-IRBIT (wild-type or I42A/F44A mutant) or the empty pEXPR-IBA103 vector as a control. Transfection was performed using FuGENE6 (Roche Molecular Biochemicals), according to the manufacturer's protocol. At 24 h after transfection, the cells were washed twice with PBS and analysed with Cell® on an Olympus IX81 fluorescence microscope.
[3H]IP3 binding
To enable phosphorylation, GST–IRBIT purified from bacteria was added to a reaction mixture containing 50 mM glycylglycine (pH 7.5), 50 μg/ml BSA and 20 μg/ml PKD and/or 30 μg/ml CK1. The reaction was started by addition of a Mg2+/ATP mixture (2 mM MgCl2 and 0.1 mM ATP), and the reaction tubes were incubated for 2 h at 30 °C. The reaction was stopped by addition of 10 mM EDTA and incubation on ice. Subsequently, PP1 was added where indicated and all samples were incubated for 10 min at 30 °C. [3H]IP3 binding was performed by incubating 1.5 μg of purified His-tagged IP3R11–581 (also termed LBS-1His; see [18]) with 1.5 nM [3H]IP3 (PerkinElmer) in IP3-binding buffer [50 mM Tris/HCl (pH 7.4), 1 mM EDTA and 10 mM 2-mercaptoethanol]. The (de)phosphorylated GST–IRBIT samples were added to a final concentration of 250 nM GST–IRBIT. After 30 min of incubation, 10 μl of γ-globulin (20 mg/ml) and 110 μl of 20% (w/v) poly(ethylene glycol) in IP3-binding buffer were added for 10 min, and the samples were rapidly filtered through glass-fibre filters. Non-specific binding was determined in the presence of 12.5 μM unlabelled IP3. Results are expressed as the percentage inhibition of IP3 binding (means±S.E.M.) compared with the control reaction in the presence of non-phosphorylated bacterially purified IRBIT. Individual groups were compared by an unpaired Student's t test (P<0.01).
RESULTS
The N-terminal K40QIQF44 sequence of IRBIT mediates a direct interaction with PP1 in vitro
The C-terminal part of mIRBIT (mouse IRBIT; GenBank® accession number AB092504), residues 105–530, is homologous with the methylation pathway enzyme AHCY (S-adenosylhomocysteine hydrolase). IRBIT was therefore also termed AHCYL1 (AHCY-like 1) [19]. The N-terminal 104 residues of mIRBIT have no homology with any other mouse protein and are likely to regulate mIRBIT function. This N-terminal region contains an in silico-predicted PEST domain, residues 65–92, which we have shown previously [7] to be essential for the interaction with the IP3R. An in silico analysis indicated several putative phosphorylation sites within the PEST domain, including the L-X-R-X-X-S/T motif (which can be phosphorylated by PKD; where X is any residue) and several pS/T-X-X-S/T motifs (which can be phosphorylated on the last residue by CK1 if the first residue is already phosphorylated) (Figure 1A). An alignment of the N-terminal region of IRBIT from Mus musculus with its orthologues from Homo sapiens (GenBank® accession number NM_006621), Xenopus laevis (GenBank® accession number BC081269), Danio rerio (GenBank® accession number NP_958450) and Drosophila melanogaster (GenBank® accession number NM_139489) showed that residues 39–104 (for mIRBIT) are well-conserved between the organisms, except for D. melanogaster in which the conservation starts only from residue 57 (mouse sequence). The L-X-R-X-X-S/T motif is conserved in all vertebrates, but not in D. melanogaster. Neither Caenorhabditis elegans nor lower eukaryotes have an IRBIT homologue.
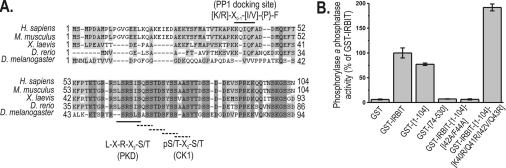
Figure 1. Conservation and identification of the PP1 docking site in IRBIT that allows in vitro binding to PP1.
(A) Sequence alignment of the unique N-terminal region of IRBIT from H. sapiens (GenBank® accession number NM_006621), M. musculus (GenBank® accession number AB092504), X. laevis (GenBank® accession number BC081269), D. rerio (GenBank® accession number NP_958450) and D. melanogaster (GenBank® accession number NM_139489). The PP1 consensus docking site, and one PKD and four CK1 (broken line) in silico phosphorylation consensus sequences, are indicated. {P} in the PP1 consensus docking site indicates that this residue can be any residue except proline [20]. Note that the in vitro CK1 phosphorylation cascade differs from the in silico-predicted cascade shown here (see the Results section). Neither the PP1 consensus docking site nor the PKD consensus docking site are conserved in D. melanogaster. (B) Purified GST, GST–IRBIT, GST–IRBIT1–104, GST–IRBIT74-530, GST–IRBIT1–104(I42A/F44A) and GST–IRBIT1–104(K40R/Q41R/I42V/Q43R) were subjected to the trypsin-releasable phosphorylase a phosphatase assay, as described in the Materials and methods section. Values are means±S.E.M. of the relative phosphorylase a phosphatase activities (n=3).
Residues 40–44, KQIQF, conform to a consensus docking site for PP1, [R/K]-X0–1-[I/V]-{P}-F (where {P} denotes any residue except proline) [20]. This sequence, also known as the RVxF motif, is conserved from H. sapiens to D. rerio, but is not found in D. melanogaster. Moreover, as in other established PP1 interactors, the RVxF motif of IRBIT is flanked N-terminally by a stretch of basic residues and C-terminally by an acidic stretch. The presence of the RVxF motif from vertebrates onwards qualifies IRBIT as a potential secondary interactor of PP1 [21] and suggests a possible role of PP1 in the control of the phosphorylation state of the unique N-terminal region.
To explore experimentally the predicted interaction between PP1 and IRBIT, we generated bacterially expressed affinity-purified GST–IRBIT fusion proteins. We assayed the ability of the GST–IRBIT fusion proteins to interact with the catalytic subunit of PP1 purified from rabbit skeletal muscle. The trypsin-releasable phosphorylase a phosphatase activity was used as a measure of the amount of PP1 bound to GST–IRBIT [13]. As shown in Figure 1(B), there was a clear interaction of PP1 with GST–IRBIT1–104 and a slightly stronger interaction with full-length GST–IRBIT. This indicates that secondary interaction motifs may be present in the C-terminus of IRBIT. We have shown previously that IRBIT is cleaved in vivo between residues 73 and 74 [7]. To investigate whether the resulting C-terminal part of IRBIT contained interaction motifs for PP1, we performed pull-down assays with GST–IRBIT74-530 and quantified associated PP1. As shown in Figure 1(B), GST–IRBIT74-530 had no significant PP1-binding activity and, hence, the presumed C-terminal secondary motifs do not represent high-affinity binding sites for PP1.
To examine the relevance of the RVxF motif of IRBIT as a docking site for PP1, we constructed the GST–IRBIT1–104(I42A/F44A) mutant. As shown in Figure 1(B), the PP1-binding activity of this mutant was reduced to background levels. This indicates that PP1 binds directly to IRBIT via its RVxF motif. RVxF variants bind with different affinities to PP1 [20]. An optimized PP1 docking motif for IRBIT would be RRVRF, according to the criteria described previously [20]. Therefore we constructed GST–IRBIT1–104(K40R/Q41R/I42V/Q43R), containing this optimized docking motif. As shown in Figure 1(B), this optimized docking mutant indeed had an approx. 2-fold increase in PP1 binding, indicating the relative strength of the RVxF variant of IRBIT for the in vitro interaction with PP1.
IRBIT and PP1 interact in vivo
To investigate whether PP1 interacted with IRBIT in intact cells, COS-1 cells were co-transfected with pEGFP-PP1α and the empty vector pTRACER (for endogenous IRBIT) or the pTRACER-IRBIT constructs [for overexpression of untagged IRBIT or IRBIT(I42A/F44A)]. After cell lysis, immunoprecipitation was performed with an antibody designed against the unique N-terminus of IRBIT. These immunoprecipitates were assayed for PP1 activity using the trypsin-released phosphorylase a phosphatase activity, as this is a measure for the amount of bound PP1. As shown in Figure 2(A), immunoprecipitates of endogenous IRBIT had a specific PP1 activity of 910±146 c.p.m. (n=3; value is mean±S.D.). This specific PP1 activity was increased over 2-fold to 1960±227 c.p.m. (n=3) by overexpression of IRBIT. Overexpression of the non-binding IRBIT(I42A/F44A) resulted in a specific PP1 activity in the immunoprecipitates of only 320±123 c.p.m. (n=3). This shows that IRBIT and PP1 interact in vivo and that this interaction is dependent on the RVxF motif of IRBIT. In addition, the non-binding IRBIT(I42A/F44A) mutant appears to act as a dominant-negative mutant for PP1 binding to endogenous IRBIT. In this respect, it should be noted that IRBIT can multimerize via its C-terminal AHCY-like domain [10]. This multimerization could be involved in the dominant-negative effect of the IRBIT(I42A/F44A) mutant, as this could reduce the binding of endogenous IRBIT with PP1. In a similar manner to the immunoprecipitation of PP1α (Figure 2A), PP1γ1 from COS-1 cells could be co-immunoprecipitated with the specific anti-IRBIT antibody (results not shown). In the latter experiment, overexpression of IRBIT also increased PP1 activity in the immunoprecipitates, indicating no difference between PP1 isoforms in the interaction with IRBIT.
It is well known that expression of PP1 interactors can lead to the subcellular redistribution of PP1 [17]. As IRBIT has been shown previously to be a mainly cytoplasmic protein [9], we investigated whether overexpression of IRBIT resulted in a cytoplasmic translocation of PP1γ1. In HeLa cells, the latter isoform of PP1 is nearly exclusively nuclear in location and is enriched in the nucleoli (Figure 2B). Overexpression of wild-type IRBIT resulted in a partial relocalization of EGFP–PP1γ1 [where EGFP is enhanced GFP (green fluorescent protein)] to the non-nucleolar nucleus and the cytoplasm, whereas the non-binding IRBIT(I42A/F44A) mutant did not alter the nucleolar EGFP–PPγ1 localization. The translocation by wild-type IRBIT was observed in approx. one-fifth of the cells in three independent experiments, each verified in 100 cells, and was not observed in the control conditions or in the cells transfected with IRBIT(I42A/F44A). This observation is compatible with an interaction between IRBIT and PP1 in vivo. PP1α could not be used for this translocation experiment, as this isoform is localized to the cytosol [17].
PP1 specifically dephosphorylates Ser68 of IRBIT
Residue 68 resides in a consensus phosphorylation site for PKD (Figure 1A) [22,23]. Interestingly, phosphorylation of Ser68 could allow for subsequent phosphorylation of Ser71, Ser74, Ser77 and Ser80 by CK1, for which the consensus phosphorylation site is pS/T-X-X-S/T (where the target residue for CK1-mediated phosphorylation is underlined, and the required preceding phosphorylated residue is indicated as pS/T). In a similar manner to the PP1 docking site, the PKD motif is conserved in all vertebrates, but not in D. melanogaster (Figure 1A).
Using in vitro phosphorylation experiments, we have shown that PKD phosphorylated IRBIT (Figure 3A). Addition of CK1 alone resulted in a weak phosphorylation of IRBIT, whereas the combination of both CK1 and PKD resulted in a synergistic phosphorylation. This was in agreement with previous findings [10], and indicates a priming function of Ser68 for subsequent phosphorylation by CK1. Using MS, we confirmed that Ser68 was phosphorylated by PKD (see Supplementary Figure S2 at http://www.BiochemJ.org/bj/407/bj4070303add.htm). Accordingly, the IRBITS68A mutant could no longer be phosphorylated by PKD, whereas the weak CK1-mediated phosphorylation was not affected (Figure 3A). As the combined phosphorylation of this mutant by PKD and CK1 was reduced to the level of phosphorylation by CK1 alone, we concluded that phosphorylation of Ser68 (by PKD) strongly promoted subsequent phosphorylation by CK1. Similar results were obtained with CaMKIV instead of PKD (see Supplementary Figure S3 at http://www.BiochemJ.org/bj/407/bj4070303add.htm).
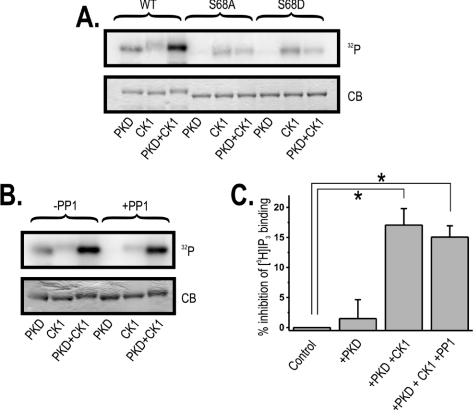
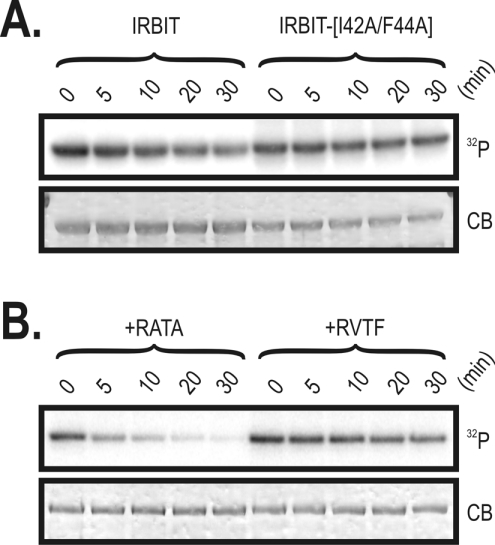
Figure 3. Phosphorylation of Ser68 enables phosphorylation by CK1 and inhibition of IP3 binding, and PP1 specifically dephosphorylates Ser68.
(A) GST–IRBIT1–104 [wild-type (WT)] and GST–IRBIT1–104(S68A) (S68A) or GST–IRBIT1–104(S68D) (S68D) mutants were phosphorylated with PKD and/or CK1 (see the Materials and methods section). The autoradiogram (indicated as 32P) and Coomassie Blue staining (indicated as CB) from a typical gel are shown (representative of three). (B) GST–IRBIT1–104 was phosphorylated with PKD and/or CK1 (see the Materials and methods section). Subsequently, buffer or PP1 (indicated as-PP1 or +PP1 respectively) was added and the sample was incubated for another 10 min at 30 °C. The autoradiogram (indicated as 32P) or Coomassie Blue staining (indicated as CB) from a typical gel are shown (representative of three). (C) The inhibition of [3H]IP3 binding to 1.5 μg of purified IP3R11–581 by 250 nM GST–IRBIT phosphorylated by PKD (+PKD) and PKD and CK1 (+PKD +CK1). Addition of non-phosphorylated IRBIT was used as a control (0% inhibition). A sample that was first phosphorylated by PKD and CK1 and subsequently incubated with PP1 (see the Materials and methods section) was also used (+PKD+CK1+PP1). Binding was measured at pH 7.4 in the presence of 1 mM EDTA and 1.5 nM [3H]IP3. Values are expressed as means±S.E.M. for three independent experiments, each assayed five times. Individual groups were compared by an unpaired Student's t test (P<0.01).
In an attempt to mimic the priming phosphorylation of Ser68 by PKD, we made two mutants in which Ser68 was replaced by an acidic residue, i.e. IRBIT1–104(S68D) and IRBIT1–104(S68E). IRBIT1–104(S68D) was incubated with CK1 in the presence of [γ-32P]ATP. As shown in Figure 3(A), this mutant was not phosphorylated by CK1. IRBIT1–104(S68E) gave a similar result (results not shown), demonstrating that CK1 requires a priming phosphorylation at Ser68, which cannot be mimicked by an acidic residue.
Our in silico screen using the CK1 phosphorylation recognition site suggested that the CK1 phosphorylation cascade could result in subsequent phosphorylation of Ser71, Ser74, Ser77 and Ser80. To identify the residues that were actually phosphorylated in vitro by CK1, IRBIT was phosphorylated by both PKD and CK1, and the sample was analysed using MS (see Supplementary Figures S4–S6 at http://www.BiochemJ.org/bj/407/bj4070303add.htm). In this sample, IRBIT was clearly phosphorylated on Ser68 (by PKD) and on Ser71 and Ser74 (by CK1). We could not detect species that were phosphorylated on residues Ser77 and/or Ser80. Therefore our results indicate that Ser71 and Ser74 are the major CK1 phosphorylation sites.
In a similar manner to the PP1 docking site, the PKD phosphorylation site of IRBIT is conserved in all vertebrates, but not in D. melanogaster (Figure 1A). We therefore examined the effect of PP1 on PKD-mediated phosphorylation of IRBIT. IRBIT was first phosphorylated by PKD in the presence of [γ-32P]ATP, and subsequently incubated with purified PP1 or buffer as a control. As shown in Figure 3(B), PP1 dephosphorylated IRBIT completely. We also investigated the effect of PP1 on IRBIT that was first phosphorylated by CK1 alone or by a mixture of PKD and CK1. As shown in Figure 3(B), the weak CK1-mediated phosphorylated of IRBIT was not significantly reduced by the addition of PP1. The strong phosphorylation by a mixture of PKD and CK1 was slightly decreased after subsequent addition of PP1. This observation again indicates that PP1 does not dephosphorylate the CK1 sites and specifically dephosphorylates Ser68. The specific dephosphorylation of Ser68 is particularly noteworthy as it excludes in vitro dephosphorylation artifacts.
Phosphorylation of Ser68 is critical for the subsequent phosphorylation at Ser71 and Ser74, but phosphorylation of the last two is sufficient to inhibit IP3 binding
We investigated whether phosphorylation by PKD and CK1 affected the capacity of IRBIT to inhibit the binding of IP3 to the IP3R. Therefore GST–IRBIT was purified from E. coli and was in vitro phosphorylated by PKD or a mixture of PKD and CK1. IRBIT was used at a final concentration of 250 nM, and the kinases were inactivated after the phosphorylation reaction by addition of EDTA (see the Materials and methods section). We found that phosphorylation by PKD alone did not enable IRBIT to inhibit the binding of IP3 to the IP3R (Figure 3C). However, the combined phosphorylation by PKD and CK1 resulted in a significant inhibition (P<0.01) of IP3 binding (Figure 3C). To analyse the relevance of the phosphorylation of Ser68 in this inhibition, the sample that was phosphorylated by PKD and CK1 was subsequently incubated with purified PP1 to allow specific dephosphorylation of Ser68 (also see Figure 3B). We found that, under these conditions, the inhibition of IP3 binding was nearly equally effective (Figure 3C), indicating that phosphorylation of Ser71 and Ser74 was sufficient to inhibit IP3 binding to the IP3R. This finding also shows that phosphorylation of Ser68 is required to promote subsequent phosphorylation of Ser71 and Ser74, but that phosphorylated Ser68 is not directly involved in the inhibition of IP3 binding by IRBIT. We have shown previously that 250 nM IRBIT purified from Sf9 insect cells inhibited 50% of the IP3 binding to the IP3R (see Figure 4 in [7]). It is well known that in vitro phosphorylation of proteins purified from bacteria does not occur with 100% efficiency. Therefore the actual concentration of bacteria-derived GST–IRBIT phosphorylated on Ser68 and, more importantly, on Ser71 and Ser74 is expected to be less than 250 nM. This can at least partially explain why the inhibition of IP3 binding is less pronounced with 250 nM in vitro phosphorylated GST–IRBIT compared with 250 nM in vivo phosphorylated IRBIT purified from Sf9 insect cells.
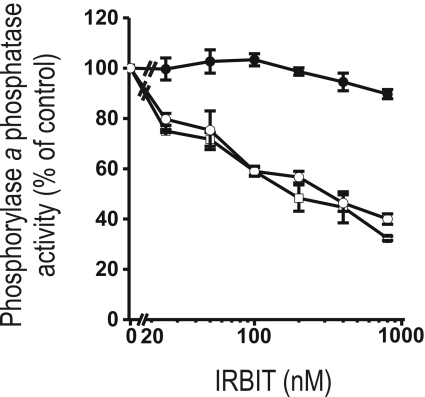
Figure 4. IRBIT inhibits the activity of PP1 towards phosphorylase a phosphatase as a substrate.
Various concentrations of purified GST–IRBIT were incubated with PP1 in the presence of 50 μM synthetic decapeptide NIPP1197-206 (RVTF; ●) or the variant NIPP1197–206(V201A/F203A) (RATA; ○; see the text for more details), or buffer (□). The spontaneous phosphorylase a phosphatase activity is shown as means±S.E.M. (n=3). Addition of buffer was used as a control.
IRBIT is a substrate specifier for PP1 and targets PP1 for dephosphorylation of Ser68
Some interactors of PP1 act as substrate specifiers. For example, the glycogen-targeting G-subunits inhibit the activity of associated PP1 towards glycogen phosphorylase (also termed phosphorylase a), but enhance the activity towards glycogen synthase [25]. IRBIT is also an inhibitor of the phosphorylase a phosphatase activity of PP1 as shown in Figure 4. IRBIT inhibited the phosphorylase a phosphatase activity of PP1 with an estimated IC50 of 148±26 nM. Remarkably, GST–IRBIT1–104 did not inhibit phosphorylase a phosphatase activity (results not shown), indicating that the inhibitory domain is located in IRBIT105–530. To verify the relevance of the RVxF motif in the N-terminal part of IRBIT for the observed inhibition of PP1, we repeated the inhibition experiment with full-length IRBIT in the presence of the synthetic decapeptide NIPP1197-206 (termed RVTF peptide; where NIPP1 is nuclear inhibitor of PP1) or the variant NIPP1197–206(V201A/F203A) (termed RATA peptide; see the Materials and methods sections) [13,20]. The RVTF peptide was used to disrupt the RVxF-mediated interaction between PP1 and its interactors, whereas the RATA peptide represented a control peptide that does not interfere with the interaction between the RVxF motif and PP1 [20]. We found that the RVTF peptide completely inhibited the IRBIT-mediated inhibition of PP1, whereas the RATA peptide had no effect (Figure 4). This shows that the RVxF motif in the N-terminus of IRBIT is essential for inhibition of the phosphorylase a phosphatase activity of PP1. Probably, the RVxF motif functions as an anchor for the initial binding of PP1 and thereby promotes lower-affinity interactions with IRBIT105–530 that are inhibitory towards phosphorylase a as a substrate [20]. Taken together, this shows that IRBIT acts as a novel substrate specifier for PP1.
We have shown previously that phosphorylation of IRBIT is essential for the interaction with and inhibition of the IP3R [7]. Therefore we addressed the question of whether IRBIT could target PP1 for its own dephosphorylation. Therefore IRBIT and IRBIT(I42A/F44A) were first phosphorylated by PKD and subsequently incubated with PP1. As shown in Figure 5(A), wild-type IRBIT was dephosphorylated in a time-dependent manner, whereas the phosphorylation of the I42A/F44A mutant, which cannot bind PP1, was only slightly decreased. Therefore we concluded that IRBIT targets PP1 for dephosphorylation of Ser68.
Figure 5. IRBIT targets Ser68 for dephosphorylation by associated PP1.
(A) GST–IRBIT and GST–IRBIT(I42A/F44A) were phosphorylated by PKD. Subsequently, PP1 was added, and samples were taken at the indicated time points. (B) GST–IRBIT was phosphorylated by PKD in the presence of the synthetic decapeptide NIPP1197-206 (RVTF) or the variant NIPP1197–206(V201A/F203A) (RATA). Subsequently, PP1 was added, and samples were taken at the indicated time points. For both panels, the autoradiogram (indicated as 32P) or Coomassie Blue staining (indicated as CB) from a typical gel is shown (n=3).
To exclude any artefacts related to an aberrant tertiary structure due to the point mutagenesis performed in IRBIT(I42A/F44A), we repeated the experiment with wild-type IRBIT that was phosphorylated by PKD, but we added one of the two following peptides to the mixture before the subsequent incubation with PP1: the RVTF decapeptide, which should disrupt the interaction with IRBIT and PP1, and the RATA decapeptide, which should not affect the interaction [20]. We found that addition of the RVTF decapeptide indeed strongly reduced the time-dependent dephosphorylation of IRBIT by PP1 compared with the control reaction in the presence of the RATA decapeptide (Figure 5B). These observations show that binding of PP1 to the RVxF motif in IRBIT is required for efficient dephosphorylation of Ser68, indicating that IRBIT is not only a substrate specifier for PP1 (Figure 4), but more specifically that IRBIT itself is a substrate of the IRBIT-associated PP1. Both the RVxF motif and the PKD consensus phosphorylation motif are conserved in all vertebrates (Figure 1A). The simultaneous introduction of both motifs during evolution is additional evidence for the relevance of PP1 as an inhibitor of the Ser68-mediated activation mechanism that acts on IRBIT.
Association of PP1 with IRBIT in vivo reduces the interaction between IRBIT and the IP3R
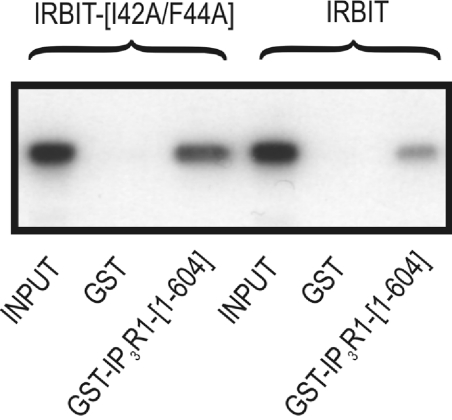
It has been shown previously that in vitro dephosphorylation of IRBIT by alkaline phosphatase prevented its interaction with the ligand-binding domain of the IP3R, also termed IP3R1–604 [7,9]. To investigate whether IRBIT is an in vivo target for dephosphorylation by associated PP1 and whether this affects its function, we expressed wild-type IRBIT or the non-binding mutant in COS-1 cells. After cell lysis, the ability of the recombinant IRBIT present in the COS-1 lysates to interact with the IP3R1–604 was assayed in a GST pull-down experiment. As shown in Figure 6, mutation of the PP1 docking site of IRBIT increased the interaction with the IP3R, presumably reflecting a higher phosphorylation level of IRBIT. This shows the in vivo relevance of this newly identified PP1 docking site on IRBIT in the regulation of the interaction between IRBIT and the IP3R. It is noteworthy that it has been shown previously that deletion of the first 59 amino acids of IRBIT, encompassing the PP1 docking site, also enhanced the interaction with the IP3R (Figure 2B in [10]). This is in full agreement with our findings that mutational inactivation of the PP1 docking site results in a decreased interaction with PP1 (Figure 1B and Figure 2) and, hence, in a decreased dephosphorylation of IRBIT (Figure 5A).
Figure 6. Binding of PP1 to IRBIT decreases the IRBIT–IP3R interaction.
COS-1 cells were transiently transfected with pEXPR-IBA103-IRBIT or pEXPR-IBA103-IRBIT(I42A/F44A). Cells were lysed, and the cleared lysate was used as a reference (indicated as INPUT). The GST pull-down assay with purified GST or GST–IP3R11–604 was performed as described in the Materials and methods section. The amount of sample used in lanes 2, 3, 5 and 6 was 5-fold higher than in lanes 1 and 4. IRBIT present in the eluates was detected using the HRP-conjugated Strep-Tactin antibody. Experiments were repeated four times and a representative blot is shown.
DISCUSSION
In the present study, we have identified PP1 as a novel regulator of the interaction between IRBIT and the IP3R. We have shown that PP1 binds directly to IRBIT both in vitro (Figure 1) and in vivo (Figure 2). Site-directed mutagenesis revealed that the interaction is mediated by the K40QIQF44 motif in the unique N-terminus of IRBIT (Figure 1). Binding of IRBIT to PP1 allows IRBIT to act as a substrate specifier for PP1: the binding of IRBIT to PP1 inhibited the dephosphorylation by PP1 of an exogenous substrate (phosphorylase a, as shown in Figure 4), but conversely stimulated the PP1-mediated dephosphorylation of IRBIT itself (Figure 5).
Using in vitro phosphorylation experiments and subsequent MS analysis, we have shown that PKD can phosphorylate IRBIT on Ser68 and that this phosphorylation enables subsequent phosphorylation on Ser71 and Ser74 by CK1 (Figure 3A, and Supplementary Figures S2 and S4–S6). In this way, phosphorylation of Ser68 can initiate a phosphorylation cascade that ultimately results in inhibition of the IP3R by IRBIT, consistent with a previous suggestion [10]. We also found that mutation of Ser68 resulted in an enhanced electrophoretic mobility of bacterially expressed IRBIT, indicating that this mutation changes the three-dimensional structure of IRBIT. As IRBIT was purified from bacteria, it seems unlikely that the enhanced electrophoretic mobility was due to a lack of phosphorylation. Inversely, phosphorylation by CK1 (on Ser71 and Ser74) was found to induce a decrease in the electrophoretic mobility (Figures 3A and 3B). Mutation of the K40QIQF44 docking site on IRBIT did not affect the electrophoretic mobility (Figure 5).
We demonstrated that IRBIT-associated PP1 specifically targeted phospho-Ser68 (Figures 3B and 5). Both the K40QIQF44 PP1-binding site and the L-X-R-X-X-S/T consensus phosphorylation motif surrounding Ser68 are conserved in all vertebrates (Figure 1A). The simultaneous introduction of both motifs during evolution is additional evidence for the relevance of PP1 as an inhibitor of the Ser68-mediated phosphorylation cascade that activates IRBIT binding to the IP3R. Moreover, we have shown that mutational inactivation of the PP1 docking site on IRBIT results in an enhanced interaction between IRBIT and the IP3R (Figure 6).
Dephosphorylation of Ser71 and Ser74 is likely to involve a phosphatase different from PP1, as these sites were not in vitro dephosphorylated by PP1 (Figure 3B). Further experiments should be conducted to investigate the putative relevance of other major serine/threonine protein phosphatases, including PP2A, PP2B and PP2C, in the dephosphorylation of these sites.
Using [3H]IP3-binding experiments, we have shown that phosphorylation of Ser71 and Ser74 of IRBIT is sufficient to inhibit IP3 binding to the IP3R (Figure 3C). Therefore we propose that Ser68 is an important regulatory residue for subsequent phosphorylation at Ser71 and Ser74, but that it is not directly involved in the inhibition of IP3 binding. In this respect, it should be noted that the residues surrounding Ser68 of IRBIT constitute a potential phosphorylation site not only for PKD, but also for many other protein kinases, including CaMKI [26,27], CaMKII [28,29], CaMKIV ([29]; also see Supplementary Figure S3), AMPK (AMP-activated protein kinase) [26] and the MAPK-activated protein kinase MK-2 (also termed MAPKAPK-2) [30,31]. Moreover, the activation of these kinases upon Ca2+ release has already been demonstrated [26,30,32–34]. Hence Ser68 could be phosphorylated upon Ca2+ release through the IP3R, and this could ultimately result in enhanced binding of IRBIT to the IP3R and blocking of additional Ca2+ release. Further experiments should be conducted to identify which kinase phosphorylates Ser68 of IRBIT in vivo. We cannot exclude that other phosphorylation sites (besides Ser71 and Ser74) are also directly involved in the inhibition of IP3 binding to the IP3R. Possible candidates would be Ser70 and Thr72, since the mutation of one of these sites strongly reduced the interaction with the IP3R in a pull-down assay (see Figure 2C in [10]). But, remarkably, these mutants did not exhibit a strongly reduced incorporation of 32P into IRBIT expressed in COS-1 cells (see Figure 2F in [10]). Other possible candidates would be Thr82, Ser84 and Ser85, which were found previously to be phosphorylated in synaptosomes [11]. However, the mutation of any of these three sites did not strongly reduce the interaction between IRBIT and the IP3R in a pull-down assay (see Figure 2C in [10]). The functional importance of these five residues on the IP3R remains elusive.
The relevance of PP1 in the regulation of IP3R-dependent Ca2+ signals, as suggested by the PP1 docking site in IRBIT, is strengthened further by findings that PP1 also binds to the IP3R either directly to its C-terminal tail [35] or indirectly via AKAP9 (A-kinase anchoring protein 9) association with the regulatory domain [36]. This makes PP1 a key player in the regulation of IP3R-dependent Ca2+ signalling. It should be noted that the other PP1 docking sites in the IP3R multiprotein complex could also be functionally relevant for the in vivo dephosphorylation of IRBIT in addition to directly bound PP1. It is, for instance, conceivable that binding of PP1 to AKAP9 or to the C-terminus of the IP3R has a scaffolding function and increases the local PP1 concentration which, in turn, increases the binding of PP1 to IRBIT.
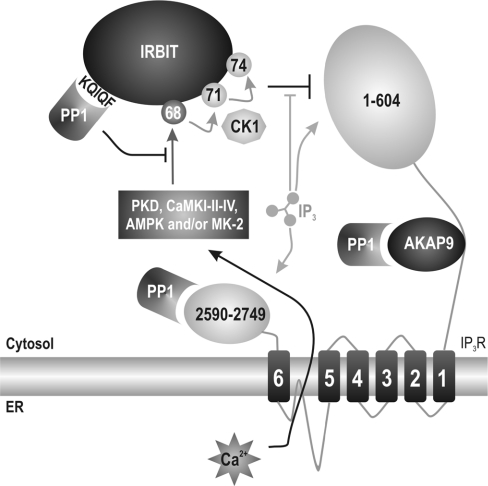
Finally, we propose a model (Figure 7) in which phosphorylation of Ser68 allows for subsequent phosphorylation of Ser71 and Ser74 by CK1 and inhibition of IP3 binding to the IP3R. Ser68 resides in an L-X-R-X-X-S/T motif and could be phosphorylated by a kinase (including PKD, CaMKI, CaMKII, CaMKIV, AMPK or MK-2) that is activated upon Ca2+ release. This could then be part of a negative-feedback mechanism attenuating IP3-induced Ca2+ release via IRBIT. Dephosphorylation of Ser68 by PP1 prevents or reverses this in vivo phosphorylation cascade, which could eventually result in a decreased interaction between IRBIT and the IP3R and a sensitization of the IP3R towards IP3.
Figure 7. Proposed functions of PP1 in IP3R signalling.
Proposed model in which IP3 competes with IRBIT for binding to the N-terminus of IP3R (residues 1–604). Binding of IP3 is favoured as it has a higher affinity for the IP3R. The binding of IP3 induces Ca2+ release from the ER (endoplasmic reticulum). Ca2+ can then activate the phosphorylation cascade on Ser68, Ser71 and Ser74. We propose that PKD, CaMKI/II/IV, AMPK and/or MK-2 are probable candidates to phosphorylate Ser68 in response to Ca2+ release. PP1 is bound to the N-terminal KQIQF motif on IRBIT, and this binding of PP1 allows for efficient dephosphorylation of Ser68. PP1 also directly interacts with the C-terminus of the IP3R (residues 2590–2749) and indirectly via AKAP9 to the regulatory domain of the IP3R.
Online data
Acknowledgments
We are grateful for excellent technical assistance by Tomas Luyten, Irène Willems, Nicole Sente and Annemie Hoogmartens, and the fruitful discussions with Dr Hugo Ceulemans, Dr Jozef Goris and Dr Katleen Lemaire (Katholieke Universiteit Leuven). In particular, we thank Dr Alex Toker (Harvard Medical School, Boston, MA, U.S.A.) for his highly appreciated contribution to this work. We thank Bénédicte Purnelle (Department of Physiological Biochemistry, Université Catholique de Louvain, Louvain, Belgium) for sequencing analysis. This work was supported by grants G.0210.03 (to H. D. S and J. B. P.), G.0282.05 (to J. V. L.) and G.0612.07 (to J. V. L.) from the Fund for Scientific Research Flanders (Belgium), GOA 2004/07 (to J. B. P., L. M. and H. D. S.) and GOA 2005/14 (to M. B.) from the Concerted Actions of the Katholieke Universiteit Leuven, the Interuniversity Attraction Poles programme P6/18 (to J. V. L.) and P6/28 (to J. B. P., L. M., M. B. and H. D. S.) of the Belgian State (Belgian Science Policy), and grant SCIE2003-53 (to J. V. L.) from the Belgian Federation against Cancer. E. W. is part of BioMacS, the Katholieke Universiteit Leuven Interfacultary Centre for Biomacromolecular Structure (http://biomacs.kuleuven.be/). B. D. is a research assistant supported by the Fund for Scientific Research Flanders, and E. S. is supported by Excellence Financing EF/95/010 (Katholieke Universiteit Leuven).
References
- 1.Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 2.Devogelaere B., Verbert L., Parys J. B., Missiaen L., De Smedt H. The complex regulatory function of the ligand-binding domain of the inositol 1,4,5-trisphosphate receptor. Cell Calcium. 2007 doi: 10.1016/j.ceca.2007.04.005. doi:10.1016/j.ceca.2007.04.005. [DOI] [PubMed]
- 3.Verbert L., Devogelaere B., Parys J. B., Missiaen L., Bultynck G., De Smedt H. Proteolytic mechanisms leading to disturbed Ca2+ signalling in apoptotic cell death. Calcium Binding Proteins. 2007;2:21–29. [Google Scholar]
- 4.Krizanova O., Ondrias K. The inositol 1,4,5-trisphosphate receptor: transcriptional regulation and modulation by phosphorylation. Gen. Physiol. Biophys. 2003;22:295–311. [PubMed] [Google Scholar]
- 5.Patterson R. L., Boehning D., Snyder S. H. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 6.Lee B., Vermassen E., Yoon S. Y., Vanderheyden V., Ito J., Alfandari D., De Smedt H., Parys J. B., Fissore R. A. Phosphorylation of IP3R1 and the regulation of [Ca2+]i responses at fertilization: a role for the MAP kinase pathway. Development. 2006;133:4355–4365. doi: 10.1242/dev.02624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devogelaere B., Nadif Kasri N., Derua R., Waelkens E., Callewaert G., Missiaen L., Parys J. B., De Smedt H. Binding of IRBIT to the IP3 receptor: determinants and functional effects. Biochem. Biophys. Res. Commun. 2006;343:49–56. doi: 10.1016/j.bbrc.2006.02.119. [DOI] [PubMed] [Google Scholar]
- 8.Cooper B. J., Key B., Carter A., Angel N. Z., Hart D. N., Kato M. Suppression and overexpression of adenosylhomocysteine hydrolase-like protein-1 (AHCYL1) influences zebrafish embryo development: a possible role for AHCYL1 in inositol phospholipid signaling. J. Biol. Chem. 2006;281:22471–22484. doi: 10.1074/jbc.M602520200. [DOI] [PubMed] [Google Scholar]
- 9.Ando H., Mizutani A., Matsu-ura T., Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J. Biol. Chem. 2003;278:10602–10612. doi: 10.1074/jbc.M210119200. [DOI] [PubMed] [Google Scholar]
- 10.Ando H., Mizutani A., Kiefer H., Tsuzurugi D., Michikawa T., Mikoshiba K. IRBIT suppresses IP3 receptor activity by competing with IP3 for the common binding site on the IP3 receptor. Mol. Cell. 2006;22:795–806. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Collins M. O., Yu L., Coba M. P., Husi H., Campuzano I., Blackstock W. P., Choudhary J. S., Grant S. G. Proteomic analysis of in vivo phosphorylated synaptic proteins. J. Biol. Chem. 2005;280:5972–5982. doi: 10.1074/jbc.M411220200. [DOI] [PubMed] [Google Scholar]
- 12.Vertommen D., Rider M., Ni Y., Waelkens E., Merlevede W., Vandenheede J. R., Van Lint J. Regulation of protein kinase D by multisite phosphorylation. Identification of phosphorylation sites by mass-spectrometry and characterization by site-directed mutagenesis. J. Biol. Chem. 2000;275:19567–19576. doi: 10.1074/jbc.M001357200. [DOI] [PubMed] [Google Scholar]
- 13.Wakula P., Beullens M., van Eynde A., Ceulemans H., Stalmans W., Bollen M. The translation initiation factor eIF2β is an interactor of protein phosphatase-1. Biochem. J. 2006;400:377–383. doi: 10.1042/BJ20060758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeGuzman A., Lee E. Y. Preparation of low-molecular-weight forms of rabbit muscle protein phosphatase. Methods Enzymol. 1988;159:356–368. doi: 10.1016/0076-6879(88)59036-5. [DOI] [PubMed] [Google Scholar]
- 15.Llorian M., Beullens M., Andres I., Ortiz J. M., Bollen M. SIPP1, a novel pre-mRNA splicing factor and interactor of protein phosphatase-1. Biochem. J. 2004;378:229–238. doi: 10.1042/BJ20030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beullens M., Stalmans W., Bollen M. The biochemical identification and characterization of new species of protein phosphatase-1. Methods Mol. Biol. 1998;93:145–155. doi: 10.1385/0-89603-468-2:145. [DOI] [PubMed] [Google Scholar]
- 17.Lesage B., Beullens M., Nuytten M., Van Eynde A., Keppens S., Himpens B., Bollen M. Interactor-mediated nuclear translocation and retention of protein phosphatase-1. J. Biol. Chem. 2004;279:55978–55984. doi: 10.1074/jbc.M411911200. [DOI] [PubMed] [Google Scholar]
- 18.Sienaert I., Nadif Kasri N., Vanlingen S., Parys J. B., Callewaert G., Missiaen L., De Smedt H. Localization and function of a calmodulin-apocalmodulin binding domain in the N-terminal part of the type 1 inositol 1,4,5-trisphosphate receptor. Biochem. J. 2002;365:269–277. doi: 10.1042/BJ20020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dekker J. W., Budhia S., Angel N. Z., Cooper B. J., Clark G. J., Hart D. N., Kato M. Identification of an S-adenosylhomocysteine hydrolase-like transcript induced during dendritic cell differentiation. Immunogenetics. 2002;53:993–1001. doi: 10.1007/s00251-001-0402-z. [DOI] [PubMed] [Google Scholar]
- 20.Wakula P., Beullens M., Ceulemans H., Stalmans W., Bollen M. Degeneracy and function of the ubiquitous RVXF motif that mediates binding to protein phosphatase-1. J. Biol. Chem. 2003;278:18817–18823. doi: 10.1074/jbc.M300175200. [DOI] [PubMed] [Google Scholar]
- 21.Ceulemans H., Stalmans W., Bollen M. Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution. BioEssays. 2002;24:371–381. doi: 10.1002/bies.10069. [DOI] [PubMed] [Google Scholar]
- 22.Hutti J. E., Jarrell E. T., Chang J. D., Abbott D. W., Storz P., Toker A., Cantley L. C., Turk B. E. A rapid method for determining protein kinase phosphorylation specificity. Nat. Methods. 2004;1:27–29. doi: 10.1038/nmeth708. [DOI] [PubMed] [Google Scholar]
- 23.Kunkel M. T., Toker A., Tsien R. Y., Newton A. C. Calcium-dependent regulation of protein kinase D revealed by a genetically-encoded kinase activity reporter. J. Biol. Chem. 2006;282:6733–6742. doi: 10.1074/jbc.M608086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rena G., Bain J., Elliott M., Cohen P. D4476, a cell-permeant inhibitor of CK1, suppresses the site specific phosphorylation and nuclear exclusion of FOXO1a. EMBO Rep. 2004;5:60–65. doi: 10.1038/sj.embor.7400048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newgard C. B., Brady M. J., O'Doherty R. M., Saltiel A. R. Organizing glucose disposal: emerging roles of the glycogen targeting subunits of protein phosphatase-1. Diabetes. 2000;49:1967–1977. doi: 10.2337/diabetes.49.12.1967. [DOI] [PubMed] [Google Scholar]
- 26.Hawley S. A., Selbert M. A., Goldstein E. G., Edelman A. M., Carling D., Hardie D. G. 5′-AMP activates the AMP-activated protein kinase cascade and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. J. Biol. Chem. 1995;270:27186–27191. doi: 10.1074/jbc.270.45.27186. [DOI] [PubMed] [Google Scholar]
- 27.Dale S., Wilson W. A., Edelman A. M., Hardie D. G. Similar substrate recognition motifs for mammalian AMP-activated protein kinase, higher plant HMG-CoA reductase kinase-A, yeast SNF1, and mammalian calmodulin-dependent protein kinase I. FEBS Lett. 1995;361:191–195. doi: 10.1016/0014-5793(95)00172-6. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimura Y., Ichinose T., Yamauchi T. Phosphorylation of tau protein to sites found in Alzheimer's disease brain is catalyzed by Ca2+/calmodulin-dependent protein kinase II as demonstrated tandem mass spectrometry. Neurosci. Lett. 2003;353:185–188. doi: 10.1016/j.neulet.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 29.White R. R., Kwon Y. G., Taing M., Lawrence D. S., Edelman A. M. Definition of optimal substrate recognition motifs of Ca2+-calmodulin-dependent protein kinases IV and II reveals shared and distinctive features. J. Biol. Chem. 1998;273:3166–3172. doi: 10.1074/jbc.273.6.3166. [DOI] [PubMed] [Google Scholar]
- 30.Kreideweiss S., Ahlers C., Nordheim A., Ruhlmann A. Ca2+-induced p38/SAPK signalling inhibited by the immunosuppressant cyclosporin A in human peripheral blood mononuclear cells. Eur. J. Biochem. 1999;265:1075–1084. doi: 10.1046/j.1432-1327.1999.00830.x. [DOI] [PubMed] [Google Scholar]
- 31.Stokoe D., Campbell D. G., Nakielny S., Hidaka H., Leevers S. J., Marshall C., Cohen P. MAPKAP kinase-2: a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 1992;11:3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jensen T. E., Rose A. J., Hellsten Y., Wojtaszewski J. F., Richter E. A. Caffeine-induced Ca2+ release increases AMPK-dependent glucose uptake in rodent soleus muscle. Am. J. Physiol. Endocrinol. Metab. 2007;293:E286–E292. doi: 10.1152/ajpendo.00693.2006. [DOI] [PubMed] [Google Scholar]
- 33.Leclerc I., Rutter G. A. AMP-activated protein kinase: a new β-cell glucose sensor?: regulation by amino acids and calcium ions. Diabetes. 2004;53(Suppl. 3):S67–S74. doi: 10.2337/diabetes.53.suppl_3.s67. [DOI] [PubMed] [Google Scholar]
- 34.Braun A. P., Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu. Rev. Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 35.Tang T. S., Tu H., Wang Z., Bezprozvanny I. Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase A and protein phosphatase-1α. J. Neurosci. 2003;23:403–415. doi: 10.1523/JNEUROSCI.23-02-00403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tu H., Tang T. S., Wang Z., Bezprozvanny I. Association of type 1 inositol 1,4,5-trisphosphate receptor with AKAP9 (Yotiao) and protein kinase A. J. Biol. Chem. 2004;279:19375–19382. doi: 10.1074/jbc.M313476200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.