Abstract
The transmembrane collagenase MT1-MMP (membrane-type 1 matrix metalloproteinase), also known as MMP-14, has a critical function both in normal development and in cancer progression, and is subject to extensive controls at the post-translational level which affect proteinase activity. As zymogen activation is crucial for MT1-MMP activity, an α1-PI (α1-proteinase inhibitor)-based inhibitor was designed by incorporating the MT1-MMP propeptide cleavage sequence into the α1-PI reactive-site loop (designated α1-PIMT1) and this was compared with wild-type α1-PI (α1-PIWT) and the furin inhibitory mutant α1-PIPDX. α1-PIMT1 formed an SDS-stable complex with furin and inhibited proMT1-MMP activation. A consequence of the loss of MT1-MMP activity was the activation of proMMP-2 and the inhibition of MT1-MMP-mediated collagen invasion. α1-PIMT1 expression also resulted in the intracellular accumulation of a glycosylated species of proMT1-MMP that was retained in the perinuclear region, leading to significantly decreased cell-surface accumulation of proMT1-MMP. These observations suggest that both the subcellular localization and the activity of MT1-MMP are regulated in a coordinated fashion, such that proMT1-MMP is retained intracellularly until activation of its zymogen, then proMT1-MMP traffics to the cell surface in order to cleave extracellular substrates.
Keywords: furin, MT1-MMP (membrane-type 1 matrix metalloproteinase), α1-PI (α1-proteinase inhibitor), trafficking
Abbreviations: BFA, brefeldin A; HRP, horseradish peroxidase; MMP, matrix metalloproteinase; MT-MMP, membrane-type MMP; PC, proprotein convertase; α1-PI, α1-proteinase inhibitor; α1-PIMT1, α1-PI with MT1-MMP propeptide cleavage sequence inserted into the reactive-site loop; α1-PIPDX, α1-PI with furin consensus cleavage sequence inserted into the reactive-site loop; α1-PIPitt, mutant of α1-PI which inhibits thrombin; α1-PIWT, wild-type form of α1-PI; PNGase, peptide N-glycosidase
INTRODUCTION
MMPs (matrix metalloproteinases) are a family of zinc-dependent metalloendopeptidases that process a variety of substrates, including proMMP zymogens, extracellular matrix proteins, growth factors and cytokines and cell adhesion proteins [1]. The MT-MMPs (membrane-type MMPs) are a subgroup of membraneanchored proteinases which possess distinct regulatory mechanisms as a result of their cell-surface localization [2]. MT1-MMP, also known as MMP-14, is the best studied MT-MMP, and mice genetically deficient in this proteinase exhibit a variety of abnormalities associated with impaired interstitial collagenolysis, including craniofacial dysmorphism, arthritis, osteopenia, dwarfism and soft-tissue fibrosis [3]. In addition to MT1-MMP playing a role in normal development, it is also up-regulated in a variety of tumour tissues, where it is proposed to function in growth regulation in the three-dimensional tissue microenvironment, as well as in the promotion of invasion of collagen-rich interstitial matrices [4,5]. The extensive involvement of MT1-MMP in normal development and in cancer progression suggests that there must be stringent regulation of proteinase activity. In addition to translational regulation of proteinase expression, MT1-MMP is subject to extensive post-translational control by processes such as glycosylation, zymogen activation, membrane trafficking, inhibition by TIMP-2 (tissue inhibitor of metalloproteinases-2), autolytic degradation and ectodomain shedding [6–9].
The zymogen form of MT1-MMP is activated in the secretory pathway by furin or furin-like PCs (proprotein convertases) that process multiple proprotein substrates including growth factors and their receptors, proteinase zymogens, cell adhesion molecules and some pathological agents by cleavage of the furin consensus sequence Arg-Xaa-Lys↓Arg-Arg (R108RKR↓YA113 in MT1-MMP) [10]. Potent peptide- and protein-based furin inhibitors have been identified, including active-site-directed chloromethane inhibitors, reversible peptide inhibitors and several protein-based inhibitors. An effective strategy for furin inhibition was based on the observation that there is a naturally occurring variant of α1-PI (α1-proteinase inhibitor), α1-PIPitt, in which of Met358 the reactive-site loop sequence (A355IPM358) is mutated to arginine, converting its specificity from an inhibitor of neutrophil elastase to a thrombin inhibitor [11]. Subsequent engineering to give α1-PIPDX, in which the α1-PI reactive-site loop contained the furin consensus cleavage sequence (R355IPR358), resulted in potent inhibition of furin and members of the PC family [12].
In the present study, α1-PIMT1, a novel α1-PI-based inhibitor was generated, in which the MT1-MMP propeptide cleavage sequence was mutated from A355IPM↓SI360 to R355RKR↓YA360, since zymogen activation is crucial for MT1-MMP activity. This inhibitor was used to evaluate the inhibition of proMT1-MMP zymogen activation relative to α1-PIWT (wild-type) and α1-PIPDX. Our results demonstrate the formation of a complex between furin and α1-PIMT1 and highlight the functional significance of furin inhibition in the context of MT1-MMP membrane trafficking, cell-surface proteolytic activity and cellular collagen invasion.
EXPERIMENTAL
Materials and reagents
Rat-tail type I collagen, monoclonal mouse anti-(FLAG M2) antibody, polyclonal rabbit anti-[MT1-MMP (hinge region)] antibody, monoclonal mouse anti-[EEA1 (early endosome antigen 1)] antibody, monoclonal mouse anti-p58 antibody (Golgi marker) and HRP (horseradish peroxidase)-conjugated goat anti-(rabbit IgG) secondary antibodies were purchased from Sigma. Alexa Fluor® 488 goat anti-(rabbit IgG) and Alexa Fluor® 546 goat anti-(mouse IgG)-conjugated secondary antibodies were purchased from Molecular Probes. Rabbit polyclonal antibodies against α3 integrin light-chain and heavy-chain were from Santa Cruz. The murine monoclonal antibody against α3 integrin (ASC-6) was purchased from Chemicon. Enhanced chemiluminescence reagent was bought from Pierce.
Construction of DNA plasmids
The human MT1-MMP cDNA with C-terminal FLAG tag (DYKDDDDK) was a gift from Dr Duanqing Pei (Department of Pharmacology, University of Minnesota Medical School, Minneapolis, MN, U.S.A.). The construction of DNA plasmids used in the present study was described previously [7]. Briefly, MT1-MMP was tagged with FLAG in the stalk region after Gly511 using an overlapping PCR method. The known and novel α1-PI variants were generated by site-directed mutagenesis using the QuikChange® kit (Stratagene) and the variants were subcloned into the pIRES-Neo3 vector (Clontech) for convenient selection of stable cell lines. The PCR reactions were performed using the high-fidelity Pfu Turbo polymerase (Stratagene), and the plasmids were verified by DNA sequencing.
Cell culture and transfections
Cell culture reagents were purchased from Mediatech. COS-7 cells (a gift from Professor Kathleen J. Green, Department of Pathology, Feinberg School of Medicine, Northwestern University, Chicago, IL, U.S.A.) and MDA-MB-231 cells (a gift from Dr V. Craig Jordan, Fox Chase Cancer Center, Philadephia, PA, U.S.A.) were maintained in DMEM (Dulbecco's modified Eagle's medium) containing 10% (v/v) fetal bovine serum. The cells were transfected using FuGENE™ 6 (Roche) following the manufacturer's instructions. Cells stably expressing the target proteins were selected and maintained in culture medium supplemented with G418.
Western blot analysis and gelatin zymography
The whole-cell lysates or purified proteins were fractionated by SDS/PAGE (10% or 5–20% gels as required). Western blot analysis was performed as described previously [7]. To perform gelatin zymography, human proMMP-2 was purified as described by Toth et al. [13]. The detailed protocol of gelatin zymography was described previously by Ellerbroek et al. [14].
Biotinylation and purification of cell-surface proteins
The cell-surface proteins were labelled with EZ-Link sulfo-NHS-LC-LC-biotin [sulfosuccinimidyl-6′-(biotinamido)-6-hexanamido hexanoate] (Pierce) as described previously [15]. The labelled proteins were immobilized on NeutrAvidin-conjugated resin (Pierce), and eluted by boiling in Laemmli sample buffer for 15 min before being resolved by SDS/PAGE.
Purification of FLAG-tagged MT1-MMP and in vitro enzymatic de-glycosylation
Transiently transfected COS-7 cells were homogenized in native lysis buffer [50 mM Tris/HCl, pH 7.5, 150 mM NaCl and 1% (v/v) Triton X-100] supplemented with 5 mM EDTA and Complete™ protease inhibitor cocktail (Roche). Immunoprecipitation of FLAG-tagged proteins was performed using anti-FLAG M2-affinity gel according to the manufacturer's instructions (Sigma) and using Handee spin cup columns (Pierce). The immunocomplexes were eluted using 200 μg/ml 3×FLAG peptide (Sigma) and enzymatic de-glycosylation of immunocomplexes was performed following the manufacturer's instructions (ProZyme). The choice of sialidase rather than PNGase (peptide N-glycosidase) for de-glycosylation was the result of our previous studies, in which the glycosylation of MT1-MMP was evaluated in detail [7]. MT1-MMP was found to be more sensitive to sialidase than other de-glycosylating enzymes, including endo-O-glycosidase, β(1–4)-galactosidase, glucosaminidase and PNGase. Briefly, the purified protein was heat-denatured in the SDS sample buffer provided, neutralized using 1% Triton X-100 and digested with 0.24 m-units of sialidase A for 24 h at 37 °C. The reaction was stopped by boiling in SDS sample buffer, and the reaction mixtures were then analysed by Western blotting, using the rabbit anti-MT1-MMP antibody [1:1000 dilution] and HRP-conjugated anti-(rabbit IgG) secondary antibody [1:10000 dilution] for detection.
FACS analysis of cell-surface proteins
The cells were trypsinized and incubated on ice with primary antibodies against MT1-MMP (hinge region) and α3 integrin (ASC-6, 1:100 dilution), followed by incubation with corresponding Alexa Fluor® 488-conjugated goat anti-(rabbit IgG) and Alexa Fluor® 546-conjugated goat anti-(mouse IgG) secondary antibodies (1:1000 dilution, Molecular Probes) respectively. The labelled live cells were analysed on the dual-channel setting of a Beckman Coulter Epics XL-MCL analyser at the Flow Cytometry Core Facility of Northwestern University.
Collagen invasion assay
Collagen invasion assays were performed as described previously [7,14,16–18]. Briefly, cells were plated on the upper surface of collagen-coated culture inserts (24-well; 8.0 μm pore size; Becton Dickinson). Cells were allowed to invade for 24 h, and non-invading cells were removed from inner wells using a cotton swab. The invading cells, which were adherent to the bottom of the membrane, were fixed and stained using a Diff-Quick staining kit (Dade AG). Invading cells were enumerated by counting 12 symmetric areas of the membrane.
Immunofluorescence
To co-immunostain MT1-MMP and p58, a Golgi marker, in MDA-MB-231 cells, the cells were fixed on coverslips using 3.7% (v/v) formalin and permeabilized with 0.1% (v/v) Triton X-100. For staining, a polyclonal rabbit anti-[MT1-MMP (hinge region)] antibody and a monclonal mouse anti-p58 antibody (1:100 dilution of both antibodies) were used, followed by Alexa Fluor® 488- and 546-conjugated secondary antibodies (1:1000 dilution) (Molecular Probes) respectively. The co-localization images were acquired in sequential mode using a Zeiss LSM510 laser-scanning confocal microscope at the Northwestern University Cell Imaging Facility. To generate overlay images, a pair of raw images were converted into 8-bit TIFF files, assigned as single layers in green and red channels respectively and recomposed in RGB mode using Adobe Photoshop 7.0 software.
RESULTS AND DISCUSSION
A novel α1-PI variant, designated α1-PIMT1, inhibits proMT1-MMP activation
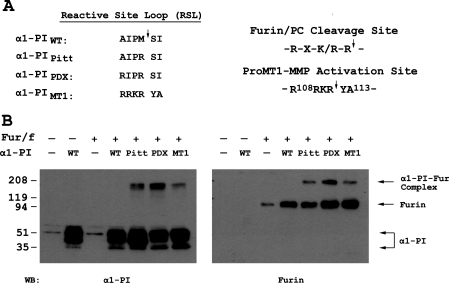
In an attempt to specifically target the furin cleavage site in proMT1-MMP, a novel α1-PI variant, α1-PIMT1, was designed in which the cleavage site of the inhibitor reactive-site loop sequence (A355IPM↓SI360) was replaced by the corresponding sequence in MT1-MMP (R355RKR↓YA360) (Figure 1A). Co-expression of FLAG-tagged furin and α1-PIWT or the various variant forms (α1-PIMT1, α1-PIPitt and α1-PIPDX) in COS-7 cells was performed and the presence of covalent enzyme–inhibitor complexes was evaluated. In both α1-PIPitt- and α1-PIPDX-transfected cells, a higher-molecular-mass species was recognized by antibodies directed against either α1-PI or furin (Figure 1B), indicating the formation of an α1-PI–furin covalent complex. In control experiments, α1-PIWT did not form a furin complex. α1-PIMT1 also exhibited complex formation with furin (Figure 1B), but this was at a lower efficiency when compared with α1-PIPDX. This may be a result of the loss of two basic residues (Arg356 and Lys357) at P2 and P3, and the aromatic hydrophobic residue (Tyr359) at P1′ found in α1-PIPDX. The latter alteration may result in α1-PIMT1 being a less favourable inhibitor of furin than α1-PIPDX, since most substrates of furin have serine or acidic residues at the P1′ postion [19]. Interestingly, a few naturally occurring and engineered mutations that prevented propeptide cleavage involved substitutions of the P1′ residue to hydrophobic amino acids [20,21].
Figure 1. A novel α1-PI variant based on the amino acid sequence flanking the proMT1-MMP activation site forms a SDS-stable complex with furin.
(A) The amino acid sequence flanking the proMT1-MMP-activation site contains the optimal cleavage motif for furin and PC proteinases. A novel α1-PI variant, designated α1-PIMT1, was designed based on the sequence (P4–P2′) flanking the proMT1-MMP activation site. The altered sequence in the reactive-site loop (RSL) of α1-PIMT1 (P4–P2′) was aligned with the corresponding sequence of α1-PIWT and those of two other known α1-PI variants, α1-PIPitt and α1-PIPDX. (B) COS-7 cells were transiently transfected with a DNA plasmid encoding FLAG-tagged furin (Fur/f) in combination with plasmids encoding α1-PIWT (WT) or one of its variants α1-PIPitt (Pitt), α1-PIPDX (PDX) and α1-PIMT1 (MT1) respectively. Empty vectors (−) were used as controls in transfection. The cell lysates were prepared 24 h after transfection as described in the Experimental section and were fractionated by SDS/PAGE (5–20% gels) in duplicate, followed by Western blot analysis using an anti-α1-PI antibody (left-hand side) and anti-FLAG antibody (right-hand side). The immunopreciptates corresponding to α1-PI and furin alone and the α1-PI–furin (α1-PI–Fur) complex are indicated with arrows on the right-hand side.
α1-PIMT1 inhibits proMT1-MMP activation, MT1-MMP-mediated proMMP-2 activation and collagen invasion
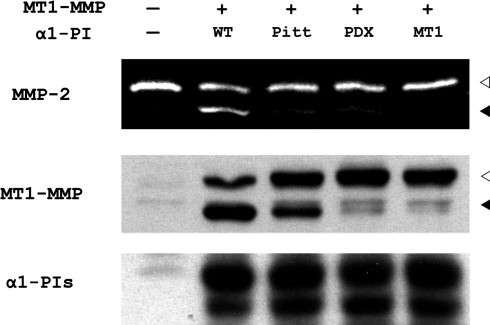
To test the functional significance of the inhibition of furin by α1-PIMT1 on MT1-MMP activity, COS-7 cells were co-transfected with proMT1-MMP and the α1-PI variants. In contrast with α1-PIWT, which does not inhibit furin activity, expression of α1-PIPitt, α1-PIPDX and α1-PIMT1 all inhibited the activation of proMT1-MMP, with α1-PIMT1 displaying the strongest effect (Figure 2, middle panel). As a result of inefficient proMT1-MMP zymogen activation, MT1-MMP proteinase activity was inhibited as demonstrated by culturing cells in medium containing the MT1-MMP substrate, proMMP-2. Whereas cells transfected with α1-PIWT activate proMMP-2 efficiently, cells co-expressing α1-PIPitt, α1-PIPDX and α1-PIMT1 exhibited a substantial reduction in proMMP-2 activation, with complete inhibition observed in α1-PIMT1-expressing cells (Figure 2, top panel). The α1-PI variants were expressed at similar levels, though subtle differences in degradation among different α1-PI variants were noticeable (Figure 2, bottom panel).
Figure 2. α1-PIMT1 inhibits proMT1-MMP activation and prevents MT1-MMP-catalysed proMMP-2 activation.
COS-7 cells were transiently transfected in duplicate with a DNA plasmid encoding FLAG-tagged MT1-MMP in combination with plasmids encoding α1-PIWT (WT) or different α1-PI variants α1-PIPitt (Pitt), α1-PIPDX (PDX) and α1-PIMT1 (MT1) respectively. The control cells (lane 1) were transfected with empty vectors (−). One set of transfected cells was incubated with 1 nM purified proMMP-2 in serum-free medium for 6 h, 18 h after transfection. The conditioned medium was analysed by gelatin zymography (top panel) as described in the Experimental section. The gelatinase activities corresponding to active MMP-2 and proMMP-2 are indicated with black and white arrowheads (top panel) respectively. The second set of transfected cells were analysed by SDS/PAGE, followed by Western blot analysis using an anti-FLAG antibody to detect MT1-MMP (middle panel) and an anti-(α1-PI) antibody (bottom panel) respectively. The immunoprecipitates corresponding to active MT1-MMP and proMT1-MMP are indicated with black and white arrowheads respectively.
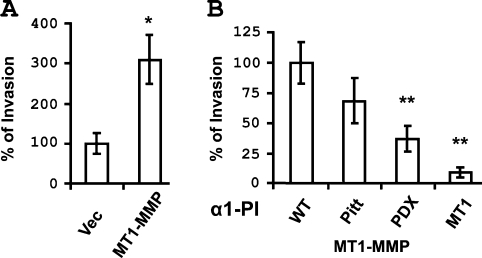
MT1-MMP is an effective interstitial collagenase and promotes cellular invasion of three-dimensional collagen gels [3,4,7,14]. To evaluate the effect of blocking proMT1-MMP activation on cellular invasion, COS-7 cells were co-transfected with MT1-MMP and the α1-PI variants and assayed for invasion of type I collagen gels in a transwell system. Expression of MT1-MMP significantly enhanced the invasion of type I collagen in comparison with vector-transfected cells (Figure 3A). However, decreased invasion was observed on co-expression of α1-PIPitt, α1-PIPDX or α1-PIMT1, with α1-PIMT1-expressing cells exhibiting the most significant inhibition of collagen invasion (Figure 3B). Together these results suggest that α1-PIMT1 is an efficient inhibitor of proMT1-MMP activation.
Figure 3. α1-PIMT1 inhibits MT1-MMP-mediated collagen invasion.
COS-7 cells were transiently transfected with (A) a vector or plasmid encoding MT1-MMP, or (B) with a plasmid encoding MT1-MMP in combination with a second plasmid encoding α1-PIWT or α1-PIPitt (Pitt), α1-PIPDX (PDX) and α1-PIMT1 (MT1) as indicated (right-hand side). After 24 h, the cells were analysed for three-dimensional collagen gel invasion as described in the Experimental section. Gel invasion is expressed as percentage invasion relative to cells transfected with vector (A) or cells transfected with both MT1-MMP and α1-PIWT (B).
ProMT1-MMP activation is coupled with its membrane trafficking
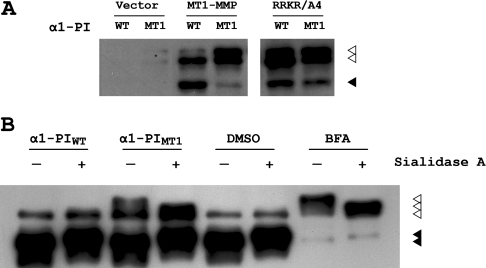
In addition to blocking proMT1-MMP zymogen activation, inhibition of furin-mediated proMT1-MMP processing by α1-PIMT1 resulted in the accumulation of a 66 kDa proMT1-MMP immunoreactive species in cells co-transfected with proMT1-MMP and α1-PIMT1 (Figure 4A, lane 1), which could potentially represent glycosylated proMT1-MMP [7]. Similar results were observed when the R108RKR111 sequence at the activation site of proMT1-MMP was mutated to alanine residues (Figure 4A, lanes 5 and 6). The electrophoretic mobility of this accumulated species is similar to that of the MT1-MMP in cells treated with BFA (brefeldin A), an inhibitor of protein translocation from the ER (endoplasmic reticulum) to the Golgi complex [22]. Furthermore, purified MT1-MMP collected from both α1-PIMT1-expressing cells and BFA-treated cells exhibit a similar mobility shift on SDS/PAGE following sialidase A digestion (Figure 4B, lanes 3, 4, 7 and 8). These results suggest that proper localization in the Golgi may be required for proMT1-MMP activation.
Figure 4. α1-PIMT1 inhibition of proMT1-MMP activation results in the accumulation of glycosylated proMT1-MMP.
(A) COS-7 cells were transiently transfected with a DNA plasmid encoding FLAG-tagged MT1-MMP or proMT1-MMP containing the activation site mutated to alanine residues (RRKR/A4), in combination with α1-PIWT or α1-PIMT1 respectively. Empty vector was used as a control. After 24 h, the cells were lysed, resolved by SDS/PAGE (10% gels) and analysed by Western blotting using an anti-FLAG antibody. The immunoprecipitates corresponding to activated MT1-MMP and proMT1-MMP are indicated with black and white arrowheads respectively. (B) COS-7 cells were transiently transfected with a DNA plasmid encoding FLAG-tagged MT1-MMP and also transfected with either α1-PIWT or α1-PIMT1, or were incubated with 10 μg/ml BFA (with DMSO used as vehicle control) respectively. The FLAG-tagged proteins were purified using anti-FLAG M2–agarose, followed by in vitro enzymatic de-glycosylation with sialydase A. The reaction mixtures were fractionated by SDS/PAGE (10% gels), followed by Western blot analysis using a polyclonal anti-[MT1-MMP (hinge region)] antibody. The immunoprecipitates corresponding to activated MT1-MMP and proMT1-MMP are indicated with black and white arrowheads respectively.
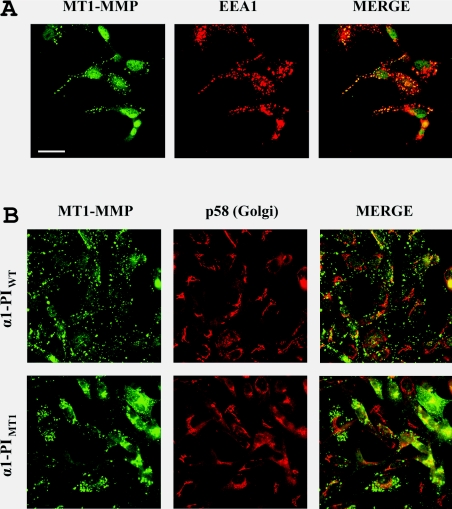
To determine the localization of the accumulated proMT1-MMP proteins resulting from α1-PIMT1 inhibition of zymogen activation, cells were analysed by immunofluorescence. In COS-7 cells, the intracellular localization of MT1-MMP could not be clearly distinguished due to the level of overexpression (results not shown). Therefore, MDA-MB-231 breast cancer cells that stably overexpress α1-PIWT and α1-PIMT1 were generated. These cells express sufficient endogenous MT1-MMP for detection by Western blotting or immunofluorescence [7]. In α1-PIWT expressing cells, the immunostaining pattern of MT1-MMP is punctate throughout the cells (Figure 5) and co-localizes with the early endosome marker EEA1 (Figure 5A). In contrast, in α1-PIMT1 expressing cells, MT1-MMP accumulates in the perinuclear region surrounding or overlapping with p58, a Golgi marker (Figure 5B), suggesting that inhibition of proMT1-MMP activation blocks its cell-surface presentation.
Figure 5. α1-PIMT1 induces perinuclear accumulation of endogenous MT1-MMP in MDA-MB-231 cells.
(A) Cells were transfected with α1-PIWT and after 24 h, the cells were fixed with 3.7% (v/v) formalin and permeablized using 0.1% (v/v) Triton X-100. and immunostained using a rabbit anti-[MT1-MMP (hinge region)] antibody and a monoclonal mouse anti-EEA1 (early endosome antigen 1) antibody (B) Cells were stably transfected with α1-PIWT or α1-PIMT1 and, after 24 h, the cells were fixed with 3.7% (v/v) formalin and permeablized using 0.1% (v/v) Triton X-100. The cells were immunostained using a rabbit anti-[MT1-MMP (hinge region)] antibody and a monoclonal mouse anti-p58 (Golgi marker) monoclonal antibody. In both (A) and (B), the primary immunostaining was followed by Alexa Fluor® 488 -conjugated goat anti-(rabbit IgG) and Alexa Fluor® 546-conjugated goat anti-(mouse IgG) secondary antibodies. The images were acquired using a Zeiss LSM510 laser-scanning confocal microscope. Overlay images (MERGE) were generated using Adobe Photoshop software. Scale bar, 20 μm.
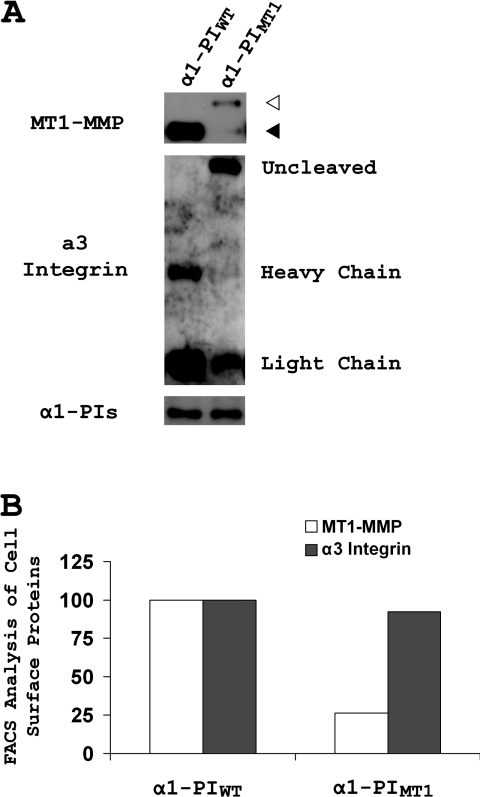
To evaluate further whether proMT1-MMP activation affects its cell-surface presentation, cell-surface proteins in α1-PIWT- and α1-PIMT1-expressing cells were labelled with a non-cell-permeant biotin and surface proteins were purified from cell lysates by streptavidin precipitation, followed by SDS/PAGE and Western blotting for MT1-MMP. The cell-surface level of MT1-MMP in α1-PIMT1-expressing cells was dramatically decreased compared with control cells transfected with α1-PIWT (Figure 6, top panel). The α3 integrin is also processed by furin-like proteinases to a heavy chain and a light chain linked by disulfide bonds [23]. Therefore, in control experiments, the cell-surface level of α3 integrin was also analysed by surface biotinylation to determine whether inhibition of pro-protein processing affects cell-surface localization. In cells expressing α1-PIWT, surface-localized α3 integrin was present as the processed form, containing both the heavy and light chains (Figure 6A, middle panel). In α1-PIMT1-expressing cells, although processing of α3 integrin was inhibited, the uncleaved full-length integrin nevertheless was prevalent on the cell surface. Surface labelling results were confirmed by FACS analysis, indicating a 75% decrease in the mean fluorescence of surface MT1-MMP in α1-PIMT-expressing cells, whereas only a 5% loss of α3 integrin was observed under similar conditions (Figure 6B). Lysates from α1-PIWT- and α1-PIMT1-expressing cells were blotted for α1-PI, demonstrating equivalent expression of both inhibitors (Figure 6A, bottom panel). Thus inhibition of pro-protein processing accompanied by intracellular protein retention is not a general phenomenom, but may represent an additional level of post-translational control of the proteolytic activity of MT1-MMP.
Figure 6. α1-PIMT1 inhibits cell-surface presentation of MT1-MMP.
(A) MDA-MB-231 cells were stably transfected with either α1-PIWT or α1-PIMT1. The cell-surface proteins were biotinylated and purified as described in the Experimental section. The purified cell-surface proteins were fractionated in duplicate by SDS/PAGE (5–20% gels) under reducing conditions, followed by Western blot analysis using an anti-[MT1-MMP (hinge region)] antibody (top) and a mixture of anti-(α3 integrin heavy chain) and anti-(α3 integrin light chain) antibodies (middle) respectively. The cell lysate inputs before purification were analysed by Western blotting using an anti-α1-PI antibody (bottom). The immunoprecipitates corresponding to activated MT1-MMP and proMT1-MMP are indicated with black and white arrowheads respectively. (B) MDA-MB-231 cells stably expressing α1-PIWT or α1-PIMT1 were labelled on ice with an anti-[MT1-MMP (hinge region)] antibody and an anti-(α3 integrin) antibody (ASC-6), followed by staining with Alexa Fluor® 488- and 546-conjugated secondary antibodies respectively. The labelled cells were analysed by FACS for fluorescence intensities on both wavelengths. The means of fluorescence intensities for MT1-MMP and α3 integrin in the α1-PIMT1 cells are are compared with α1-PIWT cells (100%).
In the present study, we explored the possibility of engineering an α1-PI-based inhibitor of furin or PCs that is more potent or specific for blocking proMT1-MMP activation by replacing the residues at P1 and P2′ positions of the active site loop of α1-PI with the residues on proMT1-MMP. Although it appeared to be less efficient for furin inhibition (less covalent proteinase–inhibitor complex in Figure 1B) than α1-PIPDX, the resultant α1-PIMT1 construct was found to be more effective in blocking proMT1-MMP activation, and subsequent MT1-MMP-mediated proMMP-2 activation and invasion of collagen were also reduced. This result is consistent with the notion that PCs, in addition to furin, could be involved in the activation of proMT1-MMP, as furin-negative cell lines were found to activate proMT1-MMP competently [8,24]. The use of protein-based inhibitors in therapeutics may still be an idea for the future, but the potential of developing specific inhibitors for research purposes is apparent. It is interesting to speculate that these inhibitors could be further engineered to contain sequence(s) that dictate trafficking to specific subcellular domains. For example, proMT1-MMP activation has been shown to take place in the trans-Golgi network (TGN) and has also been demonstrated to be associated with detergent-resistant membrane domains [25]. It is conceivable that targeting of α1-PIMT1 to such specific membrane compartments may substantially increase the potency and specificity of proMT1-MMP activation inhibition.
The inhibition of proMT1-MMP activation resulted in the intracellular retention of proMT1-MMP near the Golgi compartment, suggesting that zymogen activation of proMT1-MMP may be a prerequisite for its cell-surface presentation. Our results are consistent with a recent observation by Remacle et al. [26] which suggests that proMT1-MMP exhibits a much higher rate of endocytosis than its active form, providing a possible mechanism through which proMT1-MMP is retained intracellularly. It is interesting to speculate that such retention may be mediated by the propeptide, as it has been suggested that the propeptide of MT1-MMP may function as an intramolecular chaperone involved in protein folding and trafficking to the cell surface [27]. Therefore the retention of proMT1-MMP may allow proper folding of the enzyme before it reaches the cell surface. Consistently, propeptide-deleted MT1-MMP was found to be catalytically inactive, even if it was presented on the cell surface [28]. Alternatively, retention of the proenzyme intracellularly may provide more stringent control of pericellular proteinase activity, as it has been reported that proMT1-MMP may also be activated by serine proteases such as plasmin in vitro [29]. Thus the propeptide-mediated control of MT1-MMP trafficking may play a crucial role in proteinase regulation by preventing unregulated activation of proMT1-MMP by extracellular proteinases.
Acknowledgments
This study was generously supported by the training grant DAMD170010386 (to Y. I. W.) from the United States Army Medical Research and Materiel Command to the Feinberg School of Medicine, Northwestern University and by grant RO1CA86984 (to M. S. S.) from the NIH (National Institutes of Health)/NCI (National Cancer Institute).
References
- 1.Sternlicht M. D., Werb Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer G., Boileau G., Bendayan M. Furin interacts with proMT1-MMP and integrin αV at specialized domains of renal cell plasma membrane. J. Cell Sci. 2003;116:1763–1773. doi: 10.1242/jcs.00394. [DOI] [PubMed] [Google Scholar]
- 3.Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S. A., Mankani M., Robey P. G., Poole A. R., Pidoux I., et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 4.Hotary K., Allen E., Punturieri A., Yana I., Weiss S. J. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J. Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotary K. B., Allen E. D., Brooks P. C., Datta N. S., Long M. W., Weiss S. J. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 6.Jiang A., Lehti K., Wang X., Weiss S. J., Keski-Oja J., Pei D. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13693–13698. doi: 10.1073/pnas.241293698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Y. I., Munshi H. G., Sen R., Snipas S. J., Salvesen G. S., Fridman R., Stack M. S. Glycosylation broadens the substrate profile of membrane type 1 matrix metalloproteinase. J. Biol. Chem. 2004;279:8278–8289. doi: 10.1074/jbc.M311870200. [DOI] [PubMed] [Google Scholar]
- 8.Yana I., Weiss S. J. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol. Biol. Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toth M., Hernandez-Barrantes S., Osenkowski P., Bernardo M. M., Gervasi D. C., Shimura Y., Meroueh O., Kotra L. P., Galvez B. G., Arroyo A. G., et al. Complex pattern of membrane type 1 matrix metalloproteinase shedding. Regulation by autocatalytic cells surface inactivation of active enzyme. J. Biol. Chem. 2002;277:26340–26350. doi: 10.1074/jbc.M200655200. [DOI] [PubMed] [Google Scholar]
- 10.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan S. O., Owen M. C., Boswell D. R., Lewis J. H., Carrell R. W. Circulating proalbumin associated with a variant proteinase inhibitor. Biochim. Biophys. Acta. 1984;802:24–28. doi: 10.1016/0304-4165(84)90029-1. [DOI] [PubMed] [Google Scholar]
- 12.Anderson E. D., Thomas L., Hayflick J. S., Thomas G. Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed α1-antitrypsin variant. J. Biol. Chem. 1993;268:24887–24891. [PubMed] [Google Scholar]
- 13.Toth M., Bernardo M. M., Gervasi D. C., Soloway P. D., Wang Z., Bigg H. F., Overall C. M., DeClerck Y. A., Tschesche H., Cher M. L., et al. Tissue inhibitor of metalloproteinase (TIMP)-2 acts synergistically with synthetic matrix metalloproteinase (MMP) inhibitors but not with TIMP-4 to enhance the (membrane type 1)-MMP-dependent activation of pro-MMP-2. J. Biol. Chem. 2000;275:41415–41423. doi: 10.1074/jbc.M006871200. [DOI] [PubMed] [Google Scholar]
- 14.Ellerbroek S. M., Wu Y. I., Overall C. M., Stack M. S. Functional interplay between type I collagen and cell surface matrix metalloproteinase activity. J. Biol. Chem. 2001;276:24833–24842. doi: 10.1074/jbc.M005631200. [DOI] [PubMed] [Google Scholar]
- 15.Munshi H. G., Wu Y. I., Ariztia E. V., Stack M. S. Calcium regulation of matrix metalloproteinase-mediated migration in oral squamous cell carcinoma cells. J. Biol. Chem. 2002;277:41480–41488. doi: 10.1074/jbc.M207695200. [DOI] [PubMed] [Google Scholar]
- 16.Tam E. M., Wu Y. I., Butler G. S., Stack M. S., Overall C. M. Collagen binding properties of the membrane type-1 matrix metalloproteinase (MT1-MMP) hemopexin C domain. The ectodomain of the 44-kDa autocatalytic product of MT1-MMP inhibits cell invasion by disrupting native type I collagen cleavage. J. Biol. Chem. 2002;277:39005–39014. doi: 10.1074/jbc.M206874200. [DOI] [PubMed] [Google Scholar]
- 17.Munshi H. G., Stack M. S. Analysis of matrix degradation. Methods Cell Biol. 2002;69:195–205. doi: 10.1016/s0091-679x(02)69013-2. [DOI] [PubMed] [Google Scholar]
- 18.Munshi H. G., Wu Y. I., Mukhopadhyay S., Sassano A., Koblinski J. E., Platanias L. C., Stack M. S. Differential regulation of membrane type I-matrix metalloproteinase activity by ERK1/2- and p38 MAPK-modulated tissue inhibitor of metalloproteinase-2 expression controls TGFβ1-induced pericellular collagenolysis. J. Cell Biol. 2004;279:39042–39050. doi: 10.1074/jbc.M404958200. [DOI] [PubMed] [Google Scholar]
- 19.Molloy S. S., Anderson E. D., Jean F., Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T., Nakagawa T., Ikemizu J., Nagahama M., Murakami K., Nakayama K. Sequence requirements for precursor cleavage within the constitutive secretory pathway. J. Biol. Chem. 1992;267:8270–8274. [PubMed] [Google Scholar]
- 21.Oda K., Misumi Y., Sohda M., Takami N., Sakaki Y., Ikehara Y. Selective processing of proalbumin determined by site-specific mutagenesis. Biochem. Biophys. Res. Commun. 1991;175:690–696. doi: 10.1016/0006-291x(91)91621-i. [DOI] [PubMed] [Google Scholar]
- 22.Pelham H. R. Multiple targets for brefeldin A. Cell. 1991;67:449–451. doi: 10.1016/0092-8674(91)90517-3. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann M., Rigot V., Seidah N. G., Marvaldi J., Lissitzky J. C. Lack of integrin α-chain endoproteolytic cleavage in furin-deficient human colon adenocarcinoma cells LoVo. Biochem. J. 1996;317:803–809. doi: 10.1042/bj3170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato T., Kondo T., Fujisawa T., Seiki M., Ito A. Furin-independent pathway of membrane type 1-matrix metalloproteinase activation in rabbit dermal fibroblasts. J. Biol. Chem. 1999;274:37280–37284. doi: 10.1074/jbc.274.52.37280. [DOI] [PubMed] [Google Scholar]
- 25.Mazzone M., Baldassarre M., Beznoussenko G., Giacchetti G., Cao J., Zucker S., Luini A., Buccione R. Intracellular processing and activation of membrane type 1 matrix metalloprotease depends on its partitioning into lipid domains. J. Cell. Sci. 2004;117:6275–6287. doi: 10.1242/jcs.01563. [DOI] [PubMed] [Google Scholar]
- 26.Remacle A. G., Rozanov D. V., Fugere M., Day R., Strongin A. Y. Furin regulates the intracellular activation and the uptake rate of cell surface-associated MT1-MMP. Oncogene. 2006;25:5648–5655. doi: 10.1038/sj.onc.1209572. [DOI] [PubMed] [Google Scholar]
- 27.Cao J., Hymowitz M., Conner C., Bahou W. F., Zucker S. The propeptide domain of membrane type 1-matrix metalloproteinase acts as an intramolecular chaperone when expressed in trans with the mature sequence in COS-1 cells. J. Biol. Chem. 2000;275:29648–29653. doi: 10.1074/jbc.M001920200. [DOI] [PubMed] [Google Scholar]
- 28.Cao J., Drews M., Lee H. M., Conner C., Bahou W. F., Zucker S. The propeptide domain of membrane type 1 matrix metalloproteinase is required for binding of tissue inhibitor of metalloproteinases and for activation of pro-gelatinase. A. J. Biol. Chem. 1998;273:34745–34752. doi: 10.1074/jbc.273.52.34745. [DOI] [PubMed] [Google Scholar]
- 29.Okumura Y., Sato H., Seiki M., Kido H. Proteolytic activation of the precursor of membrane type 1 matrix metalloproteinase by human plasmin. A possible cell surface activator. FEBS Lett. 1997;402:181–184. doi: 10.1016/s0014-5793(96)01523-2. [DOI] [PubMed] [Google Scholar]