Abstract
Resveratrol, a polyphenol found in fruits, possesses chemopreventive and chemotherapeutic properties and has been shown to increase lifespan in yeast and metazoans, including mice. Genetic evidence and in vitro enzymatic measurements indicate that the deacetylase Sir2/SIRT1, an enzyme promoting stress resistance and aging, is the target of resveratrol. Similarly, down-regulation of insulin-like pathways, of which PI3K (phosphoinositide 3-kinase) is a key mediator, promotes longevity and is an attractive strategy to fight cancer. We show here that resveratrol inhibits, in vitro and in cultured muscle cell lines, class IA PI3K and its downstream signalling at the same concentration range at which it activates sirtuins. Our observations define class IA PI3K as a target of resveratrol that may contribute to the longevity-promoting and anticancer properties and identify resveratrol as a natural class-specific PI3K inhibitor.
Keywords: deacetylase, human primary myotube, insulin signalling, phosphoinositide 3-kinase, resveratrol, sirtuin
Abbreviations: AMPK, AMP-activated protein kinase; COX-1, cyclo-oxygenase 1; DAF-16, abnormal dauer formation protein-16; DMEM, Dulbecco's modified Eagle's medium; FCS, fetal calf serum; FOXO, forkhead box O; GST, glutathione S-transferase; Hck, haemopoietic cell kinase; IRS, insulin receptor substrate; LPA, lysophosphatidic acid; MAPK, mitogen-activated protein kinase; PGC-1α, peroxisome-proliferator-activated receptor γ co-activator-1α; PI, phosphoinositide; PI3K, PI 3-kinase; PKB, protein kinase B; PS, 3-sn-phosphatidyl-L-serine; SIR, silent information regulator; siRNA, short interfering RNA
INTRODUCTION
Resveratrol is a phytoalexin found in vegetal dietary sources that has been shown to possess chemopreventive and chemotherapeutic properties as well as the capability to increase stress resistance and extend lifespan in eukaryotes by acting as a caloric restriction mimetic [1].
The chemopreventive/chemotherapeutic properties of resveratrol are correlated to (i) its inhibitory action on COX-1 (cyclo-oxygenase 1) [2] and other enzymes involved in prostaglandin synthesis acting downstream of COX proteins [3], and (ii) its capability to decrease PI3K [PI (phosphoinositide) 3-kinase]-controlled glucose metabolism and the activatory phosphorylation of the anti-apoptotic kinase PKB (protein kinase B)/Akt [3,4].
The function of resveratrol as a caloric restriction mimetic is thought to occur via activation of Sir2-family proteins [SIRs (silent information regulators), collectively termed sirtuins]. Sirtuins are NAD+-dependent deacetylases involved in transcriptional silencing, genomic stability and DNA repair, p53-mediated apoptosis, lipid mobilization and gluconeogenesis [5]. These functions depend on a deacetylase activity that is not limited to histone proteins but also directed to other targets, including p53 and FOXO (forkhead box O)-family forkhead transcription factors [6,7].
Yeast Sir2 – as well as the metazoan SIR-2.1, Sir2 and SIRT1 orthologues from Caenorhabditis elegans, Drosophila melanogaster and mammalians respectively – are involved in lifespan control and define a major genetic pathway promoting aging [8]. High-throughput screening aimed at discovering sirtuin activators, using a fluorescently modified p53 peptide substrate, identified resveratrol as an in vitro activator of SIRT1 and yeast Sir2 [9]. Resveratrol administration induced lifespan extension in yeast, C. elegans, D. melanogaster and mice fed on a high-calorie diet [9–11]. Yet, a caveat on the causal relationship between resveratrol-activated SIRT1/Sir2 and lifespan prolongation exists. Indeed, while in vivo sirtuin activation by resveratrol has been related to decreased PGC-1α (peroxisome-proliferator-activated receptor γ co-activator-1α) acetylation status [12], the in vitro activatory effects of resveratrol are reportedly dependent on the fluorescently modified substrate used in the screening [13,14].
A further pathway central to the control of lifespan implicates insulin and insulin-like signalling – through the PI3K/PKB cascade and the downstream target forkhead transcription factors FOXO/DAF-16 (abnormal dawer formation protein-16) [8]. Mild mutations lowering insulin-like signalling, either at the receptor level or at intracellular signalling components, lead to sirtuin-independent lifespan prolongation in eukaryotes [8,15,16], including mammals [17,18]. FOXO/DAF-16 may represent a convergence point of lifespan control by the insulin-like and sirtuins pathways. In fact, C. elegans SIR-2.1, through interaction with 14-3-3 proteins, regulates DAF-16 and extends lifespan [19].
Besides controlling lifespan, SIRT1 acts as a regulator of insulin action. In hepatocytes, SIRT1 promotes gluconeogenesis via the induction of PGC-1α-dependent gluconeogenic genes [20]; in adipose tissue, it inhibits adipogenesis and favours non-esterified fatty acid mobilization by repressing PPARγ (peroxisome-proliferator-activated receptor γ) [21] and, in pancreatic β-cells, it improves insulin secretion via repression of the UCP2 (uncoupling protein 2) gene [22,23].
In an effort to uncover possible functions of SIRT1 in muscle insulin signalling, we treated human primary myotubes and muscle-derived cell lines with resveratrol (in the micromolar concentration range) and studied insulin signalling events. Here, we show that resveratrol inhibits the PI3K–PKB–FOXO signalling cascade induced by insulin without affecting the insulin-induced MAPK (mitogen-activated protein kinase) cascade. This inhibition, rather than depending on sirtuin activation, is due to a direct inhibitory action of resveratrol on class IA PI3K catalytic subunits p110α and p110β.
Class IA heterodimeric PI3Ks of the p85/p110 type are activated by tyrosine kinase receptor signalling and are involved in mitogenic signalling and metabolic control, while class IB PI3K p110γ is activated by G-protein-coupled receptor and is mainly involved in immune responses [24,25].
Our results define class IA PI3Ks as an additional target upon which resveratrol could act to induce lifespan extension, affect insulin action and exert its chemopreventive function.
EXPERIMENTAL
Materials
Resveratrol, recombinant human insulin, PtdIns, PtdIns(4,5)P2, bovine brain crude PIs, PS (3-sn-phosphatidyl-L-serine), sodium cholate, 2-deoxy-D-glucose and LPA (lysophosphatidic acid) were purchased from Sigma. Inositol lipids and PS were dissolved in chloroform and stored at −80 °C until use. [γ-32P]ATP (∼220 TBq/mmol) and [1,2-3H]2-deoxy-D-glucose (259 GBq/mmol) were obtained from Amersham and NEN respectively. The following antibodies were used: anti-phospho-Ser-473 PKB, anti-phospho-Thr-308 PKB and anti-phospho-FOXO1/4 (Cell Signaling Technology, Danvers, MA, U.S.A.), anti-PKB, anti-IRS1 (insulin receptor substrate 1) and anti-IRS2, anti-SIRT1 (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), anti-phospho-MAPK1/2 and anti-p85α (Upstate Biotechnology, Lake Placid, NY, U.S.A.). The adenovirus expressing constitutively activated p110αCAAX was described previously [26]. Expression plasmids for p85α, p110α, p110β, GST (glutathione S-transferase)–p110α and GST–p110γ were previously described [27–29].
Cell culture
L6 and CCL-136, RD cells (hereinafter referred to as CCL), both from A.T.C.C. (Manassas, VA, U.S.A.), were cultured by standard cell culture procedures in DMEM (Dulbecco's modified Eagle's medium), containing 4.5 g/l glucose, and supplemented with 10% (v/v) FCS (fetal calf serum); cells were starved in the same medium without FCS. Human primary myotube cultures were prepared as described in [30] and kept in DMEM, containing 1 g/l glucose, and supplemented with 10% FCS.
Western blot, immunoprecipitations and PI3K assays
Cells were lysed in a lysis buffer containing 20 mM Tris/HCl, 138 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 5% (v/v) glycerol, 1% (v/v) Nonidet P40, 5 mM EDTA, 1 mM Na3VO4 (sodium orthovanadate), 20 mM NaF, 1 mM dithiothreitol and protease inhibitor cocktail (Sigma P2714) (pH 8.0). Lysates were kept on ice for 15 min and cleared by centrifugation at 13 000 g for 15 min. Protein concentrations were determined by the Bio-Rad protein assay reagent (Bio-Rad).
When subjected to immunoprecipitation, cell lysates were incubated with Protein A–Sepharose and antibodies to p85α, IRS-1 or IRS2 as indicated.
Cell lysates (10–30 μg of protein) were separated by SDS/PAGE, transferred on to PVDF membranes (PerkinElmer) and blocked in 3% (w/v) BSA or 4% (w/v) skimmed dry milk in Tris-buffered saline/0.5% Tween 20. Membranes were incubated overnight at 4 °C with primary antibodies followed by horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Pierce) detection. PI3K protein kinase assays on overexpressed PI3Ks were performed as described in [27].
PI3K lipid kinase assays on IRS1 immunoprecipitates and overexpressed PI3Ks were performed as follows: Protein A-bound p85α/p110α, p85α/p110β and IRS1 immune complexes or glutathione–Sepharose-bound p85α/GST–p110α and GST–p110γ were washed twice with ice-cold PI3K lipid kinase buffer (10 mM Hepes and 5 mM MgCl2, pH 7.4). After washing, Protein A/glutathione–Sepharose beads containing residual lipid kinase buffer (∼5 μl) were incubated on ice in 25 μl of lipid kinase buffer containing 2× concentrated resveratrol for 30 min. Sonicated lipids (10 μl; see below for their preparation) were added for 10 min (on ice) and phosphorylation reactions were started by adding 10 μl of 50 μM ATP supplemented with 0.5 μCi/μl [γ-32P]ATP (final ATP concentration=10 μM, supplemented with 5 μCi of [γ-32P]ATP/reaction). Reactions were allowed to proceed for 20 min at room temperature (20 °C) on a shaking platform.
Lipid mixtures were prepared as follows: chloroform was removed under vacuum from stock solution. PtdIns (10 μg/reaction) was resuspended in lipid kinase buffer prior to sonication. Bovine brain crude PIs (10 μg/reaction) and PtdIns(4,5)P2/PS mixture (5 and 10 μg/reaction respectively) were resuspended in lipid kinase buffer containing 2.5% (w/v) sodium cholate prior to sonication.
Reactions were stopped by the addition of 50 μl of 1 M HCl. Phosphorylated lipids were separated from the unreacted ATP by addition of 200 μl of methanol/chloroform (1:1, v/v). After vortex-mixing and centrifugation, the lipid-containing lower phase was collected and dried under vacuum.
Extracted lipids from reactions using PtdIns(4,5)P2 or PIs as substrate were separated on oxalate-treated silica 60 TLC plates (Merck) with chloroform/acetone/methanol/acetic acid/water (80:30:26:24:14, by vol.). Extracted lipids from reactions using PtdIns as substrate were separated on silica 60 TLC plates (Merck) with chloroform/methanol/water/ammonia (45:35:8.5:1.5, by vol.). Phosphorylated lipids were detected by autoradiography on Kodak X-Omat films and quantified by phosphoimaging.
2-Deoxyglucose uptake in 3T3-L1 adipocytes
3T3-L1 preadipocytes seeded on 12-well plates were differentiated as previously described [31]. Once differentiated, cells were starved overnight in DMEM without FCS. Insulin-induced 2-deoxyglucose uptake was determined as described previously [26].
RNA interference
The siRNA (short interfering RNA) duplex targeting SIRT1 was purchased from Qiagen (Courtaboeuf, France). Transfection with siRNAs was performed by the calcium phosphate method as described in [26]. The sense sequence of the siRNA for SIRT1 was as follows: 5′-CCCUGUAAAGCUUUCAGAAdTdT.
Statistical analysis
Unless otherwise indicated, all experiments were performed at least three times. Values are expressed as means±S.D. or S.E.M. Results were analysed by an unpaired Student's t test (unless otherwise indicated) and differences were considered to be statistically significant when P<0.05.
RESULTS
Resveratrol inhibits PI3K activity and PI3K downstream targets in human primary myotubes and muscle-derived cell lines
The deacetylase SIRT1 has been implicated in the control of insulin secretion by pancreatic β-cells [22,23] and has been shown to affect insulin signalling in hepatocytes and adipocytes [20,21]. To investigate the possible function of SIRT1 in muscle insulin signalling, we used the small polyphenolic compound resveratrol, reported to activate SIRT1 [9].
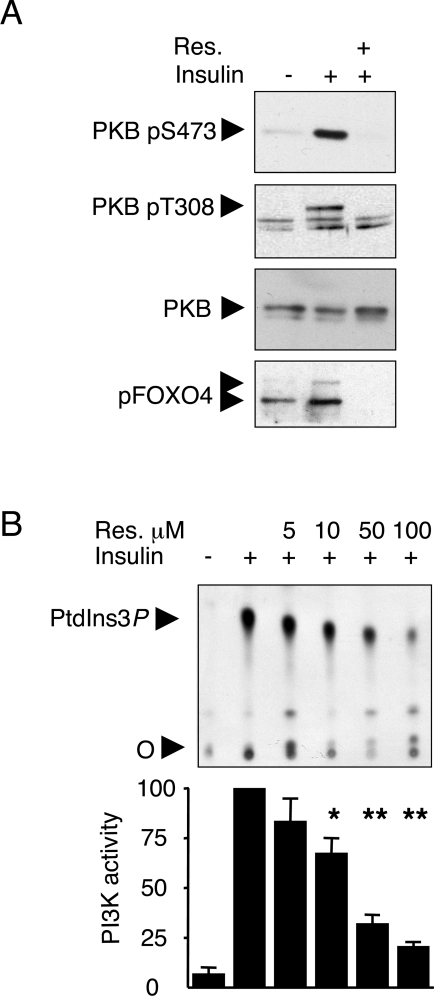
In human primary myotubes, insulin-induced PKB Ser-473 and Thr-308 phosphorylations were fully prevented by a 30 min pretreatment with 100 μM resveratrol as was Ser-193 phosphorylation of the PKB downstream target FOXO4 (Figure 1A). Such a complete inhibitory effect, obtained with resveratrol concentrations employed to activate SIRT1, is reminiscent of that observed upon treatment of cells with the PI3K inhibitors wortmannin or LY294002.
Figure 1. Resveratrol inhibits the insulin-activated PI3K–PKB pathway and IRS1-associated PI3K activity in human primary myotubes.
(A) Fully differentiated myotubes were serum-starved overnight and subsequently stimulated for 20 min with 100 nM insulin. Resveratrol (Res.) (100 μM) was added to the cells 30 min prior to insulin stimulation. Separated proteins were immunoblotted with antibodies to PKB phospho-Ser-473 (pS473), PKB phospho-Thr-308 (pT308), total PKB – to monitor equal loading – and pFoxO4. (B) Upper panel: IRS-1-associated PI3K activity was measured in IRS1 immunoprecipitates obtained from starving (–) or insulin-stimulated (+, 100 nM insulin, 20 min) cells. Immune complexes were pretreated with the indicated concentrations of resveratrol. 32P-labelled PtdIns3P was separated by TLC (‘O’, origin). Lower panel: phosphoimager quantification of PtdIns3P. PI3K activity was set at 100 for the insulin-stimulated condition. Results are means±S.E.M. (n=4). The significance of resveratrol inhibition was compared with the insulin-stimulated condition using Student's t test. *P<0.01, **P<0.001.
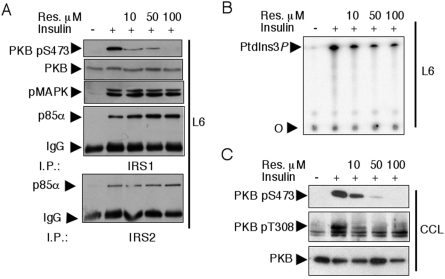
Upon insulin stimulation, PI3K associates with the IRS molecules IRS1 and IRS2 [32]. We thus evaluated the in vitro inhibitory effect of resveratrol on the IRS1–PI3K complex immunoprecipitated following insulin stimulation of primary myotubes. Increasing concentrations of resveratrol were added to IRS1 immunoprecipitates, which were subsequently subjected to a PI3K assay. Resveratrol inhibited IRS1-associated PI3K activity with an IC50 of ∼25 μM (Figure 1B). To corroborate the above findings, we tested the effect of resveratrol on insulin signalling in rat L6 and human rhabdomyosarcoma CCL muscle-derived cell lines. As in primary myotubes, 100 μM resveratrol pretreatment fully inhibited insulin-induced PKB Ser-473 and Thr-308 phosphorylations and, in both cell lines, the IC50 for such inhibition was <10 μM (Figures 2A and 2C). In L6 cells, insulin-induced activation of the MAPK pathway was only mildly affected by the highest concentration of resveratrol employed (Figure 2A), and the insulin-induced interaction between the PI3K p85α adaptor subunit and IRS1 or IRS2 was unaffected (Figure 2A), suggesting that resveratrol exerts its inhibitory action downstream of IRS1/2. Since an inhibitory effect of resveratrol on PI3K was also observed in IRS1–PI3K immune complexes from L6 cells (Figure 2B), PI3K is the likely target of resveratrol.
Figure 2. Inhibition of the PI3K–PKB pathway, but not of the MAPK pathway, by resveratrol in L6 and CCL cells.
(A) L6 myoblasts were serum-starved overnight, pretreated for 30 min with the indicated concentrations of resveratrol and subsequently stimulated for 10 min with 1000 nM insulin. Separated total proteins were immunoblotted with antibodies to PKB pS473, PKB and pMAPK. Furthermore, equal amounts of lysates were immunoprecipitated with antibodies to IRS1 and IRS2, and the associated PI3K was visualized by immunoblotting with anti-p85α antibodies. I.P., immunoprecipitation. (B) IRS1-associated PI3K activity was measured in IRS1 immunoprecipitates obtained from starving (–) or insulin-stimulated (+, 1000 nM insulin, 10 min) L6 cells. Immune complexes were pretreated with the indicated concentrations of resveratrol. 32P-Labelled PtdIns3P was separated by TLC as in Figure 1. (C) Resveratrol dose–response inhibition of insulin-induced PKB Ser-473 and Thr-308 phosphorylations in CCL cells. Equal loading was verified by immunoblotting of total PKB.
A major biological response induced by insulin via PI3K is the translocation of the glucose transporter GLUT4 at the plasma membrane and a consequential increase in glucose uptake [33]. We next tested the effect of resveratrol on insulin-induced 2-deoxyglucose uptake in 3T3-L1 adipocytes, a cell line displaying an increase in 2-deoxyglucose uptake after insulin stimulation. Insulin treatment induced a 40.0% increase of 2-deoxyglucose uptake (P<0.05, paired t test, n=6). Resveratrol (100 μM) pretreatment yielded an 82.7% decrease of insulin-induced 2-deoxyglucose transport in comparison with insulin-treated cells (P<0.01, paired t test, n=6), thus lowering the overall uptake below the basal level. This indicates that resveratrol not only affects insulin-stimulated glucose uptake but also (as previously reported in U937 and HL-60 cells [34]) the basal GLUT-1-dependent glucose transport.
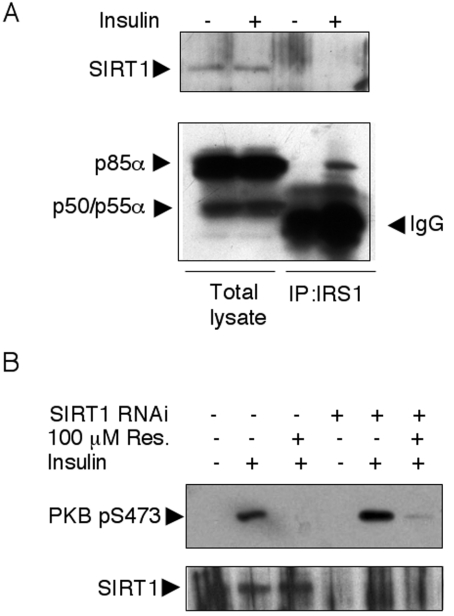
In C. elegans, the SIRT1 orthologue SIR-2.1 interacts with 14-3-3 proteins to mediate lifespan extension [19]. As 14-3-3 proteins reportedly interact with IRS1 [35,36], IRS1 could, in principle, coimmunoprecipitate SIRT1 that would be targeted by resveratrol and cause (perhaps by an indirect mechanism) PI3K inhibition. However, we have been unable to detect SIRT1 in IRS1 immune complexes either before or after insulin stimulation (Figure 7A).
Figure 7. SIRT1 is not found in IRS1 immune complexes and resveratrol inhibits the insulin-activated PKB independently of sirtuin expression.
(A) L6 myoblasts were serum-starved overnight and subsequently stimulated for 10 min with 1000 nM insulin. Separated total proteins were immunoblotted with antibodies to p85α (that also recognizes the short-spliced forms p50 and p55α) and SIRT1. In parallel, equal amounts of lysates were immunoprecipitated with antibodies to IRS1 and immunoblotted with anti-p85α and α-SIRT1 antibodies. IP, immunoprecipitation. (B) L6 myoblasts were transfected with siRNAs directed to SIRT1. After an overnight starving and a 30 min pretreatment with 100 μM resveratrol as indicated, cells were stimulated with 1000 nM insulin for 10 min. Total lysates were immunoblotted with antibodies to PKB pS473 and SIRT1.
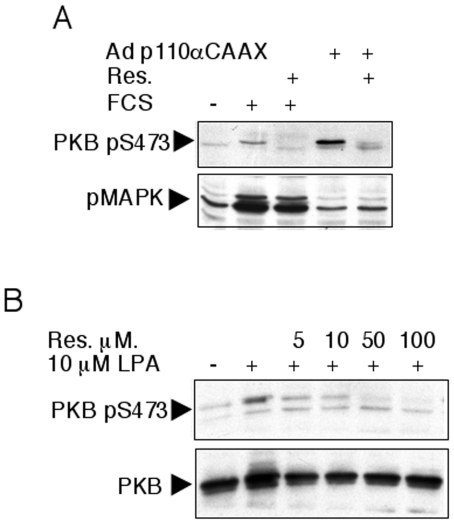
We next asked whether the inhibitory action of resveratrol on PI3K and PKB occurs when cells are subjected to a stimulus other than insulin. Adenovirus-mediated expression of a membrane-targeted constitutively active PI3K (p110αCAAX) induced a PKB Ser-473 phosphorylation – independently from an extracellular stimulation – that was inhibited by resveratrol treatment (Figure 3A), suggesting a direct inhibitory effect of resveratrol upon p110α PI3K. As observed with insulin treatment, stimulation of resveratrol-treated L6 cells with 10% FCS resulted in a complete blockade of PKB phosphorylation but not MAPK phosphorylation (Figure 3A). Likewise, resveratrol inhibited LPA-mediated PKB phosphorylation (Figure 3B).
Figure 3. Resveratrol inhibits PKB Ser-473 phosphorylation induced by various agonists.
(A) L6 cells were infected for 48 h with an adenovirus expressing a constitutively active p110α (Adp110αCAAX) as indicated. After overnight serum starvation, cells were pretreated for 30 min with 100 μM resveratrol (Res.) as indicated and subsequently stimulated with 10% FCS. Cell lysates were immunoblotted with antibodies to PKB pS473 and pMAPK. (B) CCL cells were starved overnight, pretreated for 30 min with the indicated concentrations of resveratrol and subsequently stimulated with 10 μM LPA. Cell lysates were immunoblotted with antibodies to PKB pS473 and PKB.
Resveratrol targets the class IA PI3K ATP-binding site in a competitive and reversible fashion
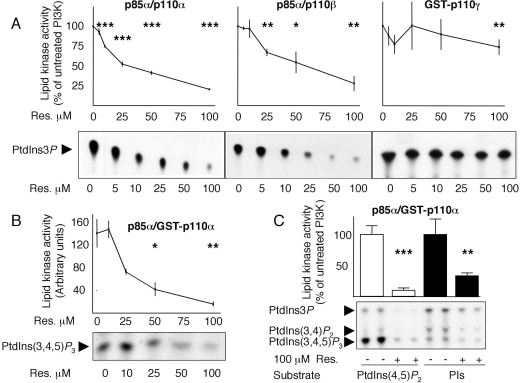
To gain insight into isoform-specificity, class IA p85α/p110α, p85α/p110β and class IB GST–p110γ were overexpressed in HEK-293 cells (human embryonic kidney cells) and their sensitivity to resveratrol was tested in in vitro lipid kinase assays by using PtdIns as the substrate. p85α/p110α was inhibited by resveratrol with an IC50 of ∼25μM, the IC50 towards p85α/p110β was ∼50 μM, while class IB GST–p110γ was less sensitive to resveratrol, retaining ∼75% of the activity when treated with 100 μM resveratrol (IC50>100 μM, Figure 4A). Although PtdIns is routinely used to test in vitro PI3K activity, the PI3K physiological substrate is PtdIns(4,5)P2 [24]. We thus tested the inhibitory action of resveratrol on p85α/GST–p110α by using PtdIns(4,5)P2 as substrate (Figure 4B), and likewise observed an IC50 of ∼25 μM and ∼90% inhibition at 100 μM resveratrol. Furthermore, comparison of the lipid kinase activities of p85α/GST–p110α with GST–p110γ by using PtdIns(4,5)P2 as substrate confirmed the modest inhibitory action of resveratrol towards GST–p110γ (see Supplementary Figures 1A and 1B at http://www.BiochemJ.org/bj/406/bj4060511add.htm). As a final approach to determine the inhibitory action of resveratrol towards PI3K, we performed lipid kinase assays using bovine brain crude PIs, which contain a mixture of PtdIns, PtdIns4P and PtdIns(4,5)P2. Inhibition of p85α/GST–p110α activity by resveratrol was observed using either substrate [PtdIns(4,5)P2 or PIs, Figure 4C]. Finally, inhibition of class IA p85α/p110α, p85α/p110β, p85α/GST–p110α and class IB GST–p110γ by 100 μM resveratrol was evaluated by using PIs as substrate. As observed with PtdIns and PtdIns(4,5)P2, inhibition was maximal for class IA p110 isoforms and moderate for the class IB p110γ isoform (Supplementary Figures 1C and 1D).
Figure 4. Resveratrol inhibits recombinant class IA, but not class IB, PI3K lipid kinase activity.
(A) Relative dose–response inhibition of class IA p85α/p110α, p85α/p110β and class IB GST–p110γ in lipid kinase assays using PtdIns as substrate. Representative TLCs for each PI3K isoform are shown at the bottom. Assays for each experimental condition were performed at least four times. Activity in the absence of resveratrol was normalized at 100%. (B) Relative dose–response inhibition of class IA p85α/GST–p110α in a lipid kinase assay using PtdIns(4,5)P2 as substrate and representative TLC. Assays for each experimental condition were performed in triplicate. (C) Inhibition of class IA p85α/GST–p110α by 100 μM resveratrol by using PtdIns(4,5)P2 or PIs as substrates. Results are means±S.D. for at least three independent assays; activity in the absence of resveratrol was normalized at 100%. The significance of inhibition was compared with the assays without resveratrol using Student's t test. *P<0.05, **P<0.01, ***P<0.001.
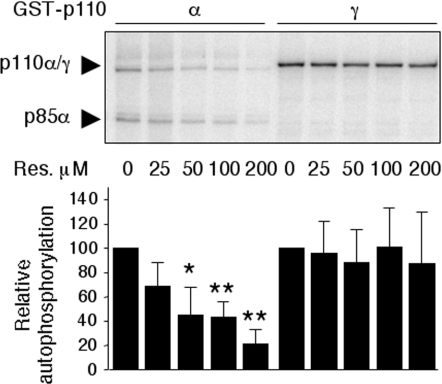
The same differential sensitivity towards inhibition by resveratrol was observed in relation to the PI3K protein kinase activities of p110α/p110β compared with p110γ. While p85α/GST–p110α autophosphorylation was inhibited by resveratrol in a concentration-dependent manner, GST–p110γ autophosphorylation was not affected by resveratrol (Figure 5 and see Supplementary Figure 2A at http://www.BiochemJ.org/bj/406/bj4060511add.htm) and both p85α/p110α [37] and p85α/p110β [28] autophosphorylation activities were inhibited by 100 μM resveratrol, albeit to a smaller extent than the inhibition observed by LY294002 treatment (Supplementary Figure 2B).
Figure 5. Resveratrol inhibits recombinant class IA, but not class IB, PI3K protein kinase activity.
Relative dose–response inhibition of class IA p85α/GST–p110α and class IB GST-p110γ autophosphorylation activities. ‘Relative autophosphorylation’ refers to the radioactivity incorporated into p85α and GST–p110γ bands relative to the autophosphorylation reaction without resveratrol. Lower panel: quantification of incorporated radioactivity from three independent experiments. Results are means±S.D. The significance of inhibition was compared with the assays without resveratrol by using Student's t test. *P<0.1, **P<0.05. p85α/GST–p110α and GST–p110γ expression levels were visualized by Coomassie Blue staining (see Supplementary Figure 2A).
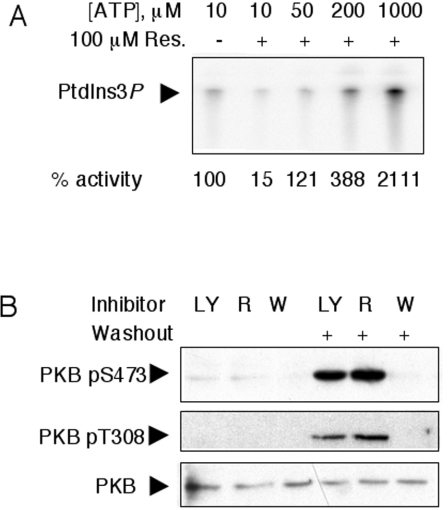
The two most widely used PI3K inhibitors, wortmannin and LY294002, differ in their mode of action. Both inhibitors target competitively the ATP/substrate-binding site. However, wortmannin binds covalently to a lysine residue involved in the phosphotransfer reaction (Lys-802 in p110α) causing an irreversible inhibition, whereas LY294002 binding to the catalytic region is reversible [38]. Pretreatment of L6 cells with 100 nM wortmannin or 50 μM LY294002 resulted in the complete inhibition of insulin-stimulated PKB phosphorylations on Ser-473 and Thr-308. Medium replacement between inhibitor treatment and insulin stimulation led to the loss of the inhibitory action of LY294002 but not wortmannin (Figure 6B). Under these experimental conditions, the mode of action of resveratrol resembles that of LY294002, and thus does not occur through a covalent binding to the p110 catalytic subunit.
Figure 6. Resveratrol targets the PI3K ATP-binding site in a non-covalent fashion.
(A) The lipid kinase activity of class IA p85α/p110α was assayed in the absence (–) or in the presence (+) of 100 μM resveratrol (Res.) and with increasing ATP concentrations as indicated. The experiment shown is representative of two independent ones in which each condition was tested in duplicate. ‘% activity’ is the mean for four independent reactions. (B) Serum-starved L6 cells were pretreated with 50 μM LY294002 (LY), 100 μM resveratrol (R) or 100 nM wortmannin (W) as indicated for 30 min. Prior to a 10 min stimulation with 1000 nM insulin, the medium was replaced in the lanes indicated (washout), thus allowing the elimination of non-covalently bound inhibitor. Separated proteins were immunoblotted with antibodies to PKB pS473, PKB pT308 and total PKB to monitor equal loading.
To assess whether resveratrol inhibits PI3K in a competitive fashion, p85α/p110α lipid kinase activity was tested in the presence of 100 μM resveratrol and increasing concentrations of ATP (Figure 6A). The inhibitory action of resveratrol was diminished by increasing ATP concentrations, indicating that resveratrol acts as a PI3K inhibitor by competing with ATP for the catalytic site.
Inhibition of PI3K by resveratrol is sirtuin-independent
As discussed above, IRS1-associated PI3K activity is inhibited by resveratrol, although SIRT1 does not co-purify in IRS1 immune complexes, suggesting an SIRT1-independent inhibition directly targeting PI3K. To demonstrate conclusively that resveratrol-mediated PI3K inhibition is sirtuin-independent, we down-regulated endogenous SIRT1 by RNA interference, obtaining a >80% decrease in expression. Under these conditions, resveratrol inhibition of insulin-stimulated PKB Ser-473 phosphorylation was unaltered (Figure 7B), demonstrating the lack of involvement of SIRT1 in the observed inhibition.
DISCUSSION
Resveratrol, a polyphenolic compound found in appreciable amounts in grapes and red wine, is currently a widely investigated molecule for its potentially beneficial effects on health and its capability to promote longevity [11].
In yeast, contrasting reports exist as to whether resveratrol administration increases replicative lifespan (reviewed in [39]). In animal models, from lower metazoans to vertebrates, including small mammals, resveratrol administration has the remarkable property to prolong lifespan [10,11,40].
Several genes have been identified that play a part in the control of lifespan, including genes implicated in insulin-like signalling and genes coding for the Sir2/SIRT1 sirtuin family of deacetylases [8]. High-throughput screening for sirtuin activators identified resveratrol as an activator of SIRT1 (13.4-fold at 100 μM) and Sir2 (2-fold at 2–5 μM) [9]. This finding, combined with genetic evidence of the involvement of Sir2/SIRT1 in lifespan prolongation, led to the suggestion that resveratrol acts through enzymatic activation of sirtuins [9,10]. Yet, recent studies suggest that resveratrol-induced increase in sirtuin enzymatic activity is dependent on the modified substrate used in the in vitro screening [13,14].
Besides sirtuins being bona fide enzymes activated by resveratrol, other targets have been proposed that may explain the aging-promoting, antioxidant and chemopreventive/chemotherapeutic properties of resveratrol, including COXs [2], intracellular signalling cascades activating nuclear factor-κB and AP-1 (activator protein-1) [41], AMPK (AMP-activated protein kinase) [11] as well as the insulin–IGF-1 (insulin-like growth factor-1) signalling pathways [42]. Intriguingly, in the report by Zhang [42] describing the inhibition of both the PKB and MAPK signalling pathways by resveratrol in primary hepatocytes and hepatocyte-derived cell lines [42], resveratrol appears to work through inhibition of the insulin-stimulated interaction between IRS1 and PI3K and between IRS-1 and Grb2 (growth-factor-receptor-bound protein 2) [42]. Contrary to this finding, we failed to observe inhibition of the IRS1/PI3K p85α interaction (Figure 2A) at concentrations up to 100 μM. Such a discrepancy is probably dependent on the different cell types investigated, and a direct comparison of the action of resveratrol in hepatocytes with that in muscle cells is warranted.
In most of the above-mentioned resveratrol-modulated pathways, the genuine molecular target has not been identified or it is controversial. In our investigation, we obtained results indicating that (i) the catalytic subunits p110α and p110β of class IA PI3Ks are inhibited in vitro by resveratrol in the micromolar concentration range with an IC50≈25 μM, and (ii) PI3K-dependent phosphorylation of downstream targets (including PKB and FOXO) is inhibited by resveratrol with an IC50<10 μM for insulin-induced PKB Ser-473 phosphorylation. This inhibition occurred in several muscle-derived cell lines, including rat L6, rhabdomyosarcoma CCL and human primary myotubes derived from several donors. The different IC50 values observed in PI3K in vitro assays compared with PKB Ser-473 measurements may be a reflection of a different affinity of resveratrol towards PI3K in a cell culture setting compared with the test tube. (iii) Finally, an insulin-dependent end-point response such as insulin-induced glucose uptake in 3T3-L1 adipocytes was likewise inhibited by resveratrol.
Both the lipid-kinase and protein-kinase activities of p110α and p110β catalytic subunits are affected by resveratrol and this inhibition explains the in vivo blockade of the PI3K–PKB signalling pathway induced by insulin, LPA or stimulation via FCS in human primary myotubes and muscle-derived cell lines.
Overexpression of both class IA and class IB PI3Ks followed by in vitro lipid-kinase and protein-kinase assays allowed us to show that resveratrol is a class IA-specific PI3K inhibitor. Nonetheless, further investigation will be necessary to define whether the class IB PI3K p110γ relative refractivity to resveratrol inhibition is maintained in intact cells. Based on competition studies with ATP, resveratrol appeared to act as a competitive inhibitor targeting the ATP-binding pocket. By comparing the inhibition of insulin-induced PKB phosphorylation by LY294002, wortmannin and resveratrol, we show that inhibition by resveratrol is reversible.
The original screening leading to the identification of resveratrol identified other molecules structurally related to resveratrol capable of activating Sir2/SIRT1, including quercetin. Quercetin is a naturally occurring flavonoid reported to be a broad-spectrum protein kinase inhibitor [43] and a PI3K inhibitor (IC50=3.8 μM) [44].
The crystal structures of the tyrosine kinase Hck (haemopoietic cell kinase) [45] and PI3K p110γ [44] complexed with quercetin showed that the 4′ hydroxy group forms a hydrogen bond to the catalytic phosphoacceptor Hck Lys-295 and p110γ Lys-833. By comparing the structures of resveratrol and quercetin, we hypothesize that the 4′ hydroxy group of resveratrol forms a hydrogen bond to the p110α and p110β catalytic lysine residues in the ATP-binding site.
Taken together, our results demonstrate that resveratrol targets class IA PI3Ks and consequently inhibits their downstream signalling molecules. However, given the broad spectrum of kinases inhibited by the related molecule quercetin, we cannot discount that other kinases might be inhibited by resveratrol with similar IC50 values as class IA PI3K. Additionally to a possible inhibitory activity on other kinases, the pleiotropic effects of resveratrol are best underscored by the fact that it also activates AMPK in C2C12 myotubes [11], a phenomenon that we also observed in human primary myotubes and L6 cells (results not shown).
In conclusion, our observation that class IA PI3K is inhibited by resveratrol in the micromolar range usually employed in lifespan extension, stress resistance and chemoprevention studies defines a further target upon which resveratrol could act and has several implications. (i) In cancer, the PI3K/PKB pathway is virtually always activated [46]. The chemopreventive and/or chemotherapeutic properties of resveratrol (in addition to its inhibitory action on COX [2]) could be consequential to the negative regulation of the PI3K pathway. (ii) Active research is currently engaged in the discovery of PI3K inhibitors with therapeutic potential. While several synthetic class- and isoform-specific PI3K inhibitors have been described [47–49], resveratrol provides an example of a natural and non-toxic molecule endowed with isoform-selectivity towards class IA PI3K. (iii) Finally, given the interplay of the insulin-like and sirtuin pathways in aging regulation, resveratrol may exert its action on both pathways. Further research is thus warranted to determine to which extent each of the two pathways is modulated by resveratrol and whether resveratrol inhibits PI3K in lower metazoans and unicellular eukaryotes.
Online data
Acknowledgments
This work has been supported by an ARD/PNRD (Association pour la Recherche sur le Diabète and Programme national de recherche sur le diabète) grant (to L.P.). We thank Bernd Nürnberg (University of Duesseldorf, Germany) for providing the pcDNA3-p110β plasmid. We thank Christine Durand for technical assistance.
References
- 1.Baur J. A., Sinclair D. A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 2.Jang M., Cai L., Udeani G. O., Slowing K. V., Thomas C. F., Beecher C. W., Fong H. H., Farnsworth N. R., Kinghorn A. D., Mehta R. G., et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 3.Sexton E., Van Themsche C., LeBlanc K., Parent S., Lemoine P., Asselin E. Resveratrol interferes with AKT activity and triggers apoptosis in human uterine cancer cells. Mol. Cancer. 2006;5:45. doi: 10.1186/1476-4598-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faber A. C., Dufort F. J., Blair D., Wagner D., Roberts M. F., Chiles T. C. Inhibition of phosphatidylinositol 3-kinase-mediated glucose metabolism coincides with resveratrol-induced cell cycle arrest in human diffuse large B-cell lymphomas. Biochem. Pharmacol. 2006;72:1246–1256. doi: 10.1016/j.bcp.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Yang T., Fu M., Pestell R., Sauve A. A. SIRT1 and endocrine signaling. Trends Endocrinol. Metab. 2006;17:186–191. doi: 10.1016/j.tem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Vaziri H., Dessain S. K., Ng Eaton E., Imai S. I., Frye R. A., Pandita T. K., Guarente L., Weinberg R. A. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 7.Brunet A., Sweeney L. B., Sturgill J. F., Chua K. F., Greer P. L., Lin Y., Tran H., Ross S. E., Mostoslavsky R., Cohen H. Y., et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 8.Guarente L., Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- 9.Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 10.Wood J. G., Rogina B., Lavu S., Howitz K., Helfand S. L., Tatar M., Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–289. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 11.Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Kaeberlein M., McDonagh T., Heltweg B., Hixon J., Westman E. A., Caldwell S. D., Napper A., Curtis R., DiStefano P. S., Fields S., et al. Substrate-specific activation of sirtuins by resveratrol. J. Biol. Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 14.Borra M. T., Smith B. C., Denu J. M. Mechanism of human SIRT1 activation by resveratrol. J. Biol. Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 15.Kaeberlein M., Powers R. W., III, Steffen K. K., Westman E. A., Hu D., Dang N., Kerr E. O., Kirkland K. T., Fields S., Kennedy B. K. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 16.Fabrizio P., Pozza F., Pletcher S. D., Gendron C. M., Longo V. D. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 17.Holzenberger M., Dupont J., Ducos B., Leneuve P., Geloen A., Even P. C., Cervera P., Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 18.Bluher M., Kahn B. B., Kahn C. R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 19.Berdichevsky A., Viswanathan M., Horvitz H. R., Guarente L. C. elegans SIR-2.1 interacts with 14-3-3 proteins to activate DAF-16 and extend life span. Cell. 2006;125:1165–1177. doi: 10.1016/j.cell.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 21.Picard F., Kurtev M., Chung N., Topark-Ngarm A., Senawong T., Machado De Oliveira R., Leid M., McBurney M. W., Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bordone L., Motta M. C., Picard F., Robinson A., Jhala U. S., Apfeld J., McDonagh T., Lemieux M., McBurney M., Szilvasi A., et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moynihan K. A., Grimm A. A., Plueger M. M., Bernal-Mizrachi E., Ford E., Cras-Meneur C., Permutt M. A., Imai S. Increased dosage of mammalian Sir2 in pancreatic β cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Wymann M. P., Zvelebil M., Laffargue M. Phosphoinositide 3-kinase signalling – which way to target? Trends Pharmacol. Sci. 2003;24:366–376. doi: 10.1016/S0165-6147(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 25.Vanhaesebroeck B., Alessi D. R. The PI3K–PDK1 connection: more than just a road to PKB. Biochem. J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 26.Pirola L., Bonnafous S., Johnston A. M., Chaussade C., Portis F., Van Obberghen E. Phosphoinositide 3-kinase-mediated reduction of insulin receptor substrate-1/2 protein expression via different mechanisms contributes to the insulin-induced desensitization of its signaling pathways in L6 muscle cells. J. Biol. Chem. 2003;278:15641–15651. doi: 10.1074/jbc.M208984200. [DOI] [PubMed] [Google Scholar]
- 27.Wymann M. P., Bulgarelli-Leva G., Zvelebil M. J., Pirola L., Vanhaesebroeck B., Waterfield M. D., Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol. Cell. Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czupalla C., Culo M., Muller E. C., Brock C., Reusch H. P., Spicher K., Krause E., Nurnberg B. Identification and characterization of the autophosphorylation sites of phosphoinositide 3-kinase isoforms β and γ. J. Biol. Chem. 2003;278:11536–11545. doi: 10.1074/jbc.M210351200. [DOI] [PubMed] [Google Scholar]
- 29.Bondeva T., Pirola L., Bulgarelli-Leva G., Rubio I., Wetzker R., Wymann M. P. Bifurcation of lipid and protein kinase signals of PI3Kγ to the protein kinases PKB and MAPK. Science. 1998;282:293–296. doi: 10.1126/science.282.5387.293. [DOI] [PubMed] [Google Scholar]
- 30.Roques M., Vidal H. A phosphatidylinositol 3-kinase/p70 ribosomal S6 protein kinase pathway is required for the regulation by insulin of the p85α regulatory subunit of phosphatidylinositol 3-kinase gene expression in human muscle cells. J. Biol. Chem. 1999;274:34005–34010. doi: 10.1074/jbc.274.48.34005. [DOI] [PubMed] [Google Scholar]
- 31.Orlicky D. J., DeGregori J., Schaack J. Construction of stable coxsackievirus and adenovirus receptor-expressing 3T3-L1 cells. J. Lipid Res. 2001;42:910–915. [PubMed] [Google Scholar]
- 32.White M. F. The insulin signalling system and the IRS proteins. Diabetologia. 1997;40:S2–S17. doi: 10.1007/s001250051387. [DOI] [PubMed] [Google Scholar]
- 33.Bryant N. J., Govers R., James D. E. Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- 34.Park J. B. Inhibition of glucose and dehydroascorbic acid uptakes by resveratrol in human transformed myelocytic cells. J. Nat. Prod. 2001;64:381–384. doi: 10.1021/np000411t. [DOI] [PubMed] [Google Scholar]
- 35.Kosaki A., Yamada K., Suga J., Otaka A., Kuzuya H. 14-3-3β protein associates with insulin receptor substrate 1 and decreases insulin-stimulated phosphatidylinositol 3′-kinase activity in 3T3L1 adipocytes. J. Biol. Chem. 1998;273:940–944. doi: 10.1074/jbc.273.2.940. [DOI] [PubMed] [Google Scholar]
- 36.Ogihara T., Isobe T., Ichimura T., Taoka M., Funaki M., Sakoda H., Onishi Y., Inukai K., Anai M., Fukushima Y., et al. 14-3-3 protein binds to insulin receptor substrate-1, one of the binding sites of which is in the phosphotyrosine binding domain. J. Biol. Chem. 1997;272:25267–25274. doi: 10.1074/jbc.272.40.25267. [DOI] [PubMed] [Google Scholar]
- 37.Dhand R., Hiles I., Panayotou G., Roche S., Fry M. J., Gout I., Totty N. F., Truong O., Vicendo P., Yonezawa K., et al. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J. 1994;13:522–533. doi: 10.1002/j.1460-2075.1994.tb06290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vlahos C. J., Matter W. F., Hui K. Y., Brown R. F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 39.Longo V. D., Kennedy B. K. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Valenzano D. R., Terzibasi E., Genade T., Cattaneo A., Domenici L., Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr. Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 41.Kundu J. K., Surh Y. J. Molecular basis of chemoprevention by resveratrol: NF-kappaB and AP-1 as potential targets. Mutat. Res. 2004;555:65–80. doi: 10.1016/j.mrfmmm.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J. Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem. J. 2006;397:519–527. doi: 10.1042/BJ20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Srivastava A. K. Inhibition of phosphorylase kinase, and tyrosine protein kinase activities by quercetin. Biochem. Biophys. Res. Commun. 1985;131:1–5. doi: 10.1016/0006-291x(85)91761-9. [DOI] [PubMed] [Google Scholar]
- 44.Walker E. H., Pacold M. E., Perisic O., Stephens L., Hawkins P. T., Wymann M. P., Williams R. L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 45.Sicheri F., Moarefi I., Kuriyan J. Crystal structure of the Src family tyrosine kinase Hck. Nature. 1997;385:602–609. doi: 10.1038/385602a0. [DOI] [PubMed] [Google Scholar]
- 46.Hennessy B. T., Smith D. L., Ram P. T., Lu Y., Mills G. B. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat. Rev. Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 47.Knight Z. A., Gonzalez B., Feldman M. E., Zunder E. R., Goldenberg D. D., Williams O., Loewith R., Stokoe D., Balla A., Toth B., et al. A pharmacological map of the PI3-K family defines a role for p110α in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson S. P., Schoenwaelder S. M., Goncalves I., Nesbitt W. S., Yap C. L., Wright C. E., Kenche V., Anderson K. E., Dopheide S. M., Yuan Y., et al. PI 3-kinase p110β: a new target for antithrombotic therapy. Nat. Med. 2005;11:507–514. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 49.Camps M., Ruckle T., Ji H., Ardissone V., Rintelen F., Shaw J., Ferrandi C., Chabert C., Gillieron C., Francon B., et al. Blockade of PI3Kγ suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat. Med. 2005;11:936–943. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.