Abstract
SP-A (surfactant protein A) is a membrane-associated SP that helps to maintain the lung in a sterile and non-inflamed state. Unlike SP-As from other mammalian species, human SP-A consists of two functional gene products: SP-A1 and SP-A2. In all the functions examined, recombinant human SP-A1 invariably exhibits lower biological activity than SP-A2. The objective of the present study was to investigate why SP-A2 possesses greater biological activity than SP-A1 and what advantage accrues to having two polypeptide chains instead of one. We analysed structural and functional characteristics of recombinant baculovirus-derived SP-A1, SP-A2 and co-expressed SP-A1/SP-A2 using a wide array of experimental approaches such as analytical ultracentrifugation, DSC (differential scanning calorimetry) and fluorescence. We found that the extent of supratrimeric assembly is much lower in SP-A1 than SP-A2. However, the resistance to proteolysis is greater for SP-A1 than for SP-A2. Co-expressed SP-A1/SP-A2 had greater thermal stability than SP-A1 and SP-A2 and exhibited properties of each protein. On the one hand, SP-A1/SP-A2, like SP-A2, had a higher degree of oligomerization than SP-A1, and consequently had lower Kd for binding to bacterial Re-LPS (rough lipopolysaccharide), higher self-association in the presence of calcium and greater capability to aggregate Re-LPS and phospholipids than SP-A1. On the other hand, SP-A1/SP-A2, like SP-A1, was more resistant to trypsin degradation than SP-A2. Finally, the importance of the supratrimeric assembly for SP-A immunomodulatory function is discussed.
Keywords: analytical ultracentrifugation, differential scanning calorimetry, immunosuppression, lipopolysaccharide, oligomerization, surfactant protein A1 (SP-A1)
Abbreviations: CHO cell, Chinese-hamster ovary cell; DPPC, dipalmitoyl phosphatidylcholine; DPPG, 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol; DSC, differential scanning calorimetry; IL-2, interleukin 2; FBS, fetal bovine serum; LAL, Limulus amoebocyte lysate; MBP, mannose-binding protein; LPS, lipopolysaccharide; Re-LPS, rough LPS; SP, surfactant protein; PHA, phytohaemagglutinin; TGF-β1, transforming growth factor-β1; TNFα, tumour necrosis factor α
INTRODUCTION
SP-A (surfactant protein A) is a large oligomeric extracellular protein present in the alveolar fluid of mammals that is primarily involved in innate lung immunity [1–3]. SP-A constitutes the major protein component of pulmonary surfactant, comprising approx. 3–4% of the total mass of surfactant. It is mainly associated with surfactant membranes in the alveolar fluid. SP-A's ability to bind to lipids (i) improves the adsorption and spreading of surfactant membranes on to an air/liquid interface; (ii) protects surfactant biophysical activity from the inhibitory action of serum proteins; and (iii) allows this protein to position and concentrate along with surfactant membranes as an initial defence barrier against inhaled pathogens or toxins.
SP-A is able to bind not only to surfactant membranes but also to pathogen-associated molecular patterns on micro-organisms and receptors on cell surfaces [1–3]. The binding of SP-A to micro-organisms results in microbial aggregation, opsonization and/or permeabilization [1–4]. This facilitates microbial clearance. The binding of SP-A to receptors on immune cells in the alveolus leads to modulation of immune cell functions such as phagocytosis of pathogens and apoptotic cells or up-regulation of cell-surface receptor expression involved in microbial recognition [1–3]. Experiments carried out using mice genetically deficient in SP-A have clarified the important role played by SP-A in host defence [5]. In general, these mice have increased susceptibility to a variety of bacterial and viral infections compared with wild-type mice.
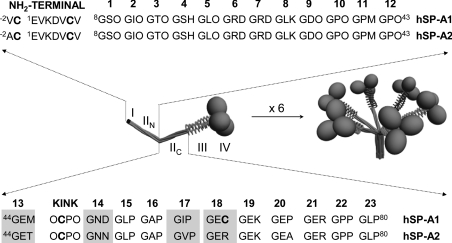
SP-A functions depend on SP-A binding capabilities (to lipids, carbohydrates and proteins), which in turn depend on its complex structure. SP-A is structurally homologous with an immune protein of the complement cascade, C1q, and belongs to the family of innate immune defence proteins known as collectins for their collagen-like and lectin domains [1–3,6]. Mature SP-A consists of 18 subunits, each of which consists of four structural domains as indicated in Figure 1. SP-A oligomerization is an intracellular process that can be conceptualized in two parts: the folding of monomeric subunits into trimers and the association of six trimers into an octadecamer [6] (Figure 1). Three domains participate in the trimerization: the neck domain, the collagen-like region and the N-terminal segment [6–8]. Supratrimeric assembly of SP-A would depend on interchain disulfide bonds and non-covalent intermolecular forces in the microfibrillar N-terminal piece [8–10].
Figure 1. Three-dimensional models of trimeric (right) and oligomeric (left) forms of SP-A and sequence of the N-terminal segment and the collagen-like domain of human SP-A1 and SP-A2.
The four structural domains of the human SP-A polypeptide chain are shown: (I) N-terminal segment involved in intermolecular disulfide bond formation; (II) collagen-like domain characterized by 23 Gly-Xaa-Yaa triplets with a sequence irregularity (kink), which divides the collagen-like domain into two parts: N-terminal (IIN) and C-terminal (IIC) portions (the triplet number is shown at the top of IIN and IIC sequences); (III) neck region between the collagen and the globular domain; and (IV) C-terminal globular domain. Amino acid differences between SP-A1 and SP-A2 at residues 46, 53, 61 and 65 are shaded. Cysteine residues are shown in boldface. The letter ‘O’ represents hydroxyproline.
In contrast with SP-As from other mammalian species, baboon and human SP-As consist of two polypeptide chains, SP-A1 and SP-A2 [11]. Both SP-A1 and SP-A2 genes are expressed in alveolar type II cells [11]. However, only the SP-A2 gene is expressed in tracheal and bronchial submucosal gland cells [12]. From a structural point of view, the ‘core’ differences that distinguish mature SP-A1 and SP-A2 are located at residues 46 (13th triplet), 53 (14th triplet), 61 (17th triplet) and 65 (18th triplet) of mature human SP-A [11], which are mostly beyond the kink in the C-terminal portion of the collagen domain (Figure 1). Despite the fact that there are only four amino acids that differ among the most common SP-A1 and SP-A2 alleles [11], several differences in their biochemical and functional properties have been observed. In all the functions examined, recombinant human SP-A2 invariably shows higher biological activity than SP-A1 [13–18]. For instance, recent studies show that SP-A2 is more effective than SP-A1 in stimulating association of Pseudomonas aeruginosa with rat alveolar macrophages and promoting its phagocytosis, suggesting that individuals whose lungs contain a higher amount of SP-A1 than SP-A2 allele might be more susceptible to bacterial pneumonia caused by Ps. aeruginosa [17,18].
The objective of the present study was to investigate why human SP-A1 possesses lower activity than SP-A2 [13–18] and what advantage accrues to having two polypeptide chains instead of one. Here, we analysed the extent of supratrimeric assembly of baculovirus-derived human SP-A1, SP-A2 and co-expressed SP-A1/SP-A2 by analytical ultracentrifugation and native electrophoresis. We also analysed their structural and functional characteristics by CD, DSC (differential scanning calorimetry), fluorescence, and absorption spectroscopy, as well as their susceptibility to proteolytic degradation. Furthermore, we explored immunomodulatory properties of these proteins expressed in insect cells in comparison with native human SP-A and trimeric and supratrimeric forms of recombinant SP-A1 expressed in mammalian cells.
EXPERIMENTAL
Materials
Synthetic phospholipids, DPPC (dipalmitoyl phosphatidylcholine) and DPPG (1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol) were purchased from Avanti Polar Lipids (Birmingham, AL, U.S.A.). Fluorescein and FITC (isomer I) were obtained from Molecular Probes (Eugene, OR, U.S.A.). Re-LPS [rough LPS (lipopolysaccharide)] from Salmonella minnesota (serotype Re-595), trypsin from bovine pancreas, PMA and PHA (phytohaemagglutinin) were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). SDS/PAGE molecular mass markers were from Amersham Biosciences (Piscataway, NJ, U.S.A.). The organic solvents (methanol and chloroform) used to dissolve lipids were HPLC-grade (Scharlau, Barcelona, Spain). RPMI 1640 medium was purchased from Invitrogen (Breda, NL, U.S.A.). Heat-inactivated FBS (fetal bovine serum) and LAL (Limulus amoebocyte lysate) kit were obtained from BioWhittaker (Walkersville, MD, U.S.A.). Serum-free Insect Express medium was from PAA (Marburg, Germany). Gradient PAGE gels were from Bio-Rad (Hercules, CA, U.S.A.). ELISA kit for TNFα (tumour necrosis factor α) and IL-2 (interleukin 2) immunoassays, anti-TGF-β1 (transforming growth factor-β1) antibody and TGF-β1 were obtained from BD PharMingen (San Diego, CA, U.S.A.). Macrophage-like cell line U937 and lymphocyte-like cell line (Jurkat T-cells) were supplied by the A.T.C.C. (Manassas, VA, U.S.A.). All other reagents were of analytical grade and obtained from Merck.
Expression and purification of baculovirus-derived recombinant human SP-A1, SP-A2 and co-expressed SP-A1/SP-A2
The recombinant forms of human SP-A1 (allele 6A2), SP-A2 (allele 1A0) and co-expressed SP-A1/SP-A2 (alleles 1A0/6A2) were expressed in SF21 cells using serum-free Insect Express medium as reported previously [19]. The cDNA for human SP-A1 and SP-A2 contained in the plasmids pMTE HS10/5 and pMTE HS10/4 were subcloned into the EcoRI site of the baculovirus expression vector pVL1393. Proteins were purified from the medium by mannose-affinity chromatography. Endotoxin content of recombinant proteins was in the range of 50–200 pg of LPS/mg of SP-A as determined by LAL test. All SP-A samples were stored in 5 mM Tris/HCl buffer (pH 7.4) (buffer A) at −20 °C. In some Figures, baculovirus-derived SP-A1, SP-A2 and the co-expressed product are referred to as A1, A2 and A1/A2 respectively.
Expression and purification of CHO cell (Chinese-hamster ovary cell)-derived recombinant human SP-A1 and SP-A1C6S mutant
The cloning, expression and purification of both wild-type form of human SP-A1 (6A2 allele) and a Cys−1- and Cys6-deficient mutant (SP-A1ΔAVC,C6S) was performed as described in [10]. SP-A1 expressed in mammalian cells is referred to as SP-A1m. This protein had supratrimeric structure and was N187-glycosylated and prolyl-hydroxylated [19]. The cysteine-deficient mutant is referred to as SP-A1mC6S or C6S. This protein is secreted as hydroxylated full-length trimers by CHO cells [10]. The endotoxin content of SP-A1m and SP-A1mC6S was 200 and 50 pg/mg of SP-A respectively as determined by the LAL assay. CHO cell-derived SP-As were stored in buffer A at −20 °C.
Isolation of native human SP-A
Broncho-alveolar lavages from alveolar proteinosis patients were used as a source of native human SP-A. SP-A was purified from isolated surfactant using sequential butanol and n-octylglucoside extractions [19]. Endotoxin content of native human SP-A was approx. 300 pg of endotoxin/mg of SP-A as determined by LAL test. Native human SP-A (referred to as nSP-A) was stored in buffer A at −20 °C.
PAGE and Western-blot analysis
Purity and apparent molecular mass of monomeric SP-As were determined by SDS/12%-(w/v)-PAGE under reducing conditions (5% 2-mercaptoethanol). For native conditions, electrophoresis was performed at 4 °C with a 4–20%-(w/v)-polyacrylamide gradient gel. To determine the assembly of disulfide-linked polypeptide chains, SP-A samples were separated on an SDS/4–15% gradient PAGE gel under non-reducing conditions. Proteins were visualized by silver staining. Possible contamination of TGF-β1 in recombinant or native human SP-A preparations was detected by Western-blot analysis. TGF-β1 was detected with an anti-TGF-β1 antibody. Bound antibodies were visualized by using chemiluminescence detection.
CD measurements
CD spectra were obtained on a Jasco J-715 spectropolarimeter fitted with a 150 W xenon lamp as previously described [10,19]. The acquired spectra were corrected by subtracting the appropriate blanks, subjected to noise-reduction analysis, and presented as molar ellipticities (degrees·cm2·dmol−1) assuming 110 Da as the average molecular mass per amino acid residue. The analysis of thermal stability of the collagen-like domain of recombinant human SP-As was performed as described in [10,19]. The temperature where the collagen triple helix of SP-A was 50% unfolded (F=0.5) was taken as the melting temperature (Tm). All measurements were typically performed in buffer A. SP-A concentrations were 120 μg/ml, and quartz cells with 1 mm path length were used.
DSC
Calorimetric measurements of SP-A were performed in a Microcal VP differential scanning calorimeter (Microcal, Northampton, MA, U.S.A.) [20]. SP-A (0.18 mg/ml) dissolved in 20 mM phosphate buffer (pH 7.4) was loaded in the sample cell of the microcalorimeter, with buffer in the reference cell. All solutions used for DSC were degassed just before loading into the calorimeter. Calorimetric scans were collected from each sample between 15 and 80 °C at a heating rate of 0.5 °C/min. The reversibility of the thermal transition was evaluated by several cycles of heating and cooling between 15 and 65 °C. The standard Microcal Origin software was used for data acquisition and analysis. The excess heat capacity functions were obtained after subtraction of the buffer–buffer baseline.
Analytical ultracentrifugation
To assess the degree of oligomerization of recombinant and native SP-A, samples (0.2 mg/ml) were assayed by sedimentation velocity. These experiments were carried out at 35000 rev./min and 25 °C in a Beckman XL-I ultracentrifuge (Beckman Coulter) with a UV–visible optics detector, using an An-60Ti rotor and double-sector centrepieces of Epon-charcoal. Sedimentation profiles were registered every 5 min at the appropriate wavelength. The sedimentation coefficient (s) distributions were calculated by least-squares boundary modelling of sedimentation-velocity data using the c(s) method [21], as implemented in the SEDFIT program, from which the corresponding s values of the main sedimenting species were determined.
Trypsin digestion
To determine SP-A susceptibility to trypsin degradation, proteins (2 μg) were incubated with trypsin (0.13 μg) in buffer A as described in [10,22]. The mixtures were incubated during 30 min at 25 and 37 °C. The digestion was stopped by addition of reducing SDS/PAGE sample buffer [62.5 mM Tris/HCl, pH 6.8, 2% (w/v) SDS, 25% (v/v) glycerol and 0.01% Bromophenol Blue] containing PMSF followed by boiling. Samples were analysed by SDS/PAGE followed by silver staining.
Fluorescence assays to determine the binding of SP-A to FITC–Re-LPS
A fluorescent Re-LPS derivative (FITC–Re-LPS) in which the phosphoethanolamine groups of Re-LPS were bound to FITC was prepared as previously described [10,23]. Fluorescence measurements were carried out using an SLM-Aminco AB-2 spectrofluorimeter with a thermostatically controlled cuvette holder (±0.1 °C), using 5 mm×5 mm path-length quartz cuvettes. Fluorescence emission spectra and fluorescence emission anisotropy measurements of FITC–Re-LPS (4×10−7 M) or free fluorescein (10−6 M) were performed in the presence and absence of SP-A in buffer A containing 100 mM NaCl and 2 mM EDTA at 25 °C exactly as previously described [23]. The apparent dissociation constant (Kd) for FITC–Re-LPS–SP-A complexes was obtained by analysing the time dependence of the fluorescence change when 2×10−8 M FITC–Re-LPS reacted with various concentrations of SP-A (0–250 μg/ml) at 25 °C [23].
SP-A self-association assay
The capability of SP-A to self-associate was determined as previously described [22,24] by measuring the Ca2+-dependent change in protein absorbance at 360 nm in a Beckman DU-800 spectrophotometer. Self-association was also determined by centrifugation at 12000 g in a Hettich microtitre centrifuge for 15 min as described in [25].
Re-LPS and phospholipid vesicle aggregation assays
LPS aggregation or DPPC/DPPG vesicle aggregation induced by SP-A was studied at 25 °C by measuring the change in absorbance at 400 nm in a Beckman DU-800 spectrophotometer as previously reported [10,19,23]. For LPS aggregation assay, final concentrations of Re-LPS, SP-A, Ca2+ and EDTA were 40 μg/ml, 20 μg/ml, 2.5 mM and 5 mM respectively. For phospholipid vesicle aggregation, final concentrations of phospholipids, SP-A, calcium and EDTA were 100 μg/ml, 20 μg/ml, 2.5 mM and 5 mM respectively. The potential contribution of SP-A self-association to the light absorption at 400 nm was routinely checked under the experimental conditions in which Re-LPS or DPPC/DPPG vesicle aggregation assays were performed [10,23].
Cell assays
Jurkat T-cells (A.T.C.C., TIB-152), a CD4+ human lymphoblastoid cell line, were grown in RPMI 1640 supplemented with 10% heat-inactivated FBS, 2 mM glutamine and penicillin G sodium (100 units/ml)/streptomycin sulfate (100 μg/ml), and 0.25 μg/ml amphotericin B at 37 °C under an air/CO2 (19:1) humidified atmosphere as previously reported [26]. Jurkat T-cells (seeded at 5×105 cells/ml) were stimulated with PMA (10 nM) and PHA (1 μg/ml) and cultured in 24-well microtitre plates for 48 h in the presence or absence of different amounts of SP-A preparations. Cell viability and measurement of IL-2 production were determined as in [26]. Four different T-cell cultures were used. The assays from each Jurkat T-cell culture were performed in triplicate, the triplicate values were averaged, and their mean was treated as a single point. The results are presented as the means (±S.E.M.) from four cell cultures.
Human monocyte-like U937 cells (A.T.C.C., CRL-1593.2) were grown as reported previously [10,23]. U937 cells were dispensed into 24-well plates at 1×106 cells/ml and differentiated into macrophages by incubation with 10 nM PMA for 24 h at 37 °C under a 5%-CO2 humidified atmosphere. Then adhered cells were washed with medium and, 24 h later, washed and pretreated with different SP-A preparations for 10 min prior to 4 h Re-LPS (4×10−8 M) stimulation in the presence of 5% heat-inactivated FBS at 37 °C. Measurement of TNFα production was performed as in [10,23].
For statistical analysis, mean comparison between groups was done by one-way ANOVA followed by Bonferroni post hoc analysis; a confidence level of 95% or greater (P<0.05) was considered significant.
RESULTS
Structural characteristics
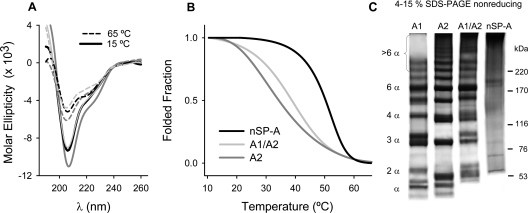
Recombinant human SP-A2 and co-expressed SP-A1/SP-A2 had CD spectra comparable with those of native SP-A (Figure 2A), as previously reported for SP-A1 [19], indicating that they have similar secondary structure. We previously demonstrated that the change in ellipticity of porcine SP-A during the thermal transition from 20 to 70 °C can be fitted to a structural transition between two components, which closely follows the denaturation profile of collagen [24]. Figure 2(B) shows the thermal denaturation of the collagen triple helix of SP-A2 and co-expressed SP-A1/SP-A2 monitored by CD spectroscopy, along with that of nSP-A, which has been reported recently [19]. The collagen triple helix of recombinant SP-A2 and SP-A1/SP-A2 melted at a much lower temperature than that of nSP-A, as was previously found for SP-A1 [19]. This is probably because SP-As expressed in insect cells are deficient in proline hydroxylation, and the content of hydroxyproline is related to the Tm of collagens [27].
Figure 2. Structural characteristics of recombinant and native SP-A.
(A) CD spectra of SP-A2 (dark-grey line), co-expressed SP-A1/SP-A2 (light-grey line), and native SP-A (black line) at 10 (continuous lines) and 70 °C (broken lines). (B) Melting curves were monitored at 207 nm, while the sample temperature was raised from 10 to 70 °C, with an average heating rate of 0.2 °C/min. (C) Electrophoretic analysis of SP-A on SDS/4–16% PAGE under non-reducing conditions to identify disulfide-linked polypeptide chains. Proteins were visualized by silver staining. Molecular-mass markers in kDa are indicated to the right of the gel. The results shown are from a representative one of three experiments.
We previously found that the lack of prolyl hydroxylation influences the arrangement of disulfide bonding on human SP-A1 [19]. To determine whether the assembly of disulfide-linked polypeptide chains was also altered in SP-A2 and SP-A1/SP-A2, we performed electrophoretic analysis of these proteins on gradient SDS/PAGE under non-reducing conditions. Figure 2(C) shows that whereas native SP-A consisted mainly of large disulfide-linked oligomeric structures that hardly entered the gel, recombinant SP-As consisted of a mixture of disulfide-linked polypeptide chains. SP-A2 and co-expressed SP-A1/SP-A2 favour the formation of higher disulfide-linked oligomers than SP-A1.
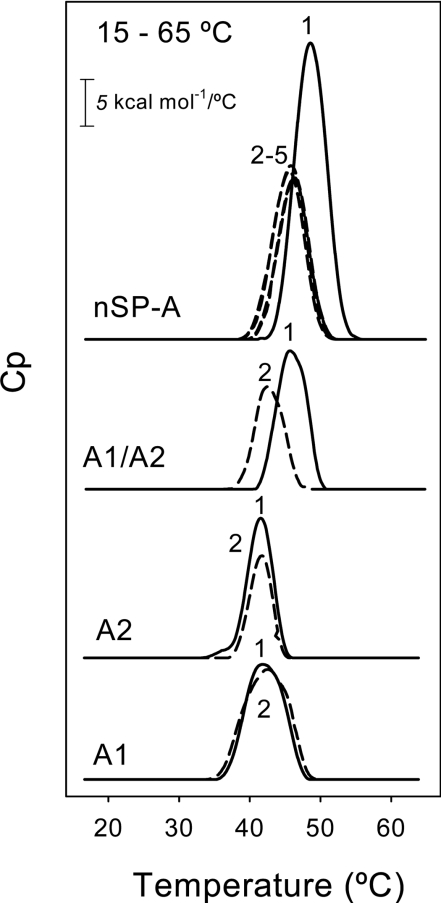
DSC of native and recombinant SP-A was used as an alternative approach to determining the stability of their collagen-like domain in the context of the full molecule. The calorimetric measurements were performed at neutral pH and low ionic strength to avoid aggregation of the samples under study. Figure 3 shows the melting curves of native and recombinant SP-A at a concentration of 0.18 mg/ml. Under these conditions, the melting curves always displayed one heat absorption peak over a temperature range of 15–80 °C. The apparent Tm value for native SP-A was 48.5±0.3 °C (n=3). This value was identical with that obtained by CD (Figure 2B) and was also similar to the Tm for the collagen-like domains of C1q and MBP (mannose-binding protein) determined by DSC [28,29]. When native SP-A was submitted to several cycles of heating and cooling over a temperature range of 15–65 °C, the triple-helix-to-coil transition was partially reversible. The heat-capacity curves at the second, third and subsequent scans completely overlapped. However, the transition occurred at a reduced temperature (46 °C), indicating that the protein did not completely achieve a native conformation after cooling.
Figure 3. Thermal unfolding of recombinant and native SP-A determined by DSC.
The temperature dependence of specific heat capacity at constant pressure, Cp, was determined at an SP-A concentration of 0.18 mg/ml in 20 mM phosphate buffer (pH 7.4) (heating rate 0.5 °C/min). Calorimetric scans were collected between 15 and 80 °C. The reversibility of the thermal transition was evaluated by several cycles of heating and cooling between 15 and 65 °C (broken lines). The results shown are from a representative one of two or three experiments.
On the other hand, DSC of baculovirus-derived SP-As revealed that the triple-helix-to-coil transition occurred at the same temperature for SP-A1 (42±0.5 °C, n=3) and SP-A2 (41.8±0.3 °C, n=3). These apparent Tm values were higher than those obtained by CD (probably because of the higher scan rate, 30 °C/h, utilized in DSC), but similar to those of type I collagen under fibril-non-forming conditions (i.e. in 0.007 M acetic acid, pH 3.0) determined by DSC (heating rate 1 °C/min) [30]. The Tm of fibril-forming type I collagen is 55 °C [30]. Refolding experiments with SP-A1 and SP-A2 samples (Figure 3, dashed lines) showed roughly similar areas under the melting curve. The reversibility of unfolding of SP-A1 or SP-A2 collagen-like domain might reside in the adjacent α-helical coiled-coil domain and the interchain disulfide bonds at the N-terminus of the triple helix (Figure 1).
DSC experiments revealed that co-expressed SP-A1/SP-A2, with an apparent Tm of 45.5±0.01 °C (n=3), was significantly more stable than SP-A1 or SP-A2, indicating that the combination of both gene products (SP-A1 and SP-A2) may be important for reaching a fully native conformation. Refolding experiments (Figure 3, dashed lines) revealed that in the second and third heating scans, the transition peak for co-expressed SP-A1/SP-A2 took place at a slightly reduced temperature (42 °C), similar to that of SP-A1 or SP-A2, but the area under the curve remained roughly the same, suggesting that the triple-helix structure was re-formed.
Supratrimeric oligomerization
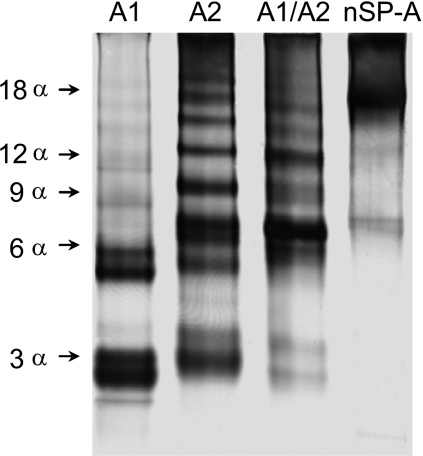
We previously showed that the degree of oligomerization of SP-A markedly influences its function [10]. Here, we studied whether differences in the extent of oligomerization between SP-A1 and SP-A2 might explain why SP-A1 has lower biological activity than SP-A2 [13–18]. The relative extent of oligomerization was first assessed by electrophoresis under native conditions (Figure 4). This electrophoresis is performed at 4 °C and the samples were not treated with SDS, reducing agents, or heat. Thus proteins retain their natural conformation. While in SDS/PAGE the electrophoretic mobility of proteins depends primarily on their molecular mass, in native PAGE the mobility depends on the protein's charge, size and shape. As observed in Figure 4, native SP-A shows low mobility, consistent with its high supratrimeric structure, whereas baculovirus-derived SP-As migrate as several bands of different mobility, indicating that they are composed of a mixture of oligomers of different sizes. While SP-A1 migrated as two bands, compatible with trimers (3α) and hexamers (6α) as previously shown [19], SP-A2 and co-expressed SP-A1/SP-A2 were composed of a mixture of oligomers in which large oligomeric forms were visualized. Given that CD (Figure 2) and DSC (Figure 3) experiments indicated that baculovirus-derived SP-A1, SP-A2 and co-expressed SP-A1/SP-A2 possessed an intact collagen-like domain, we assumed that, under native conditions, SP-A samples must consist of three or three-multiplied polypeptide chains. Thus we assumed that the bands visualized in the native gel correspond to trimers, hexamers, nonamers etc. No monomers (one polypeptide chain) of recombinant SP-As expressed in insect cells were visualized by native PAGE in the absence of reducing agents, as previously shown for SP-A1 [19].
Figure 4. Patterns of oligomerization of native and recombinant human SP-A under native conditions.
Samples (1 μg of each SP-A preparation) were subjected to 4–20% gradient PAGE under native conditions at 4 °C and visualized by silver staining. The arrows on the left indicate bands that would be compatible with trimers (3α), hexamers (6α), nonamers (9α) etc. The results shown are from a representative one of three experiments.
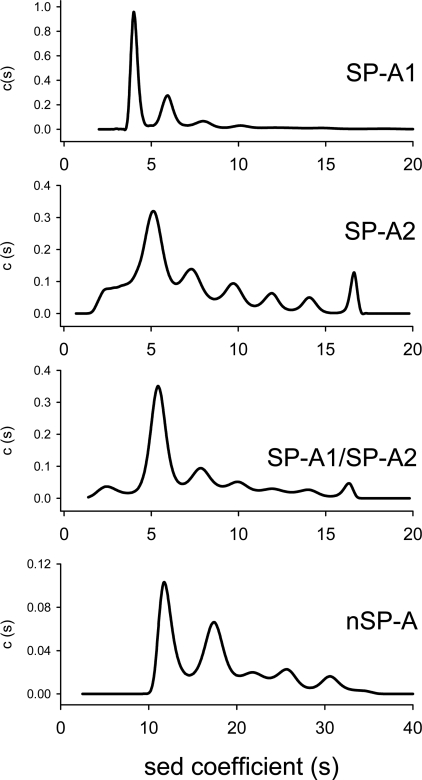
To investigate whether the patterns of oligomerization among SP-A1, SP-A2 and co-expressed SP-A1/SP-A2 differ in solution, the proteins were submitted to analytical ultracentrifugation. Figure 5 shows the sedimentation-coefficient distributions c(s) of each protein. This analysis revealed that the sedimentation behaviour of SP-A1 can be well explained if the protein is a mixture of two main species with s values of 4.1±0.2 and 5.8±0.3 S (which correspond to approx. 50 and 30% of the initial loading concentration respectively), and a third minor species with an s value of 7.6±0.3 S (which amounts to less than 8% of the protein concentration). The two main sedimenting species are compatible with the hydrodynamic behaviour of trimers and hexamers of the protein, having a non-spherical overall shape (frictional ratios of approx. 2). This would seem to be a realistic assumption, if the sedimentation studies of related proteins, such as MBP, are considered. Sedimentation coefficients of 3.7 and 5.5 were found for trimers and hexamers of MBP [31].
Figure 5. Sedimentation-velocity analysis of native and recombinant human SP-A.
Data were obtained with 0.2 mg/ml SP-A subjected to centrifugation at 35000 rev./min in buffer A, at 25 °C. Concentration distribution versus sedimentation coefficient shows different oligomerization states for recombinant SP-As, which would be compatible with trimers and hexamers (for SP-A1), and higher supratrimeric assemblies (for SP-A2 and SP-A1/SP-A2). Native human SP-A is highly oligomerized and multimerized in solution. The protein exhibited two major species peaks with sedimentation coefficients of 12 and 17.5 S. Results are consistent with the presence of heterogeneity. The results shown are from a representative one of two or three experiments.
The sedimentation-velocity behaviour of SP-A2 also shown in Figure 5 is typical of a sample highly polydisperse in size that agrees well with the electrophoretic results described in Figure 4. In this case, the sedimentation coefficient distribution is much more complex than the one obtained for SP-A1 and yields a much broader distribution of s values, with the 5.0±0.3 S being the more predominant species (approx. 40%). The latter might be compatible with an elongated hexamer; however, the high polydispersity of SP-A2 in solution precludes a reliable assignment of these s values to certain oligomeric species of the protein. With respect to co-expressed SP-A1/SP-A2, its pattern of oligomerization was between those of SP-A1 and SP-A2. Taken together, the results indicate that the degree of oligomerization was greater for SP-A2 and co-expressed SP-A1/SP-A2 than for SP-A1.
On the other hand, native human SP-A is highly oligomerized and multimerized in solution. At 35000 or 20000 rev./min, nSP-A sedimented as two main species with s values of 12.1±0.4 and 17.5±0.5 S. Sedimentation coefficients of 12.5 and 14 were reported for C1q [32] and dog SP-A [33] respectively.
SP-A self-association and SP-A-induced lipid vesicle aggregation
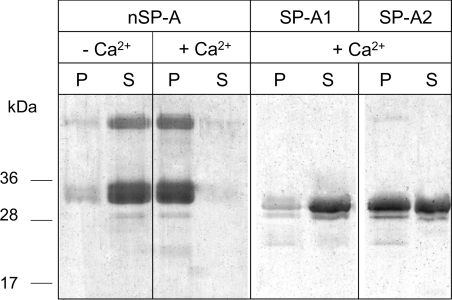
We previously reported that the ability of SP-A to self-associate in the presence of calcium depends on its degree of supratrimeric assembly [10] and requires a structurally intact collagen domain [19,24]. To determine whether the different degree of oligomerization of SP-A1 and SP-A2 correlates with its capability to self-associate in the presence of calcium, we analysed Ca2+-dependent self-association by centrifugation at 12000 g for 15 min at 25 °C, a temperature below the Tm for these proteins at which their collagen domains are correctly folded. The entire pellet fractions and supernatants were then subjected to SDS/PAGE under reducing conditions, followed by staining of the gels. Figure 6 shows that native SP-A was recovered in the supernatant fraction in the absence of calcium. However, the protein was recovered in the pellet upon addition of Ca2+, indicating that Ca2+-dependent self-association of native SP-A occurred. In contrast, recombinant SP-A1 was mainly recovered in the supernatant fraction upon addition of Ca2+, signifying that the protein did not self-associate. Recombinant SP-A2 was partly recovered in the pellet and the supernatant fraction, indicating that only part of the protein self-associated in the presence of calcium. These results indicate that small oligomeric forms of baculovirus-derived SP-As (likely trimers and hexamers) were unable to self-associate in the presence of calcium.
Figure 6. Ca2+-dependent self-association of SP-A1 and SP-A2 determined by centrifugation.
Proteins (20 μg/ml) were incubated in the absence or presence of calcium. After centrifugation at 12000 g for 15 min, the pellets (P) and the supernatants (S) were subjected to SDS/PAGE and the gel was stained with Coomassie Blue. Numbers on the left denote molecular mass.
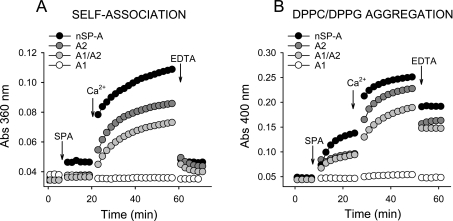
The capabilities of different SP-As to self-associate and to induce lipid vesicle aggregation in the presence of calcium were also studied by light absorption at 360 and 400 nm respectively (Figure 7). The relative abilities of different SP-As to undergo these two phenomena at 25 °C were as follows:
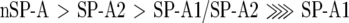 |
These results indicate that the ability of different SP-As to self-associate and to induce lipid aggregation was correlated with their degree of supratrimeric assembly. These two processes seem to be related phenomena, since both processes depend on the degree of SP-A oligomerization (the present study and [10]), are parallel inhibited by exposure of SP-A to ozone [34], and possess similar calcium activation constants (KaCa2+) in saline buffers [22,24]. However, at least for human SP-A, while EDTA completely reversed the calcium-mediated SP-A self-association (Figure 7A), it partly reversed phospholipid vesicle aggregation induced by the protein (Figure 7B).
Figure 7. SP-A self-association and SP-A-induced lipid vesicle aggregation.
(A) The Figure shows kinetics of Ca2+-dependent self-association of different SP-A samples. SP-As (20 μg/ml) were added to the sample cuvette filled with buffer A. The turbidity change at 360 nm was monitored at 25 °C at 1 min intervals. After stabilization, 5 mM Ca2+ (final concentration) was added to both the sample and reference cuvette, and the turbidity changes were monitored again. Addition of EDTA (10 mM, final concentration) dissociated SP-A aggregates induced by Ca2+. (B) The Figure shows kinetics of DPPC/DPPG vesicle aggregation induced by SP-A in the presence of calcium at 25 °C. In (A, B), one representative experiment of three experiments is shown.
SP-A binding to LPS, and LPS aggregation induced by SP-A
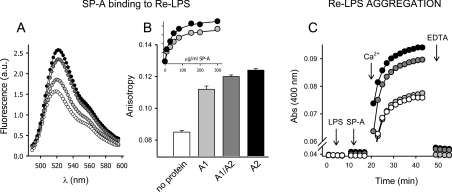
The binding of different SP-As expressed in insect cells to Re-LPS in solution was monitored by changes in FITC–Re-LPS fluorescent properties such as intensity and anisotropy. These experiments were performed in the presence of EDTA. Figure 8(A) shows that addition of different SP-As produced an increase in fluorescence emission intensity and a red-shift of the emission maximum of FITC–Re-LPS, as previously reported for nSP-A [23]. The magnitude of the fluorescence change depended on the type of recombinant SP-A, with SP-A2 and co-expressed SP-A1/SP-A2 having a greater effect than SP-A1. For each protein, the magnitude of the fluorescence change increased as a function of SP-A concentration and was saturable. The apparent equilibrium dissociation constant (Kd) for FITC–LPS–SP-A complexes, calculated from the saturation curve, was 4.4×10−8 M for native SP-A, 3.5×10−7 M for co-expressed SP-A1/SP-A2, 3.0×10−7 M for SP-A2 and 5.6×10−7 M for SP-A1, considering a molecular mass of 105 kDa for trimeric SP-A, which is the basic oligomeric unit. Collectively, these results indicate that recombinant SP-As produced in insect cells bind to LPS in a Ca2+-independent manner and that the binding is higher for SP-A2 and SP-A1/SP-A2 than for SP-A1.
Figure 8. SP-A binding to LPS and LPS aggregation induced by SP-A.
Binding of SP-A to Re-LPS (A, B) was monitored by changes in FITC–Re-LPS fluorescent properties. (A) The Figure shows the fluorescence emission (λex=470) spectra of FITC–Re-LPS in the absence (white circles) and presence of recombinant human SP-A1 (light-grey circles), SP-A2 (black circles), and SP-A1/SP-A2 (dark-grey circles) in buffer A containing 100 mM NaCl and 2 mM EDTA. One representative experiment of three experiments is shown. (A) The Figure shows that recombinant human SP-As expressed in insect cells induce a significant increase in fluorescence anisotropy upon binding to FITC–Re-LPS. All data points are the means±S.D. for experiments performed in triplicate. The inset of (B) shows that the magnitude of FITC–Re-LPS anisotropy increases as a function of SP-A1 (light grey circles) and SP-A2 (black circles) concentration and was saturable. (C) The Figure shows kinetics of Ca2+-dependent Re-LPS aggregation in the absence and presence of different SP-A samples at 25 °C (symbols as in A). One representative experiment of three experiments is shown.
We also examined the binding of different SP-As to LPS by measuring fluorescence emission anisotropy of FITC–Re-LPS in the absence and presence of SP-A (Figure 8B). We found that the formation of complexes between LPS and different SP-As led to an increase in fluorescence emission anisotropy, indicating that the binding of these proteins to Re-LPS caused mechanical restrictions of the rotational mobility of the dye. The magnitude of FITC–Re-LPS anisotropy increased as a function of SP-A concentration and was saturable. The ability of different SP-As to increase FITC–Re-LPS anisotropy is greater for SP-A2 and SP-A1/SP-A2 than for SP-A1. This is consistent with results from fluorescence intensity measurements described above. Figure 8(C) shows that despite the fact that SP-A1 was able to bind to Re-LPS, SP-A1 was not able to induce Re-LPS aggregation. Taken together, these results indicate that the capability of SP-A to induce LPS aggregation in the presence of calcium depends not only on the protein affinity for LPS but also on the degree of SP-A supratrimeric assembly (Figure 5).
Susceptibility to proteolytic cleavage by trypsin
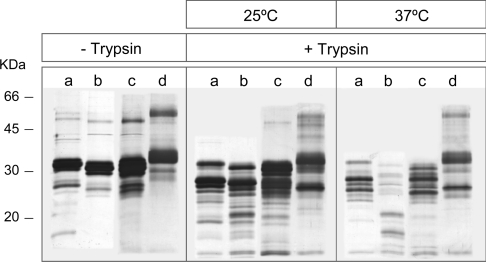
Figure 9 shows that whereas the susceptibility to trypsin of native SP-A was quite similar at 25 and 37 °C (temperatures below its Tm=48 °C), that of non-hydroxylated baculovirus-derived SP-As was much lower at 25 °C (a temperature below their collagen melting temperatures) than at 37 °C (a temperature near the Tm of these proteins). This is consistent with previous studies that demonstrated that SP-A is more susceptible to trypsin degradation following incubation at temperatures close to or above its collagen melting temperature than below it [10]. In addition, Figure 9 indicates that SP-A1 was more resistant to trypsin degradation than SP-A2 at 37 °C (a temperature at which the collagen domain of these two proteins might be partially unfolded), and the correct folding of the collagen domain at 25 °C seemed to decrease the susceptibility to trypsin. The polypeptide chains of SP-A1 and SP-A2 contain 15 similar trypsin cleavage targets in the N-terminal segment, neck, and globular domains. However, trypsin cleavage sites differ in the collagen-like domain. Given that SP-A1 contains five trypsin cleavage targets in the collagen domain, whereas SP-A2 contains six (Figure 1), differences in protease susceptibility between SP-A2 and SP-A1 at 37 °C should be attributed to the accessibility of trypsin to an additional cleavage site (Arg65) at the collagen domain of SP-A2. This is supported by the fact that the presence of SP-A1 polypeptide chains in co-expressed SP-A1/SP-A2 made this protein less susceptible to trypsin degradation than SP-A2 at 37 °C. The collagen stability of human SP-A may be altered in various disease states or by exposing the lungs to environmental pollutants (e.g. ozone, SO2 or nitrogen oxides), and SP-A2 polypeptide chains might be more susceptible to proteolytic degradation.
Figure 9. SP-A susceptibility to trypsin degradation.
The Figure shows a silver-stained 12% reducing SDS/PAGE gel of SP-A1 (a), SP-A2 (b), SP-A1/SP-A2 (c) and nSP-A (d) incubated in the absence (–) or presence (+) of trypsin (SP-A/trypsin; 15:1, w/w) for 30 min, at 25 and 37 °C. Numbers on the left denote molecular masses.
Immunomodulatory properties
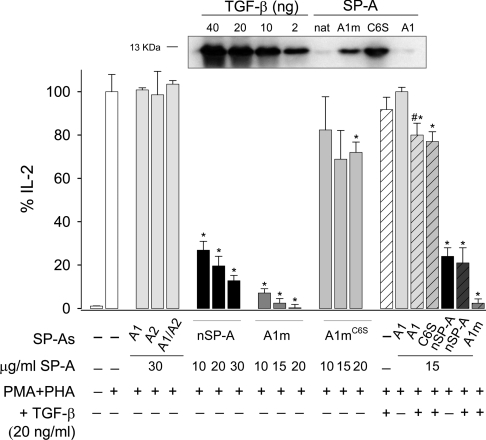
SP-A inhibits human T-lymphocyte proliferation and IL-2 secretion in vitro [35], and this immunomodulatory activity depends on SP-A collagen domain [36]. The results in Figure 10 illustrate that nSP-A showed a concentration-dependent inhibitory effect on IL-2 secretion by PMA/PHA-stimulated Jurkat T-cells after 48 h of culture. In contrast, none of the recombinant baculovirus-derived SP-A proteins had any effect on IL-2 secretion at concentrations as high as 30 μg/ml. The main differences between native SP-A and baculovirus-derived SP-As are the state of oligomerization, proline hydroxylation, and glycosylation. To investigate the effect of the degree of supratrimeric assembly on SP-A immunosuppressive properties, we compared the effect of hydroxylated supratrimeric SP-A1m expressed in CHO cells [10,19] with a hydroxylated full-length mutant SP-A1m molecule (SP-A1mC6S) that is secreted as a trimer, utilizing a CHO cell expression system [10]. Results indicated that while SP-A1m showed a marked dose-dependent inhibitory effect on IL-2 secretion, SP-A1mC6S mutant had a modest immunosuppressive effect (Figure 10).
Figure 10. Effect of SP-A on IL-2 production by stimulated Jurkat T-cells.
Jurkat T-cells stimulated with PMA (10 nM) and PHA (1 μg/ml) were incubated for 48 h in the absence (white bars) and presence of native SP-A (black bars), baculovirus-derived SP-As (light-grey bars) and CHO cell-derived SP-As (dark-grey bars). Some experiments were performed in the presence of 20 ng/ml TGF-β1 (striped bars). Supernatants were collected after 48 h of culture and the levels of IL-2 were measured by ELISA. The results are presented as the means (±S.E.M.) from four different cell cultures. Results are expressed as percentages of the control level of IL-2 production by stimulated cells in the absence of SP-A. *P<0.05 compared with response elicited by PMA/PHA in the absence of SP-A. #P<0.05, SP-A+TGF-β1 compared with SP-A alone. Upper panel: detection of TGF-β1 in native SP-A (supratrimeric), CHO-cell-derived SP-A1m (supratrimeric), CHO-cell-derived SP-A1C6S (trimeric) and baculovirusderived SP-A1 (trimeric/hexameric) by Western-blot analysis using an anti-TGF-β1 antibody. The amount of SP-A samples was 150 μg. Increasing concentrations of recombinant TGF-β1 were also detected by Western blot to estimate roughly the amount of TGF-β1 bound to SP-A.
It has been recently reported that recombinant SP-A1 produced by mammalian cells (SP-A1m) is bound to TGF-β1 present in the culture medium and that the presence of TGF-β1 in SP-A1m preparations influences immunosuppressive properties of SP-A1m on T-lymphocytes [37]. Here, we show that SP-A1mC6S mutant, which had a small inhibitory effect on IL-2 secretion, also contained TGF-β1. Western-blot analysis revealed that the relative amount of TGF-β1 bound to SP-A1C6S mutant seemed to be greater than that bound to SP-A1m (Figure 10). Thus both supratrimeric and trimeric forms of SP-A1 expressed in mammalian cells were bound to TGF-β1, but only supratrimeric SP-A1m showed a marked inhibitory effect on IL-2 secretion. These results clearly indicate the importance of the supratrimeric assembly for SP-A modulation of IL-2 secretion. However, the possibility cannot be excluded that the low thermal stability of the collagen domain of SP-A1mC6S (Tm=32.7 °C) is responsible for its low immunosuppressive activity, since its collagen domain must be partly unfolded at 37 °C. Similarly, the low immunosuppressive activity of baculovirus-derived SP-As might be explained by their low supratrimeric assembly and low thermal stability of their nonhydroxylated collagen domains.
To know whether the binding of TGF-β1 to SP-A preparations might strengthen the immunosuppressant action of SP-A, we incubated TGF-β1 with different SP-As at a TGF-β1 concentration of 20 ng/ml. At this concentration, TGF-β1 showed a modest immunosuppressive effect on IL-2 secretion by stimulated T-cells (Figure 10). The combination of TGF-β1 with baculovirus-derived SP-A1 showed a small immunosuppressive effect, which was slightly greater than that of TGF-β1 or the protein alone, which had no effect. Similar results were found for baculovirus-derived SP-A2 or SP-A1/SP-A2 (results not shown). However, the combination of TGF-β1 with native SP-A, supratrimeric SP-A1m and trimeric SP-A1mC6S had the same effect on IL-2 secretion as adding these proteins alone. CHO-cell-derived SP-As already contained TGF-β1. These results further support the idea that the immunosuppressive action of SP-A on IL-2 secretion depends on the degree of its supratrimeric structure and/or thermal stability of the collagen domain, and that the presence of small amounts of TGF-β1 might contribute to increase the immunosuppressive effect of some SP-A preparations.
Finally, we compared the effects of baculovirus-derived and native SP-A on TNFα production by macrophage-like cells stimulated with Re-LPS. We found that SP-A1, SP-A2 and co-expressed SP-A1/SP-A2 showed an inhibitory effect on LPS-induced TNFα secretion without significant differences among them (results not shown). This inhibitory effect is shown at high protein concentrations (30 μg/ml), but not at low concentrations (5 μg/ml), at which native SP-A has an inhibitory effect [23]. Likewise, we previously found that higher concentrations of trimeric SP-A1mC6S mutant than that of wild-type supratrimeric SP-A1m are required to inhibit TNFα secretion by LPS-stimulated macrophages [10]. Taken together, these results indicate that the degree of oligomerization contributes to the immunomodulatory properties of SP-A.
DISCUSSION
In the present study, we explore structural and functional differences between baculovirus-derived SP-A1 and SP-A2 that allow us to understand why SP-A1 invariably presents lower biological activity than SP-A2 [13–18]. For instance, it has been reported that baculovirus-derived SP-A2 is more effective than SP-A1 in inducing phospholipid vesicle aggregation [13], binding to carbohydrates [14], eliciting the production of TNFα and IL-8 by THP-1 cells [15], inhibiting ATP-stimulated phosphatidylcholine secretion from type II cells [16], stimulating association of Ps. aeruginosa with rat alveolar macrophages [17], and promoting phagocytosis of Ps. aeruginosa [18]. Recombinant SP-A1 and SP-A2 produced in insect cells provide a model for the study of various biological functions of human SP-A, despite the lack of some post-translational modifications (hydroxyproline and complete N-glycosylation), which positively influence SP-A biological functions [10,16,18,19].
SP-A1 and SP-A2 had similar structural characteristics studied by CD and fluorescence spectroscopy [13]. CD and DSC experiments indicated that SP-A1 and SP-A2 possessed an intact collagen-like domain with similar thermal stability (Tm=42±0.5 °C for SP-A1 and 41.8±0.3 for SP-A2), which was comparable with that of type I collagen under fibril-non-forming conditions [33]. Thus, under native conditions, SP-A1 and SP-A2 proteins must consist of three or three-multipled polypeptide chains. SP-A supratrimeric assembly depends on interchain disulfide bonds and non-covalent intermolecular forces in the N-terminal portion of the collagen domain and N-terminal segment [8–10]. We analysed whether SP-A1 and SP-A2 differ in their degree of supratrimeric assembly by analytical centrifugation and native electrophoresis. Our results clearly show that the extent of supratrimeric assembly is much higher in SP-A2 than SP-A1 as well as the extent of disulfide-linked oligomers detected by SDS/PAGE under non-reducing conditions. This explains why SP-A properties linked with the degree of oligomerization, such as SP-A binding to ligands (Re-LPS or carbohydrates) or SP-A-induced ligand aggregation (such as phospholipid, LPS or bacterial aggregation), were much higher in recombinant SP-A2 than SP-A1 expressed in insect cells. On the other hand, whereas native SP-A completely self-associates in the presence of calcium, we found that SP-A1 hardly self-associated and only part of SP-A2 self-associated. Self-association of SP-A also depends on its degree of supratrimeric assembly, and here we show that trimeric and hexameric forms of SP-A were unable to undergo Ca2+-dependent self-association. The biological significance of SP-A self-association is not clearly understood, but it is thought that SP-A protein networks stabilize large surfactant aggregates and decrease surfactant inactivation in the presence of serum protein inhibitors [3].
We do not know why the extent of supratrimeric oligomerization is lower in SP-A1 than in SP-A2. The ‘core’ differences that distinguish SP-A1 and SP-A2 are located at residues 46, 53, 61 and 65 of mature human SP-A (Figure 1). The presence of the extra Cys65 in SP-A1 is intriguing. Cysteine, in its reduced form, has moderate stability, and yet is never found in fibril-forming collagens except under pathological conditions. For instance, the human immunodeficiency associated with MBP may occur as a consequence of the Arg23 for cysteine substitution in the collagen-like domain that results in an almost complete failure to form supratrimeric oligomers [29], and a human cartilage disorder occurs as a consequence of the Arg-α1-519 for cysteine substitution in collagen II [27]. Mutagenesis studies of Cys65 will be necessary to understand the function of this Cys65, which is arginine for SP-A2 and SP-As from other species.
Although the function of Cys65 in human SP-A1 remains obscure, we show here that one advantage of having Cys65 instead of arginine is to increase SP-A1 resistance to trypsin degradation. SP-A1 was less susceptible to trypsin degradation than SP-A2 at 37 °C. Given that SP-A1 and SP-A2 contain similar trypsin cleavage targets in the N-terminal segment, neck, and globular domains and only differ at the Arg65 trypsin-cleavage site in SP-A2, it is reasonable to conclude that the presence of Cys65 decreases SP-A1 susceptibility to trypsin-like proteases. Interestingly, the development of an SP-A1 gene-specific antibody was recently reported [38]. Using this antibody, the SP-A1 content has been evaluated according to lung status [38]. The SP-A1/SP-A ratio is significantly higher in cystic fibrosis patients compared with alveolar proteinosis patients and healthy subjects, even though SP-A1 and total SP-A decrease in cystic fibrosis relative to other groups [38]. Cystic fibrosis is a chronic inflammatory disease of the airways with recurrent infections and lung deterioration. The imbalance of proteases and anti-proteases in cystic fibrosis patients is responsible for the low SP-A levels in these patients. The high SP-A1/SP-A ratio in cystic fibrosis in comparison with other groups supports our conclusion that SP-A1 is less susceptible to trypsin degradation than SP-A2. Both the higher SP-A1/SP-A ratio and the reduced total SP-A content may contribute to the impaired host defence in these patients.
SP-A1 and SP-A2 are expressed in alveolar type II cells, and native alveolar SP-A is thought to consist of hetero-oligomers of SP-A1 and SP-A2 [11]. To understand what advantage accrues to having two polypeptide chains instead of one, we have compared hetero-oligomers of co-expressed SP-A1/SP-A2 with homooligomers of SP-A1 or SP-A2. DSC analyses showed greater thermal stability of co-expressed SP-A1/SP-A2 over SP-A derived from one gene (SP-A1 or SP-A2). This may be explained by the fact that co-expressed SP-A1/SP-A2 contained a greater proportion of disulfide-linked hexamers (6α) over trimers (3α), and interchain disulfide cross-linking within the most N-terminal portion of human SP-A substantially increases the thermal stability of SP-A collagen-like domain [10]. Thus the combination of both gene products seems to favour a configuration in which the collagen stems would be closer together and shrink on cross-linking. However, baculovirus-derived SP-A1/SP-A2, as well as SP-A2 or SP-A1, showed incomplete oligomerization as a consequence of the lack of proline hydroxylation. The greater thermal stability of native SP-A over co-expressed SP-A1/SP-A2 is explained by both its high hydroxyproline content and its high disulfide- bond cross-linking, drawing collagen stems closer together in the microfibrillar part of the molecule.
Our results reveal that co-expressed SP-A1/SP-A2 exhibited properties of each protein. On the one hand, co-expressed SP-A1/SP-A2, like SP-A1, is more resistant to trypsin degradation than SP-A2. On the other hand, SP-A1/SP-A2 exhibits a greater degree of oligomerization, lower Kd for binding to bacterial Re-LPS, and greater capability to self-associate in the presence of calcium and aggregate Re-LPS than SP-A1, but less than SP-A2. It seems that the presence of SP-A1 polypeptide chains in co-expressed products modulates structural and functional properties of co-expressed SP-A, which are between those of SP-A1 and SP-A2.
Bronchial submucosal gland cells express only SP-A2 [12]. SP-A2 secreted by the submucosal glands of the conducting airways might be effective in innate host defence, facilitating the binding and agglutination of pathogens and particles, thus improving their transport to the pharynx by ciliary motion. Jack et al. [39] recently reported that the Q203K substitution in the CRD (carbohydrate recognition domain) of mature SP-A2 is linked with an increased risk of both meningococcal disease and death due to meningococcal disease in British patients. This finding is important, because it associates a new mucosal barrier gene with this disease. On the other hand, the presence of SP-A1 in alveolar SP-A might decrease SP-A–ligand aggregation activity but increase SP-A resistance to trypsin. It is unclear, however, whether the presence of SP-A1 in alveolar SP-A holds any advantage with regard to inflammatory responses. Our results with SP-As produced by insect cells cannot answer this question, since the immunosuppressive activity of baculovirus-derived SP-A1, SP-A2 or co-expressed SP-A1/SP-A2 on T-cells is non-existent in comparison with native SP-A or CHO-cell-derived SP-A1m, suggesting the importance of post-translational modifications and the extent of oligomerization in SP-A biological functions. We demonstrated here the importance of the degree of supratrimeric oligomerization for the ability of SP-A to inhibit IL-2 secretion from stimulated Jurkat T-cells by comparing the activity of hydroxylated supratrimeric SP-A1m with a hydroxylated full-length mutant SP-A1mC6S molecule secreted as a trimer. Results indicated that while SP-A1m showed a dose-dependent inhibitory effect on IL-2 secretion, SP-A1mC6S mutant had a modest immunosuppressive effect, despite the fact that both supratrimeric and trimeric forms of SP-A1 expressed in mammalian cells were bound to TGF-β, and the presence of TGF-β1 in SP-A preparations seems to influence SP-A immunosuppressive properties on T-lymphocytes [37].
We also analysed the effect of baculovirus-derived SP-As on the anti-inflammatory activity of SP-A, which is responsible for maintaining low alveolar inflammation in the resting lung by causal stimuli such as low concentrations of inhaled LPS. Unlike native SP-A or CHO-cell-derived SP-A1m, baculovirus-derived SP-As, as well as SP-A1C6S trimers [10], showed an inhibitory effect on LPS-induced TNFα secretion only at high but not at low protein concentrations. Taken together, these studies strengthen the concept that supratrimeric oligomerization is important for the host defence function and the immunosuppressive activity of SP-A. The extent of oligomerization of human collectins may be altered in various disease states and can vary among individuals. In addition, it is possible that mutations in SP-A compromise supratrimeric oligomerization and lead to increased susceptibility to bacterial and viral infections and/or chronic inflammation. Leth-Larsen et al. [40] recently reported that a polymorphic variation in the N-terminal segment of SP-D (M11T) influences SP-D oligomerization and function. The extent of oligomerization of the recombinant and serum Met/Met11 variant is higher than that of the Thr/Thr11 variant. The SP-D allele coding for Thr11 is associated with higher susceptibility to tuberculosis, and there is increasing evidence that this relatively common Thr/Thr11 polymorphism has important implications for innate host defence against infection. For MBP, individuals who are heterozygous or homozygous for (R23C) variant allele are immunocompromised [29].
In summary, the present study shows that the extent of supratrimeric oligomerization is lower in recombinant SP-A1 than SP-A2 expressed in insect cells. Consequently, SP-A properties linked with the degree of oligomerization decrease in SP-A1 compared with SP-A2. Co-expressed SP-A1/SP-A2 has greater thermal stability than SP-A1 and SP-A2, and exhibited properties of each protein. On one hand, co-expressed SP-A1/SP-A2, like SP-A1, is more resistant to trypsin degradation than SP-A2. On the other hand, SP-A1/SP-A2 exhibits a greater degree of oligomerization, lower Kd for binding to bacterial Re-LPS and greater capability to aggregate Re-LPS than SP-A1. Finally, the comparison of the immunosuppressive effect of recombinant baculovirus- and CHO-cell-derived SP-A molecules, bound or unbound to TGF-β1 and with different degrees of oligomerization, indicated the importance of the supratrimeric assembly for SP-A immunomodulatory function.
Acknowledgments
We acknowledge Dr C. Alfonso (Centro de Investigaciones Biológicas) for his advice and support on the analytical ultracentrifugation of native and baculovirus-derived SP-A. The present work was supported by the Ministerio de Educación y Ciencia (SAF2006-04434), Instituto de Salud Carlos III (Ciberes-CB06/06/0002), Comunidad Autonoma de Madrid (S-BIO-0260-2006), and Fundación Médica MM.
References
- 1.Wright J. R. Immunoregulatory functions of surfactant proteins. Nat. Rev. Immunol. 2005;5:58–68. doi: 10.1038/nri1528. [DOI] [PubMed] [Google Scholar]
- 2.Kishore U., Greenhough T. J., Waters P., Shrive A. K., Ghai R., Kamran M. F., Bernal A. L., Reid K. B., Madan T., Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol. Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Casals C., Garcia-Verdugo I. Molecular and functional properties of surfactant protein A. In: Nag K., editor. Developments in Lung Surfactant Dysfunction in Lung Biology in Health and Disease. New York: Marcel Dekker; 2005. pp. 55–84. [Google Scholar]
- 4.Wu H., Kuzmenko A., Wan S., Schaffer L., Weiss A., Fisher J. H., Kim K. S., McCormack F. X. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J. Clin. Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeVine A. M., Whitsett J. A. Pulmonary collectins and innate host defense of the lung. Microbes Infect. 2001;3:161–166. doi: 10.1016/s1286-4579(00)01363-0. [DOI] [PubMed] [Google Scholar]
- 6.Voss T., Eistetter H., Schafer K. P., Engel J. Macromolecular organization of natural and recombinant lung surfactant protein SP 28-36. Structural homology with the complement factor C1q. J. Mol. Biol. 1988;201:219–227. doi: 10.1016/0022-2836(88)90448-2. [DOI] [PubMed] [Google Scholar]
- 7.Head J. F., Mealy T. R., McCormack F. X., Seaton B. A. Crystal structure of trimeric carbohydrate recognition and neck domains of surfactant protein A. J. Biol. Chem. 2003;278:43254–43260. doi: 10.1074/jbc.M305628200. [DOI] [PubMed] [Google Scholar]
- 8.McCormack F. X., Pattanajitvilai S., Stewart J., Possmayer F., Inchley K., Voelker D. R. The Cys6 intermolecular disulfide bond and the collagen-like region of rat SP-A play critical roles in interactions with alveolar type II cells and surfactant lipids. J. Biol. Chem. 1997;272:27971–27979. doi: 10.1074/jbc.272.44.27971. [DOI] [PubMed] [Google Scholar]
- 9.Haas C., Voss T., Engel J. Assembly and disulfide rearrangement of recombinant surfactant protein A in vitro. Eur. J. Biochem. 1991;197:799–803. doi: 10.1111/j.1432-1033.1991.tb15974.x. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Barbero F., Strassner J., Garcia-Canero R., Steinhilber W., Casals C. Role of the degree of oligomerization in the structure and function of human surfactant protein A. J. Biol. Chem. 2005;280:7659–7670. doi: 10.1074/jbc.M410266200. [DOI] [PubMed] [Google Scholar]
- 11.Floros J., Hoover R. R. Genetics of the hydrophilic surfactant proteins A and D. Biochim. Biophys. Acta. 1998;1408:312–322. doi: 10.1016/s0925-4439(98)00077-5. [DOI] [PubMed] [Google Scholar]
- 12.Saitoh H., Okayama H., Shimura S., Fushimi T., Masuda T., Shirato K. Surfactant protein A2 gene expression by human airway submucosal gland cells. Am. J. Respir. Cell Mol. Biol. 1998;19:202–209. doi: 10.1165/ajrcmb.19.2.3239. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Verdugo I., Wang G., Floros J., Casals C. Structural analysis and lipid-binding properties of recombinant human surfactant protein a derived from one or both genes. Biochemistry. 2002;41:14041–14053. doi: 10.1021/bi026540l. [DOI] [PubMed] [Google Scholar]
- 14.Oberley R. E., Snyder J. M. Recombinant human SP-A1 and SP-A2 proteins have different carbohydrate-binding characteristics. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284:L871–L881. doi: 10.1152/ajplung.00241.2002. [DOI] [PubMed] [Google Scholar]
- 15.Wang G., Phelps D. S., Umstead T. M., Floros J. Human SP-A protein variants derived from one or both genes stimulate TNF-alpha production in the THP-1 cell line. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;278:L946–L954. doi: 10.1152/ajplung.2000.278.5.L946. [DOI] [PubMed] [Google Scholar]
- 16.Wang G., Bates-Kenney S. R., Tao J. Q., Phelps D. S., Floros J. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry. 2004;43:4227–4239. doi: 10.1021/bi036023i. [DOI] [PubMed] [Google Scholar]
- 17.Mikerov A. N., Umstead T. M., Huang W., Liu W., Phelps D. S., Floros J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L150–L158. doi: 10.1152/ajplung.00135.2004. [DOI] [PubMed] [Google Scholar]
- 18.Mikerov A. N., Wang G., Umstead T. M., Zacharatos M., Thomas N. J., Phelps D. S., Floros J. SP-A2 variants expressed in CHO-cells stimulate phagocytosis of Pseudomonas aeruginosa more than SP-A1. Infect. Immun. 2007;75:1403–1412. doi: 10.1128/IAI.01341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Verdugo I., Sanchez-Barbero F., Bosch F. U., Steinhilber W., Casals C. Effect of hydroxylation and N187-linked glycosylation on molecular and functional properties of recombinant human surfactant protein A. Biochemistry. 2003;42:9532–9542. doi: 10.1021/bi0347196. [DOI] [PubMed] [Google Scholar]
- 20.Saenz A., Canadas O., Bagatolli L. A., Sanchez-Barbero F., Johnson M. E., Casals C. Effect of surfactant protein A on the physical properties and surface activity of KL4-surfactant. Biophys. J. 2007;92:482–492. doi: 10.1529/biophysj.106.090217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruano M. L., Miguel E., Perez-Gil J., Casals C. Comparison of lipid aggregation and self-aggregation activities of pulmonary surfactant-associated protein A. Biochem. J. 1996;313:683–689. doi: 10.1042/bj3130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Verdugo I., Sanchez-Barbero F., Soldau K., Tobias P. S., Casals C. Interaction of SP-A (surfactant protein A) with bacterial rough lipopolysaccharide (Re-LPS), and effects of SP-A on the binding of Re-LPS to CD14 and LPS-binding protein. Biochem. J. 2005;391:115–124. doi: 10.1042/BJ20050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruano M. L., Garcia-Verdugo I., Miguel E., Perez-Gil J., Casals C. Self-aggregation of surfactant protein A. Biochemistry. 2000;39:6529–6537. doi: 10.1021/bi000188z. [DOI] [PubMed] [Google Scholar]
- 25.Ruano M. L., Perez-Gil J., Casals C. Effect of acidic pH on the structure and lipid binding properties of porcine surfactant protein A. Potential role of acidification along its exocytic pathway. J. Biol. Chem. 1998;273:15183–15191. doi: 10.1074/jbc.273.24.15183. [DOI] [PubMed] [Google Scholar]
- 26.Canadas O., Guerrero R., Garcia-Canero R., Orellana G., Menendez M., Casals C. Characterization of liposomal tacrolimus in lung surfactant-like phospholipids and evaluation of its immunosuppressive activity. Biochemistry. 2004;43:9926–9938. doi: 10.1021/bi036227z. [DOI] [PubMed] [Google Scholar]
- 27.Prockop D. J., Kivirikko K. I. Collagens: molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 28.Tischenko V. M., Ichtchenko A. M., Andreyev C. V., Kajava A. V. Thermodynamic studies of the collagen-like region of human subcomponent C1q. A water-containing structural model. J. Mol. Biol. 1993;234:654–660. doi: 10.1006/jmbi.1993.1618. [DOI] [PubMed] [Google Scholar]
- 29.Wallis R., Shaw J. M., Uitdehaag J., Chen C. B., Torgersen D., Drickamer K. Localization of the serine protease-binding sites in the collagen-like domain of mannose-binding protein: indirect effects of naturally occurring mutations on protease binding and activation. J. Biol. Chem. 2004;279:14065–14073. doi: 10.1074/jbc.M400171200. [DOI] [PubMed] [Google Scholar]
- 30.Tiktopulo E. I., Kajava A. V. Denaturation of type I collagen fibrils is an endothermic process accompanied by a noticeable change in the partial heat capacity. Biochemistry. 1998;37:8147–8152. doi: 10.1021/bi980360n. [DOI] [PubMed] [Google Scholar]
- 31.Wallis R., Drickamer K. Molecular determinants of oligomer formation and complement fixation in mannose-binding proteins. J. Biol. Chem. 1999;274:3580–3589. doi: 10.1074/jbc.274.6.3580. [DOI] [PubMed] [Google Scholar]
- 32.Poon P. H., Schumaker V. N. Measurement of macromolecular interactions between complement subcomponents C1q, C1r, C1s, and immunoglobulin IgM by sedimentation analysis using the analytical ultracentrifuge. J. Biol. Chem. 1991;266:5723–5727. [PubMed] [Google Scholar]
- 33.King R. J., Simon D., Horowitz P. M. Aspects of secondary and quaternary structure of surfactant protein A from canine lung. Biochim. Biophys. Acta. 1989;1001:294–301. doi: 10.1016/0005-2760(89)90114-8. [DOI] [PubMed] [Google Scholar]
- 34.Oosting R. S., van Greevenbroek M. M., Verhoef J., van Golde L. M., Haagsman H. P. Structural and functional changes of surfactant protein A induced by ozone. Am. J. Physiol. 1991;261:L77–L83. doi: 10.1152/ajplung.1991.261.2.L77. [DOI] [PubMed] [Google Scholar]
- 35.Borron P., Veldhuizen R. A., Lewis J. F., Possmayer F., Caveney A., Inchley K., McFadden R. G., Fraher L. J. Surfactant associated protein-A inhibits human lymphocyte proliferation and IL-2 production. Am. J. Respir. Cell Mol. Biol. 1996;15:115–121. doi: 10.1165/ajrcmb.15.1.8679215. [DOI] [PubMed] [Google Scholar]
- 36.Borron P., McCormack F. X., Elhalwagi B. M., Chroneos Z. C., Lewis J. F., Zhu S., Wright J. R., Shepherd V. L., Possmayer F., Inchley K., Fraher L. J. Surfactant protein A inhibits T-cell proliferation via its collagen-like tail and a 210-kDa receptor. Am. J. Physiol. 1998;275:L679–L686. doi: 10.1152/ajplung.1998.275.4.L679. [DOI] [PubMed] [Google Scholar]
- 37.Kunzmann S., Wright J. R., Steinhilber W., Kramer B. W., Blaser K., Speer C. P., Schmidt-Weber C. TGF-beta1 in SP-A preparations influence immune suppressive properties of SP-A on human CD4+ T-lymphocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L747–L756. doi: 10.1152/ajplung.00401.2005. [DOI] [PubMed] [Google Scholar]
- 38.Tagaram H. R., Wang G., Umstead T. M., Mikerov A. N., Thomas N. J., Graff G. R., Hess J. C., Thomassen M. J., Kavuru M. S., Phelps D. S., Floros J. Characterization of a human surfactant protein A1 (Sp-A1) gene-specific antibody; Sp-A1 content variation among individuals of varying age and pulmonary health. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:L1052–L1063. doi: 10.1152/ajplung.00249.2006. [DOI] [PubMed] [Google Scholar]
- 39.Jack D. L., Cole J., Naylor S. C., Borrow R., Kaczmarski E. B., Klein N. J., Read R. C. Genetic polymorphism of the binding domain of surfactant protein-A2 increases susceptibility to meningococcal disease. Clin. Infect. Dis. 2006;43:1426–1433. doi: 10.1086/508775. [DOI] [PubMed] [Google Scholar]
- 40.Leth-Larsen R., Garred P., Jensenius H., Meschi J., Hartshorn K., Madsen J., Tornoe I., Madsen H. O., Sorensen G., Crouch E., Holmskov U. A common polymorphism in the SFTPD gene influences assembly, function, and concentration of surfactant protein D. J. Immunol. 2005;174:1532–1538. doi: 10.4049/jimmunol.174.3.1532. [DOI] [PubMed] [Google Scholar]