Abstract
The Arabidopsis acn (acetate non-utilizing) mutants were isolated by fluoroacetate-resistant germination and seedling establishment. We report the characterization of the acn2 mutant. Physiological analyses of acn2 showed that it possessed characteristics similar to those of the mutants cts (COMATOSE)-1 and pxa [peroxisomal ABC (ATP-binding-cassette) transporter]1. The acn2 locus was mapped to within 3 cM of the CTS gene on the bottom arm of chromosome IV using CAPS (cleavage amplification polymorphism) and SSLP (simple sequence-length polymorphism) markers. Crossing acn2 and cts-1 failed to restore the fluoroacetate-sensitive phenotype, suggesting that these mutations were allelic. Sequencing of the ACN2 locus revealed a C→T nonsense mutation in exon 13, which would have resulted in the elimination of the C-terminal hemitransporter domain of the encoded protein. Neither the full-length CTS protein nor the truncated protein was detected on immunoblots using either C-terminal- or N-terminal-specific anti-CTS antibodies respectively, demonstrating the absence of the entire CTS protein in acn2 mutants. Emerged seedlings of both cts-1 and pxa1 alleles displayed increased resistance to FAc (monofluoroacetic acid) compared with the corresponding wild-type seedlings. Complementation studies showed that mutation of the CTS gene was responsible for the FAc-resistant phenotype, as when the wild-type protein was expressed in both the cts-1 and pxa1 mutant lines, the strains became FAc-sensitive. Feeding studies confirmed that both acn2 and cts-1 mutants were compromised in their ability to convert radiolabelled acetate into soluble carbohydrate. These results demonstrate a role for the ABC protein CTS in providing acetate to the glyoxylate cycle in developing seedlings.
Keywords: acetyl-CoA, Arabidopsis, COMATOSE, glyoxylate cycle, lipid mobilization, β-oxidation
Abbreviations: 2,4-D, 2,4-dichlorophenoxyacetic acid; 2,4-DB, 2,4-dichlorophenoxybutyric acid; AAE, acyl-activating enzyme; ABC, ATP-binding-cassette; ABCD, D subgroup of ABC protein family; AcetCS, acetyl-CoA synthetase; ACN, acetate non-utilizing; ALD, adrenoleucodystrophy; ALDP, ALD protein; CAPS, cleavage amplification polymorphism; CTS, COMATOSE, FAc, monofluoroacetic acid; IBA, indole-3-butyric acid; JA, jasmonic acid; LACS, long-chain acyl-CoA synthetase; Ler, Landsberg erecta ecotype; MS, Murashige and Skoog; NBF, nucleotide-binding fold; ped, peroxisome-defective; PGS, principal growth stage; PMP, peroxisomal membrane protein; pxa, peroxisomal ABC transporte; RT, reverse transcription; SSLP, simple sequence-length polymorphism; TAG, triacylglycerol; TMD, transmembrane domain; ttg, transparent testa glabra; VLCS, very-long-chain acyl-CoA synthetase
INTRODUCTION
The classic gluconeogenic model of lipid mobilization is the conversion of fatty acids into sucrose during establishment in order to support the growing seedling. Acetyl-CoA from β-oxidation enters the glyoxylate cycle for conversion into organic acids for transportation to mitochondria, which subsequently produce sucrose through the TCA (tricarboxcylic acid) cycle and gluconeogenesis [1]. Establishing the path of carbon flow through the glyoxylate cycle has relied almost exclusively on feeding radiolabelled acetate to seeds and seedling tissues [2–4]. Gluconeogenic models derived from the use of exogenous acetate instead of labelled fatty acids must include the activation of acetate to acetyl-CoA and the process of acetate and/or acetyl-CoA entry into glyoxysomes. However, the specific proteins involved have remained unidentified until recently. Through characterization of an Arabidopsis mutant resistant to the toxic acetate analogue FAc (monofluoroacetic acid) [5], we determined that the AMP-binding protein AAE (acyl-activating enzyme) 7/ACN (acetate non-utilizing) 1 [6,7], was a glyoxysomal short-chain AcetCS (acetyl-CoA synthetase) responsible for the activation of acetate [7]. This suggested that free acetate had to enter glyoxysomes for activation, but it remained unclear whether uptake of acetate occurred by diffusion, ion-trapping or active transport by some protein component.
CTS (COMATOSE) is a member of the ABC (ATP-binding-cassette) superfamily of proteins, which is ubiquitous in cellular organisms. CTS is a full-sized transporter characterized by two NBFs (nucleotide-binding folds) and two TMDs (transmembrane domains), in a forward TMD1–NBF1–TMD2–NBF2 orientation. In surveys of the ABC protein family of Arabidopsis, it was noted that CTS is similar to both yeast and mammalian PMPs (peroxisomal membrane proteins) [8,9]. The ABCD (D subgroup of ABC protein family) subfamily of yeast contains two members, PXA1 and PXA2, which are half-transporters that heterodimerize [10] to facilitate the transportation of long-chain fatty acyl-CoA across the peroxisomal membrane [11]. The ABCD subfamily of peroxisomal transporters in humans is composed of four members: ABCD1–4, of which ABCD3 (PMP70) and ABCD1 {ALDP [ALD (adrenoleucodystrophy) protein]} appear to transport long-chain [12] and very-long-chain fatty acyl-CoA respectively [13] for chain-shortening by β-oxidation. Elimination of ALDP in humans by mutation causes X-linked ALD, a progressive genetic illness [14] which results in increased levels of very-long-chain fatty acids in nerve cells, owing to impaired β-oxidation [15]. The consequences of this mutation can be severe, with those afflicted with childhood cerebral ALD perhaps surviving only into their teens [16]. Peroxisomal ABC transporters have been the focus of a recent review [17].
The cts mutant of Arabidopsis was isolated in a forward genetic screen to identify lines that possessed reduced germination potential [18]. Footitt et al. [19] reported that the mutation responsible for the reduced germination potential phenotype resided in the gene encoding the ABC protein homologue of human ALDP. Just prior to this, Zolman et al. [20] described a mutation in the same gene, which was isolated from a screen to identify mutants resistant to IBA (indole-3-butyric acid), an auxin analogue [21]. Mutant alleles for this gene, ped (peroxisome-defective) 3, were isolated in a screen for mutants resistant to 2,4-DB (2,4-dichlorophenoxybutyric acid), an auxin analogue, as early as 1998 [22], but the mutant loci were not reported until later [23]. The nature of the screens by which the mutants were isolated, the reduced lipid mobilization and the accumulation of acyl-CoAs in young seedlings [19] suggested that the function of the protein was to supply fatty acid and/or acyl-CoAs for peroxisomal β-oxidation. Evidence that JA (jasmonic acid) levels were reduced in both basal and wounded cts-1 led Theodoulou et al. [24] to conclude that CTS is responsible for transporting fatty acid/CoA substrates of at least four carbons in length, encompassing a wide variety of straight-chain or cyclic derivatives. The isolation of the FAc-resistant mutant acn2, which exhibited characteristics similar to cts-1 [19], pxa (peroxisomal ABC transporter) 1 [21] and ped3 [23], raised the possibility that the protein may also function as an acetate transporter. FAc exerts its toxicity through the inhibition of aconitase on conversion into fluorocitrate by the sequential action of AcetCS and citrate synthase [25]. Therefore a mutation that would prevent FAc entering the glyoxylate cycle would result in enhanced tolerance to FAc. Using a physiological, genetic and biochemical approach, we demonstrate that CTS is integral to acetate metabolism in glyoxysomes in developing seedlings, and present evidence to support the speculation that unesterified acetate is the compound transported.
EXPERIMENTAL
Plant material
All seed batches used in the experiments were surface-sterilized and were allowed to imbibe in the dark at 4 °C for 4 days before being sown on to agar plates. For all experimental procedures, seeds were germinated at 20 °C with constant illumination at 70 μmol of photons/m2 per s, except for the immunoblot analysis, where seeds were germinated and grown with a day length of 9 h. Standard agar medium plates contained 0.8% agar and half-strength MS (Murashige and Skoog) salts [26], to which sucrose was added to a concentration of 20 mM where specified. Prior to the addition of agar and subsequent autoclaving, all media were adjusted to pH 5.7 using 0.1 M KOH. To test FAc resistance, seeds were sown on to standard agar medium plates containing 0.5 mM sodium FAc (Sigma–Aldrich). FAc was prepared as a concentrated stock solution, filter-sterilized and added to autoclaved standard agar medium. The auxin analogues 2,4-D (2,4-dichlorophenoxyacetic acid) and 2,4-DB were prepared as concentrated stocks in ethanol and added to autoclaved standard agar medium. The concentrations used experimentally were 250 mM, 500 nM, 1 μM, 3 μM and 5μM for 2,4-DB, and 20 nM, 80 nM, 120 nM and 240 nM for 2,4-D. For root growth measurements, surface-sterilized seeds were sown on to standard agar medium plates containing 20 mM sucrose. After 4 days of incubation in the dark at 4 °C, the plates were transferred to the growth room. After 4 further days in the growth room, ungerminated seeds of acn2 and cts-1 were moved to selective plates and damaged by nicking the testa with forceps. Wild-type seeds were moved from the cold to growth conditions at this time. All growth and feeding studies were conducted on acn2 lines that had been back-crossed three times. Each successive round of back-crossing entailed screening the F2 generation for sucrose-dependent germination in the presence of 0.5 mM FAc. Whole plants were grown at 20 °C on a 9 h light/15 h dark cycle.
Characterization of the acn2 locus and gene and protein expression analysis
For mapping purposes, F2 generation acn2 plants homozygous for FAc resistance were crossed to Ler (Landsberg erecta ecotype) reciprocally. FAc-resistant F2 generation seedlings (approx. 120) were rescued by sowing on to soil and growing in order to harvest leaf material for DNA extraction. Genomic DNA was isolated from leaf material using the PUREGENE® DNA isolation kit (Gentra Systems). Primer combinations and thermocycling conditions for analysing CAPS (cleavage amplification polymorphisms) were performed as specified by Konieczny and Ausubel [27] and Baumbusch et al. [28]. The analysis of SSLPs (simple sequence-length polymorphisms) was conducted as described by Bell and Ecker [29]. PCR set-up and thermocycling was conducted on an MWG Automated Biosystem RoboSeq™ 4204 with an integrated 96-well thermocycler.
To sequence the acn2 locus, primer pairs were designed to amplify overlapping regions of the ACN2 gene (At4g39850) from the sequence in the TAIR (The Arabidopsis Information Resource) database website (http://www.arabidopsis.org). The resulting PCR products were isolated from agarose gels, purified and sequenced commercially by the John Innes Genome Laboratory. A portion of exon 13 from both Col-0 and ACN2 DNA and cDNA templates was amplified using the forward primer 5′-AAGTGTAGTGTTGCCTCGTTTTC-3′ and the reverse primer 5′-AAAGAGGCTATTCGGTCAGAGAT-3′. ACN2 cDNA was prepared by reverse transcription from total RNA isolated from newly emerged seedlings at PGS (principal growth stage) 0.7 [30], which had been germinated on standard agar medium containing 20 mM sucrose. Total RNA was isolated using the PURESCRIPT® RNA isolation kit (Gentra Systems), modified with the insertion of a phenol/chloroform/isoamyl alcohol (25:24:1) wash step prior to isopropanol precipitation.
For expression analysis of CTS in wild-type and mutant seedlings, vernalized seeds were sown on to standard agar medium plates with or without 20 mM sucrose. Germinated seedlings were removed after 4 days, and the remaining seeds damaged by nicking the seed coat with forceps under a microscope. PolyA mRNA was isolated from either ten seeds from mutant lines plated on to standard agar medium, or from five PGS 0.7 seedlings plated on to standard agar medium containing 20 mM sucrose. PolyA mRNA was isolated using Dynabeads (Dynal AS) according to the manufacturer's instructions. Reverse transcription was performed as described by Laval et al. [31]. PCR was conducted using the CTS specific forward primer 5′-ACGGATGCTGAAATTGATTCAGT-3′ and the reverse primer 5′-TGCTGAGTTCACTCTGTTGTCT-3′.
For the immunoblot analysis of CTS expression, vernalized seeds were plated on standard agar medium plates containing 20 mM sucrose. Germinated seeds were removed after 4 days, and ungerminated mutant seeds were damaged by nicking the testa. Protein was extracted from PGS 0.7 seedlings as described by Footitt et al. [19], except the quantities of PMSF and Sigma protease inhibitor cocktail used were increased to 5 mM and to 5% (v/v) respectively. Protein aliquots of 20 μg were resolved by SDS/PAGE (7.5% gel), blotted on to a PVDF membrane and stained with Ponceau S to ensure equal protein loading. The membranes were then probed with either an antibody against the C-terminal portion of CTS (raised as a recombinant protein containing amino acids 1112–1337 of CTS) or the N-terminal of CTS (raised using a peptide containing amino acids 10–25 of CTS). The C-terminal antibody was purified by affinity chromatography before use. After probing with the C-terminal antibody, the blot was stripped in 60 mM Tris/HCl, pH 6.7, 2% (w/v) SDS and 0.7% (v/v) 2-mercaptoethanol at 50 °C for 30 min and reprobed with the N-terminal antibody. Detection of immunoreactive proteins was performed using the ECL® (enhanced chemiluminescence)+Plus™ Western blotting detection kit (Amersham Biosciences) according to the manufacturer's instructions.
Radiolabelled acetate feeding
The [14C]acetate feeding experiments were adapted from those described by Eastmond et al. [3] with the following modifications. A total of 100 PGS 0.7 Arabidopsis seedlings were bubbled for 4 h in a 1.5 ml microcentrifuge tube containing 0.2 ml of 1 mM sodium [2-14C]acetate (20.5 MBq/mmol) and 50 mM Mes, pH 5.2. Two consecutive 150 μl aliquots of 5 M KOH were used to trap the released CO2. Both fractions were combined for liquid-scintillation counting. After 4 h of bubbling, the seedlings were washed, extracted and fractionated [2]. The proportion of radioactivity in each component was determined on a Wallac 1409 liquid-scintillation counter using 10 ml of PerkinElmer Optiphase HiSafe3 liquid-scintillation cocktail. The ethanol-insoluble material was combusted using a Biological Material Oxidizer OX400 (R. J. Harvey Instrument Corporation). The CO2 was trapped in 15 ml of Oxosol™ C14 (National Diagnostics) and counted directly.
Subcellular fractionation and enzyme assays
Fractionation of seedling organelles was conducted as described by Eastmond et al. [3] with the modifications specified by Turner et al. [7]. AcetCS activity was measured spectrophotometrically in a coupled reaction with citrate synthase and malate deydrogenase as described by Millerd and Bonner [32]. Short-chain acyl-CoA oxidase activity was measured as described by Hyrb and Hogg [33].
RESULTS
Phenotypic characteristics of acn2
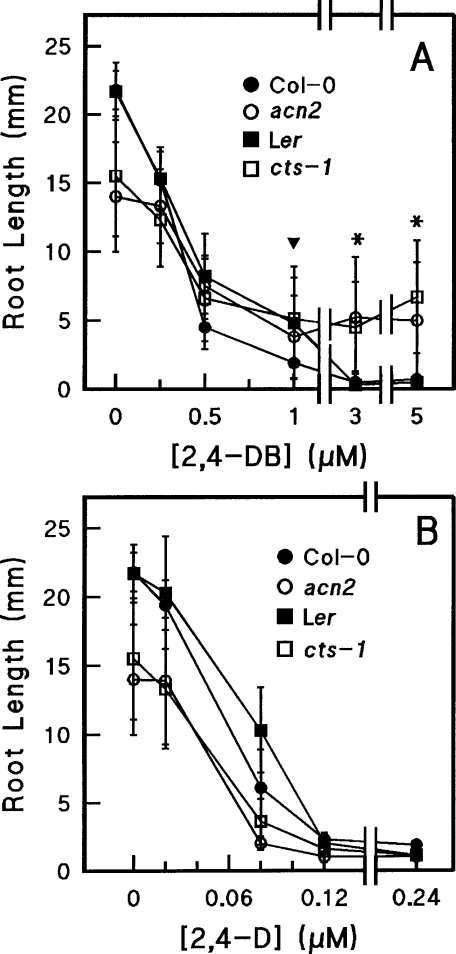
The synthetic auxin analogues 2,4-DB and IBA have been used to select for defects in glyoxysomal function, including biogenesis and fatty acid β-oxidation [21,22]. A general effect of auxin analogues on sensitive genotypes is a reduction in root growth, because the compounds are able to be converted into their more bioactive derivatives [20,22]. Therefore we compared the root growth of acn2 seedlings (Col-0 background) with that of the 2,4-DB resistant cts-1 seedlings (Ler background), in the presence of increasing concentrations of 2,4-DB (Figure 1A). In the absence of 2,4-DB, root growth of the mutants was less than that of the corresponding wild-type seedling. Mutant root growth decreased with increasing concentrations of 2,4-DB, but not as dramatically as either wild-type strain. Significantly greater root growth for the mutant strains compared with wild-type strains was observed for both acn2 and cts-1 starting at 1 and 3 μM 2,4-DB respectively. At 5 μM 2,4-DB, root growth of the wild-type seedlings was abolished, whereas a large number of mutant seedlings exhibited substantial root growth. Like cts-1, acn2 was found to be sensitive to the degradation product 2,4-D, consistent with the possibility that conversion of 2,4-DB into 2,4-D is disrupted in acn2 (Figure 1B).
Figure 1. Comparison of 2,4-DB resistance of mutants and wild-type.
Seeds were treated as described in the Experimental section. Root lengths were measured 6 days after transfer to growth conditions. Results are means±S.D. for at least ten seedlings. (A) Root growth in the presence of 2,4-DB. Student's t tests were conducted on data from roots from each mutant and respective wild-type at concentrations of 1, 3 and 5 μM. Symbols: ▼, P<0.01 for acn2 and P<0.1 for cts-1 compared with Ler; *, P<0.0001 for both acn2 and cts-1. (B) Root growth in the presence of 2,4-D. Col-0 and acn2 were significantly different (P<0.0001) at all concentrations. Ler and cts-1 were statistically different at concentrations below 0.12 μM (P<0.0001), but statistically non-significant at concentrations of 0.12 μM and 0.24 μM (P>0.1).
The presence of short-chain fatty acids had been reported to remove dormancy and instigate germination of cts-1 [19]. For cts-1 germination, we found a significant effect of propionate and butryrate at concentrations of 0.01 and 0.1 mM respectively. Neither acetate nor other concentrations of propionate or butyrate tested had any effect (results not shown). No effect on acn2 seed germination was apparent in the presence of fatty acids (results not shown). Some of the vegetative phenotypes observed for acn2 are exhibited by pxa1 [20], such as poor initiation of lateral root formation and smaller rosettes with fewer leaves. In addition, the leaves were crinkled and waxy, a phenotype reported previously for the ped3 alleles [23]. The delay in flowering time was not as dramatic for acn2 as observed for pxa1 (results not shown). Such phenotypic variation for cts alleles is not unusual and has been discussed recently [17].
Allelism of acn2 and cts-1
The chromosomal position of the mutant locus was determined by a map-based PCR approach using CAPS markers [27,28] and SSLPs [29]. The acn2 locus was found to lie within 2 cM of the CAPS marker DHS1, and to within 3 cM of the SSLP marker NGA1107. These map distances placed the acn2 locus very close to the position of the CTS gene on chromosome IV. No other gene in the vicinity of CTS would be expected to give the phenotypes observed for acn2 when disrupted by mutation. Reciprocal crosses of acn2 with cts-1 were conducted in order to test complementation of the genotypes. Crosses with cts-1 as the pollen donor were more successful and produced more seeds, which corresponded to a reduced fertility of acn2. The same occurred with Ler and acn2 crosses. The F1 generation of the cts-1×acn2 and Ler×acn2 crosses were tested for dormancy and FAc resistance (Table 1). A small proportion of seeds from the cts-1×acn2 crosses germinated in the absence of sucrose, similar to that observed for either the cts-1 or acn2 single mutant, whereas 100% of the seeds from the Ler×acn2 and Col-0×acn2 crosses germinated. As with the single mutants, a substantial increase in the proportion of seeds from the mutant crosses germinated when transferred on to sucrose plates, and F1 generation seed from the crosses established well in the presence of FAc and sucrose once the testa was damaged or removed. Although listed in Table 1 as being FAc-sensitive, the F1 generation Ler×acn2 crosses possessed a slightly elevated FAc resistance, as previously reported for the F1 generation of the backcross of acn2 to its parental wild-type strain [5].
Table 1. Phenotypic analysis of the F1 generation of acn2 and cts-1 crosses.
The phenotypes tested were the proportion of total seeds germinated by 4 days after transfer to the growth chamber and establishment in the presence of FAc. FAcR, fluoroacetate resistance; NA, not applicable; suc, sucrose.
| Genotype | MS* (%) | MS+Suc† (%) | FAcR‡ (%) |
|---|---|---|---|
| Col-0 (n=3) | 99±1 | NA | 0 |
| Ler (n=3) | 100 | NA | 0 |
| cts-1 (n=3) | 11±4 | 53±2 | >99 |
| acn2 (n=3) | <1 | 35±4 | >99 |
| cts-1×acn2 (n=6) | 5±2 | 49±17 | >99 |
| Ler×acn2 (n=3) | 100 | NA | 0 |
| Ler×cts-1 (n=3) | 100 | NA | 0 |
| Col-0×acn2 (n=3) | 96±2 | NA | 0 |
*Approx. 100 seeds were sown on to agar plates containing half-strength MS salts, pH 5.7. The plates were incubated in the dark for 4 days before being transferred to the growth cabinet.
†Seeds that had not germinated were tranferred on to agar plates containing half-strength MS salts and 20 mM sucrose.
‡Seeds were sown directly on to agar plates containing half-strength MS salts, pH 5.7, 20 mM sucrose and 0.5 mM FAc. Seeds from mutants and mutant crosses were nicked 24 h after transfer to the growth cabinet.
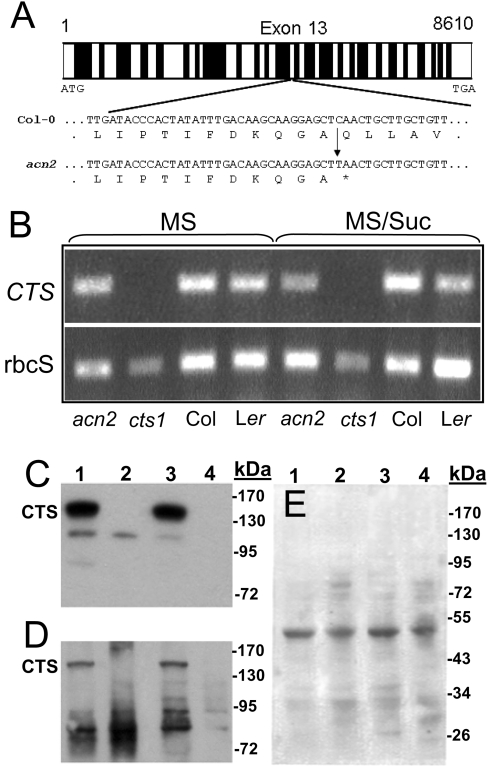
DNA was isolated from acn2 plants exhibiting sucrose-dependent establishment in the presence of FAc, to ensure that only a homozygous locus would be sequenced. Comparison of the first-pass sequences of overlapping PCR-amplified fragments of the CTS gene with the full-length genomic sequence present in the TAIR database, At4g39850, revealed a single mutation, which was a C→T substitution in exon 13 (Figure 2A). When compared with the coding sequence for At4g39850, the mutation lies at nucleotide position 2284, which introduces a stop codon at Gln762, truncating the protein in the first predicted TMD of the second hemitransporter domain. A detailed description of the gene and a comprehensive comparison of the protein primary sequence with human and Saccharomyces cerevisiae ABC transporters is presented in Zolman et al. [20].
Figure 2. Molecular characterization of acn2.
(A) The acn2 locus. DNA and total RNA were isolated from Col-0 and acn2 mutants. All exons of the acn2 locus were sequenced once, and the mutation was confirmed by re-sequencing of exon 13 and the corresponding region of the cDNA. Exons and introns are represented by open and closed bars respectively. (B) An ethidium-bromide-stained agarose gel showing the expression of CTS as determined by RT–PCR. rbcS represents the small subunit of ribulose bisphosphate carboxylase. (C–E) Analysis of CTS protein in PGS 0.7 seedlings germinated on standard agar medium plates containing 20 mM sucrose using either C-terminal- (C) or N-terminal- (D) specific antibodies. Lane 1, Ler; Lane 2, cts-1; Lane 3, Col-0; Lane 4, acn2. Protein (20 μg) was loaded into each well, and equal loading was verified by staining with Ponceau S (E).
RT (reverse transcription)–PCR analysis of the expression of CTS in acn2 seedlings was performed compared with cts-1 and their respective wild-type parents (Figure 2B). The expression of CTS in wild-type seedlings from seeds germinated in the presence of sucrose, and in seeds left on standard agar medium plates for 24 h (no radical emergence) is similar to that previously reported by Footitt et al. [19]. No CTS mRNA was expected to be present in cts-1, since the choice of primers used for PCR amplification reside downstream of the chromosome V translocation site within exon 10 [19]. Using the same primer pair, CTS mRNA was expressed within the acn2 mutant in both newly emerged seedlings and un-germinated seeds that had been allowed to imbibe. Although ACN2 was expressed, no immunoreactive protein was observed using CTS-specific polyclonal antibodies [19] raised against either the C-terminal or N-terminal portion of CTS (Figures 2C–2E). Although the specificity of the N-terminal antibody is less than that of the affinity-purified C-terminal one, it is apparent that the lack of an approx. 84 kDa protein (predicted molecular mass of the truncated protein) indicated that the entire CTS protein is missing in acn2. All protein bands observed in the mutant strains between 72 and 95 kDa were present also in the wild-type strains, suggesting either non-specific binding of the anti-CTS antibody or the presence of contaminating antibodies within the antiserum.
FAc resistance of CTS mutants
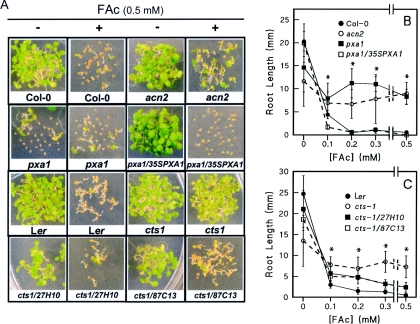
The acn2 mutant was isolated based on FAc-resistant germination and emergence [5]. Interestingly, the subsequent generation of seed from the initial plant did not establish, except after the seed coat was damaged or removed in the presence of sucrose. The discovery of the mutant was quite fortuitous, because we did not select specifically for sucrose-dependent germination and establishment, and isolation of the mutant resulted from spontaneous germination owing to the inclusion of sucrose within FAc-containing standard agar medium plates. We also reported that acn2 appeared to be dominant, with F1 generation seeds from the Col-0×acn2 backcrosses showing slightly elevated resistance to FAc. Seedlings from the F2 generation seed batches exhibited three different levels of FAc resistance, which is indicative of the three possible genotypes at the acn2 locus. This mirrors the dominance reported for the various ped3 alleles [22] and the pxa1 allele [20] for resistance to 2,4-DB and IBA respectively. In contrast with FAc, the phenotype of sucrose-dependent establishment was recessive in acn2, as reported for the other CTS mutants. The FAc-resistance phenotypes of acn2, pxa1 and cts-1 are demonstrated in Figure 3. Signs which were indicative of resistance include greening, expansion of the cotyledons and, in particular, root growth, which does not occur with either wild-type strain. The effects of FAc on root growth of each genotype are shown in Figures 3(B) and 3(C). Significant differences between the mutant and wild-type seedlings were observed at FAc concentrations as low as 0.1 mM. Interestingly, the spontaneously germinated seeds of undamaged homozygous acn2 mutants exhibited a wild-type FAc-sensitive phenotype, as shown by yellow cotyledons and no root growth (results not shown). The resistance of cts-1 is in stark contrast with the sensitive phenotypes of its wild-type parent, Ler, and the mutant that had been transformed with the wild-type gene [19], i.e. cts-1/27H10 and cts-1/87C13 (Figure 3A). The pxa1 mutant, expressing the full-length PXA1 cDNA (pxa1/35SPXA1), was also sensitive to FAc (Figures 3A and 3B). These results demonstrate that CTS plays a role in mediating FAc sensitivity in wild-type plants.
Figure 3. FAc resistance of CTS mutants.
Seeds of wild-type and complemented mutants were not nicked. The labels 27H10 and 87C13 represent clone designations from the GeTCID (Gene Transfer Clone Identification and Distribution Service) (John Innes Centre), provided for in planta expression as part of the GARNet (Genomic Arabidopsis Resource Network) facilities. pxa1/35SPXA1 represents the mutant line expressing the PXA1 cDNA under the control of the CaMV (cauliflower mosaic virus) 35S promoter [26]. (A) Seeds were treated as described in the Experimental section, except that spontaneously germinated mutants were not removed prior to nicking the testa. All seeds were sown on to standard agar medium containing 20 mM sucrose with (+) or without (−) 0.5 mM FAc. Photographs were taken 8 days after transfer to growth conditions. Root growth of Col-0-related genotypes (B) and Ler-related genotypes (C) in the presence of increasing FAc concentrations. Seeds were treated with FAc as described in the Experimental section. Root lengths were measured 6 days after nicking the testae of the mutants. Results are means±S.D. for at least ten seedlings. Lines have been included to clarify connections for obscured data points. Based on Student's t test statistics, wild-type root growth was significantly less than the mutant seedling (P<0.0001) at FAc concentrations of 0.1 mM and greater as indicated by *. Average root growth for complementation genotypes cts-1/155A23 and cts-1/159N01 was intermediate between Ler and the complementation genotypes shown.
CTS mutants show reduced incorporation of acetate into soluble carbohydrate
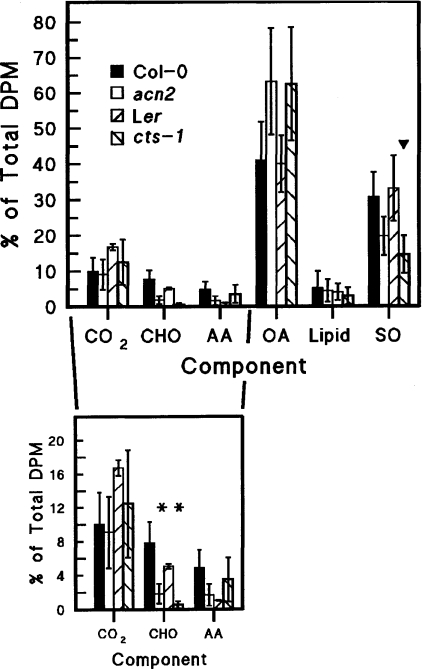
In order to establish the metabolic consequences caused by disruption to glyoxysomal acetate metabolism, as suggested by FAc resistance, we conducted [14C]acetate feeding studies on acn2 and cts-1 strains compared with parental wild-type strains. The profiles of radioactivity recovered within the different analytical fractions are characteristic of the disruption of acetate entry into the glyoxylate cycle, as observed for the icl (isocitrate lyase) [3,7] and acn1 mutants [7]. Dramatic reductions were observed in the quantity of radiolabel measured within the soluble carbohydrate fraction, whereas only minor differences were observed in the other fractions. The proportion of radiolabel in the carbohydrate fraction of the mutants was, on average, less than 20% of that present in the same fraction of wild-type seedlings (Figure 4). The ability of cts-1 and acn2 to assimilate some proportion of acetate may explain why wild-type sensitivity to FAc is observed in seedlings that spontaneously germinate. The reduction in the conversion of exogenous acetate into carbohydrate in the acn2 and cts-1 mutants was similar to that detected for acn1 [7]. The reduced radioactivity observed within the ethanol-insoluble fractions for cts-1 and acn2 is likely to be a result of less radiolabelled carbohydrates being converted into complex structural and storage polysaccharides. The small, but significant, increase in the labelled organic acid pools within the mutants probably reflects the difference in acetate assimilation. Similar speculation for the incorporation of amino acids into proteins cannot be made, since the relative labelling of amino acid pools between cts-1 and Ler was reversed compared with acn2 and Col-0. It is evident that both the cts-1 and acn2 mutants retain the capability to assimilate radiolabelled acetate by other mechanisms.
Figure 4. Comparison of exogenous [2-14C]acetate utilization by acn2 and cts-1 seedlings.
Results are means±S.D., n=3 for Col-0 and Ler and n=6 for acn2 and cts-1. The seedlings were maintained under the growth conditions of light and temperature during the incubation period described in the Experimental section. The total amounts of radiolabel taken up over the 4 h incubation period were 53000±14200, 65800±29700, 50300±10300 and 37800±10400 d.p.m. (disintegrations per min) for Col-0, acn2, Ler, and cts-1 respectively. Student's t test differences between mutant and wild-type are shown: *, P<0.0001; ▼, P<0.01. All other differences were insignificant at P>0.05. AA, amino acids (basic fraction); CHO, soluble carbohydrates (neutral fraction); CO2, KOH-trapped label; Lipid, ether-soluble label; OA, organic acids (acidic fraction); SO, ethanol-insoluble label from sample oxidizer.
Some reports have suggested that the human ALDP protein may function by stabilizing VLCS (very-long-chain acyl-CoA synthetase), since the defect demonstrated in X-linked ALD1 cells is the activation of long-chain fatty acids to very-long-chain acyl-CoA [15]. Although characterized VLCS mutations are not associated with X-ALD [34] and current evidence suggests that ALDP does not stabilize or physically associate with VLCS [17], we decided to verify that the labelling results observed for cts were not a result of a reduction in AcetCS activity. Glyoxysomes were isolated from mutant and wild-type seedlings on sucrose density gradients and assayed for AcetCS activity [7]. Short-chain acyl-CoA oxidase activity was used as a co-localization control. The glyoxysomes from the lower fraction of the sucrose gradient were gently disrupted either by a freeze–thaw cycle or by passage through a small-bore syringe needle. Thus any physical interaction between CTS and ACN1, which is the only enzyme causing AcetCS activity in seedling glyoxysomes [7], was unlikely to be disrupted. AcetCS activity was detected at a level of 7.5±3.8 nmol/mg of protein per h (n=3) for wild-type Ler, which was similar to that for Col-0 (∼6 nmol/mg of protein per h [13]). A trial of cts-1 gave an activity of 18 nmol/mg of protein per h. It was clear that to obtain sufficient material, thousands of cts-1 seeds needed to be damaged, which would not permit the precise staging of dark-growth development. Therefore the cts-1/ttg (transparent testa glabra) 1–1 double mutant was also tested, since the ttg mutation facilitates normal germination of seeds in the cts background [19]. AcetCS activity was also apparent in the cts-1/ttg1-1 double mutant at 40±16 nmol/mg of protein per h (n=3). The greater apparent specific activity of the cts-1/ttg1-1 double mutant may be attributed to differences in the background genotype in which the ttg1-1 mutation resides. Short-chain ACX (acyl-CoA oxidase) specific activities were similar between the cts-1/ttg1-1 double mutant and Ler, and did not suggest a differential stability of glyoxysomes (results not shown). These results indicate that the decrease in carbohydrate labelling is not caused by compromised glyoxysomal AcetCS activity in plants lacking CTS.
DISCUSSION
Mutant CTS alleles have been identified from a number of forward genetic screens based on the tolerance to fatty acid analogues, including IBA [21] and 2,4-DB [22]. cts was first identified as a germination factor, whose expression overcame dormancy-promoting programmes [18]. Analysis of fatty acid and acyl-CoA profiles of germinating cts seeds revealed that the mobilization of TAG (triacylglycerol) is greatly compromised in the mutants; high levels of long-chain fatty acids in TAG remain and long-chain acyl-CoAs accumulate [19]. These observations led to the conclusion that CTS is involved in the transport of fatty acyl-CoAs into peroxisomes for β-oxidation, in a manner analogous to that of the yeast PXA1/PXA2 and mammalian ABCD1 and ABCD3 (for recent reviews, see [17,35]) proteins. A recent important finding is that the level of JA is substantially reduced in cts alleles, thereby also implicating CTS in the transport of OPDA [(9S, 13S)-12-oxophytodienoic acid], which is converted into JA within peroxisomes [24]. Alterations in fatty acid and acyl-CoA profiles and disruption of the metabolism of JA, 2,4-DB and IBA in cts alleles was the basis for speculation that substrates for CTS transport were at least four carbons long, and could include ring moieties [20,24]. Our results suggest that CTS has the potential to facilitate the transport of compounds as small as acetate.
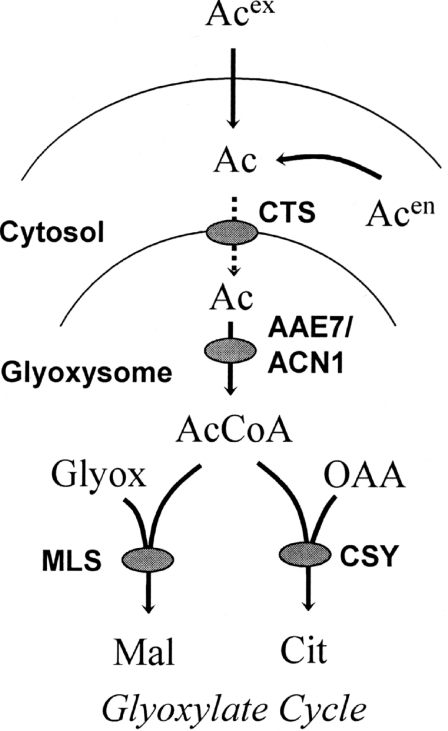
Although mammalian and yeast models suggest that CoA derivatives are transported, and the accumulation of long-chain acyl-CoAs in cts-1 is consistent with the concept that the Arabidopsis CTS transports acyl-CoAs [19], there is evidence that CTS may transport fatty acids as well. Single or multiple gene mutations in every step of peroxisomal β-oxidation, acyl-CoA oxidase [36,37], multifunctional protein [38] or thiolase [22,39], produces the phenotype of sucrose-dependent germination and accumulation of fatty acyl-CoA [37–39]. Thus the acyl-CoA accumulation observed for cts alleles is not unique to disruption of a particular transport step, and no direct conclusion about substrate transport can be drawn [17]. Elimination of the glyoxysomal LACSs (long-chain acyl-CoA synthetases) LACS6 and LACS7 in Arabidopsis, produces sucrose-dependent establishment, similar to that observed for the CTS mutants [40]. Such a severe phenotype at this step suggests that the majority of fatty acids are activated into their CoA esters directly within glyoxysomes. Fulda et al. [40] presented two models for fatty acid supply to LACS6/LACS7, one which involved CTS/PXA1/PED3 and the other which used a currently unidentified fatty acid transporter. Although distinction between the two models is not possible for unesterified long-chain fatty acids, our results support the CTS/PXA1/PED3 model for the transport of unesterified acetate. The reduction in the amount of radiolabelled acetate appearing in the soluble carbohydrate fraction for the two cts alleles is similar to the reduction observed for the acn1 mutant [7]. If acetate was activated into acetyl-CoA within the cytosol and subsequently transported into peroxisomes by CTS, then it would not be possible to explain the similarly large reduction in labelled soluble carbohydrate observed for acn1. We cannot exclude the possibility that acetate or FAc is activated to (F)acetyl-CoA in the cytosol, and that the CoA moiety is removed during the transport process. In either case, it would be unesterified acetate that is transported. The presence of AcetCS activity in glyoxysomes of seedlings lacking CTS eliminates the alternative explanation of a negative effect on ACN1 activity, thus the explanation of compromised acetate transport is more plausible. The model for the involvement of CTS and AAE7/ACN1 in the glyoxylate cycle is given in Scheme 1. It shows that acetate (either exogenous or endogenous recycled acetate) enters the glyoxysome via CTS, is activated by AAE7/ACN1 and subsequently partitioned between malate synthase and citrate synthase.
Scheme 1. Scheme of acetate transport and activation for entry into the glyoxylate cycle in developing seedlings.
The broken arrow indicates the hypothesis that acetate is transported in the free, unesterified, form. Ac, acetate; AcCoA, acetyl-CoA; Acen, endogenous acetate; Acex, exogenously supplied acetate; Cit, citrate; CSY, citrate synthase; Glyox, glyoxylate; Mal, malate; MLS, malate synthase; OAA, oxaloacetate.
It has been proposed that CTS functions as a signalling molecule during the initiation of germination and metabolic pathways during seedling establishment [24]. During germination, CTS may either produce a germination-promoting compound or remove a germination inhibitor, and its role subsequently alters to provide fatty acids for β-oxidation during seedling establishment. Comparative germination studies of cts-1, cts-2 and pxa1-1 with other β-oxidation and glyoxylate cycle-deficient mutants indicated that a lack of CTS results in reduced germination potential, as opposed to increased dormancy, since cts seeds after-ripen normally [41]. Comparision of the germination potential of cts with kat (3-oxoacyl-CoA thiolase) 2-1, a mutant lacking peroxisomal 3-oxoacyl-CoA thiolase, led to the conclusion that reduced germination was not specifically a consequence of compromised β-oxidation, and that CTS was likely to play other metabolic roles as well. Our results support the conclusion of potential multiple metabolic roles for CTS. They emphasize the potential involvement of CTS in distinct metabolic pathways, since acetate would bypass β-oxidation and enter the glyoxylate cycle directly once activated to acetyl-CoA. Is it possible that CTS and the glyoxylate cycle have a specific secondary function to re-assimilate acetate, as well as to facilitate lipid mobilization? Despite extensive research into reserve mobilization within oilseeds, surprisingly little is known about the intermediate steps in carbon reserve partitioning and potential carbon recycling during seedling development. A comparative investigation of time-course labelling patterns appearing in metabolite fractions and specific intermediates from isotopically labelled acetate and fatty acids, which employs the existing glyoxylate cycle and other mutants at various stages of germination and establishment, is essential in order to remedy our lack of knowledge concerning seedling carbon nutrition.
Acknowledgments
We thank Professor Michael Holdsworth and Dr Steven Footitt for providing cts-1, complemented lines and the cts-1/ttgl-1 double mutant, and Professor Bonnie Bartel for providing the pxa1 and pxa1/35SPXA1 lines. We also thank Dr Alison Baker, Professor Steven Smith and Professor Ian Graham for their insightful discussion and helpful advice with the manuscript. We thank the John Innes Centre Genome Laboratory for doing the DNA sequencing. We thank the E. C. (European Commission) for the award made to S. J. to fund his participation in the Erasmus exchange programme. This work was supported by the U.K. Biotechnology and Biological Sciences Research Council Grant 5/P14659) and postgraduate student funding from the University of Wales Bangor.
References
- 1.Beevers H. The role of the glyoxylate cycle. In: Stumpf P. K., editor. The Biochemistry of Plants, vol. 4. New York: Academic Press; 1980. pp. 117–130. [Google Scholar]
- 2.Canvin D. T., Beevers H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J. Biol. Chem. 1961;236:988–995. [PubMed] [Google Scholar]
- 3.Eastmond P. J., Germain V., Lange P. R., Bryce J. H., Smith S. M., Graham I. A. Postgerminative growth and lipid catabolism in oilseeds lacking the glyoxylate cycle. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5669–5674. doi: 10.1073/pnas.97.10.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornah J. E., Germain V., Ward J. L., Beale M. H., Smith S. M. Lipid utilization, gluconeogenesis, and seedling growth in Arabidopsis mutants lacking the glyoxylate cycle enzyme malate synthase. J. Biol. Chem. 2004;279:42916–42923. doi: 10.1074/jbc.M407380200. [DOI] [PubMed] [Google Scholar]
- 5.Hooks M. A., Turner J. E., Murphy E., Graham I. A. Acetate non-utilizing mutants of Arabidopsis: evidence that organic acids influence carbohydrate perception in germinating seedlings. Mol. Genet. Genomics. 2004;271:249–256. doi: 10.1007/s00438-004-0985-9. [DOI] [PubMed] [Google Scholar]
- 6.Shockey J. M., Fulda M. S., Browse J. Arabidopsis contains a large superfamily of acyl-activating enzymes. Phylogenetic and biochemical analysis of a new class of acyl-coenzyme A synthetases. Plant Physiol. 2000;132:1065–1076. doi: 10.1104/pp.103.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner J. E., Greville K., Murphy E. C., Hooks M. A. Characterization of Arabidopsis fluoroacetate resistant mutants reveals the principal mechanism of acetate activation for entry into the glyoxylate cycle. J. Biol. Chem. 2005;280:2780–2787. doi: 10.1074/jbc.M407291200. [DOI] [PubMed] [Google Scholar]
- 8.Davies T. G. E., Coleman J. O. D. The Arabidopsis thaliana ATP-binding cassette proteins: an emerging superfamily. Plant Cell Environ. 2000;23:431–443. [Google Scholar]
- 9.Sanchez-Fernandez R., Davies T. G. E., Colman J. O. D., Rea P. A. The Arabidopsis thaliana ABC protein superfamily, a complete inventory J. Biol. Chem. 2001;276:30231–30244. doi: 10.1074/jbc.M103104200. [DOI] [PubMed] [Google Scholar]
- 10.Shani N., Valle D. A Saccharomyces cerevisiae homolog of the human adrenoleukodystrophy transporter is a heterodimer of two half ATP-binding cassette transporters. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11901–11906. doi: 10.1073/pnas.93.21.11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verleur N., Hettema E. H., van Roermund C. W. T., Tabak H. F., Wanders R. J. A. Transport of activated fatty acids by the peroxisomal ATP-binding-cassette transporter Pxa2 in a semi-intact yeast cell system. Eur. J. Biochem. 1997;249:657–661. doi: 10.1111/j.1432-1033.1997.00657.x. [DOI] [PubMed] [Google Scholar]
- 12.Imanaka T., Aihara K., Takano T., Yamashita A., Sato R., Suzuki Y., Yokota S., Osumi T. Characterization of the 70-kDa peroxisomal membrane protein, an ATP binding cassette transporter. J. Biol. Chem. 1999;274:11968–11976. doi: 10.1074/jbc.274.17.11968. [DOI] [PubMed] [Google Scholar]
- 13.Guimaraes C. P., Sa-Maranda C., Azevedo J. E. Probing substrate-induced conformational alterations in adrenoleukodystrophy protein by proteolysis. J. Hum. Genet. 2005;50:99–105. doi: 10.1007/s10038-004-0226-4. [DOI] [PubMed] [Google Scholar]
- 14.Mosser J., Douar A. M., Sarde C. O., Kioschis R., Feil H., Moser A. M., Poustka J. L., Mandel J. L., Aubourg P. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- 15.Smith K. D., Kemp S., Braiterman L. T., Lu J. F., Wei H.-M., Geraghty M., Stetten G., Bergin J. S., Pevsner J., Watkins P. A. X-linked adrenoleukodystrophy: genes, mutations, and phenotypes. Neurochem. Res. 1999;24:521–535. doi: 10.1023/a:1022535930009. [DOI] [PubMed] [Google Scholar]
- 16.Moser H. W., Smith K. D., Moser A. B. X-linked adrenoleukodystrophy. In: Skriver C. R., Beaudet A. L., Sly W. S., Valle D., editors. The Metabolic and Molecular Basis of Inherited Disease. 7th edn. York: McGraw-Hill; 1994. pp. 2325–2349. [Google Scholar]
- 17.Theodoulou F. L., Holdsworth M., Baker A. Peroxisomal ABC transporters. FEBS Lett. 2006;580:1139–1155. doi: 10.1016/j.febslet.2005.12.095. [DOI] [PubMed] [Google Scholar]
- 18.Russell L., Larner V., Kurup S., Bougourd S., Holdsworth M. The Arabidopsis COMATOSE locus regulates germination potential. Development. 2000;127:3759–3767. doi: 10.1242/dev.127.17.3759. [DOI] [PubMed] [Google Scholar]
- 19.Footitt S., Slocombe S. P., Larner V., Kurup S., Wu Y., Larson T., Graham I., Baker A., Holdsworth M. Control of germination and lipid mobilization by COMATOSE, the Arabidopsis homologue of human ALDP. EMBO J. 2002;21:2912–2922. doi: 10.1093/emboj/cdf300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zolman B. K., Silva I. D., Bartel B. The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol. 2001;127:1266–1278. [PMC free article] [PubMed] [Google Scholar]
- 21.Zolman B. K., Yoder A., Bartel B. Genetic analysis of indole-3-butyric acid responses in Arabidopsis reveals four mutant classes. Genetics. 2000;156:1323–1337. doi: 10.1093/genetics/156.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi M., Toriyama K., Kondo M., Nishimura M. 2,4-Dichlorophenoxy acid-resistant mutants of Arabidopsis have defects in glyoxysomal fatty acid β-oxidation. Plant Cell. 1998;10:183–195. doi: 10.1105/tpc.10.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayashi M., Nito K., Takei-Hoshi R., Yagi M., Kondo M., Suenaga A., Yamaya T., Nishimura M. Ped3p is a peroxisomal ATP-binding cassette transporter that might supply substrates for fatty acid β-oxidation. Plant Cell. Physiol. 2002;43:1–11. doi: 10.1093/pcp/pcf023. [DOI] [PubMed] [Google Scholar]
- 24.Theodoulou F. L., Job K., Slocombe S. P., Footitt S., Holdsworth M., Baker A., Larson T. R., Graham I. A. Jasmonic acid levels are reduced in COMATOSE ATP-binding cassette transporter mutants. Implications for transport of jasmonate precursors into peroxisomes. Plant Physiol. 2005;137:835–840. doi: 10.1104/pp.105.059352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quastel J. H. Inhibitions of the citric acid cycle. In: Hochester J. H., Quastel J. H., editors. Metabolic Inhibitors: A Comprehensive Treatise vol. 2. New York: Academic Press; 1963. pp. 473–502. [Google Scholar]
- 26.Murashige T., Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 1962;15:473–496. [Google Scholar]
- 27.Konieczny A., Ausubel F. M. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- 28.Baumbusch L. O., Sundal I. K., Hughes W., Galau G. A., Jakobsen K. S. Efficient protocols for CAPS-based mapping in Arabidopsis. Plant Mol. Biol. Rep. 2001;19:137–149. [Google Scholar]
- 29.Bell C. J., Ecker J. R. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- 30.Boyes D. C., Zayed A. M., Ascenzi R., McCaskill A. J., Hoffman N. E., Davis K. R. Growth stage-based phenotypic analysis of Arabidopsis: a model for high throughput functional genomics in plants. Plant Cell. 2001;13:1499–1510. doi: 10.1105/TPC.010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laval V., Koroleva O. A., Murphy E., Lu C. G., Milner J. J., Hooks M. A., Tomos A. D. Distribution of actin gene isoforms in the Arabidopsis leaf measured in microsamples from intact individual cells. Planta. 2002;215:287–292. doi: 10.1007/s00425-001-0732-y. [DOI] [PubMed] [Google Scholar]
- 32.Millerd A., Bonner J. Acetate activation and acetoacetate formation in plant systems. Arch. Biochem. Biophys. 1954;49:343–355. doi: 10.1016/0003-9861(54)90204-0. [DOI] [PubMed] [Google Scholar]
- 33.Hryb D. J., Hogg J. F. Chain length specificities of peroxisomal and mitochondrial β-oxidation in rat liver. Biochem. Biophys. Res. Commun. 1979;87:1200–1206. doi: 10.1016/s0006-291x(79)80034-0. [DOI] [PubMed] [Google Scholar]
- 34.Jia Z. Z., Pei Z. T., Li Y. Y., Wei L. M., Smith K. D., Watkins P. A. X-linked adrenoleukodystrophy: role of very long-chain acyl-CoA synthetases Mol. Genet. Metab. 2004;83:117–127. doi: 10.1016/j.ymgme.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Pohl A., Devaux P. F., Herrman A. Function of prokaryotic and eukaryotic ABC proteins in lipid transport. Biochim. Biophys. Acta. 2005;1733:29–52. doi: 10.1016/j.bbalip.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Adham A. R., Zolman B. K., Millius A., Bartel B. Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in β-oxidation. Plant J. 2005;41:859–874. doi: 10.1111/j.1365-313X.2005.02343.x. [DOI] [PubMed] [Google Scholar]
- 37.Pinfield-Wells H., Rylott E. J., Gilday A. D., Graham S., Job K., Larson T. R., Graham I. A. Sucrose rescues seedling establishment but not germination of Arabidopsis mutants disrupted in peroxisomal fatty acid. Plant J. 2005;43:861–872. doi: 10.1111/j.1365-313X.2005.02498.x. [DOI] [PubMed] [Google Scholar]
- 38.Rylott E. L., Eastmond P. J., Gilday A. D., Slocombe S. P., Larson T. R., Baker A., Graham I. A. The Arabidopsis thaliana multifunctional protein gene (MFP2) of peroxisomal β-oxidation is essential for seedling establishment. Plant J. 2006;45:930–941. doi: 10.1111/j.1365-313X.2005.02650.x. [DOI] [PubMed] [Google Scholar]
- 39.Germain V., Rylott E. L., Larson T. R., Sherson S. M., Bechtold N., Carde J-P., Bryce J. H., Graham I. A., Smith S. M. Requirement for 3-ketoacyl-CoA thiolase-2 in peroxisome development, fatty acid β-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J. 2001;28:1–12. doi: 10.1046/j.1365-313x.2001.01095.x. [DOI] [PubMed] [Google Scholar]
- 40.Fulda M., Schurr J., Abbadi A., Heinz E., Browse J. Peroxisomal acyl-CoA synthetase activity is essential for seedling development in Arabidopsis thaliana. Plant Cell. 2004;16:394–405. doi: 10.1105/tpc.019646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Footitt S., Marquez J., Schmuths H., Baker A., Theodoulou F. L., Holdsworth M. Analysis of the role of COMATOSE and peroxisomal β-oxidation in the determination of germination potential in Arabidopsis. J. Exp. Bot. 2006;57:2805–2814. doi: 10.1093/jxb/erl045. [DOI] [PubMed] [Google Scholar]