Abstract
PLA2 (phospholipase A2) enzymes play critical roles in membrane phospholipid homoeostasis and in generation of lysophospholipid growth factors. In the present study, we show that the activity of the cytosolic iPLA2 (calcium-independent PLA2), but not that of the calcium-dependent cPLA2 (cytosolic PLA2), is required for growth-factor-independent, autonomous replication of ovarian carcinoma cells. Blocking iPLA2 activity with the pharmacological inhibitor BEL (bromoenol lactone) induces cell cycle arrest in S- and G2/M-phases independently of the status of the p53 tumour suppressor. Inhibition of iPLA2 activity also leads to modest increases in apoptosis of ovarian cancer cells. The S- and G2/M-phase accumulation is accompanied by increased levels of the cell cycle regulators cyclins B and E. Interestingly, the S-phase arrest is released by supplementing the growth factors LPA (lysophosphatidic acid) or EGF (epidermal growth factor). However, inhibition of iPLA2 activity with BEL remains effective in repressing growth-factor- or serum-stimulated proliferation of ovarian cancer cells through G2/M-phase arrest. Down-regulation of iPLA2β expression with lentivirus-mediated RNA interference inhibited cell proliferation in culture and tumorigenicity of ovarian cancer cell lines in nude mice. These results indicate an essential role for iPLA2 in cell cycle progression and tumorigenesis of ovarian carcinoma cells.
Keywords: calcium-independent phospholipase A2, cell cycle, growth autonomy, lysophosphatidic acid, ovarian cancer cell, tumorigenesis
Abbreviations: BEL, bromoenol lactone; EGF, epidermal growth factor; FBS, foetal bovine serum; GFP, green fluorescent protein; HEK-293 cells, human embryonic kidney cells; LPA, lysophosphatidic acid; PLA2, phospholipase A2; cPLA2, cytosolic PLA2; iPLA2, calcium-independent PLA2; shRNA, small-hairpin RNA; siRNA, short interfering RNA
INTRODUCTION
Cancer cells exhibit reduced dependence on exogenous growth factors for continuous proliferation and survival [1,2]. The development of growth advantages is a critical step towards oncogenic and metastatic phenotype of tumour cells. Human ovarian cancer cells represent a prototype model of growth autonomy [3–6]. In culture, most ovarian cancer cell lines proliferate in the absence of exogenous growth factors, leading to a slow yet steady increase in cell populations [3–6]. The mechanism for the growth factor-independent replication of ovarian cancer cells is poorly understood. It has been suggested that the growth autonomy of ovarian cancer cells is mediated by constitutive production of LPA (lysophosphatidic acid) and other autocrine growth factors or by genetic alterations that bypass the requirement of exogenous stimuli for growth [1–6].
PLA2 (phospholipase A2) enzymes have been implicated in activation of cell migration as well as in production of LPA in ovarian carcinoma cells [7–11]. PLA2 catalyses the hydrolysis of glycerophospholipids at the sn-2 ester bond, producing unesterified fatty acids such as arachidonic acid and 2-lysophospholipids [12]. The PLA2 enzymes are classified into three major categories: the sPLA2 (secreted PLA2), the Group IV calcium-dependent cPLA2 (cytosolic PLA2) and the Group VI cytosolic iPLA2 (calcium-independent PLA2) [12]. In the present study, we examined the potential involvement of PLA2 enzymes in growth regulation of ovarian cancer cells. The autonomous replication and growth-factor-stimulated proliferation of ovarian cancer cells are highly sensitive to inhibition of iPLA2 but are refractory to inhibition of cPLA2. When ovarian cancer cells were grown under growth-factor-independent conditions, suppression of iPLA2 activity led to accumulation of cell populations in both S- and G2/M-phases. Supplementation of exogenous growth factors such as LPA, EGF (epidermal growth factor) or serum to culture released the S-phase arrest but did not affect the G2/M arrest associated with inhibition of iPLA2. In addition to the prominent effect on cell cycle, inhibition of iPLA2 also induced weak to modest increases in apoptosis. Down-regulation of iPLA2β with lentivirus-mediated RNAi (RNA interference) targeting iPLA2β expression inhibited cell proliferation in culture and decreased tumorigenicity of ovarian cancer cell lines in athymic nude mice. These results revealed a pivotal role for iPLA2 in driving cell cycle progression and tumorigenesis of ovarian cancer cells.
EXPERIMENTAL
Reagents
BEL (bromoenol lactone) [13] was purchased from Cayman Chemicals, and 1 mM stock solution was made in DMSO. The cPLA2 inhibitor pyrrolidine N-{(2S,4R)-4-(biphenyl-2-ylmethyl-isobutyl-amino)-1-[2-(2,4-difluorobenzoyl)-benzoyl]-pyrrolidin-2-ylmethyl}-3-[4-(2,4-dioxothiazolidin-5-ylidenemethyl)-phenyl]-acrylamide, HCl [14] was obtained from Calbiochem and dissolved as 1 mM in water. LPA (18:1, oleoyl) was obtained from Avanti Polar Lipids and dissolved as 21.8 mM stock solution in chloroform. Before use, chloroform was evaporated in a tissue culture hood and LPA was resuspended in PBS containing 0.5% fatty-acid-free BSA. EGF (tissue culture grade) was obtained from Sigma. Antibodies against iPLA2β, cyclin B, cyclin E and GFP (green fluorescent protein) were purchased from Santa Cruz Biotechnology. Anti-c-Myc, cPLA2 and tubulin antibodies were obtained from Cell Signaling Technology.
Cells
The sources of ovarian cancer cell lines have been described previously [15,16]. They were maintained in culture in RPMI 1640 medium supplemented with 10% (v/v) FBS (foetal bovine serum). All lines were frozen at early passages and used for less than 10 weeks in continuous culture.
iPLA2 activity assay
iPLA2 activity was assayed according to the method described previously [17]. Briefly, cell homogenates were prepared in 10 mM Hepes (pH 7.4), 0.34 M sucrose, 10 μg/ml leupeptin, 10 μg/ml aprotinin and 1 mM PMSF, and were lysed by sonication on ice. The cell lysates were incubated for 2 h at 40 °C with a substrate consisting of 100 μM DPPC (dipalmitoyl phosphatidylcholine) and 400 μM Triton X-100, with 0.02 μCi L-α-dipalmitoyl[2-palmitoyl-1-14C]phosphatidylcholine added as a tracer. The released palmitic acid was quantified as described by Dole [18]. The assays were performed under conditions where substrate hydrolysis was linear with respect to protein and time.
Western blot
Cells in culture plates were washed with PBS and lysed with SDS sample buffer. After boiling for 5 min, cell lysates were resolved by SDS/PAGE, transferred on to Immobilon (PVDF) and immunoblotted with antibodies following the protocols of the manufacturers. Immunocomplexes were visualized with an ECL® (enhanced chemiluminescence) detection kit (GE Healthcare) by using appropriate horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology).
Growth assay
The propagation of ovarian cancer cell lines in culture was analysed by colorimetric staining with Crystal Violet or by quantification of cell numbers with a Coulter counter. For Crystal Violet staining, the cells were cultured in 6- or 12-well plates, washed with PBS and incubated with 0.5% Crystal Violet at room temperature (22 °C) for 20 min. The staining solution was removed and the cells were washed four times with deionized water. After the plates were air-dried, the Crystal Violet was solubilized with the Sorenson's Buffer [0.1 M sodium citrate, pH 4.2, and 50% (v/v) ethanol] and the D570 was measured using a spectrophotometric plate reader (BioTek Instruments).
Apoptosis assay
Both floating and attached cells were harvested and washed twice with PBS before staining with the Vybrant Apoptosis Assay kit #3 (Invitrogen). Briefly, cells (2×105) were resuspended in 0.2 ml of staining buffer (10 mM Hepes, pH 7.4, 140 mM NaCl and 2.5 mM CaCl) and incubated for 20 min at room temperature with 10 μl of FITC-conjugated Annexin V. After the incubation, 200 μl of the staining buffer was added. The percentages of Annexin V-positive cells were determined with a fluorescence microscope by counting four randomly selected fields totalling 200 or more cells. The results are the means±S.D. for triplicate assays, representative of three independent experiments.
Cell cycle analysis
Cells were plated and cultured in complete medium in 100 mm culture dishes. At approx. 40% confluence, the cells were incubated with RPMI 1640 medium supplemented with or without growth factors in the presence of indicated concentrations of BEL. The attached cells were harvested by trypsinization, washed twice with PBS and resuspended at a concentration of 1×106 cells/ml in a fluorochrome staining solution (3.8 mM sodium citrate, 0.05 mg/ml propidium iodide, 0.1% Triton X-100 and 7 Kunitz units/ml RNase B) and incubated on ice for 3 h or kept at 4 °C for up to 2 weeks before flow cytometric analysis.
siRNA (short interfering RNA) knockdown of cPLA2
cPLA2α siRNA or non-target control siRNA (2.25 μg) (Dharmacon RNA Technologies) was transfected into ovarian cancer cell lines with Amaxa nucleofector II (Kit V/program V-05 for SKOV-3 and Kit T/program T-30 for Dov-13). The electroporated cells were cultured in 12-well plates in complete medium. After 24 h of recovery, the cells were fed serum-free RPMI 1640 medium and incubated for 1–3 days for growth assay and for Western blot analysis of cPLA2 protein expression.
Lentivirus-mediated shRNA (small-hairpin RNA)
Lentivirus-based U6-promoted iPLA2β shRNA constructs were generated by cloning PCR products carrying sense and antisense oligonucleotides of iPLA2β into the pLL3.7 vector as described previously [19,20]. Three iPLA2 shRNA constructs were made that target the nucleotide sequence 40–60 (construct 1), 810–831 (construct 2) and 1673–1693 (construct 3) of the human iPLA2β cDNA (GenBank® accession number NM_003560) [21]. The viral construct carrying a non-target shRNA sequence (CTTGTTAACGCGCGGTGACCC) was used as a control. The viruses were packaged in HEK-293 (human embryonic kidney) FT cells following the method of Rubinson et al. [19].
Ovarian cancer cell lines in six-well plates at approx. 50% confluence were incubated with 1 ml of the viral supernatants in the presence of polybrene (8 μg/ml). The infected cells were then kept in regular complete medium for 48 h. Inspection with fluorescence microscopy confirmed the presence of more than 70% of GFP-positive cells after viral infection. Efficient down-regulation of iPLA2β expression in infected cells was also confirmed by immunoblotting for iPLA2β protein and by enzymatic analysis of cellular iPLA2 activity. Both construct 1 and construct 2 strongly inhibited iPLA2β expression in testing experiments, and construct 1 was used for further experiments in the study.
Tumorigenesis assay in nude mice
Uninfected and lentivirus-infected cells were cultured in 100-mm dishes. The cells in exponential growth stage were trypsinized, washed twice with serum-free RPMI 1640 medium and resuspended in the serum-free medium. The cells (1×106 of SKOV-3 and 2×106 of OVCAR-3) were injected subcutaneously on the right flank of Balb/c nude mice (Nu/Nu, female, 5–6 weeks old). The formation of subcutaneous tumours was monitored and measured with a digital calliper. The tumour volumes were calculated based on the formula lw2/2 where l is the length and w is the shortest width of the tumour. All procedures for animal studies were conducted in compliance with the policies and regulations of Virginia Commonwealth University Institutional Animal Care and Use Committee.
Statistics
All numerical results are presented as means±S.D. The statistical significance of differences was analysed using Student's t test, where P<0.05 was considered to be statistically significant.
RESULTS
Growth autonomy of ovarian cancer cells requires cellular iPLA2 activity
Most ovarian carcinoma cell lines are capable of multiplying autonomously under serum-free conditions, leading to a continuous increase in cell numbers (Figure 1A). In the absence of exogenous growth factors, ovarian cancer cell lines displayed a majority of cells in G1 and low percentages of populations in S- and G2/M-phases (results not shown, also see Figure 3), indicating that the cell number increases result from slow yet continuous cell cycling. To explore the mechanism of the uncontrolled proliferation of ovarian cancer cells and potential therapeutic approaches for intervention, we examined whether cellular PLA2 activities are required for the growth-factor-independent propagation of ovarian cancer cells. PLA2 enzymes are potentially involved in migration of ovarian cancer cells and in generation of lipid growth factors that may constitute an autocrine stimulation loop to drive proliferation of ovarian cancer cells [2,7–11].
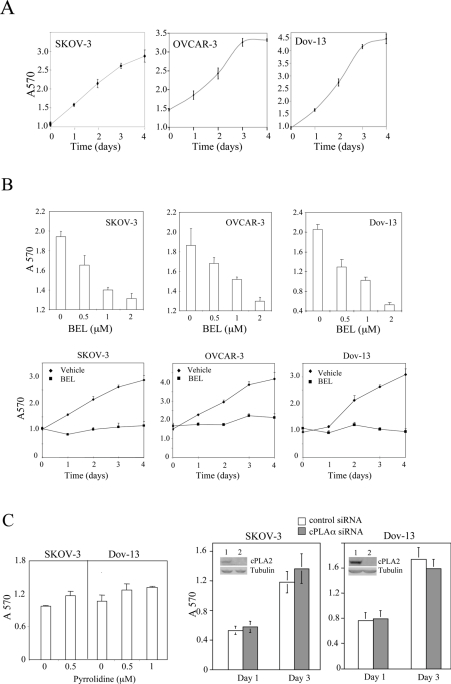
Figure 1. Suppression of growth-factor-independent replication of ovarian cancer cells by inhibition of cellular iPLA2 activity.
(A) Ovarian cancer cell lines proliferate independently of exogenous growth factors. SKOV-3, OVCAR-3 and Dov-13 cells were plated on to six-well plates with the complete medium. At approx. 40% confluence, the cells were washed and incubated with serum-free medium for the indicated periods of time (days). Cells were stained with Crystal Violet and the staining was quantified by measuring the D570 (arbitrary units). (B) The iPLA2 inhibitor BEL suppressed the replication of ovarian cancer cell lines. SKOV-3, OVCAR-3 and Dov-13 cells were incubated for 2 days in serum-free medium with the indicated concentrations of BEL (upper panel) or the cells were incubated in serum-free medium with 2 μM BEL or DMSO (vehicle) for the indicated periods of time (days) (lower panel). (C) The cPLA2 inhibitor pyrrolidine (left panel) or siRNA knockdown of cPLA2 expression (right panel) did not inhibit autonomous growth of ovarian cancer cell lines. At 1 day after transfection with control siRNA or cPLA2α-specific siRNA, the cells were switched to serum-free medium and maintained for 1–3 days for growth assay with Crystal Violet staining as described in (A). Knockdown of cPLAα expression by cPLA2α siRNA (lane 2) compared with control siRNA-transfected cells (lane 1) was confirmed by Western blotting (insets of right panels). All numerical results are means±S.D. for triplicates, representative of three independent experiments.
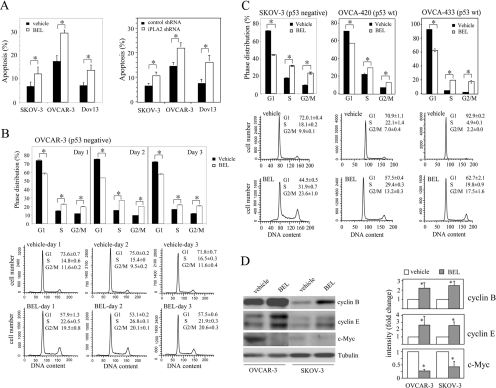
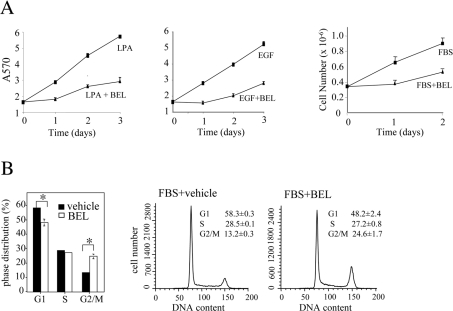
Figure 3. The effect of iPLA2 inhibition on viability and cell cycle progression of ovarian cancer cells.
(A) Treatment of ovarian cancer cell lines with BEL or knockdown of iPLA2β expression with lentivirus-mediated shRNA led to modest increases in apoptotic cell death. Ovarian cancer cell lines were incubated for 2 days with BEL (2 μM) or DMSO (vehicle) in serum-free medium (left panel). Alternatively, the cells were infected with control virus or iPLA2β shRNA virus and then switched to serum-free conditions for two more days before analysis of apoptosis (right panel). Both floating and attached cells were harvested and stained with FITC-conjugated Annexin V as described in the Experimental section. Shown are percentages of Annexin V-positive, apoptotic cells (mean±S.D. for triplicates), representative of three independent assays. (B, C) Inhibition of iPLA2 with BEL caused cell cycle arrest in S- and G2/M-phases in both p53-positive and p53-negative ovarian carcinoma cell lines. The p53-deficient cell lines OVCAR-3 (B) and SKOV-3 (C) and the p53-wild-type cell lines OVCA-420 (C) and OVCA-433 (C) were incubated in serum-free medium with DMSO (vehicle) or BEL (2 μM) for 1–3 days in (B) or for 2 days in (C). The cells were harvested by trypsinization, washed with PBS and stained with propidium iodide for flow cytometric analysis. Upper panel: means±S.D. for three determinations. Lower panel: flow cytogram, representative of three independent assays. The statistical significance (P<0.05) of differences between vehicle and BEL-treated cells is indicated with an asterisk. (D) Cell cycle arrest in S- and G2/M-phases caused by iPLA2 inhibition was associated with increased abundance of cyclins B and E and with down-regulation of c-Myc. The cells were incubated with vehicle (DMSO) or BEL (2 μM) for 2 days before immunoblotting analysis of the cyclins and c-Myc. The band intensities from three independent experiments were determined by densitometry and presented as relative values with that of vehicle-treated cells (control) defined as 1 arbitrary unit. The statistical significance (P<0.05) of differences in band intensities is indicated with an asterisk.
We examined the effect of specific pharmacological inhibitors of iPLA2 and cPLA2 on the proliferation of ovarian cancer cells under serum-free conditions. The iPLA2 inhibitor BEL [13] strongly blocked the growth of various ovarian cancer cell lines including OVCAR-3, SKOV-3 and Dov-13 (Figure 1B). The inhibitory effect of BEL in ovarian cancer cell lines was dose dependent. A significant effect was detected when as low as 0.5 μM BEL was applied (Figure 1B). Treatment of ovarian cancer cell lines with BEL at 2 μM, a concentration 5–10-fold lower than the doses used by others in previous publications [22–24], strongly inhibited cell growth during a 4 day incubation time (Figure 1B). The results suggest that cellular iPLA2 activity is critical for growth-factor-independent replication of ovarian cancer cells.
In contrast with BEL, the cPLA2 inhibitor pyrrolidine [14] at pharmacologically relevant concentrations (0.5–1.0 μM) did not have an inhibitory effect on these cells as analysed by the colorimetric staining assay (Figure 1C). Consistent with this, knockdown of cPLAα expression with specific siRNA did not affect the growth-factor-independent proliferation of these cells (Figure 1C).
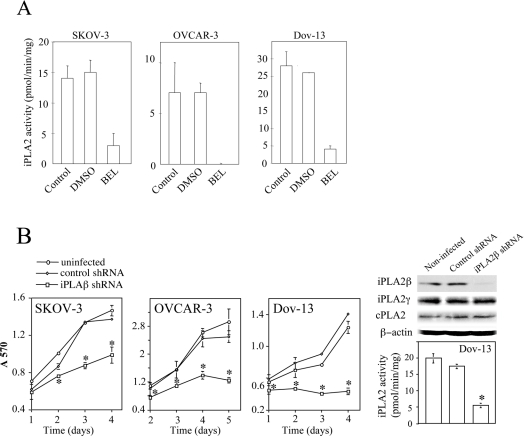
To confirm that BEL indeed exerts its effect through targeting iPLA2, we measured cellular iPLA2 activity using in vitro enzymatic assay. All ovarian cancer cell lines examined showed significant iPLA2 activity which was blocked by addition of BEL to cell lysates (Figure 2A). Although BEL does not affect other PLA2s, it has been shown to inhibit phosphatidic acid phosphohydrolase [25]. Therefore, to confirm the involvement of iPLA2 in cell proliferation, we used lentivirus-mediated shRNA to down-regulate expression of iPLA2β, the major iPLA2 isoform potentially involved in regulation of proliferation of other types of cell [26,27]. As demonstrated in Figure 2(B), iPLA2β knockdown led to strong growth inhibition in OVCAR-3, SKOV-3 and Dov-13 cells compared with uninfected or non-target control virus-infected cells. In Dov-13 and OVCAR-3 cells, significant decrease in colorimetric staining of shRNA-treated cells was detected 1 day after culturing under serum-free conditions. Significant growth decreases in SKOV-3 cells were observed after 2 days in culture.
Figure 2. Inhibition of iPLA2 enzymatic activity by BEL and down-regulation of iPLA2β expression by lentivirus-mediated shRNA.
(A) SKOV-3, OVCAR-3 and Dov-13 cells exhibited iPLA2 activity that was inhibited by BEL. The iPLA2 enzymatic activity in cell lysates was assayed in the absence or presence of BEL or DMSO as detailed in the Experimental section. (B) siRNA down-regulation of iPLA2β expression caused growth inhibition. SKOV-3, OVCAR-3 and Dov-13 cells were infected with no virus (uninfected), control virus or with iPLA2β-specific shRNA virus. At 3 days after infection, cell lysates were analysed for expression of iPLA2β protein and other PLA2 isoforms (iPLA2γ and cPLA2). The enzymatic activity of iPLA2 in uninfected, control virus and iPLA2 shRNA virus-infected cells was measured as detailed under the Experimental section. Parallel cultures were incubated in serum-free medium for the indicated periods of time for growth analysis as described in Figure 1. The statistical significance of differences of data in Figures 2–6 was determined by Student's t test, where P<0.05 was considered statistically significant and marked with an asterisk.
Inhibition of iPLA2 activity results in a modest increase in cell apoptosis
A decrease in cell viability could potentially contribute to the observed growth suppression associated with inhibition of iPLA2 activity. To evaluate this possibility, we determined whether inhibition of iPLA2 with BEL induces apoptotic cell death. Most ovarian cancer cell lines are largely resistant to serum deprivation-triggered apoptosis [2,16]. After 2 days of incubation under serum-free conditions, only low percentages of OVCAR-3, SKOV-3 or Dov-13 cells underwent apoptosis as analysed by fluorescence-conjugated Annexin V staining (Figure 3A). Inclusion of 2 μM BEL in culture medium to inhibit cellular iPLA2 led to modest yet consistent increases in percentages of apoptotic cells in each of these cell lines (Figure 3A). Knockdown of iPLA2β expression with shRNA also modestly increased apoptosis of ovarian cancer cell lines cultured under serum-free conditions (Figure 3A).
Inhibition of iPLA2 activity induces cell cycle arrest in S- and G2/M-phases in a p53-independent manner
The cellular iPLA2 activity is regulated in a cell cycle-dependent manner [17,22]. The highest iPLA2 activity is observed at the G2/M- and late S-phase in proliferating T-cells and CHO-K1 (Chinese-hamster ovary K1) cells [17,22], suggesting that appropriate regulation of iPLA2 activity is necessary for cell cycle progression. Previous studies in the INS-1 insulinoma cells indicated that disruption of G1-phase phospholipid turnover by inhibition of iPLA2 induced G1-phase arrest in a p53 tumour suppressor-dependent manner [23]. We examined whether growth suppression in ovarian cancer cells by inhibition of iPLA2 was also mediated by cell cycle arrest. Ovarian cancer cell lines were incubated with 2 μM BEL or vehicle in serum-free medium for 1–3 days. The cells were harvested on a daily basis for cell cycle analysis by flow cytometry. In contrast with previous studies in INS-1 cells [23], BEL treatment did not induce G1 arrest in these ovarian cancer cell lines. Instead, we observed marked accumulation of cells in S- and G2/M-phases accompanied by a concordant decrease in G1 populations in OVCAR-3 (Figure 3B), SKOV-3 (Figure 3C) and Dov-13 (results not shown). In the absence of BEL, OVCAR-3 and SKOV-3 showed a majority of cells in G1-phase (70–75%) and low percentages of populations in S-phase (15–20%) and G2/M-phase (approx. 10%) after 2–3 days in culture (Figures 3B and 3C). The results demonstrate that iPLA2 activity is required for the growth-factor-independent cell cycle progression through S- and G2/M-phases.
The G1 cell cycle arrest induced by inhibition of iPLA2 in the INS-1 insulinoma cells is dependent on the function of the p53 tumour suppressor gene [23]. OVCAR-3 and SKOV-3 are deficient in p53 [28,29], which may explain the lack of G1 arrest in these cells treated with BEL. We therefore extended to examine other ovarian cancer cell lines known to have normal p53 such as OVCA-420 and OVCA-433 [28,29]. Cell cycle analysis indicated that treatment of these two p53-positive ovarian cancer cell lines with BEL also led to cell cycle arrest in S- and G2/M-phases, but not in G1 (Figure 3C), confirming a p53-independent effect of iPLA2 on cell cycle transit through S- and G2/M-phases.
Consistent with the significant accumulation of cells in S- and G2/M-phases in BEL-treated cells, immunoblotting analysis showed increases in expression levels of S-phase and G2/M-phase-associated cell cycle regulators such as cyclin B and cyclin E (Figure 3D) [30,31]. SKOV-3 and OVCAR-3 cells showed low levels of cyclin D1 that were not altered by treatment with BEL (results not shown). We also observed down-regulation of c-Myc levels in BEL-treated cells (Figure 3D). Since c-Myc expression is highest at late G1 promoting G1-to S-transition [30], the decreased expression of c-Myc seen in BEL-treated cells may reflect the partial loss of G1 cells following inhibition of iPLA2.
The S-phase arrest is released by supplying exogenous growth factors
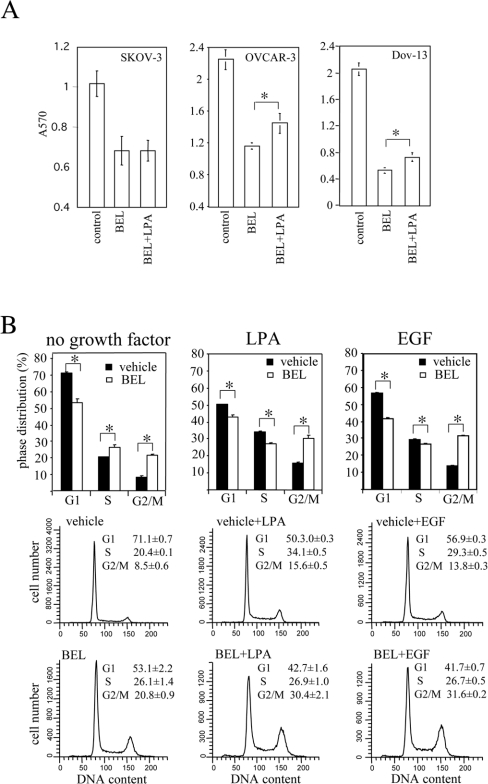
In addition to the membrane phospholipid turnover, iPLA2 may also participate in generating lysophospholipids, particularly LPA, which is a potent growth factor towards ovarian cancer cells [2,7–11]. Inhibition of iPLA2 activity may block autocrine production of LPA, leading to loss of replicative stimulation and growth arrest at S- and G2/M-phases. To assess this possibility, we added LPA to cultures to determine whether LPA could rescue ovarian carcinoma cells from BEL-induced growth arrest. As shown in Figure 4(A), LPA had a weak reversal effect on BEL-mediated growth suppression in OVCAR-3 and Dov-13 cells but had no effect in SKOV-3 cells, suggesting that potential inhibition of LPA production by BEL is not likely to be the major determinant of BEL-induced growth repression of ovarian cancer cells.
Figure 4. The effect of iPLA2 inhibition on growth and cell cycle progression of ovarian cancer cells maintained with exogenous growth stimuli.
(A) Exogenous LPA failed to reverse the inhibitory effect of BEL on cell division. SKOV-3, OVCAR-3 and Dov-13 cells were incubated for 2 days with BEL (2 μM) in serum-free medium supplemented with 10 μM LPA (BEL+LPA) or without LPA (BEL). The growth of SKOV-3 and OVCAR-3 cells under these experimental conditions was assayed as described in Figure 1. (B) The S-phase arrest, but not the G2/M arrest was released by LPA or EGF. OVCAR-3 cells were treated with or without BEL (2 μM) for 24 h before addition of LPA (10 μM), EGF (50 ng/ml) or no exogenous growth factor. After a further incubation for 42 h, the cells were stained with propidium iodide for flow cytometric analysis.
We next asked whether BEL inhibited cell cycle progression at S and G2/M transit when exogenous growth factors such as LPA or EGF were present. To this end, we added LPA or EGF to OVCAR-3 cells that had been arrested in S- and G2/M-phases by BEL (Figure 4B). Cell cycle parameters were analysed 42 h after addition of EGF or LPA. As shown in Figure 4(B), LPA and EGF were each able to release the S-phase accumulation in BEL-treated cells. However, BEL remained effective in arresting cells at G2/M in cultures supplemented with LPA or EGF. The control cells maintained without exogenous growth factor were arrested by BEL in both S- and G2/M-phases (Figure 4B).
Inhibition of iPLA2 suppresses growth factor- or serum-stimulated mitogenesis through cell cycle arrest in G2/M-phase
To address whether the G2/M arrest enforced by inhibition of iPLA2 also suppresses the growth factor-driven proliferation of ovarian cancer cells, we incubated OVCAR-3 cells in medium supplemented with EGF or LPA. As shown in Figure 5(A), EGF- and LPA-induced proliferation of OVCAR-3 cells was strongly decreased by addition of 2 μM BEL (Figure 5A). Cell cycle analysis indicated that in the presence of EGF or LPA, OVCAR-3 cells were growth arrested at G2/M by treatment with BEL (results not shown), similar to the results presented in Figure 4(B).
Figure 5. BEL suppression of growth factor- and serum-stimulated proliferation of ovarian cancer cells through cell cycle arrest in the G2/M-phase.
(A) Inhibition of iPLA2 with BEL suppressed LPA, EGF or FBS-stimulated growth. OVCAR-3 cells were incubated in medium supplemented with LPA (10 μM), EGF (50 ng/ml) or FBS (10%) in the presence or absence of indicated concentrations of BEL (2 μM for LPA and EGF and 15 μM for FBS). The growth of cells was assayed with Crystal Violet staining as described in Figure 1 or by quantification of cell numbers with a Coulter counter. (B) Inhibition of iPLA2 with BEL induced cell cycle arrest at G2/M in OVCAR-3 cells grown with serum. The cells were incubated in the complete medium containing 10% FBS with BEL (15 μM) or vehicle for 42 h before cell cycle analysis.
We next determined whether inhibition of iPLA2 suppresses the growth of ovarian cancer cells maintained in the complete medium containing 10% FBS. BEL, which at 1–2 μM strongly inhibited division of ovarian cancer cell lines under serum-free conditions or in LPA- or EGF-supplemented medium, had little or minimum effect in ovarian cancer cell lines maintained in the complete medium (results not shown), consistent with reduced stability and/or efficacy of BEL in the presence of serum. However, 10–20 μM BEL, a concentration range similar to that used by others in previous studies [22–24], strongly inhibited the proliferation of OVCAR-3 (Figure 5A) and other ovarian cancer cell lines. Similar to the cells grown with LPA or EGF, OVCAR-3 cells supported by serum were arrested at G2/M by the high doses of BEL (Figure 5B). Thus inhibition of cellular iPLA2 activity suppresses growth factor or serum-stimulated propagation of ovarian cancer cells through a G2/M checkpoint mechanism.
siRNA knockdown of iPLA2β expression decreases tumorigenicity of ovarian cancer cells in nude mice
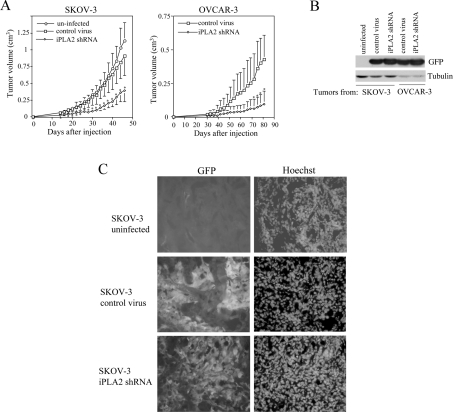
To explore further the biological significance of iPLA2 in regulation of proliferation of ovarian cancer cells in vivo, we examined the effect of down-regulation of iPLA2 on tumorigenicity of SKOV-3 and OVCAR-3 cells. Dov-13 cells do not grow in nude mice [32], precluding evaluation of the in vivo effect of iPLA2 down-regulation on this line. Expression of iPLA2β was stably decreased by lentivirus-mediated shRNA as demonstrated in Figure 2(B). As demonstrated in Figure 6(A), uninfected SKOV-3 cells and SKOV-3 cells infected with control virus carrying a non-targeting sequence were highly tumorigenic in nude mice after subcutaneous injection. However, the cells infected with iPLA2β shRNA virus became less tumorigenic as reflected by reduced tumour volumes and tumour growth rate (Figure 6). iPLA2β knockdown also significantly decreased the tumorigenicity of OVCAR-3 cells in nude mice (Figure 6A). The co-expressed GFP was present in the tumours derived from virus-infected cells as demonstrated by Western blot analysis (Figure 6B) and fluorescence microscopy (Figure 6C), confirming the long-term stable expression of the shRNA in vivo. These results indicate that inhibition of iPLA2 could offer a novel strategy to control tumour growth and progression in vivo.
Figure 6. Inhibition of tumorigenicity of ovarian cancer cell lines by shRNA knockdown of iPLA2β expression.
SKOV-3 and OVCAR-3 cells were infected with lentivirus carrying non-target control or iPLA2β-specific shRNA. These infected cells and uninfected SKOV-3 cells were injected subcutaneously on the right flank of Balb/c nude mice (four mice/group). Shown are volumes of tumours (mean±S.D.) grown from uninfected SKOV-3 cells, control virus-infected cells and the iPLA2β shRNA virus-infected cells (A). The statistical significances of differences in tumorigenicity between iPLA2β knockdown cells and control virus-infected cells are indicated with an asterisk if P<0.05. The expression of GFP in tumours derived from the virus-infected cells was confirmed by Western blot analysis (B) and by fluorescence microscopy (C).
DISCUSSION
In the present study, we demonstrated that iPLA2 activity is required for cell cycle progression and tumorigenesis of ovarian cancer cells. Inhibition of iPLA2 activity with the irreversible and mechanism-based specific inhibitor BEL [13] strongly blocked the growth-factor-independent and stimulated proliferation of ovarian cancer cells. In the absence of exogenous stimuli, inhibition of iPLA2 induced cell cycle arrest in S- and G2/M-phases. However, the S-phase accumulation could be reversed by providing a growth factor such as LPA or EGF in culture. When ovarian cancer cells are maintained with the support of a defined growth factor or serum, inhibition of iPLA2 remained effective in arresting cells in G2/M-phase, leading to efficient suppression of cell division. Under these experimental conditions, we noticed that inhibition of iPLA2 also induced apoptotic cell death albeit modestly, which may contribute to the observed growth suppression associated with iPLA2 inhibition. Furthermore, intact iPLA2 activity seems to be crucial in maintaining proliferation and survival of ovarian cancer cells in vivo as stable siRNA down-regulation of iPLA2 expression dramatically decreased tumorigenicity of ovarian cancer cell lines in athymic nude mice.
Phosphatidylcholine is a major membrane phospholipid in mammalian cells [33]. During the cell cycle, cells must double their phospholipid mass and reassemble the membrane to form daughter cells. Therefore the metabolism of phospholipids must be co-ordinately regulated throughout the cell cycle, which is achieved by the opposing actions of CTP:phosphocholine cytidylyltransferase and iPLA2 [34,35]. The phospholipid turnover rate is high in the G1-phase and decreases during the S-phase, which coincides with the doubling of PC mass [17]. Recently, Zhang et al. [23] reported that iPLA2 activity was essential for the entry of G1-phase cells into the S-phase. Blockade of iPLA2-mediated phospholipid turnover induced cell cycle arrest in G1 in the INS-1 insulinoma cells in a p53-dependent manner [23]. In ovarian cancer cell lines, however, we consistently observed suppression of cell cycle in G2/M- and S-phases, but not in G1-phase upon inhibition of iPLA2 activity in both p53-positive and p53-negative cell lines. It is apparent that the p53 status is an unlikely determinant of the unique pattern of cell cycle arrest in our model system. We cannot exclude the possibility that the mode of iPLA2 regulation of the cell cycle is cell type-specific. Indeed, Atsumi et al. [36] reported that iPLA2 inhibitors suppress Fas- and TNF (tumour necrosis factor)-induced apoptosis of the U937 human monocytic leukaemia cells, while overexpression of a highly active variant of iPLA2 suppresses proliferation of HEK-293 cells.
We tested the effect of BEL on replication of ovarian cancer cell lines maintained in a medium supplemented with FBS. It required >5-fold higher concentrations of BEL to achieve a similar magnitude of growth inhibition compared with cells cultured under serum-free conditions or in a medium chemically defined with growth factors such as EGF or LPA. The requirement of high levels of BEL for growth inhibition may reflect a decreased stability and/or efficacy of BEL in the presence of serum components. In addition, serum contains high levels of lysophospholipids such as lysophosphatidylcholine that can be utilized as a substrate for PC synthesis to affect membrane phospholipid metabolism as seen in cells cultured in choline-deficient medium or treated with CTP:phosphocholine cytidylyltransferase inhibitors [37,38]. Furthermore, activity or expression of enzymes involved in phospholipid homoeostasis, such as CTP:phosphocholine cytidylyltransferase and other isoforms of PLA2, may be modulated by sterol or diverse serum factors in compensation for enforced inhibition of iPLA2 activity [24,39].
The findings described in our study suggest that iPLA2 activity is especially required for S- and G2/M-phase transition, which is consistent with the fact that iPLA2 activity is low at the G1/S border and reaches a peak at late S- and G2/M-phases [17,22]. Our results of experiments in nude mice suggest that iPLA2 activity is also necessary for cell cycle progression in vivo. It is yet to be determined how inhibition of iPLA2 activity culminates in cell cycle arrest in G2/M- or S-phases. Since iPLA2 is involved in generation of LPA, a potent lysophospholipid growth/survival factor in ovarian cancer [2,7–11], inhibition of iPLA2 may cause discontinuation of autocrine LPA production in ovarian cancer cells. Other investigators reported that BEL suppresses LPA production in ovarian cancer cells [7,8]. However, exogenous LPA was incapable of rescuing ovarian cancer cell lines from BEL-mediated growth inhibition in spite of the prominent effect on the S-phase transit. Taken together, these results indicate that the role of iPLA2 in cell division is more than the generation of lysophospholipid growth factors. Most likely, inhibition of iPLA2 may induce alterations in the contents and function of membrane phospholipids that could trigger activation of intracellular signalling pathways to halt cell cycle progression.
Acknowledgments
We thank Dr Rick Schnellmann (Medical University of South Carolina) for providing anti-iPLA2γ antibody. This work has been supported in part by the NIH (National Institutes of Health)/NCI (National Cancer Institute) grant CA102196 (X. F.), the U.S. Department of Defense career development award W81XWH0410103 (X. F.), the NSF (National Science Federation) grant MCB 0212213 (S. E. B.) and the Massey Cancer Center pilot project grant (X. F. and S. E. B.). P. W. was supported by an individual predoctoral fellowship from NSF (F31NS051090). The flow cytometry facility has been supported in part by the NIH grant P30 CA16059 to Massey Cancer Center.
References
- 1.Sherbet G. V., Patil D. Genetic abnormalities of cell proliferation, invasion and metastasis, with special reference to gynaecological cancers. Anticancer Res. 2003;23:1357–1371. [PubMed] [Google Scholar]
- 2.Fang X., Schummer M., Mao M., Yu S., Tabassam F. H., Swaby R., Hasegawa Y., Tanyi J. L., LaPushin R., Eder A., et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim. Biophys. Acta. 2002;1582:257–264. doi: 10.1016/s1388-1981(02)00179-8. [DOI] [PubMed] [Google Scholar]
- 3.Conover C. A., Hartmann L. C., Bradley S., Stalboerger P., Klee G. G., Kalli K. R., Jenkins R. B. Biological characterization of human epithelial ovarian carcinoma cells in primary culture: the insulin-like growth factor system. Exp. Cell Res. 1998;238:439–449. doi: 10.1006/excr.1997.3861. [DOI] [PubMed] [Google Scholar]
- 4.Hirte H. W., Kaiser J. S., Bacchetti S. Establishment and characterization of four human epithelial ovarian carcinoma cell lines. Cancer. 1994;74:900–906. doi: 10.1002/1097-0142(19940801)74:3<900::aid-cncr2820740317>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Gotlieb W. H., Bruchim I., Gu J., Shi Y., Camirand A., Blouin M. J., Zhao Y., Pollak M. N. Insulin-like growth factor receptor I targeting in epithelial ovarian cancer. Gynecol. Oncol. 2006;100:389–396. doi: 10.1016/j.ygyno.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Stromberg K., Collins T. J., IV, Gordon A. W., Jackson C. L., Johnson G. R. Transforming growth factor-α acts as an autocrine growth factor in ovarian carcinoma cell lines. Cancer Res. 1992;52:341–347. [PubMed] [Google Scholar]
- 7.Sengupta S., Xiao Y. J., Xu Y. A novel laminin-induced LPA autocrine loop in the migration of ovarian cancer cells. FASEB J. 2003;17:1570–1582. doi: 10.1096/fj.02-1145fje. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X., Wang D., Zhao Z., Xiao Y., Sengupta S., Xiao Y., Zhang R., Lauber K., Wesselborg S., Feng L., et al. Caspase-3-dependent activation of calcium-independent phospholipase A2 enhances cell migration in non-apoptotic ovarian cancer cells. J. Biol. Chem. 2006;281:29357–29368. doi: 10.1074/jbc.M513105200. [DOI] [PubMed] [Google Scholar]
- 9.Shen Z., Belinson J., Morton R. E., Xu Y., Xu Y. Phorbol 12-myristate 13-acetate stimulates lysophosphatidic acid secretion from ovarian and cervical cancer cells but not from breast or leukemia cells. Gynecol. Oncol. 1998;71:364–368. doi: 10.1006/gyno.1998.5193. [DOI] [PubMed] [Google Scholar]
- 10.Luquain C., Singh A., Wang L., Natarajan V., Morris A. J. Role of phospholipase D in agonist-stimulated lysophosphatidic acid synthesis by ovarian cancer cells. J. Lipid Res. 2003;44:1963–1975. doi: 10.1194/jlr.M300188-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Eder A. M., Sasagawa T., Mao M., Aoki J., Mills G. B. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin. Cancer Res. 2000;6:2482–2491. [PubMed] [Google Scholar]
- 12.Six D. A., Dennis E. A. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim. Biophys. Acta. 2000;1488:1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 13.Hazen S. L., Zupan L. A., Weiss R. H., Getman D. P., Gross R. W. Suicide inhibition of canine myocardial cytosolic calcium-independent phospholipase A2. J. Biol. Chem. 1991;266:7227–7232. [PubMed] [Google Scholar]
- 14.Seno K., Okuno T., Nishi K., Murakami Y., Watanabe F., Matsuura T., Wada M., Fujii Y., Yamada M., Ogawa T., et al. Pyrrolidine inhibitors of human cytosolic phospholipase A2. J. Med. Chem. 2000;43:1041–1044. doi: 10.1021/jm9905155. [DOI] [PubMed] [Google Scholar]
- 15.Fang X., Yu S., Bast R. C., Liu S., Xu H. J., Hu S. X., LaPushin R., Claret F. X., Aggarwal B. B., Lu Y., Mills G. B. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J. Biol. Chem. 2004;279:9653–9661. doi: 10.1074/jbc.M306662200. [DOI] [PubMed] [Google Scholar]
- 16.Lee Z., Swaby R. F., Liang Y., Yu S., Liu S., Lu K. H., Bast R. C., Jr, Mills G. B., Fang X. Lysophosphatidic acid is a major regulator of growth-regulated oncogene α in ovarian cancer. Cancer Res. 2006;66:2740–2748. doi: 10.1158/0008-5472.CAN-05-2947. [DOI] [PubMed] [Google Scholar]
- 17.Manguikian A. D., Barbour S. E. Cell cycle dependence of group VIA calcium-independent phospholipase A2 activity. J. Biol. Chem. 2004;279:52881–52892. doi: 10.1074/jbc.M410659200. [DOI] [PubMed] [Google Scholar]
- 18.Dole V. P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J. Clin. Invest. 1956;35:150–154. doi: 10.1172/JCI103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubinson D. A., Dillon C. P., Kwiatkoski A. V. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat. Genet. 2003;33:401–406. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 20.Hu W. H., Pendergast J. S., Mo X. M., Brambilla R., Bracchi-Ricard V., Li F., Walters W. M., Blits B., He L., Schaal S. M., Bethea J. R. NIBP, a novel NIK and IKKβ-binding protein that enhances NF-κB activation. J. Biol. Chem. 2005;280:29233–29241. doi: 10.1074/jbc.M501670200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang J., Kriz R. W., Wolfman N., Shaffer M., Seehra J., Jones S. S. A novel cytosolic calcium-independent phospholipase A2 contains eight ankyrin motifs. J. Biol. Chem. 1997;272:8567–8575. doi: 10.1074/jbc.272.13.8567. [DOI] [PubMed] [Google Scholar]
- 22.Roshak A. K., Capper E. A., Stevenson C., Eichman C., Marshall L. A. Human calcium-independent phospholipase A2 mediates lymphocyte proliferation. J. Biol. Chem. 2000;275:35692–35698. doi: 10.1074/jbc.M002273200. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X. H., Zhao C., Seleznev K., Song K., Manfredi J. J., Ma Z. A. Disruption of G1-phase phospholipid turnover by inhibition of calcium-independent phospholipase A2 induces a p53-dependent cell-cycle arrest in G1 phase. J. Cell Sci. 2006;19:1005–1015. doi: 10.1242/jcs.02821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez T., Moreno J. J. The effect of high molecular phospholipase A2 inhibitors on 3T6 fibroblast proliferation. Biochem. Pharmacol. 2001;61:811–816. doi: 10.1016/s0006-2952(01)00555-x. [DOI] [PubMed] [Google Scholar]
- 25.Fuentes L., Perez R., Nieto M. L., Balsinde J., Balboa M. A. Bromoenol lactone promotes cell death by a mechanism involving phosphatidate phosphohydrolase-1 rather than calcium-independent phospholipase A2. J. Biol. Chem. 2003;278:44683–44690. doi: 10.1074/jbc.M307209200. [DOI] [PubMed] [Google Scholar]
- 26.Balsinde J., Balboa M. A. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell. Signalling. 2005;17:1052–1062. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Saavedra G., Zhang W., Peterson B., Cummings B. S. Differential roles for cytosolic and microsomal Ca2+-independent phospholipase A2 in cell growth and maintenance of phospholipids. J. Pharmacol. Exp. Ther. 2006;318:1211–1219. doi: 10.1124/jpet.106.105650. [DOI] [PubMed] [Google Scholar]
- 28.Havrilesky L. J., Elbendary A., Hurteau J. A., Whitaker R. S., Rodriguez G. C., Berchuck A. Chemotherapy-induced apoptosis in epithelial ovarian cancers. Obstet. Gynecol. 1995;85:1007–1010. doi: 10.1016/0029-7844(95)00058-y. [DOI] [PubMed] [Google Scholar]
- 29.Gibb R. K., Taylor D. D., Wan T., O'Connor D. M., Doering D. L., Gercel-Taylor C. Apoptosis as a measure of chemosensitivity to cisplatin and taxol therapy in ovarian cancer cell lines. Gynecol. Oncol. 1997;65:13–22. doi: 10.1006/gyno.1997.4637. [DOI] [PubMed] [Google Scholar]
- 30.Sherr C. J. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- 31.Porter L. A., Donoghue D. J. Cyclin B1 and CDK1: nuclear localization and upstream regulators. Prog. Cell Cycle Res. 2003;5:335–347. [PubMed] [Google Scholar]
- 32.Hu L., Hofmann J., Lu Y., Mills G. B., Jaffe R. B. Inhibition of phosphatidylinositol 3′-kinase increases efficacy of paclitaxel in in vitro and in vivo ovarian cancer models. Cancer Res. 2002;62:1087–1092. [PubMed] [Google Scholar]
- 33.Lykidis A., Jackowski S. Regulation of mammalian cell membrane biosynthesis. Prog. Nucleic Acid Res. Mol. Biol. 2001;65:361–393. doi: 10.1016/s0079-6603(00)65010-9. [DOI] [PubMed] [Google Scholar]
- 34.Barbour S. E., Kapur A., Deal C. L. Regulation of phosphatidylcholine homeostasis by calcium-independent phospholipase A2. Biochim. Biophys. Acta. 1999;1439:77–88. doi: 10.1016/s1388-1981(99)00078-5. [DOI] [PubMed] [Google Scholar]
- 35.Baburina I., Jackowski S. Cellular responses to excess phospholipid. J. Biol. Chem. 1999;274:9400–9408. doi: 10.1074/jbc.274.14.9400. [DOI] [PubMed] [Google Scholar]
- 36.Atsumi G., Murakami M., Kojima K., Hadano A., Tajima M., Kudo I. Distinct roles of two intracellular phospholipase A2s in fatty acid release in the cell death pathway. J. Biol. Chem. 2000;275:18248–18258. doi: 10.1074/jbc.M000271200. [DOI] [PubMed] [Google Scholar]
- 37.Cui Z., Houweling M., Chen M. H., Record M., Chap H., Vance D. E., Terce F. A genetic defect in phosphatidylcholine biosynthesis triggers apoptosis in Chinese hamster ovary cells. J. Biol. Chem. 1996;271:14668–14671. doi: 10.1074/jbc.271.25.14668. [DOI] [PubMed] [Google Scholar]
- 38.Cui Z., Houweling M. Phosphatidylcholine and cell death. Biochim. Biophys. Acta. 2002;1585:87–96. doi: 10.1016/s1388-1981(02)00328-1. [DOI] [PubMed] [Google Scholar]
- 39.Seashols S. J., del Castillo Olivares A., Gil G., Barbour S. E. Regulation of group VIA phospholipase A2 expression by sterol availability. Biochim. Biophys. Acta. 2004;1684:29–37. doi: 10.1016/j.bbalip.2004.05.003. [DOI] [PubMed] [Google Scholar]