Abstract
The identification of gene promoter methylation is a useful tool for the molecular diagnosis of human diseases. We have developed a new PCR-based technique for detecting the methylation status of CpG islands of gene promoters. This new method, named methyl-sensitive dimethyl sulfoxide-PCR (Ms-DMSO-PCR), is based on the finding that methylated and unmethylated DNAs show a different sensitivity to the amount of DMSO used in the PCR reaction. For the amplification of methylated DNA, more DMSO is required in comparison to unmethylated DNA. This finding resulted in the development of a simple PCR screening of CpG islands with addition of DMSO in the range from 0 to 8% (v/v), and the same pair of primers is sufficient for distinguishing hyper- or hypomethylated gene promoters from normally methylated sequences. This new technique is a one-step procedure and does not require any modifications of DNA or expensive equipment. Therefore, Ms-DMSO-PCR has the potential to be widely used for clinical applications as well in basic research.
Changes in gene expression occur in many physiological and pathological conditions including carcinogenesis.1,2 The main epigenetic event responsible for these changes is the alteration of the DNA methylation pattern. Both local increases in DNA methylation and global decreases in genomic methylation are extremely common in human cancer.3 In particular, the hypermethylation of CG-rich promoter regions of tumor suppressor genes is a frequent event in carcinogenesis.4,5 Although the DNA hypermethylation causing gene silencing and tumor progression has been intensively studied,4,5 the role of demethylation is only beginning to be clarified. Cancer-associated aberrant demethylation has been detected in DNA sequences that are methylated in normal tissues and the hypomethylation of repeated sequences in genome has been shown to increase the homologous recombination and rearrangements of chromosomes.6 Demethylation also changes expression of imprinted genes, tissue-specific genes, proto-oncogenes, and genes associated with invasion and metastases.6 Evidence is therefore accumulating that not only local hypermethylation but also global demethylation are involved in the neoplastic process.
The increased interest for DNA methylation has led to the development of many sophisticated techniques. Currently, two main methods are used to study the degree of DNA methylation: nonbisulfite and bisulfite methods. The first relies on the digestion of DNA with methylation-sensitive restriction enzymes followed by Southern blot or PCR. The second technique is based on the bisulfite modification of tested DNA. Bisulfite converts unmethylated cytosine to uracil, whereas methylated cytosine remains unaltered. Subsequently, the modified DNA can be analyzed by different methods including methyl-sensitive PCR, combined bisulfite restriction analyses, methylation-sensitive single nucleotide primer extension, methylation-sensitive single-stranded conformational polymorphism, and others.7
This article describes a new technique, the methylation-sensitive dimethyl sulfoxide-PCR (Ms-DMSO-PCR), which allows the one-step methylation analysis of the entire CpG island of gene promoter region. The developed method is quick, inexpensive, and does not need any DNA manipulation before the test. It is based on the different sensitivity of methylated and unmethylated DNAs to the amount of DMSO used in PCR reaction. We developed and tested the new method using DAPK, RASSF1A, TIMP3, and MGMT gene promoters, which are known as methylation markers of cancer. DAPK is a positive mediator of apoptosis, and the methylation of its gene promoter has been associated with early recurrence of tumors.8 The hypermethylation of MGMT, a gene involved in repair of O6-methylguanine, has been shown to be a useful predictor of the responsiveness of tumors to alkylating agents,9 whereas the methylation of RASSF1A in serum DNA is associated with poor cancer prognosis.10
Materials and Methods
Cell Culture and Tissues
Cervical cancer-derived cell lines C33 [human papillomavirus (HPV)-negative], SiHa, and CaSki (containing 1 copy and 500 copies of HPV16 genome, respectively) were grown under standard conditions.11 Normal human keratinocytes were isolated from biopsies of uterine cervix and cultivated as described before.11 Frozen biopsy specimens, cell scrapings, and formalin-fixed, paraffin-embedded tissue (FFPE) corresponding to cervical squamous cell carcinoma (SCC) and normal tissues were retrieved from the Tumor Bank of the University Hospital of Liege. The histological and cytological diagnoses were confirmed after hematoxylin and eosin and Papanicolaou staining.
Treatment with 5-Aza-2′-Deoxycytidine
SiHa cells (1 × 105) were seeded in 6-cm tissue dishes. One hundred μmol/L 5-aza-dC (Sigma-Aldrich, Schnelldorf, Germany) in DMSO (Sigma-Aldrich) was added the next day. For control, cells were mock-treated with the identical volume of DMSO. The cells were cultured for 6 days, and the media with 5-aza-dC or DMSO were changed every second day. At the end, cells were collected and chromosomal DNA was isolated.
DNA Isolation
Chromosomal DNA from cell lines, cervical scrapes, frozen biopsies, and FFPE blocks were isolated using QIAmp DNA mini kit (Qiagen, Venlo, The Netherlands) according to the manufacturer’s recommendations. After purification DNA was extracted with phenol-chloroform, precipitated with ethanol, and dissolved in water.
Methylation in Vitro
Methylation in vitro was performed with site-specific SssI (CG) methylase and S-adenosylmethionine (New England Biolabs, Leusden, The Netherlands). Two μg of DNA were methylated in 50 μl of reaction mixture containing 6 U of SssI and 640 μmol/L S-adenosylmethionine at 37°C for 30, 60, or 120 minutes. Methylation efficiency was monitored by HpaII digestion and gel electrophoresis. The DNA was purified by phenol extraction followed by ethanol precipitation and dissolved in water.
Restriction of DNA with MspI/HpaII Endonucleases
Two hundred μg of genomic DNA were digested with restriction endonucleases in 20 μl of reaction mixture containing 10 U of MspI or HpaII (both from Fermentas, St. Leon-Rot, Germany) at 37°C for 16 hours. Restriction digestion was checked by gel electrophoresis. One μl of reaction mixture was taken for PCR. PCR was performed in 25 μl containing 50 pmol of each primer, 6% DMSO, and 0.5 U of Taq polymerase (Fermentas). Thermocycling included 30 cycles at annealing temperature of 58°C for 30 seconds and extension at 72°C for 45 seconds. Five μl of PCR mixtures were analyzed by 2% agarose gel electrophoresis. The sequences of primers for PCR are shown in Table 1.
Table 1.
Primer Sequences
| Gene name | GenBank no. | Primers and annealing temperature (Tann) | Location* | Size† (bp) |
|---|---|---|---|---|
| Death-associated protein kinase 1 | NT_023935.17 | F:5′-CAGTACACATCTCGGGACGG-3′ | −371+24 | 395 |
| (DAPK) | R:5′-GTCCCCGGCGTTGGCTCG-3′ | |||
| Tann = 58°C | ||||
| O-6-methylguanine-DNA | AL355531 | F:5′-GGTCGCTTGCACGCCCGC-3′ | −435−102 | 333 |
| methyltransferase (MGMT) | R:5′-AAGAGAGCGCGCGGGGGC-3′ | |||
| Tann = 70°C | ||||
| Ras association domain family 1, | NM_007182 | F:5′-CGCTGAAGTCGGGGCCCG-3′ | +45+371 | 326 |
| transcript variant A (RASSF1A) | R:5′-CAGGCCTTTGCGCACGACG-3′ | |||
| Tann = 62°C | ||||
| Tissue inhibitor of metalloproteinase 3 | NT_011520.10 | F:5′-CCCCAACAGCCCGAGGCG-3′ | +825+1192‡ | 367 |
| (TIMP3) | R:5′-TTGCCGCTGCTGCCGCCG-3′ | |||
| Tann = 63°C |
Location of CpG island to the start of transcription.
Size of PCR product.
Start of translation is at 1193 bp from start of transcription.
Ms-DMSO-PCR
The location of CpG islands in gene promoters, the sequences of primers, and their annealing temperatures for PCR are shown in Tables 1and 2. The DMSO-PCR was performed in 25 μl containing 1× Taq buffer with (NH4)2SO4, 0.2 mmol/L of each dNTP, 1.5 mmol/L MgCl2 (for DAPK, TIMP3, and RASSF1) or 2 mmol/L MgCl2 (for MGMT1), 0 to 8% DMSO, 5 to 15 ng of DNA template, 50 pmol of each primer, and 0.5 U Taq polymerase (Fermentas). PCR cycling conditions for all of the amplified fragments and the temperatures used for primer hybridization (Table 1) were the same: 95°C for 9 minutes, then 30 cycles with denaturation at 95°C for 30 seconds, annealing for 30 seconds, and polymerization at 72°C for 30 to 60 seconds. All the PCR products were analyzed by a 2% agarose gel electrophoresis.
Table 2.
Primers and Characterization of PCR Products of DAPK Promoter
| Size of PCR product (bp) | Location of PCR product to the start of transcription | Primer name and annealing temperature (Tann) | Number of CG in primer | Number of CG in PCR product | C + G content in PCR product (%) |
|---|---|---|---|---|---|
| 161 | −137 | Up 3 | 1 | 16 | 74.5 |
| +24 | Down 2 | 3 | |||
| Tann = 60°C | |||||
| 395 | −371 | Up 2 | 2 | 45 | 72.4 |
| +24 | Down 2 | 3 | |||
| Tann = 58°C | |||||
| 448 | −424 | Up 1 | 0 | 47 | 70.3 |
| +24 | Down 2 | 3 | |||
| Tann = 58°C | |||||
| 649 | −137 | Up 3 | 1 | 65 | 73.2 |
| +512 | Down 1 | 0 | |||
| Tann = 64°C | |||||
| 883 | −371 | Up 2 | 2 | 95 | 72.6 |
| +512 | Down 1 | 0 | |||
| Tann = 60°C | |||||
| 936 | −424 | Up 1 | 0 | 97 | 71.6 |
| +512 | Down 1 | 0 | |||
| Tann = 60°C |
Results
Direct Detection of DNA Methylation by Ms-DMSO-PCR
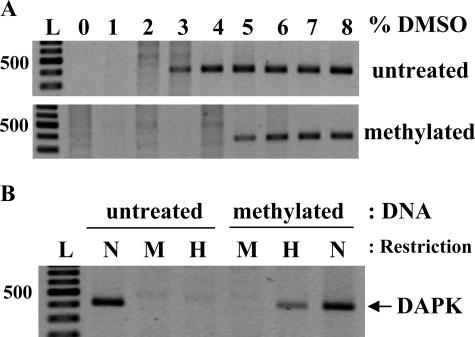
The DNA extracted from normal human keratinocytes was split in two parts. One remained untreated whereas the other one was methylated in vitro by SssI methylase as described in the Materials and Methods. In an attempt to find optimal PCR conditions for the amplification of DAPK promoter, we observed that methylated and untreated DNAs showed a different sensitivity to the amount of DMSO used in the PCR mixture (Figure 1A). For untreated DNA, PCR products appeared at 3% DMSO whereas at least 5% DMSO was necessary for the methylated DNA. The same results were observed with DNAs from C33 and CaSki cell lines (data not shown). To confirm that this different sensitivity to DMSO was related to methylation, we used an enzymatic method for detecting DNA methylation. After digestion of untreated and in vitro methylated DNAs with the methylation-sensitive enzyme HpaII and subsequent PCR, amplified products were observed only in the case of methylated DNA (Figure 1B).
Figure 1.
Analysis of DAPK promoter methylation in human cervical keratinocytes. Isolated DNA was methylated in vitro by SssI methylase and compared with the same untreated DNA preparation. A: Ms-DMSO-PCR: 15 ng of each DNA template were taken for each PCR reaction with the concentration of DMSO ranging from 0 to 8%. B: PCR of MspI- and HpaII-digested DNA. N, noncut DNA; M, MspI digest; H, HpaII digest; L, 100-bp DNA ladder (Fermentas).
Methylation Analysis of TIMP3, RASSF1A, and MGMT Gene Promoters
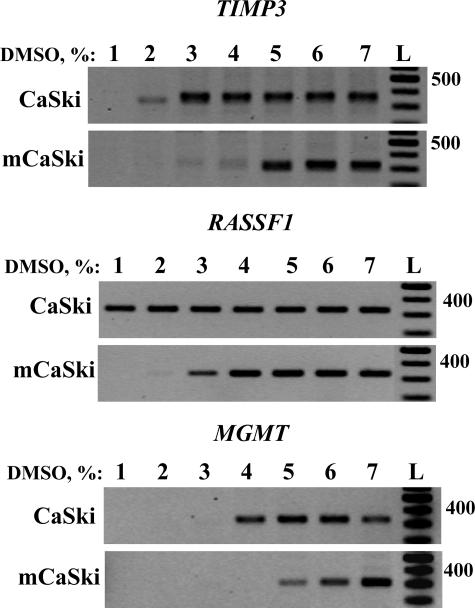
To verify whether this different sensitivity to DMSO is a general feature of methylated DNA, we performed Ms-DMSO-PCR of TIMP3, RASSF1A, and MGMT gene promoters. Chromosomal DNA was isolated from CaSki cells and methylated in vitro by SssI methylase. Again, we found that DNA methylation changed the sensitivity to DMSO for all tested promoters (Figure 2), although the amplified gene products appeared at different DMSO concentrations. Indeed, the PCR signal for the TIMP3 promoter started to be seen at 2% DMSO (Figure 2A), whereas those of RASSF1A (Figure 2B) and MGMT (Figure 2C) were detected at 1% and 4% DMSO, respectively. Interestingly in the case of methylated and untreated DNA for DAPK, TIMP3, and RASSF1A gene promoters, the difference in the sensitivity to DMSO was the same (∼2%). A lower difference in the sensitivity to DMSO of methylated and untreated DNA from CaSki cells was observed in the case of MGMT (Figure 2C). However, the promoter of MGMT gene was found to be already methylated in cancer cells,12 and additional methylation in vitro did not change significantly the promoter configuration.
Figure 2.
Ms-DMSO-PCR of TIMP3, RASSF1A, and MGMT gene promoters. CaSki cell DNA was methylated in vitro by SssI methylase and compared with the same untreated DNA preparation. Ten ng of each DNA template were taken for each PCR reaction with the concentration of DMSO ranging from 1 to 7%. L, 100-bp DNA ladder.
Influence of the Length or C+G Content of Amplified Fragments and Primer Design on Ms-DMSO-PCR
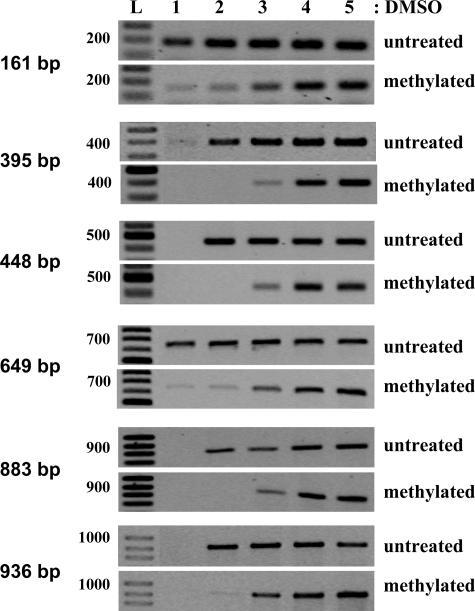
To understand the effect of DMSO in the amplification of methylated versus unmethylated DNA templates, we tested the method for DAPK promoter fragments ranging from 161 to 940 bp (Figure 3, Table 2). Although the amplified DNA fragments contained a high percentage of C+G because of their locations in CpG islands, they had a different number of CG nucleotides, the substrate for methylation in eukaryotes. In addition, the primers used for PCR contained or not CG sequences (Table 2). We found that DNA methylation changed the sensitivity to DMSO for all the tested fragments (Figure 4). The best results were observed for the fragments of 395, 448, 883, and 940 bp. For these PCR products, the amplification of untreated DNA started at 2% DMSO, and the intensity of the bands did not change with increased DMSO concentration. In contrast, the PCR signals for the methylated DNA started to be weakly seen at 3% DMSO and reached the maximal intensity at 4% DMSO (Figure 4). Interestingly, strong amplification signals of untreated DNA for the fragments of 161 bp (which is a part of the 395- and 448-bp fragments, Table 2) and 649 bp (part of 883- and 936-bp fragments) were detected at 1% DMSO. The PCR products of in vitro methylated DNA for the 161- and 648-bp fragments were weak at 1% DMSO, but the maximum intensity was detected at 4% DMSO as for the other tested methylated DNA fragments. In these experiments, we found that the differential sensitivity to DMSO was not linearly dependent on the C+G content of amplified fragments. As shown in Table 2 and Figure 3, the highest C+G content was in the short 161-bp fragment (74.5%), but the PCR signal started to be detected earlier than for the fragment of 448 bp which has only 70.3% C+G. In addition, we demonstrated that primers designed for the test do not need to contain CG dinucleotides. Accordingly, the primers used for amplification of the 936-bp fragment do not contain any CGs, whereas the primers allowing the amplification of the 395-bp fragment have two or three CGs (Figure 3, Table 2). Because the sensitivity to DMSO was the same for both fragments (Figure 4), DNA internal secondary structures of methylated and unmethylated DNA fragments are likely to be more critical for the DMSO effect than the presence of CG dinucleotides in primer sequences.
Figure 3.
Sequence region of the DAPK promoter. All potentially methylated CG sites are labeled with an asterisk. The primer sequences used for the amplification of different parts of the promoter and the starting point of transcription are indicated.
Figure 4.
Methylation analysis of different fragments of DAPK promoter. CaSki cell DNA was methylated in vitro by SssI methylase and compared with the same untreated DNA preparation. Ms-DMSO-PCR: 5 to 10 ng of each DNA template were taken for each PCR reaction with the concentration of DMSO ranging from 1 to 5%. L, 100-bp DNA ladder. Primers and their location in promoter region are shown in Figure 3 and Table 2.
Detection of Naturally Occurring DNA Methylation by Ms-DMSO-PCR
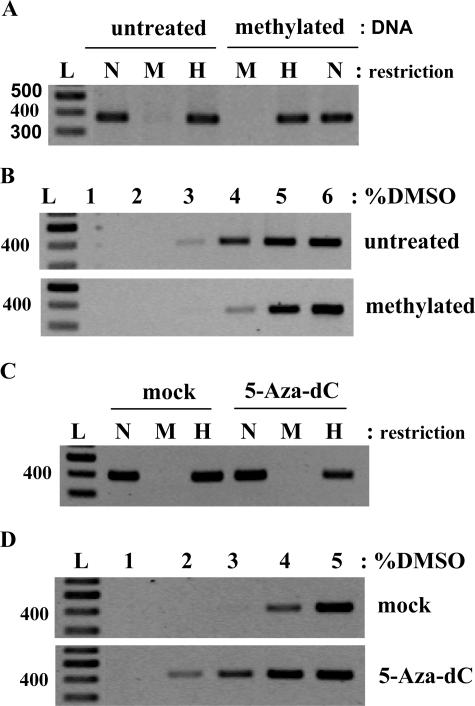
To demonstrate that this method can be used for detecting DNA methylated in vivo, Ms-DMSO-PCR of the 395-bp fragment of the DAPKpromoter was performed in native DNA extracted from SiHa cells. The methylation of DAPKpromoter in these cells was confirmed by the enzymatic method (Figure 5A). Agarose gel analysis of Ms-DMSO-PCR showed that amplification signal starts to be clearly seen at 4% and reached maximal intensity at 5% DMSO (Figure 5B), whereas the PCR products of the same fragment were detected at 3% DMSO for DNA isolated from normal keratinocytes (Figure 1A) and 2% DMSO for CaSki cells (Figure 4) and C33 cells (data not shown). There was no methylation of DAPK gene promoter detected by the enzymatic method in normal keratinocytes (Figure 1B), CaSki, and C33 cells (data not shown). To better characterize the level of DAPK promoter methylation in SiHa cells, we induced additional methylation of isolated DNA by SssI methylase. Interestingly, the Ms-DMSO-PCR technique showed a further shift of detected amplification signal toward a higher percentage of DMSO (Figure 5B), suggesting that the DAPK promoter is only partially or heterogeneously methylated in living SiHa cells. We also treated SiHa cells with an inhibitor of DNA methyl-transferases (5-aza-dC) for 6 days. Because 5-aza dC was dissolved in DMSO, the cells were mock-treated with the same amount of DMSO as control. After treatment with 5-aza-dC, a PCR product of DAPK promoter appeared at 2% DMSO (Figure 5D), whereas after treatment with DMSO alone the appearance of the PCR product was not changed in comparison to untreated cells (Figure 5, B and D). The methylation analysis of DAPK promoter from treated SiHa cells by the enzymatic method also confirmed the demethylation of DNA (Figure 5C). In this experiment we still observed some level of methylation by both used methods, but it should be mentioned that complete demethylation is difficult to obtain in vivo. These data therefore suggest that the same Ms-DMSO-PCR is able to detect not only the methylation of DNA induced by DNA methyl-transferases in living cells but also the demethylation of specific gene promoters.
Figure 5.
Analysis of DAPK promoter methylation in the SiHa cell line. A: PCR of MspI- and HpaII-digested DNA of methylated in vitro by SssI methylase and the same untreated preparation. N, Noncut DNA; M, MspI digest; H, HpaII digest; L, 100-bp DNA ladder. B: Ms-DMSO-PCR of the same samples: 10 ng of each DNA template were taken for each PCR reaction with the concentration of DMSO ranging from 1 to 6%. C: PCR of MspI- and HpaII-digested DNA from 5-aza-dC or mock-treated SiHa cells. N, Noncut DNA; M, MspI digest; H, HpaII digest; L, 100-bp DNA ladder. D: Ms-DMSO-PCR of the same samples: 10 ng of each DNA template were taken for each PCR reaction with the concentration of DMSO ranging from 1 to 5%.
Influence of Methylation Heterogeneity in DNA Samples on Ms-DMSO-PCR
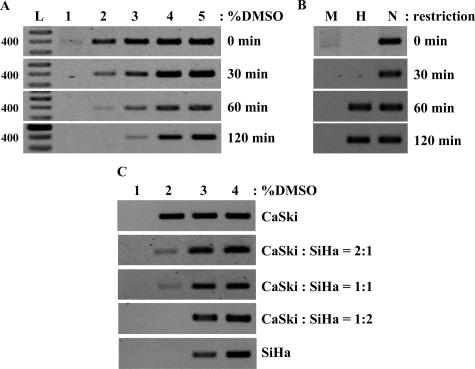
To understand the sensitivity of Ms-DMSO-PCR to methylation heterogeneity in DNA samples, we performed two sets of experiments (Figure 6). We first performed a time course in vitro methylation of DNA isolated from CaSki cells and analyzed DAPK promoter methylation by Ms-DMSO-PCR. After 30 minutes of methylation the shift in sensitivity to DMSO was not observed, but the intensity of PCR signal at 2% DMSO for methylated DNA was significantly lower compared to the unmethylated one (Figure 6A). DNA methylation was not detected by the enzymatic method in the same preparation (Figure 6B). A clear shift from to 2 to 3% DMSO appeared after 1 hour of methylation, and after 2 hours this shift increased to 4% DMSO (Figure 6A). The enzymatic method showed that DAPK promoter was methylated in both samples, but no differences in methylation level were observed (Figure 6B).
Figure 6.
Sensitivity of Ms-DMSO-PCR to time course methylation and detection of methylation in CaSki and SiHa DNA mixtures. A: Ms-DMSO-PCR of DAPK promoter of DNA from CaSki cells methylated in vitro for 0, 30, 60, and 120 minutes: 10 ng of each DNA template were taken for each PCR reaction with the concentration of DMSO ranging from 1 to 5%. B: PCR of MspI- and HpaII-digested DNA of the same samples. N, Noncut DNA; M, MspI digest; H, HpaII digest. C: Ms-DMSO-PCR of DAPK promoter from CaSki and SiHa DNA mixtures: 10 ng of each DNA template or mixture were taken for each PCR reaction with the concentration of DMSO ranging from 1 to 4%.
In a second set of experiments, we tested the ability of Ms-DMSO-PCR to detect the methylated gene against a background of the same, but unmethylated, gene. We mixed, in different proportions, the DNA isolated from CaSki cells (unmethylated DAPK promoter) with DNA isolated from SiHa cells (naturally partially methylated gene). As shown in Figure 6C, the PCR product was detected at 3% DMSO when the methylated gene was present in the DNA mixture at a percentage higher than 50%. When the proportion of methylated DNA was less than 50%, the strong PCR signal was detected also at 3% DMSO, but the weak signal corresponding to unmethylated gene was also detected at 2% DMSO.
Detection of DNA Hyper- and Hypomethylation in Clinical Samples
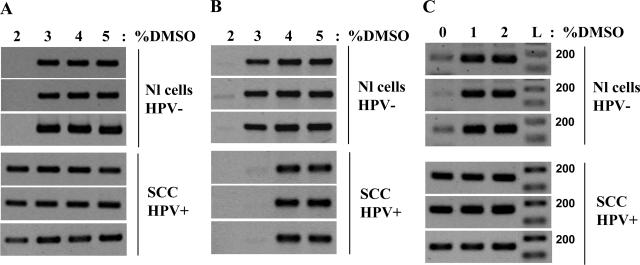
To check whether this method is also efficient in the analysis of clinical samples, we extracted chromosomal DNA from cervical scrapes and frozen biopsies and performed DMSO-PCR of DAPK and MGMT gene promoters. As shown in Figure 7, the DAPK promoter was found to be hypomethylated in cancer samples compared with normal tissues (Figure 7A), whereas MGMT1 promoter was hypermethylated at the same time (Figure 7B), demonstrating that cancer cells may have concomitant hyper- and hypomethylation of promoter regions of specific genes. The technique for DAPK, MGMT, RASSF1A, and TIMP3 gene promoters was also tested on DNA isolated from other tissues including thyroid and nervous system cancers and gave similar results (data not shown).
Figure 7.
Ms-DMSO-PCR of DAPK and MGMT promoters in clinical samples of the uterine cervix: 10 ng of each DNA template were taken for each PCR reaction with the concentration of DMSO ranging from 2 to 5% for frozen biopsies and 0 to 2% for FFPE tissue. Nl cells, HPV−, normal cells, HPV-negative; SCC HPV+, squamous cell carcinoma, HPV-positive. A: Demethylation of DAPK promoter in frozen biopsies of SCC. B: Methylation of MGMT promoter in frozen biopsies of SCC. C: Demethylation of DAPK promoter in FFPE tissues of SCC.
To test the possible application of the new method for the methylation analysis of DNA isolated from FFPE tissues, we extracted the DNA from cervical FFPE blocks and performed the Ms-DMSO-PCR of the DAPK promoter. Because extracted DNA was significantly degraded because of tissue fixation in formalin and the average size of DNA fragments was ∼200 bp, we amplified the 161-bp fragment of the DAPK promoter (Table 2). Ms-DMSO-PCR showed that this fragment of the DAPK promoter was hypomethylated in cancer samples compared with normal tissues (Figure 7C). These results were in agreement with the data obtained with DNA isolated from frozen biopsies (Figure 7A).
Discussion
In the last decade, many researchers reported that the methylation status of individual or multiple genes can be associated with clinically useful information, suggesting that the discovery of DNA methylation markers and development of new techniques for promoter methylation studies are of potential interest for the clinical diagnosis, prognosis, and therapeutics of cancer and other diseases.13
DMSO helps the melting of DNA template and the proper primer annealing. Therefore, it is widely used in PCR to improve the specificity and efficiency of the amplification.14 For the first time, we showed that DMSO can act differently depending on the secondary structure of DNA caused by methylation of CpG islands. The fact that cytosine methylation affects the melting of DNA structure is shown by the possibility to separate methylated and unmethylated DNA fragments by denaturing gradient gel electrophoresis.15 These differences of DNA structure caused by methylation can be easily monitored by performing PCR with different amounts of DMSO (from 0 to 8% v/v) and subsequent agarose gel electrophoresis of PCR products. This new method, named methyl-sensitive DMSO-PCR (Ms-DMSO-PCR), allows the fast methylation analysis of CpG islands of gene promoters.
To better characterize the chemical basis of the DMSO effect in the amplification efficiency of methylated DNA templates, we tested the influence of C+G content or length of amplicons and primer design on Ms-DMSO-PCR. All the analyzed DNA fragments contained a high percentage of C+G (70 to 74%) because they were included in CpG island of promoter area. However, the fragments with the highest C+G content (more than 73%) were efficiently amplified with lower percentages of DMSO and methylated DNA showed a slightly lower sensitivity to DMSO in comparison to fragments of the same gene promoter with 70 to 73% C+G. When this technique was tested for detecting methylation in promoters of different genes (DAPK, TIMP3, and RASSF1A), the difference in sensitivity to DMSO was the same (∼2%), although the PCR signals started to be seen at different DMSO concentrations. This different start of amplification probably reflects the level of promoter methylation in the cells and does not depend on the C+G-content of DNA. Accordingly, DAPK and TIMP3 gene promoters contain 72 and 77% GCs, but PCR signals started to be seen at 3 and 2% DMSO, respectively. In contrast, RASSF1A and MGMT gene promoters have the same CG content (75% CGs), but amplification started to be observed at 1 and 4% DMSO, respectively. These data suggest that the DNA secondary structure is more crucial for PCR efficiency than the simple calculation of C+G content and/or number of potentially methylated bases.
The application of modern methylation-sensitive techniques is restricted to the usage of short (up to 200 bp) DNA fragments and analysis of very few methylated cytosines, raising the question about the biological meaning of methylation of individual DNA bases and their role in epigenetic regulation of gene transcription. In our experiments we found that the new technique is applicable for fragments with lengths ranging from 161 to 936 bp, suggesting that short fragments as well as entire CpG islands may be analyzed with this method. There is accumulating evidence that methylation leads to global changes of promoter structures by recruiting complexes of methylation-binding proteins and histone deacetylases, which cause the blocking of transcription.16,17,18 However, it is likely that not only methyl-binding proteins change the promoter structure but also that DNA methylation itself causes formation of different structures of entire CpG islands. Most probably, the differences in sensitivity to DMSO reflect structural changes of promoter regions in relation to their methylation status, rather than methylation of separate bases within CpG islands. The method described here is the first technique allowing detection of methylation-induced structural DNA changes that may have more biological significance than methylated individual cytosines. Interestingly, the DMSO sensitivity of methylated versus unmethylated DNA was strongly correlated with changes of gene transcription. Accordingly, after treatment of SiHa cells with demethylation agent, we observed a notable shift from 4 to 2% DMSO when we analyzed DAPK promoter methylation by Ms-DMSO-PCR, and we also detected the appearance of significant amounts of DAPK mRNA in the treated cells (unpublished data).
We also showed that Ms-DMSO-PCR can be successfully used for detecting partial and/or heterogeneously methylated gene promoters. The methylation analysis of DAPK gene promoter in CaSki and C33 cells showed a strong PCR signal at 2% DMSO, suggesting that in these cells the promoter was not or only slightly methylated. After in vitro methylation, the amplification signal of DAPK promoter was clearly detected only at 5% DMSO. In SiHa cells, we observed the PCR signal of the analyzed fragment at 3% (very weak band) and 4% (strong band) of DMSO. These results, which are in agreement with the data obtained using the methylation-sensitive enzymatic method, suggest that DAPK promoter in SiHa cells is more methylated than in CaSki and C33 cells. However, in vivo-methylated DNA did not reach the maximal possible shift in sensitivity to DMSO, probably because of different DNA methylation pattern in individual cells. Time course in vitro methylation experiments also confirmed that this technique can be used for the detection of partial and/or heterogeneous methylation of gene promoters. However, it should be noted that the differences in DMSO sensitivity during the PCR amplification probably reflect the structural differences of methylated versus unmethylated DNA. Therefore, we believe that the DMSO dependence for in vitro-methylated DNA might not be the same as for in vivo-methylated genes.
Experiments with mixtures of methylated and unmethylated DNA showed that Ms-DMSO-PCR allows the detection of methylated gene in a background of the same, but unmethylated, genes. We were able to detect methylation in samples containing only 30% methylated DNA. This issue is crucial for clinical application because biopsies normally represent mixtures of tumor and normal cells. Unfortunately, we could not determine the correspondence of DMSO sensitivity to the number of methylated bases in DNA because of the absence of suitable methods for methylation analysis of long fragments. Bisulfite conversion causes denaturation and significant degradation (up to 95%) of DNA making impossible the analysis of DNA fragments longer than 200 bp.19 In contrast to bisulfite-based methods, Ms-DMSO-PCR is a quick and qualitative test able to detect methylation of entire (up to 1 kb) CpG island of gene promoters.
This method also has several other advantages over existing methylation-detection techniques. In contrast to widely used protocols, Ms-DMSO-PCR does not require any additional DNA modification before methylation analysis. This is an important point because a limited amount of DNA is often available from biopsies and other medical materials. The technique described here can be performed with nanograms (25 to 50 ng) of DNA directly after isolation from analyzed tissues. In addition, this one-step procedure does not need expensive reagents and equipment and can be used widely in medical and research laboratories. Accordingly, the shift in DMSO sensitivity in clinical samples from cervical scrapes and frozen biopsies, corresponding to a mixture of normal and cancer cells, suggests that this new technique can be applied for the molecular diagnosis of cancer and other methylation-related diseases. Another interesting feature of Ms-DMSO-PCR is that it allows the concomitant detection of hyper- and hypomethylation. Only one reaction is indeed necessary to find any methylation changes occurring in the DNA. No additional steps and sets of primers are required, in contrast to other methods based on bisulfite conversion of DNA bases.
In conclusion, the new method described in this report should help to find and to re-evaluate new and existing markers for different diseases based on both DNA hypermethylation and hypomethylation events in the cells.
Footnotes
Supported by the Belgian Fund for Medical Scientific Research, the Walloon Region (grant NeoAngio N 616476), the L. Fredericq Fund, and the Centre Anti-Cancereux pres l’Universite de Liege.
P.D. is a senior research associate of the Belgian National Fund for Scientific Research.
References
- Ehrlich M. Expression of various genes is controlled by DNA methylation during mammalian development. J Cell Biochem. 2003;88:899–910. doi: 10.1002/jcb.10464. [DOI] [PubMed] [Google Scholar]
- Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- Paz MF, Fraga MF, Avila S, Guo M, Pollan M, Herman JG, Esteller M. A systematic profile of DNA methylation in human cancer cell lines. Cancer Res. 2003;63:1114–1121. [PubMed] [Google Scholar]
- Esteller M. Dormant hypermethylated tumour suppressor genes: questions and answers. J Pathol. 2005;205:172–180. doi: 10.1002/path.1707. [DOI] [PubMed] [Google Scholar]
- Kisseljova NP, Kisseljov FL. DNA demethylation and carcinogenesis. Biochemistry (Moscow) 2005;70:743–752. doi: 10.1007/s10541-005-0179-z. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Esteller M. DNA methylation: a profile of methods and applications. BioTechniques. 2002;33:632–664. doi: 10.2144/02333rv01. [DOI] [PubMed] [Google Scholar]
- Tada Y, Wada M, Taguchi K, Mochida Y, Kinugawa N, Tsuneyoshi M, Naito S, Kuwano M. The association of death-associated protein kinase hypermethylation with early recurrence in superficial bladder cancers. Cancer Res. 2002;62:4048–4053. [PubMed] [Google Scholar]
- Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- Müller HM, Widschwendter A, Fiegl H, Ivarsson L, Goebel G, Perkmann E, Marth C, Widschwendter M. DNA methylation in serum of breast cancer patients: an independent prognostic marker. Cancer Res. 2003;63:7641–7645. [PubMed] [Google Scholar]
- Havard L, Rahmouni S, Boniver J, Delvenne P. High levels of p105 (NFKB1) and p100 (NFKB2) proteins in HPV16-transformed keratinocytes: role of E6 and E7 oncoproteins. Virology. 2005;331:357–366. doi: 10.1016/j.virol.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Liu L, Gerson SL. Targeted modulation of MGMT: clinical implications. Clin Cancer Res. 2006;12:328–331. doi: 10.1158/1078-0432.CCR-05-2543. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Ushijima T. Diagnostic and therapeutic applications of epigenetics. Jpn J Clin Oncol. 2005;35:293–301. doi: 10.1093/jjco/hyi088. [DOI] [PubMed] [Google Scholar]
- Varadaraj K, Skinner DM. Denaturants or cosolvents improve the specificity of PCR amplification of a G + C-rich DNA using genetically engineered DNA polymerases. Gene. 1994;140:1–5. doi: 10.1016/0378-1119(94)90723-4. [DOI] [PubMed] [Google Scholar]
- Collins M, Myers M. Alterations in DNA helix stability due to base modifications can be evaluated using denaturing gradient gel electrophoresis. J Mol Biol. 1987;198:737–744. doi: 10.1016/0022-2836(87)90214-2. [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Gonzales FA, Jones PA. Altered chromatin structure associated with methylation-induced gene silencing in cancer cells: correlation of accessibility, methylation. MeCP2 binding and acetylation. Nucleic Acids Res. 2001;29:4598–4606. doi: 10.1093/nar/29.22.4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrner JA, Eguchi S, Herman JG, Baylin SB. Dependence of histone modifications and gene expression on DNA hypermethylation in cancer. Cancer Res. 2002;62:7213–7218. [PubMed] [Google Scholar]
- Stirzaker C, Song JZ, Davidson B, Clark SJ. Transcriptional gene silencing promotes DNA hypermethylation through a sequential change in chromatin modifications in cancer cells. Cancer Res. 2004;64:3871–3877. doi: 10.1158/0008-5472.CAN-03-3690. [DOI] [PubMed] [Google Scholar]
- Grunau C, Clark SJ, Rosenthal A. Bisulfite genomic sequencing: systematic investigation of critical experimental parameters. Nucleic Acids Res. 2001;29:e65. doi: 10.1093/nar/29.13.e65. [DOI] [PMC free article] [PubMed] [Google Scholar]