Abstract
Annexin A1 (ANXA1) has an important role in cell-cell communication in the host defense and neuroendocrine systems. In both systems, its actions are exerted extracellularly via membrane-bound receptors on adjacent sites after translocation of the protein from the cytoplasm to the cell surface of adjacent cells. This study used molecular, microscopic, and pharmacological approaches to explore the mechanisms underlying the cellular exportation of ANXA1 in TtT/GF (pituitary folliculo-stellate) cells. LPS caused serine-phosphorylation of ANXA1 (ANXA1-S27-PO4) and translocation of the phosphorylated protein to the cell membrane. The fundamental requirement of phosphorylation for membrane translocation was confirmed by immunofluorescence microscopy on cells transfected with wild-type or mutated (S27/A) ANXA1 constructs tagged with enhanced green fluorescence protein. The trafficking of ANXA1-S27-PO4 to the cell surface was dependent on PI3-kinase and MAP-kinase. It also required HMG-coenzyme A and myristoylation. The effects of HMG-coenzyme A blockade were overcome by mevalonic acid (the product of HMG-coenzyme A) and farnesyl-pyrophosphate but not by geranyl-geranylpyrophosphate or cholesterol. Together, these results suggest that serine-27 phosphorylation is essential for the translocation of ANXA1 across the cell membrane and also identify a role for isoprenyl lipids. Such lipids could target consensus sequences in ANXA1. Alternatively, they may target other proteins in the signal transduction cascade (e.g., transporters).
Keywords: phosphorylation, green fluorescence protein, lipidation, signaling
Annexin A1 (ANXA1) is a Ca2+- and phospholipids-binding protein that acts both intracellularly and extracellularly to modulate cell signaling. Data from our laboratory have shown that it has a particularly important role in the regulation of the host defense and neuroendocrine systems and that ANXA1 mimetics may provide a novel therapeutic approach to the control of inflammatory disease (reviewed in ref 1). The regulatory actions of ANXA1 in both systems appear to be exerted mainly extracellularly, via membrane-bound cell surface receptors (2-4). Access of endogenous ANXA1 to these receptors is thus dependent on the translocation of the protein from its cytoplasmic stores to the cell surface (1). These conclusions are supported by evidence that within the neuroendocrine system ANXA1 is expressed in cells adjacent to its target cells (5), and it is concentrated at sites where the cells make contact with endocrine cells (6) and translocated to the cell surface in response to appropriate stimuli (7, 8). Further evidence of the paracrine/juxtacrine mode of ANXA1 action is provided by evidence that drugs that prevent the cellular exportation of the protein ablate the regulatory actions of endogenous ANXA1 within the neuroendocrine system (6, 7, 9, 10) as also do compounds that destroy the cell surface ANXA1 receptors (2). Substantial evidence supports the view that membrane translocation is also critical to the actions of ANXA1 within the host defense system, for example, for the modulation of neutrophil migration (4). Furthermore, the phenomenon may have more widespread significance, as ANXA1 has been detected in rat peritoneal exudates (11), human lung lavage fluid from asthmatic patients, (12, 13), and seminal fluid (14), as well as in culture medium from primary tissue (15) and cell lines (16).
The mechanisms by which ANXA1 is translocated from the cytoplasm to the cell membrane are poorly understood, although our previous studies have shown it to be independent of the classical endoplasmic reticulum/Golgi pathway of exocytosis (17) and de novo protein synthesis (7, 18) and to require a signaling cascade involving protein kinase C (PKC) and other kinases leading to ANXA1 phosphorylation (10, 18, 19). Other data support a role for ATP-binding cassette (ABC) transporter proteins and ATP-sensitive K+ channels (20).
In the present study, we have used a murine folliculo-stellate (FS) cell line and a combination of molecular, microscopic, and pharmacological approaches to advance our understanding of the role of phosphorylation in the membrane translocation of ANXA1. In addition, we have explored for the first time the potential role of lipids in facilitating the translocation of ANXA1 to the cell surface. Our study data clearly show that the translocation induced by a proinflammatory stimulus (LPS) is dependent on phosphorylation of ANXA1 at serine-27. In addition, they support a role of lipidation and phosphorylation in ANXA1 membrane trafficking.
MATERIALS AND METHODS
Drugs
The following drugs were used: LPS (from Escherichia coli serotype 055:B5) and mevalonic acid, the product of 3-hydroxy-3-methylglutaryl coenzyme A (CoA) reductase (HMG-CoA, both from Sigma-Aldrich, Poole, Dorset, UK); lovastatin, an inhibitor of HMG-CoA (MK-803), a PI3 kinase inhibitor (LY 294002), and a MAP-kinase inhibitor (PD98059; all from Calbiochem, CN Bioscience, Beeston, Nottingham, UK); farnesyl-pyrophosphate triammonium, geranyl-geranylpyrophosphate triammonium, substrate for farnesylation and geranylation of the protein, and 2-hydroxymyristic acid, an inhibitor of myristoylation (all from Biomol, Plymouth, PA). The drugs were diluted in incubation medium immediately before use; the final concentration of the original diluents did not exceed 0.04%; 2-hydroxymyristic acid was delivered to cells as complex with BSA, exactly as described previously (21).
Antisera
Primary antisera
Total ANXA1 protein was detected using a polyclonal antiserum [polyclonal antibody (pAb)] raised in rabbit against human recombinant ANXA1 (hrANXA11-346; ref 22-24). Phosphorylated forms of ANXA1 were detected using purified pAbs raised in rabbit against ANXA115-31 phosphorylated on the serine residue at position 27 (anti ANXA1-S27-PO4, pAb) (Neosystem, Strasbourg, France; ref 18). An anti-α-tubulin monoclonal antibody (mAb) was purchased from Sigma-Aldrich (Poole, UK) and a neutralizing anti-mouse interleukin (IL)-6 pAb raised in rabbit from R&D Systems (Abingdon, UK). A FITC-labeled anti-CD14 mAb raised against human CD14 (clone MΦP9, Becton-Dickinson, BD Biosciences Oxford, UK) and anti-TLR 2 and anti-TLR 4 pAbs (eBioscience, San Diego, CA) were also used.
Secondary antisera
A peroxidase-conjugated goat anti-rabbit antiserum (Sigma-Aldrich) was used for Western blot analysis. FITC-coupled rabbit anti-mouse or goat anti-rabbit antisera (Chemicon International, Harrow, UK) were employed for fluorescence activated cell (FAC) analysis.
Cell culture
A pituitary FS cell line (TtT/GF), derived from a murine pituitary thyrotrophic tumor (25), was maintained at 37°C in DMEM-Glutamax (Invitrogen, Paisley, UK) enriched with 10% fetal calf serum (PAA Laboratories, Yeovil, UK), and 0.1% gentamicin sulfate (Sigma, Pool, UK) in a 5% CO2 humidified atmosphere. The cells were plated at 106/well and incubated for 24 h. They were then challenged with LPS (100 ng/ml) for various periods (5 min to 24 h) and processed for protein, RNA, or microscopic analysis as reported below. Where appropriate, drugs were added to the culture medium either 30 min (kinase inhibitors, anti-IL-6 mAb) or 16 h (modifiers of lipidation) before LPS stimulation; controls were incubated in medium alone. A second cell line (murine corticotroph AtT20, subclone D16:D16) was used to examine the subcellular distribution of ANXA1 proteins expressed by transfected ANXA1 cDNA constructs before and after challenge with LPS as described above. Unlike the TtT/GF cell line, AtT20 cells do not express ANXA1 mRNA or protein constitutively (26). They thus resemble their primary counterparts (5) and make a useful model in which to map the trafficking of transfected ANXA1.
RNA isolation and reverse transcriptase-polymerase chain reaction
Total RNA was isolated by RNeasy (QIAGEN, Crawley, West Sussex, UK) according to the manufacturer’s directions. Expression of mouse Anxa 1 (ORF 1.040 bp, accession number: NM_010730, NCBI), mouse CD14 (ORF 1.1 bp, accession number NM_009841 NCBI), mouse Toll receptor 4 (TLR4, 300 bp between 1770 bp and 2068 from the ATG, accession number AF110133 NCBI), and Toll receptor 2 (TLR2, 499 bp between 1265 and 1764 from ATG, accession number NM_011905 NCBI) were analyzed by reverse transcriptase-polymerase chain reaction (RT-PCR) as described previously using GAPDH as an internal control (18). The PCR products were subjected to 0.02% ethidium bromide (Sigma) agarose gel electrophoresis (1%), and the images were captured with a Syngene imaging System (Cambridge, UK).
Western blot analysis
Expression of total ANXA1 protein and serine 27 phosphorylated ANXA1 (ANXA1-S27-PO4) by the TtT/GF cells was examined by SDS-PAGE and Western blotting. Briefly, total protein was extracted from the cells by freeze/thawing (23), the protein content was estimated (27), and the sample was analyzed either immediately or after storage at -80°C. For those experiments in which cell surface and intracellular ANXA1 were measured, the cells were first washed for 10 min on ice in HEPES buffer (25 mM) containing protease and phosphatase inhibitors (1 mM PMSF, 1 mg/ml leupeptin, 1 mg/ml pepstatin, 1 mg/ml aprotinin, 1 mM Na3VO4, 1 mM NaF; all from Sigma-Aldrich), and a Ca2+ chelating agent (1 mM EDTA-EGTA, Sigma-Aldrich), which removes ANXA1 attached to the cell surface. The washes were retained for ANXA1 measurement (cell surface ANXA1). Protein extracts of the washed cells were then prepared as described above for analysis of the remaining ANXA1 in the cells (intracellular ANXA1). In some cases, ANXA1 released into the incubation medium was also determined. For this procedure, the cells were centrifuged gently (800 g for 5 min) and 1 ml aliquots of the supernatant were collected and concentrated in Micron 10 kDa cut-off concentrators (Millipore Watford, UK).
For ANXA1 analysis, samples (10 μg protein per well for both intracellular and EDTA wash samples or 1 ml supernatant concentrate) were loaded onto 10% acrylamide gels and separated by SDS-PAGE in denaturing conditions at 50 mA for 90 min. The separated proteins were then transferred electrophoretically (100 mA. per blot 70 min; 2117 MULTIPHOR II, Pharmacia LKB, Bromma, Sweden) to nitrocellulose paper (Hybond-P; GE Healthcare, Buckinghamshire, UK) soaked in transfer buffer (25 mM Tris, 192 mM glycine; Sigma-Aldrich) and 20% methanol v/v (VWR Int., Poole, UK). Nonspecific binding was blocked by incubation of the blots in 5% milk powder (Marvel, Knighton Edbaston, Stafford) in TBS-Tween (50 mM Tris; 150 mM NaCl and 0.1% Tween v/v (all from Sigma-Aldrich) for 60 min. After being washed, the blots were incubated overnight at 4°C in primary antibody (Ab; diluted 1:1000) and then for 1 h at room temperature with the appropriate secondary Ab (diluted 1:25,000). Immunoreactive protein bands were detected by chemiluminescence using enhanced chemiluminescence reagents and exposed to Hyperfilm (both from GE Healthcare). The blots were then scanned and analyzed (HP Scanjet 5200 with Adobe Photodeluxe Business Edition v1.1).
Flow cytometric analysis
Aliquots of the TtT/GF cell suspension (106 cells in 100 μl) were added to 96-flat well plates in triplicate and incubated at 4°C for 1 h in the presence or absence (controls) of anti-ANXA1-S27-PO4 pAb (1:300), anti-CD14 mAb (2 mg/100 ml of 106 cell suspension), anti-TLR-2 pAb or anti-TLR pAb (2 mg/100ml of 106 cell suspension) in a buffer A, containing HEPES (25 mM), CaCl2 (1 mM), and MgCl2 (1 mM); nonspecific binding was minimized by the addition of mouse immunoglobulin (1.6 mg/ml, Roche, Bell Lane, Lewes UK). The cells were then washed in HEPES buffer (as previously reported) and incubated on ice for 30 min with F(ab′)2 fragments of goat anti-rabbit IgG FITC conjugated secondary Ab (diluted 1:600). After a further wash in buffer A, the cell surface fluorescence was analyzed immediately by flow cytometry. In the majority of experiments, cell membrane integrity was assessed by sequential incubation of the cells with anti β-actin mAb (diluted 1:20) and FITC-conjugated rabbit anti-mouse IgG (diluted 1:200). In some experiments, preimmune serum was employed as a further control.
Construction of expression plasmids and site directed mutagenesis
A mouse ANXA1 cDNA construct with a mutation in a key phosphorylation site (S27) was prepared; this site was selected on the basis of our previous studies that implicated its importance in the exportation process (10, 18). In addition, cDNA constructs for C-terminal enhanced green fluorescent protein (EGFP)-tagged Anxa 1 protein were prepared by attaching pEGFP-N1 (Gene Bank #U55762, from Clontech, Palo Alto, CA) to the WT construct between the restriction sites XhoI at the start of the coding region and the BamHI site replacing the stop codon. Amino acid replacement S27/A was introduced by sequential site-directed mutagenesis reactions employing the QuikChange site-directed mutagenesis method (Stratagene, Amsterdam, Netherlands). Synthetic oligonucleotide primers containing the desired mutation were extended in temperature cycling reactions with plaque-forming units of DNA polymerase. The products were DpnI-treated to digest the parental template and transformed in E. coli. Plasmids were amplified in E. coli and purified with QIAGEN Plasmid Purification Kit (QIAGEN).
Transfection of TtT/GF cells and fluorescence microscopy
TtT/GF cells were kept in culture for no longer than 20 passages. The cells were plated on Lab-Tek II Chamber Slide System (Nalgene Nunc International Naperville, IL) at a concentration of 3000/well in DMEM. Transient transfection was performed the following day, using fugene transfection reagent (Roche, Bell Lane, Lewes, UK) according to the manufacturer’s instructions. After 30 h, the cells were stimulated with LPS (100 ng/ml, for 5 min), fixed in 4% paraformaldehyde (PFA), and stained with rhodamine-fluorescent-phalloidin (1:40) or TO-PRO 3 (monomeric cyanine, 1:100, both from Molecular Probes Leiden, the Netherlands) to visualize the cytoskeleton and nuclei respectively. The slides were mounted in Mowiol (Sigma). Fluorescence micrographs were obtained with a Cool-Snap-Procf color camera (Media Cybernetics, Finchapstead, Berkshire, UK) and image processor (Image Pro Plus 4.5 software) linked to a Nikon eclipse E800 microscope. The filter used to detect GFP-fluorescence consisted of an excitation band-pass filter (450-490 nM), a dichroic mirror (510 nM), and an emission band filter (515-560 nM). Deconvolved images were collected on an inverted epifluorescence TE2000 U Nikon microscope using Openlab 4.0.3 software (Improvision) and nearest neighbor deconvolution.
Transfection and immunogold electron microscopic analysis of AtT20 cells
AtT20 cells were transfected as described above with constructs expressing either WT ANXA1 (pRCM/ANXA1-WT; ref 28) or a mutant ANXA1 in which serine27 was replaced with alanine. Cells transfected with pRCMV empty plamid were used as controls. After 48 h, cells were treated with LPS (100 ng/ml, 5 min) and fixed in 3% paraformaldehyde, 0.05% gluteraldheyde; freeze substituted in methanol; and processed into LGR resin H (London resin gold, Basingstoke, UK); cells not exposed to LPS were used as controls. Ultrathin sections were stained for ANXA1 by immunogold labeling using a well characterized polyclonal anti-ANXA1 antiserum raised in sheep against full length human recombinant ANXA1 and examined morphologically as described previously (5).
Statistical analysis
Statistical significance was assessed by ANOVA followed by Bonferroni’s t test, and P < 0.05 was taken as significant.
RESULTS
Characterization of the TtT/GF cells
LPS is the major mediator of the organism’s response to Gram-negative bacterial invasion. In the pituitary gland, LPS targets the FS cells causing them to release IL-6 and other cytokines that target gp130 cytokine receptors (e.g., LIF); these cytokines act locally to modulate the secretory activity of the endocrine cells (29) and thereby play an important part in mediating the neuroendocrine response to bacterial infection (1). We have previously shown that the inhibitory effect of glucocorticoids on ACTH release from the corticotrophs is dependent, at least in part, on the externalization of ANXA1 from FS cells (5, 7). Other data have shown that IL-6 up-regulates the transcriptional activity of the Anxa1 promoter (22), raising the possibility that LPS may also influence the cellular disposition of ANXA1 in FS cells. To address this question, we used RT-PCR and FACS analysis to confirm that TtT/GF cells express the necessary machinery to respond to LPS. Our data clearly show that these cells express mRNA and protein for each of the following: CD14, TLR2, and TLR4 (Fig. 1A-B).
Figure 1.
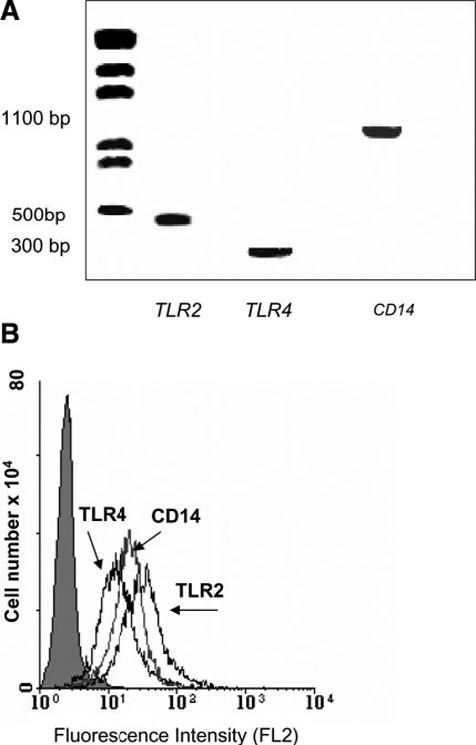
Detection of Toll receptors in TtT/GF cells by RT-PCR and flow cytometry. A) Representative RT-PCR for TLR2, TLR4, and CD14 in TtT/GF cells (see Materials and Methods); B) FACS profiles demonstrating expression on plasma membrane of the above Toll receptors immunostained with specific antibodies indicated by second Ab, fluorescence intensity due to second Ab alone.
Effect of LPS on the expression and phosphorylation of ANXA1 in TtT/GF cells
LPS (100 ng/ml) increased the expression of Anxa1 mRNA (Fig. 2A) and ANXA1 protein in the cytoplasm (Fig 2B); its effects were first evident after 8 h contact and declined thereafter. By contrast, LPS had no effect on the expression of ANXA1 protein expression on the cell membrane (Fig. 2C). Together these data suggest that LPS augments the synthesis of ANXA1 but has no effect on its cellular disposition. The Ab employed for these studies does not differentiate between phosphorylated and unphosphorylated species of ANXA1. Our subsequent studies that used an Ab that recognizes ANXA1 only when phosphorylated on serine 27 (ANXA1-S27-PO4) revealed a different profile of data. Within 5 min, LPS treatment induced a marked increase in the expression of ANXA1-S27-PO4 on the cell surface (Fig. 3A). This effect was transient and no longer apparent after 30 min contact. However, a secondary rise in membrane ANXA1-S27-PO4 was evident at 24 h (Fig 3A), coincident with a rise in the intracellular pool of the phosphorylated protein (Fig 3B). Confirmation that the Ab recognizes only the phosphorylated form of ANXA1 is provided in Fig. 3C, which demonstrates that treatment of the cell surface protein fraction with alkaline phosphatase (which removes 5′ phosphate groups from proteins) reduces markedly the amount of cell surface ANXA1-S27-PO4 detected.
Figure 2.
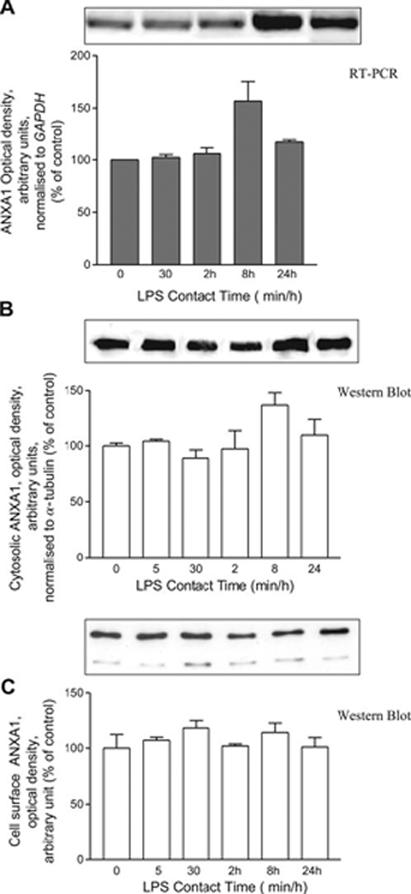
Time-dependent effect of LPS on ANXA1 expression. Cells were treated with LPS (100 ng/ml). Cells were harvested for Anxa1 mRNA (A normalized on GAPDH) analysis, ANXA1 cytosolic protein (B normalized to α-tubulin) analysis, and membrane bound ANXA1 (C EDTA extracted). Gels/blots shown represent 3 independent experiments; histograms are mean of 3 independent experiments [data expressed as intensity of absorbance normalized for each internal control: GAPDH (PCR) or tubulin (Western blot)].
Figure 3.
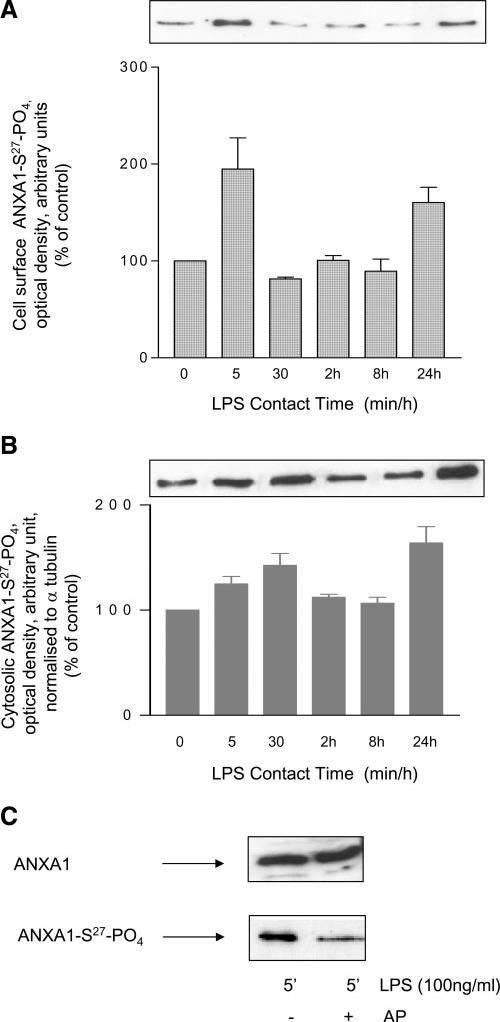
Effect of LPS stimulation on ANXA1 phosphorylation. Membrane attached (A) and cytosolic fraction (B) of ANXA1 was analyzed for its phosphorylation on serine 27. Western blot analysis expressed as % of unstimulated cells (C) as optical density arbitrary units. C) Equal amount of EDTA detached ANXA1 from LPS-stimulated cells were treated with 10 U alkaline phosphatase for 1 h at 37°C. Then, Western blot with 2 different antibodies: one of which discriminates between the phosphorylated or not was performed. One representative of at least 3 independent experiments is shown.
Blockade of the LPS-induced translocation of ANXA1-S27-PO4 to the cell surface by inhibitors of PI3 kinase and MAP kinase
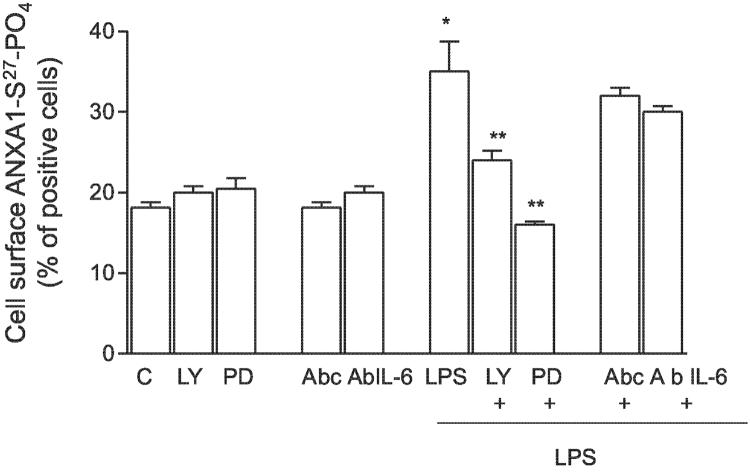
To investigate the signal transduction pathway involved in the early effects (5 min) of LPS on the cellular disposition of ANXA1-S27-PO4 in TtT/GF cells, we examined the effect of specific inhibitors of PI3-kinase (LY294002), MAP kinase (PD98059), and IL-6 (anti-interleukin-6 mAb) on the translocation of ANXA1-S27-PO4 to the cell membrane, using concentrations of known efficacy (18). Both LY294002 (500 nM) and PD98059 (5 mM) inhibited the response to LPS (Fig. 4). By contrast, anti-IL-6 mAb was without effect although it did suppress the rise in intracellular ANXA1 apparent after 8 h contact (data not shown), a finding that supports a role for IL-6 in the regulation of the synthesis but not the post-translational serine- phosphorylation of ANXA1.
Figure 4.
Effects of PI3 kinase and MAPK inhibitors on ANXA1-S27 phosphorylation. Cells were pretreated for 30 min with LY 294002 (LY, 1.4 mM) or PD 98059 (PD, 5 mM) or with a neutralizing Ab against IL-6 (50 ng/ml) and then LPS was added for further 5 min. ANXA1-S27-PO4 was measured by FACS; % of positive cells to immunostaining is reported. A control Ab (matched isotype) was used as an internal control. *P < 0.025 vs. unstimulated, **P < 0.01 vs. LPS stimulated (ANOVA and Bonferroni test).
Mutation of serine27 impairs the translocation of ANXA1 to the cell membrane
To investigate further the role of serine27 in the translocation of ANXA1 to the plasma membrane, we examined the impact of mutating this site on the subcellular distribution of ANXA1 using GFP-tagged constructs. WT EGFP-ANXA1 (ANXA1-WT EGFP) was strongly expressed in the cytoplasm and nucleus of the transfected cells (vs. mock-transfected control) and translocated to the plasma membrane, with an accumulation on one pole of the cell membrane and also to the nuclear membrane on stimulation with LPS (Fig. 5A and B, row 2). The mutant protein (ANXA1-S27/A-EGFP), in which serine27 was replaced with alanine, was also abundant in the cytoplasm and nucleus (Fig. 5A, row 3). However, unlike the WT, after LPS stimulation this protein appeared to accumulate in patches at a submembrane location as well as in the nucleus (Fig. 5B, row 3). The accumulation at submembrane level was revealed by costaining with phalloidin (see Fig. 1B; ANXA1-S27/A-EGFP merged Supplementary Materials). This distribution was further confirmed by deconvolution xz reconstruction (0.5 μM, Fig. 6A and B), which demonstrated the contrasting membrane localization of ANXA1-WT-EGFP and punctuate distribution of ANXA1-S27/A-EGFP after LPS stimulation.
Figure 5.
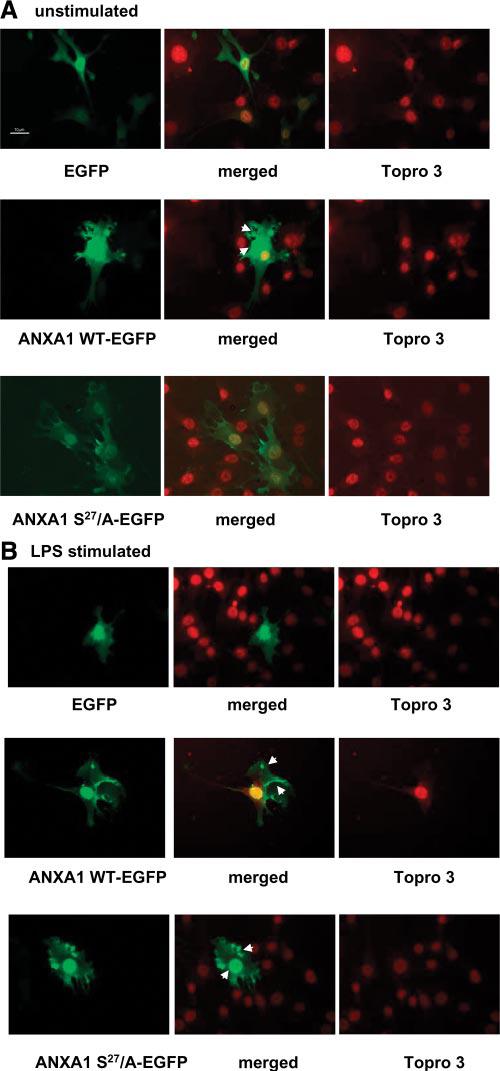
Cellular localization of ANXA1-wild-type-enhanced GFP and ANXA1-S27/A-enhanced GFP mutant in TtT/GF cells. A) Unstimulated cells transfected with empty plasmid EGFP, ANXA1 WT-enhanced GFP, or ANXA1-S27/A-enhanced GFP. Nuclei were stained with topro 3. Merged images represent the EGFP-topro3. Cells were fixed and moviol mounted. B) Cells stimulated with LPS (100 ng/ml, 5 min). All images were collected at similar exposure time. All image files were processed for presentation using Image Pro plus software (Media Cybernetics) and imported directly in PowerPoint in publication format. Arrows indicate area of ANXA1 localization after LPS treatment.
Figure 6.
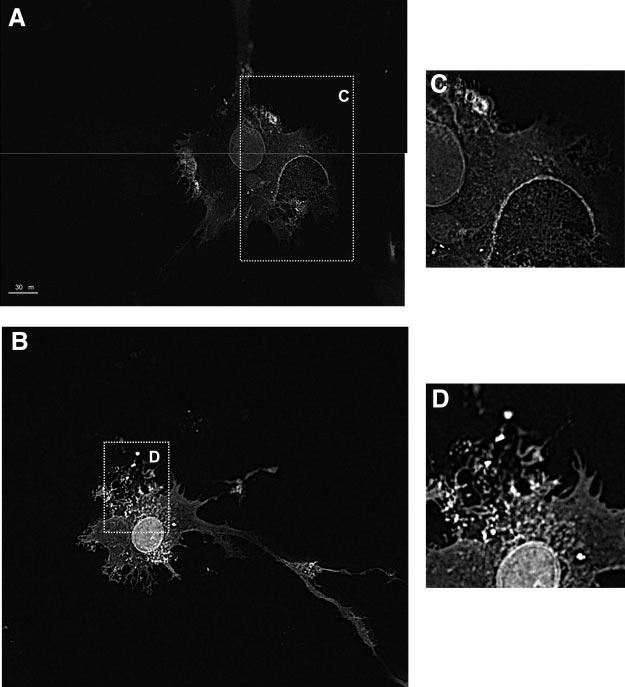
ANXA1 distribution in LPS treated cells. TtT/GF cells were transfected with ANXA1-wild-type-enhanced GFP (A) or ANXA1-S27/A-enhanced GFP (B) and stimulated for 5 min with LPS (100 ng/ml). Fluorescence was visualized by epifluorescence inverted microscopy, and xz-reconstructions were obtained by deconvolution using open-Lab software and nearest neighbor deconvolution (C) and region has been magnified (D) ×2.
We next analyzed the effect of mutation of serine27 on the subcellular distribution of ANXA1 in AtT-20 cells by immunogold electron microscopy (EM). ANXA1 was undetectable in untransfected cells and cells transfected with the empty pRCMV plasmid (Fig. 7A-C). By contrast, cells transfected with pRCMV-ANXA1 WT showed immunogold staining in the cytoplasm and in association with the plasma membrane as indicated by the arrows in Fig. 7E but not in association with the dense core vesicles. Exposure of the cells to LPS resulted in a marked increase in external membrane-bound ANXA1 (Fig. 7F). The cells transfected with the mutant for serine27 (pRCMV ANXA1 S27/A) also showed strong cytoplasmic immunogold staining. However, there was little ANXA1 associated with the membrane and no staining in the vesicles (Fig. 7G). Furthermore, the profile of positive ANXA1 staining was not modified by the treatment with LPS (Fig. 7H). These data strongly support for a role of phosphorylation of serine27 in the translocation of ANXA1 from the cytoplasm to the cell surface and thus support the data obtained using GFP-tagged proteins in the TtT/GF cell line.
Figure 7.
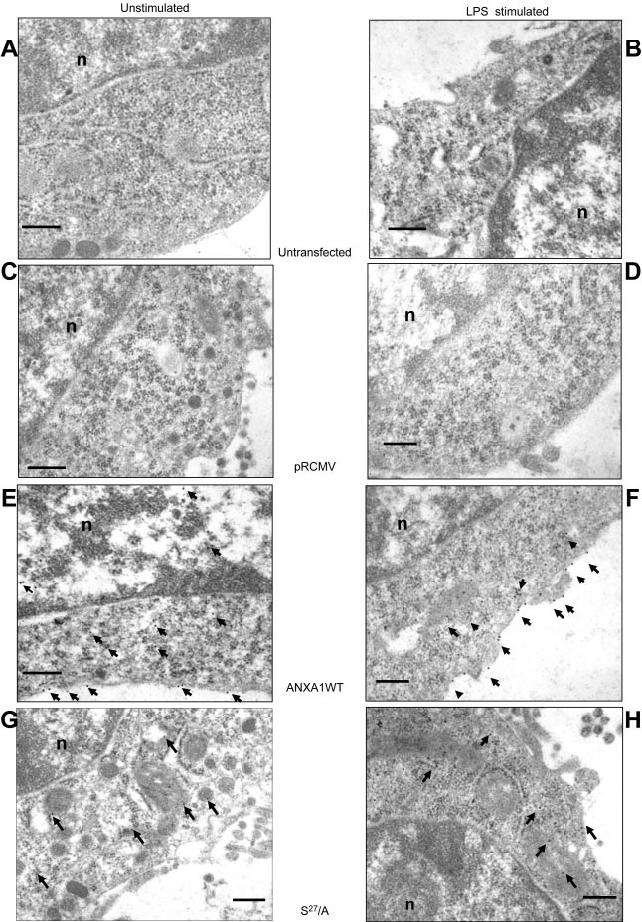
EM analysis of ANXA1 localization in AtT20 cell line. Immunogold microscopy showing ANXA1-ir in AtT20 D16 -16 cell line (A, untransfected-unstimulated; B, LPS 100 ng/ml stimulated 5 min) transfected with different constructions (control plasmid pRCM, C-D) expressing ANXA1/wild-type (E-F) or its S27/A mutant (G-H). Nucleotide indicates nucleus on pictures; arrows indicate gold particle. 0.25 μM scale bar.
Role of lipidation in the translocation of ANXA1 to the cell membrane
Lipidation (see Fig. 2, Supplementary Materials; S-prenylation, S-palmytoylation, and N-myristoylation) is involved in the maturation of many proteins in both prokaryotic and eukaryotic cells (30). Both myristoylated and prenylated proteins interact with the negative phospholipid head groups in the lipid bilayer to fine-tune the association with the membrane (see Fig. 3, Supplementary Materials; ref 30-32). ANXA1 is well known to interact with negatively charged membrane phospholipids, and inspection of its sequence shows that it possesses potential sites for myristoylation and prenylation. To explore the potential role of lipidation in the translocation of ANXA1 to the cell surface, we examined the effects of pretreatment of the cells with lovastatin (an inhibitor of HMG-coenzyme A, 10 mM) on the response to LPS (100 ng/ml, 5 min). Lovastatin abolished the significant increase cell surface ANXA1-S27-PO4 induced by LPS, as determined by FACS (Fig. 8A). Its effects were overcome by mevalonic acid (the product of HMG-coenzyme A, 10 μM) and farnesylpyrophosphate (a substrate for protein farnesylation, FPP 5 μM) but not by geranyl-geranylpyrophosphate (GGPP, 5 μM) or cholesterol (200 μM; Fig. 8A and also Fig. 4, Supplementary Materials). These data were confirmed by Western blot analysis (Fig. 8B), which also indicated that lovastatin alone causes a marked reduction in the cell surface of ANXA1-S27-PO4 (Fig. 8B). In addition, the LPS-induced translocation of ANXA1-S27-PO4 to the plasma membrane was prevented by treatment of the cells with 2-hydroxymyristic acid (an inhibitor of myristoylation, 1 mM, 16 h, Fig. 9).
Figure 8.
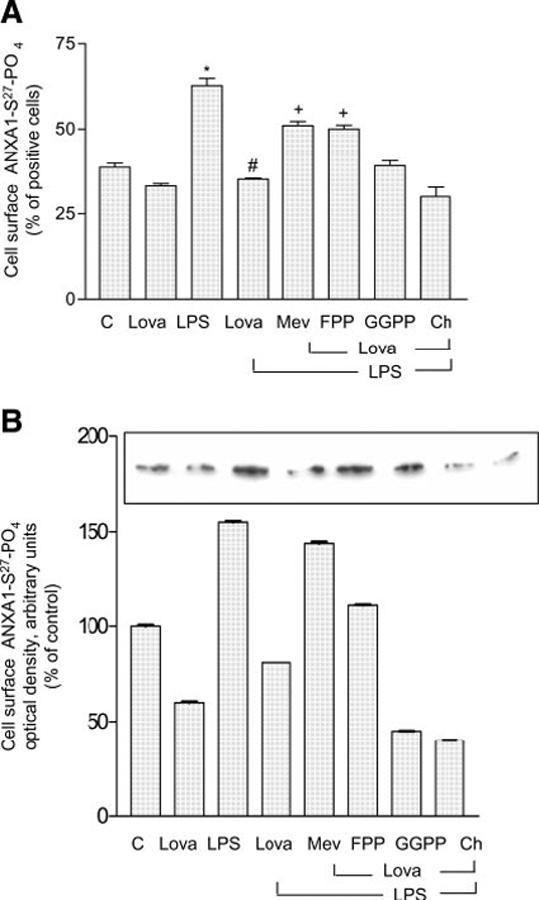
Lovastatin inhibits the access of LPS induced phosphorylated ANXA1 on the cell-surface. FACS analysis (A) and Western blot (B) of ANXA1-S27-PO4 expression after lovastatin treatment (10 μM, 16 h). Cells were pretreated with lovastatin (lova) or lovastatin and mevalonic acid (Mev, 10 μM), lovastatin and FPP (5 μM), lovastatin and GGPP (5 μM), lovastatin, and cholesterol (CH, 200 μM) followed by 5 min of LPS stimulation. Cells were harvested and processed both for FACS (A) or Western blot (B, see Materials and Methods). *P < 0.01 vs. unstimulated cells; #P < 0.025 vs. LPS stimulated cells; +P < 0.025 vs. lovastatin+LPS treated cells (ANOVA and Bonferroni test)
Figure 9.
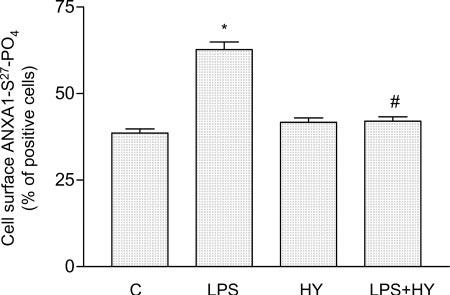
Chemical inhibition of myristoylation affect phosphorylated concentration on ANXA1 expression on TtT/GF cell surface. Flow cytometric analysis (FACS) of ANXA-S27-PO4 expression on the cell membrane was measured with a specific Ab directed against the S27-PO4 of ANXA1 region. Cells were treated or not with 2-hydroxymyristic acid 1 mM (HY) for 16 h before LPS stimulation for further 5 min; 1 representative experiment (performed in triplicate) out of 3 independent experiments is shown. *P < 0.01 vs. unstimulated and #P < 0.025 vs. LPS-stimulated cells (ANOVA and Bonferroni test)
DISCUSSION
This study provides molecular, microscopic, and pharmacological evidence to support the premise that the trafficking of ANXA1 from the cytoplasm to the cell surface is dependent on posttranslational modification and, specifically, phosphorylation of serine27. It also provides novel evidence to suggest that lipidation is an important component of the signaling sequence effecting the membrane translocation of ANXA1-S27-PO4.
LPS is a potent activator of the HPA axis in experimental animals and in humans, exerting its effects at multiple loci within the axis, including the pituitary FS cells (33, 34). These cells express the LPS receptor Tlr-4 and respond to the toxin by releasing cytokines (e.g., IL-6), which augment the ACTH response to CRH (35). In the same vein, the FS cell line used here (TtT/GF) expresses the signaling molecules necessary to respond to LPS. We have previously shown that ANXA1 is an important paracrine/juxtacrine mediator of the negative feedback actions of glucocorticoids in the hypothalamo-pituitary-adrenocortical axis, particularly in conditions of immune/inflammatory insults, and that its actions in the pituitary gland are dependent on translocation of the protein from the cytoplasm to the surface of FS cells (26). Our finding that LPS mimics the actions of glucocorticoids in promoting ANXA1 release from our FS cell line is thus paradoxical. However, the temporal characteristics of the responses are very different, with the toxin triggering a rapid and transient increase in membrane ANXA1-S27-PO4 (maximal at 5 min) and the steroids inducing a response that is relatively slow in onset but sustained for several hours (7, 18). As any increase in membrane ANXA1-S27-PO4 would be expected to suppress ACTH release (1), it is feasible that the transient response to LPS contributes to the delay in the HPA response to the toxin that is evident in vivo. It may thus form part of the protective mechanism that allows the pro-inflammatory host defense response to develop before it is contained within appropriate limits by the hypersecretion of the anti-inflammatory glucocorticoids (36).
Our studies in which membrane ANXA1 was detected by FACS or Western blot analysis using an Ab, which specifically recognizes ANXA1-S27-PO4, showed clearly that LPS promotes the membrane translocation of a species of ANXA1, which is phosphorylated at serine27. They thus accord with our previous studies in which ANXA1 release was elicited by glucocorticoids (18). Further evidence of the importance of serine27 phosphorylation for the cellular exportation of ANXA1 was provided by immunofluorescence analysis of the distribution of wild type and mutant GFP-tagged ANXA1 proteins. Our initial data showed that, like the native protein (5, 6, 37, 38), the WT GFP-fused protein is distributed throughout the cytoplasm and the nucleus; however, in contrast to data on J-774A.1 cells undergoing phagocytosis (39), it does not appear to associate with actin (see Fig. 1, Supplementary Materials). Exposure of the cells to LPS caused prompt translocation of the tagged WT protein to the cell surface; this response occurred preferentially at one pole of cells, as has also been reported in other conditions for the native protein (6). Together, these data suggest that the trafficking of GFP-tagged ANXA1 mirrors that of the native protein and that the tagged proteins are thus valid markers of the native protein. Importantly, our data also revealed that the mutant ANXA1-S27/A-EGFP protein shows a very different distribution in the cells, particularly after LPS stimulation when the mutant protein accumulates beneath the plasma membrane in a “patch-like” formation. These findings, together with our data based on Western blot, FACS analysis, and immunogold labeling, provide firm evidence that serine27 phosphorylation is critical to the translocation of ANXA1 to the cell surface. They also support the premise that both PI3-kinase and MAP-kinase are critical to this event (18) as also is PKC (10, 18). Further studies are now required to ascertain whether phosphorylation at other sites, e.g., serine (5) (40) is also required.
A further important novel finding was that blockade of HMG-CoA reductase with lovastatin prevents the LPS-induced membrane translocation of ANXA1-S27-PO4. This effect was specific (it was overcome by mevalonic acid, the product of HMG-CoA reductase) and thus indicates for the first time that lipidation is also an important factor in the translocation of ANXA1 to the cell membrane. Since the effects of lovastatin were overcome by both FPP and 2-hydroxymyristic acid, although not by GGPP and cholesterol, both prenylation and myristoylation may be important in this regard (41). The target(s) for lipidation remain to be identified. The sequence of ANXA1 itself includes potential sites for both modifications (see Fig. 3, Supplementary Materials). However, the addition of lipid moieties would be expected to alter the molecular mass and electrophorectic mobility ANXA1 and no such changes were evident in our Western blot analyses. Thus, although we cannot exclude the possibility that the lipidation of ANXA1 is a transient event, perhaps essential for the passage of ANXA1 across the plasma membrane (Fig. 10); it seems more likely that the targets are signaling intermediates, for example RAS and PKC, which are substrates for farnesylation and myristoylation, respectively (42, 43).
Figure 10.
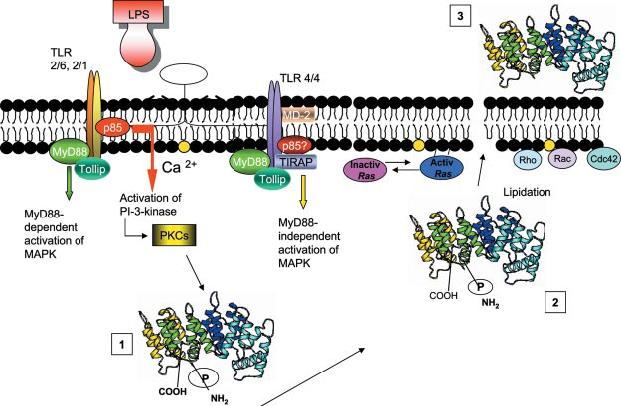
Schema representing our working hypothesis. ANXA1 is post-translationally modified by LPS triggered signal (1). Such modification induces a translocation of the protein to the cellular membrane where it is further membrane transported (2). Both phosphorylation and lipidation (directly on the protein or other molecules e.g., signal transduction proteins) may contribute (possibly through a conformational change) to the exportation outside the cell surface (3).
In conclusion, the results presented in this study demonstrate that the LPS-induced translocation of ANXA1 across the cell membrane is dependent on serine-27 phosphorylation and lipidation. The lipid moieties may attach to consensus sequences in ANXA1. Alternatively, they may target other proteins in the signal transduction cascade, e.g., Ras and PKC.
Acknowledgments
We thank the Wellcome Trust for generous financial support (Grant N:069234/B/02/Z) and C. Rantle for technical support.
REFERENCES
- 1.John CD, Christian HC, Morris JF, Flower RJ, Solito E, Buckingham JC. Annexin 1 and the regulation of endocrine function. Trends Endocrinol. Metab. 2004;15:103–109. doi: 10.1016/j.tem.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Christian HC, Taylor AD, Flower RJ, Morris JF, Buckingham JC. Characterization and localization of lipocortin 1-binding sites on rat anterior pituitary cells by fluorescence-activated cell analysis/sorting and electron microscopy. Endocrinology. 1997;138:5341–5351. doi: 10.1210/endo.138.12.5593. [DOI] [PubMed] [Google Scholar]
- 3.Goulding NJ, Jefferiss CM, Pan L, Rigby WF, Guyre PM. Specific binding of lipocortin-1 (annexin I) to monocytes and neutrophils is decreased in rheumatoid arthritis. Arthritis Rheum. 1992;35:1395–1397. doi: 10.1002/art.1780351126. [DOI] [PubMed] [Google Scholar]
- 4.Perretti M, Chiang N, La M, Fierro IM, Marullo S, Getting SJ, Solito E, Serhan CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A(4) receptor. Nat. Med. 2002;8:1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traverso V, Christian HC, Morris JF, Buckingham JC. Lipocortin 1 (annexin 1): a candidate paracrine agent localized in pituitary folliculo-stellate cells. Endocrinology. 1999;140:4311–4319. doi: 10.1210/endo.140.9.7008. [DOI] [PubMed] [Google Scholar]
- 6.Chapman L, Nishimura A, Buckingham JC, Morris JF, Christian HC. Externalization of annexin I from a folliculo-stellate-like cell line. Endocrinology. 2002;143:4330–4338. doi: 10.1210/en.2002-220529. [DOI] [PubMed] [Google Scholar]
- 7.Taylor AD, Cowell AM, Flower J, Buckingham JC. Lipocortin 1 mediates an early inhibitory action of glucocorticoids on the secretion of ACTH by the rat anterior pituitary gland in vitro. Neuroendocrinology. 1993;58:430–439. doi: 10.1159/000126572. [DOI] [PubMed] [Google Scholar]
- 8.Loxley H, Cowell A-M, Flower R, Buckingham J. Modulation of the hypothalamo-pituitary-adrenocortical responses to cytokines in the rat by lipocortin 1 and glucocorticoids: a role for lipocortin 1 in the feedback inhibition of CRF-41 release. Neuroendocrinology. 1993;57:801–814. doi: 10.1159/000126439. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AD, Christian HC, Morris JF, Flower RJ, Buckingham JC. An antisense oligodeoxynucleotide to lipocortin 1 reverses the inhibitory actions of dexamethasone on the release of adrenocorticotropin from rat pituitary tissue in vitro. Endocrinology. 1997;138:2909–2918. doi: 10.1210/endo.138.7.5260. [DOI] [PubMed] [Google Scholar]
- 10.John C, Cover P, Solito E, Morris J, Christian H, Flower R, Buckingham J. Annexin 1-dependent actions of glucocorticoids in the anterior pituitary gland: roles of the N-terminal domain and protein kinase C. Endocrinology. 2002;143:3060–3070. doi: 10.1210/endo.143.8.8965. [DOI] [PubMed] [Google Scholar]
- 11.Pepinsky RB, Sinclair LK, Browning JL, Mattaliano RJ, Smart JE, Chow EP, Falbel T, Ribolini A, Garwin JL, Wallner BP. Purification and partial sequence analysis of a 37-kDa protein that inhibits phospholipase A2 activity from rat peritoneal exudates. J. Biol. Chem. 1986;261:4239–4246. [PubMed] [Google Scholar]
- 12.Ambrose MP, Hunninghake GW. Corticosteroids increase lipocortin I in alveolar epithelial cells. Am. J. Respir. Cell Mol. Biol. 1990;3:349–353. doi: 10.1165/ajrcmb/3.4.349. [DOI] [PubMed] [Google Scholar]
- 13.DeCaterina R, Sicari R, Giannessi D, Paggiaro P, Paoletti P, Lazzerini G, Bernini W, Solito E, Parente L. Macrophage-specific eicosanoid synthesis inhibition and lipocortin-1 induction by glucocorticoids. J. Appl. Physiol. 1993;75:2368–2374. doi: 10.1152/jappl.1993.75.6.2368. [DOI] [PubMed] [Google Scholar]
- 14.Christmas P, Callaway J, Fallon J, Jones J, Haigler HT. Selective secretion of annexin 1, a protein without a signal sequence, by the human prostate gland. J. Biol. Chem. 1991;266:2499–2507. [PubMed] [Google Scholar]
- 15.Smith T, Flower R, Buckingham J. Lipocortins 1, 2 and 5 in the central nervous system and pituitary gland of the rat: selective induction by dexamethasone of lipocortin 1 in the anterior pituitary gland. Molecular Neuropharmacology. 1993;3:45–55. [Google Scholar]
- 16.Solito E, Raugei G, Melli M, Parente L. Dexamethasone induces the expression of the mRNA of lipocortin 1 and 2 and the release of lipocortin 1 and 5 in differentiated, but not undifferentiated U-937 cells. FEBS Lett. 1991;291:238–244. doi: 10.1016/0014-5793(91)81293-h. [DOI] [PubMed] [Google Scholar]
- 17.Philip JG, Flower RJ, Buckingham JC. Blockade of the classical pathway of protein secretion does not affect the cellular exportation of lipocortin 1. Regul. Pept. 1998;73:133–139. doi: 10.1016/s0167-0115(97)01077-x. [DOI] [PubMed] [Google Scholar]
- 18.Solito E, Mulla A, Morris JF, Christian HC, Flower RJ, Buckingham JC. Dexamethasone induces rapid serine-phosphorylation and membrane translocation of annexin 1 in a human folliculostellate cell line via a novel nongenomic mechanism involving the glucocorticoid receptor, protein kinase C, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase. Endocrinology. 2003;144:1164–1174. doi: 10.1210/en.2002-220592. [DOI] [PubMed] [Google Scholar]
- 19.Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptordependent, transcription-independent mechanism. Br. J. Pharmacol. 2000;130:289–298. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne J, Morris J, Solito E, Buckingham J. Modulators of the SUR2B/Kir6.1 ATP-sensitive K+channel regulate annexin 1 release in the TtT/GF folliculostellate cell line.; 24th Joint Meeting of the British Endocrine Societies 9; 2005. OC 22. [Google Scholar]
- 21.Galbiati F, Guzzi F, Magee AI, Milligan G, Parenti M. Chemical inhibition of myristoylation of the G-protein Gi1 alpha by 2-hydroxymyristate does not interfere with its palmitoylation or membrane association. Evidence that palmitoylation, but not myristoylation, regulates membrane attachment. Biochem. J. 1996;313(Pt 3):717–720. doi: 10.1042/bj3130717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solito E, de Coupade C, Parente L, Flower R, RussoMarie F. IL-6 stimulates Annexin 1 expression and translocation and suggests a new biological role as class II acute phase protein. Cytokine. 1998;10:514–521. doi: 10.1006/cyto.1997.0325. [DOI] [PubMed] [Google Scholar]
- 23.Solito E, de Coupade C, Parente L, Flower RJ, Russo-Marie F. Human annexin 1 is highly expressed during the differentiation of the human epithelial cell line A 549. Involvement of NF-IL6 in phorbol ester induction of annexin 1. Cell Growth Diff. 1998;9:327–336. [PubMed] [Google Scholar]
- 24.Solito E, Raguenes-Nicol C, de Coupade C, Bisagni-Faure A, Russo-Marie F. U 937 cells deprived of annexin 1 demonstrate an increased PLA2 activity. Br. J. Pharmacol. 1998;124:1675–1683. doi: 10.1038/sj.bjp.0701991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasumura Y. Retention of differentiated function in clonal animal cell lines, particularly hormone-secreting cultures. Am. Zool. 1968;8:285–305. doi: 10.1093/icb/8.2.285. [DOI] [PubMed] [Google Scholar]
- 26.Tierney T, Christian HC, Morris JF, Solito E, Buckingham JC. Evidence from studies on co-cultures of TtT/GF and AtT20 cells that annexin 1 acts as a paracrine or juxtacrine mediator of the early inhibitory effects of glucocorticoids on ACTH release. J. Neuroendocrinol. 2003;15:1134–1143. doi: 10.1111/j.1365-2826.2003.01111.x. [DOI] [PubMed] [Google Scholar]
- 27.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 28.de Coupade C, Gillet R, Bennoun M, Briand P, Russo-Marie F, Solito E. Annexin 1 expression and phosphorylation are upregulated during liver regeneration and transformation in antithrombin III SV40 T large antigen transgenic mice. Hepatology. 2000;31:371–380. doi: 10.1002/hep.510310217. [DOI] [PubMed] [Google Scholar]
- 29.Beishuizen A, Thijs LG. Endotoxin and the hypothalamo-pituitary-adrenal (HPA) axis. J. Endotoxin Res. 2003;9:3–24. doi: 10.1179/096805103125001298. [DOI] [PubMed] [Google Scholar]
- 30.Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 31.Farazi TA, Waksman G, Gordon JI. The biology and enzymology of protein N-myristoylation. J. Biol. Chem. 2001;276:39501–39504. doi: 10.1074/jbc.R100042200. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin S, Aderem A. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem. Sci. 1995;20:272–276. doi: 10.1016/s0968-0004(00)89042-8. [DOI] [PubMed] [Google Scholar]
- 33.Berridge M, Bootman M, Lipp P. Calcium-a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 34.Gloddek J, Lohrer P, Stalla J, Arzt E, Stalla GK, Renner U. The intrapituitary stimulatory effect of lipopolysaccharide on ACTH secretion is mediated by paracrine-acting IL-6. Exp. Clin. Endocrinol. Diabetes. 2001;109:410–415. doi: 10.1055/s-2001-18995. [DOI] [PubMed] [Google Scholar]
- 35.Lohrer P, Gloddek J, Nagashima AC, Korali Z, Hopfner U, Pereda MP, Arzt E, Stalla GK, Renner U. Lipopolysaccharide directly stimulates the intrapituitary interleukin-6 production by folliculostellate cells via specific receptors and the p38alpha mitogen-activated protein kinase/nuclear factor-kappaB pathway. Endocrinology. 2000;141:4457–4465. doi: 10.1210/endo.141.12.7811. [DOI] [PubMed] [Google Scholar]
- 36.Munck A, Naray-Fejes-Toth A. Glucocorticoids and stress: permissive and suppressive actions. Ann. N. Y. Acad. Sci. 1994;746:115–130. doi: 10.1111/j.1749-6632.1994.tb39221.x. [DOI] [PubMed] [Google Scholar]
- 37.Rescher U, Zobiack N, Gerke V. Intact Ca(2+)-binding sites are required for targeting of annexin 1 to endosomal membranes in living HeLa cells. J. Cell Sci. 2000;113(Pt 22):3931–3938. doi: 10.1242/jcs.113.22.3931. [DOI] [PubMed] [Google Scholar]
- 38.Mulla A, Christian HC, Solito E, Mendoza N, Morris JF, Buckingham JC. Expression, subcellular localization and phosphorylation status of annexins 1 and 5 in human pituitary adenomas and a growth hormone-secreting carcinoma. Clin. Endocrinol. 2004;60:107–119. doi: 10.1111/j.1365-2265.2004.01936.x. [DOI] [PubMed] [Google Scholar]
- 39.Kusumawati A, Liautard JP, Sri Widada J. Implication of annexin 1 in phagocytosis: effects of n-terminal domain deletions and point mutations of the phosphorylation site Ser-27. Cell Biol. Int. 2001;25:809–813. doi: 10.1006/cbir.2000.0704. [DOI] [PubMed] [Google Scholar]
- 40.Dorovkov MV, Ryazanov AG. Phosphorylation of annexin I by TRPM7 channel-kinase. J. Biol. Chem. 2004;279:50643–50646. doi: 10.1074/jbc.C400441200. [DOI] [PubMed] [Google Scholar]
- 41.Monick MM, Powers LS, Butler NS, Hunninghake GW. Inhibition of Rho family GTPases results in increased TNF-alpha production after lipopolysaccharide exposure. J. Immunol. 2003;171:2625–2630. doi: 10.4049/jimmunol.171.5.2625. [DOI] [PubMed] [Google Scholar]
- 42.Del Villar K, Dorin D, Sattler I, Urano J, Poullet P, Robinson N, Mitsuzawa H, Tamanoi F. C-terminal motifs found in Ras-superfamily G-proteins: CAAX and C-seven motifs. Biochem. Soc. Trans. 1996;24:709–713. doi: 10.1042/bst0240709. [DOI] [PubMed] [Google Scholar]
- 43.Aderem AA, Albert KA, Keum MM, Wang JK, Greengard P, Cohn ZA. Stimulus-dependent myristoylation of a major substrate for protein kinase C. Nature. 1988;332:362–364. doi: 10.1038/332362a0. [DOI] [PubMed] [Google Scholar]