Abstract
Rationale: An increase in the number of mononuclear phagocytes in lung biopsies from patients with idiopathic pulmonary fibrosis (IPF) worsens prognosis. Chemokines that recruit mononuclear phagocytes, such as CC chemokine ligand 2 (CCL2), are elevated in bronchoalveolar lavage (BAL) fluid (BALF) from patients with IPF. However, little attention is given to the role of the mononuclear phagocyte survival and recruitment factor, macrophage colony-stimulating factor (M-CSF), in pulmonary fibrosis.
Objectives: To investigate the role of mononuclear phagocytes and M-CSF in pulmonary fibrosis.
Methods: Wild-type, M-CSF−/−, or CCL2−/− mice received intraperitoneal bleomycin. Lung inflammation and fibrosis were measured by immunohistochemistry, ELISA, collagen assay, BAL differentials, real-time polymerase chain reaction, and Western blot analysis. Human and mouse macrophages were stimulated with M-CSF for CCL2 expression. BALF from patients with IPF was examined for M-CSF and CCL2.
Measurements and Main Results: M-CSF−/− and CCL2−/− mice had less lung fibrosis, mononuclear phagocyte recruitment, collagen deposition, and connective tissue growth factor (CTGF) expression after bleomycin administration than wild-type littermates. Human and mouse macrophages stimulated with M-CSF had increased CCL2 production, and intratracheal administration of M-CSF in mice induced CCL2 production in BALF. Finally, BALF from patients with IPF contained significantly more M-CSF and CCL2 than BALF from normal volunteers. Elevated levels of M-CSF were associated with elevated CCL2 in BALF and the diagnosis of IPF.
Conclusions: These data suggest that M-CSF contributes to the pathogenesis of pulmonary fibrosis in mice and in patients with IPF through the involvement of mononuclear phagocytes and CCL2 production.
Keywords: bleomycin, CC chemokine ligand 2, macrophage colony-stimulating factor, mononuclear phagocytes, pulmonary fibrosis
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Although mononuclear phagocytes have been shown to play an important role in pulmonary fibrosis, the mechanisms underlying their contributions have not been identified.
What This Study Adds to the Field
M-CSF contributes to the pathogenesis of pulmonary fibrosis in mice and in patients with IPF through the involvement of mononuclear phagocytes and CCL2 production.
Macrophage colony-stimulating factor (M-CSF) is a hematopoietic growth factor responsible for the survival and proliferation of monocytes and macrophages (mononuclear phagocytes) and the differentiation of monocytes into macrophages. In its biologically active form, M-CSF is a glycosylated 40–70 kD homodimer produced by somatic cells, such as endothelial cells, fibroblasts, and mononuclear phagocytes (1–3). M-CSF mediates its effects via binding the M-CSF receptor expressed on monocytes, macrophages, proliferating smooth muscle cells, umbilical vein endothelial cells, and cells of the female reproductive tract (4–6). Of the circulating leukocytes, M-CSF selectively targets mononuclear phagocytes.
As evidence for a critical role for M-CSF in normal development and in models of human disease, M-CSF−/− mice have reduced numbers of monocytes and macrophages and are protected against vascular disease, such as atherosclerosis and transplant-associated vascular sclerosis (7, 8). The protection from coronary heart disease in M-CSF−/− mice is attributed to reduced monocyte survival and recruitment to the atherosclerotic plaques. Similarly, mice deficient in CC chemokine ligand 2 (CCL2) or the receptor for CCL2, CC chemokine receptor 2 (CCR2), are protected from atherosclerosis (9, 10), potentially linking the functions of M-CSF and CCL2. In transgenic mouse models of breast cancer, M-CSF−/− mice exhibit less tumor metastasis and mononuclear phagocyte recruitment to the tumor, which, in part, involves angiogenesis (11). Accordingly, CCL2 and CCR2 also play important roles via inducing the chemotaxis of endothelial cells and the formation of blood vessels (12). Thus, in murine models of atherosclerosis and breast cancer, M-CSF and CCL2 appear causally linked. In addition, macrophages are also important in these diseases, with foam cells and tumor-associated macrophages, respectively, contributing to disease progression.
Interstitial lung diseases, such as idiopathic pulmonary fibrosis (IPF), are characterized by elevated levels of inflammatory cytokines, including CCL2 and CCL3 (13–16), which may direct the recruitment of cells to the lung and enhance cytokine production (17–26). Various cells, such as eosinophils, alveolar macrophages, T cells, neutrophils, and natural killer cells, are implicated in the pathogenesis of IPF, with alveolar macrophages contributing to the disease via cytokine release (27). Similar to mouse models of atherosclerosis, mouse models of pulmonary fibrosis also highlight the importance of CCL2 and CCR2 in the pathogenesis of this disease. Specifically, antibody therapy neutralizing CCL2 attenuates bleomycin-induced pulmonary fibrosis in mice (28), and CCR2−/− mice are protected from bleomycin-induced pulmonary fibrosis (29–31).
The role of alveolar macrophages in pulmonary fibrosis has been extensively studied (27, 32, 33), and M-CSF has been identified as a growth factor that plays a role in the proliferation of alveolar macrophages in fibrotic lung disorders (34). However, a functional connection between M-CSF, CCL2, and alveolar macrophages has not been made. By using a mouse model of pulmonary fibrosis, in vitro and in vivo analysis of mononuclear phagocyte function, and samples from patients with IPF, this study defines a functional relationship between M-CSF, CCL2, and mononuclear phagocytes in the pathogenesis of pulmonary fibrosis.
METHODS
Materials
Recombinant human M-CSF and all ELISA kits were purchased from R&D Systems (Minneapolis, MN), fetal bovine serum (FBS) from Hyclone (Logan, UT), and RPMI 1640 medium from Cellgro/Mediatech, Inc. (Herndon, VA). SYBR Green Master Mix was obtained from Applied Biosystems (Foster City, CA). Primers for real-time polymerase chain reaction (PCR) were purchased from Invitrogen (Carlsbad, CA). Anti-mouse CD68/mouse microsialin was from Serotec (Raleigh, NC), and Diff-Quik stain from Fisher Scientific (Pittsburgh, PA). Bleomycin was obtained from Sicor Pharmaceuticals (Irvine, CA). The Sircol Collagen Assay was acquired from Accurate Chemical and Scientific Corp. (Westbury, NY). Immunohistochemical processing and staining with hematoxylin and eosin (H&E) and Masson's trichrome was done at Histotechniques (Powell, OH). Staining for CD68 was performed at the Histology Core at the Ohio State University Medical Center. Antibodies for connective tissue growth factor (CTGF) and actin were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Mice and Bleomycin Administration
B6C3Fe a/a-Csf1op/J (M-CSF mice) breeding pairs were purchased from the Jackson Laboratory (Bar Harbor, ME). CCL2+/+ and CCL2−/− mice were a kind gift from Dr. Bethany Moore (University of Michigan, Ann Arbor, MI) and Dr. Barrett Rollins (Dana-Farber Cancer Institute, Boston, MA). Mice (knockout and wild-type littermate control animals) underwent intraperitoneal injection with 0.035 U bleomycin/g or vehicle control on Days 1, 4, 8, 11, 15, 18, 22, and 25. One week after the last injection, mice were killed and the lungs were inflated at 20 cm pressure. The left lobe of the lung was placed in 10% formalin for immunohistochemical processing, and the right lobes were snap frozen in liquid nitrogen for RNA and protein processing.
Analysis of Lung Fibrosis: Subjective Pathologic Assessment
A board-certified veterinary pathologist subjectively analyzed samples in a blinded manner and assessed them for the amount of inflammation, the number of alveolar macrophages, and the amount of fibrous connective tissue present within the H&E- and trichrome-stained slides, and scored them accordingly. A longitudinal section of the caudal lung lobe from each mouse was evaluated for lesions and subjectively assigned a score using a 5-point scheme. Subpleural fibrosis and the intensity of cellular response of both lymphocytes and alveolar macrophages were evaluated separately. The severity of the lesion was scored as 0 when the lung was completely normal, and 0.5 when the lesions were minimal or had only scattered foci. Lesions more severe than that were categorized as mild, moderate, or marked, and were graded from 1+ to 3+. Severity ranged from scattered foci beneath the pleura to extensive areas that extended more deeply into the lung lobe toward the hilus. Percent pleural involvement was determined by measuring thickened pleural areas of H&E-stained sections relative to total linear length of the visible pleura.
Real-Time PCR
Total RNA from cells was purified using the Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA), according to the manufacturer's instructions. Total RNA was extracted from lung tissue by freeze fracture and trizol extractions. Reverse transcription was done to amplify only mRNA by using oligo-dT primers (Invitrogen). cDNA was analyzed and used for real-time PCR with SYBR Green. All primers were designed using Primer Express software (Applied Biosystems). Primer sequences: mouse CCL2: 5′-TCTTCCTCCACCACCATGC-3′ (forward); 5′-TCATTGGGATCATCTTCGTGG-3′ (reverse); mouse GAPDH: 5′-GCACAGTCAAGGCCGAGAAT-3′ (forward); 5′-GCCTTCTCCATGGTGGTGAA-3′ (reverse); mouse type I collagen: 5′-ATGGATTCCCGTTCGAGTACG-3′ (forward); 5′-TCAGCTGGATAGCGACATCG-3′ (reverse); mouse type III collagen: 5′-CACCCTTCTTCATCCCACTCTTA-3′ (forward); 5′-ACCAAGGTGGCTGCATCC-3′ (reverse); mouse CTGF: 5′-AAAGTGCATCCGGACACCTAA-3″ (forward); 5′-TGCAGCCAGAAAGCTCAAACT-3′ (reverse); mouse CCL12: 5′-TGGCTGGACCAGATGCG-3′ (forward); 5′-GACGTGAATCTTCTGCTTAACAACA-3′ (reverse); human CCL2: 5′-GCTCAGCCAGATGCAATCAA-3′ (forward); 5′-CCTTGGCCACAATGGTCTTG-3′ (reverse); human GAPDH: 5′-CGGAGTCAACGGATTTGGTC-3′ (forward); 5′-GAATTTGCCATGGGTGGAAT-3′ (reverse); real-time PCR analysis was done using an ABI 7700 machine (Applied Biosystems) with GAPDH as an internal control.
Bronchoalveolar Lavage Analysis
Mice were anesthetized via inhaled isoflurane and underwent tracheal cut-down. Two instillations of 0.5 ml of phosphate-buffered saline (PBS) were performed, and the bronchoalveolar lavage (BAL) fluid (BALF) was collected. The total number of cells isolated via BAL was calculated. BALF was then centrifuged to pellet the cells, and the supernatant (BALF) was collected and stored. Cells were resuspended in 100 μl of PBS, cytospun on slides, Diff-Quik stained (according to the manufacturer's protocol), and analyzed under a microscope for cell differentials.
Sircol Collagen Assay
Mouse lungs were homogenized in 1.5 ml of 0.5 M acetic acid and rocked overnight at 4°C. Samples were centrifuged for 10 minutes at 2,000 rpm, and the resulting supernatant was assessed for collagen according to the manufacturer's instructions.
Derivation of Mononuclear Phagocytes
We have previously described the derivation of mouse bone marrow–derived macrophages (BMMs) (35). Briefly, wild-type C57BL/6J mice were killed via CO2 asphyxiation, and the femurs flushed with RPMI 1640 medium. Cells were maintained in RPMI supplemented with 2% FBS and 20 ng/ml M-CSF. Fresh M-CSF was added every 2 days or until cells were confluent.
For human monocyte–derived macrophages, mononuclear cells were isolated from Buffy Coats obtained from the American Red Cross (Columbus, OH) via the clumping method, as previously described (36). Monocytes were collected, washed with RPMI, and resuspended in RPMI supplemented with 2% FBS. A total of 4 × 106 cells/well were plated in a 12-well tissue culture plate and incubated at 37°C, 5% CO2, for 60 minutes to allow the monocytes to bind the plate. All nonadherent cells were removed by washing the plate with RPMI, and fresh RPMI supplemented with 2% FBS and 20 ng/ml M-CSF was added to the adherent monocytes. Fresh M-CSF was added every 2 days, and the cells were incubated at 37°C, 5% CO2, for 5–6 days to allow differentiation. Cells were analyzed via flow cytometry, and were shown to be 95.95 (± 2.65) % (average ± SEM) positive for both CD14-PE-Cy7 and mannose receptor–fluorescein isothiocyanate.
Intratracheal M-CSF Installation and BAL
Mice were anesthetized via inhaled isoflurane and underwent tracheal cut-down for the installation of M-CSF. Four hours after installation (200 μg/kg), mice were killed, and 0.5 ml increments of PBS were added and withdrawn from the lungs three times. Samples were concentrated using YM-10 Centricon filters (Millipore Corporation, Beverly, MA), according to the manufacturer's instructions, and analyzed via ELISA for CCL2.
ELISA Analysis
Analysis was done using an ELx808 Microplate Reader (Bio-Tek Instruments, Winooski, VT). A standard curve r2 value of no less than 0.98 was considered acceptable for sample analysis. Blinded analysis of BALF from normal subjects and patients with IPF/usual interstitial pneumonitis (UIP) via ELISA was performed.
IPF Disease Classification
Patients were identified as part of the National Heart, Lung, and Blood Institute's Specialized Centers of Research Program in Occupational and Immunologic Lung Disease. The diagnosis of IPF was based on accepted American Thoracic Society criteria (37). Of the 68 patients with IPF, 46 had open lung biopsies, 13 had transbronchial biopsies, and the remaining 9 fulfilled all of the clinical criteria required for this diagnosis (37–39).
Human BAL Protocol
Fiberoptic bronchoscopy was performed with informed consent on healthy volunteer control subjects or patients with IPF. Fiberoptic bronchoscopy was performed through an endotracheal tube while the patient was ventilated on 100% oxygen at the University of Iowa. A total of 5 sequential 20-ml aliquots of normal saline were instilled and removed by gentle suctioning. Recovered BALF was placed in a sterile container on ice. Fluid was centrifuged at 1,200 rpm for 10 minutes at 4°C. Aliquots were stored in sterile polypropylene tubes at −80°C until processed.
Statistical Methods
For all ELISA and real-time PCR analyses, we used Minitab software (State College, PA), with analysis of variance and Tukey's post hoc testing. Student's t test was used for analysis of cell differentials from mouse BALF. For the human BAL data, cytokine levels were compared using the Kruskal-Wallis test for nonparametric comparisons and Spearman's rank correlation for measures of correlation. Logistic regression was used to determine the cytokine most closely associated with the sample origin (patient with IPF/UIP versus normal volunteer). The strength of association was determined by the Wald test, and cytokines were considered for inclusion in a regression model using stepwise selection techniques. These analyses were completed using SAS for Windows version 9.1 (SAS Institute, Cary, NC). A p value less than 0.05 was considered to be statistically significant for all comparisons.
RESULTS
M-CSF−/− Mice Are Protected from Bleomycin-induced Pulmonary Fibrosis
To determine the relevance of M-CSF in a mouse model of pulmonary fibrosis, M-CSF−/− and M-CSF+/+ mice were challenged with bleomycin. We used M-CSF−/− mice because they have fewer numbers of monocytes and macrophages (40), and bleomycin was used to generate pulmonary fibrosis (28–31, 41). In patients with IPF, lung infiltrates are predominantly subpleural and bibasilar (37). A recent review by Chua and colleagues discusses the significance of repeated bleomycin dosing and the subpleural lung involvement associated with intraperitoneal administration of bleomycin in mice (42), pathologically resembling IPF. As a result, we administered bleomycin via intraperitoneal injection to M-CSF+/+ and M-CSF−/− mice.
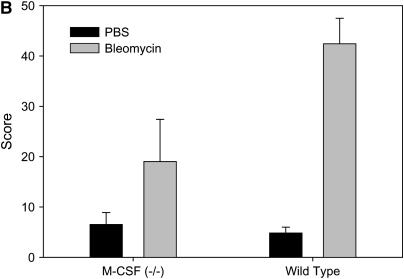
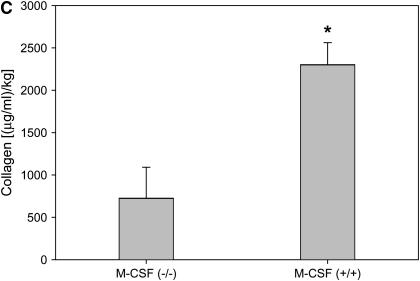
As shown in Figure 1, M-CSF−/− mice were protected from bleomycin-induced pulmonary fibrosis when compared with M-CSF+/+ littermates. M-CSF−/− mice had reduced influx of cells to the lungs and reduced interstitial thickening (Figure 1A, top panels), and reduced subpleural collagen deposition identified by trichrome staining (Figure 1A, bottom panels). A blinded pathologic assessment of lung fibrosis confirmed that M-CSF−/− mice had less subpleural lung involvement (Figure 1B) and less fibrosis (data not shown) than M-CSF+/+ mice after bleomycin treatment. Further analysis revealed that, after bleomycin treatment, M-CSF−/− mice had less mRNA for type I and type III collagen (Figure 2A) and CCL2 (Figure 2B) than M-CSF+/+ mice. Quantitation of lung collagen in mice treated with bleomycin was also performed (Figure 2C), and was consistent with the mRNA analysis.
Figure 1.
Macrophage colony-stimulating factor (M-CSF)−/− mice are protected from bleomycin-induced pulmonary fibrosis. (A) M-CSF−/− (left panels) and M-CSF+/+ (right panels) mice were treated with intraperitoneal bleomycin. Lungs were harvested, sectioned, and stained with hematoxylin and eosin (H&E) (top panels) or trichrome (bottom panels). Photomicrographs are representative of at least three mice per condition. Original magnification: ×20 (H&E) and ×10 (trichrome). (B) Blinded, subjective pathologic assessment of subpleural lung involvement as described in Methods. Data are representative of at least three mice per condition. PBS = phosphate-buffered saline.
Figure 2.
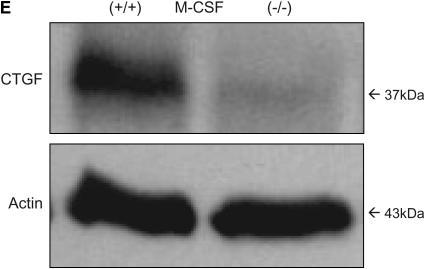
Macrophage colony-stimulating factor (M-CSF)−/− mice have reduced CC chemokine ligand 2 (CCL2), collagen, and connective tissue growth factor (CTGF) expression in response to bleomycin treatment. Real-time polymerase chain reaction (PCR) for type I and type III collagen (A), CCL2 (B), and CTGF (D) was performed on lung tissue from M-CSF+/+ and M-CSF−/− mice treated with bleomycin. (C) Sircol collagen assay on lungs from M-CSF−/− and M-CSF+/+ mice treated with bleomycin. Data represent mean and SEM of at least three mice per condition. *p < 0.05 versus M-CSF−/−. (E) Protein was isolated from lung tissue from bleomycin-treated M-CSF−/− and M-CSF+/+ mice, separated via sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotted for CTGF (top). The same blot was also probed for actin (bottom) to ensure equal loading.
To understand which growth factors were important in the pulmonary fibrosis induced by bleomycin, we assessed CTGF concentrations in lung tissue. CTGF can function downstream of active transforming growth factor (TGF)-β to drive collagen synthesis and fibrogenesis (43), and is implicated in bleomycin-induced pulmonary fibrosis in mice (44) and in humans with IPF (45). As shown in Figures 2D and 2E, lungs from M-CSF−/− mice had reduced CTGF expression compared with lungs from M-CSF+/+ mice treated with bleomycin, as shown by mRNA and protein analysis. However, analysis of active TGF-β in BALF of mice treated with bleomycin revealed no significant difference between M-CSF−/− and M-CSF+/+ mice (mean value of 34.3 ± 16.6 pg/ml for M-CSF−/− mice vs. 55.5 ± 41.2 pg/ml for M-CSF+/+ mice; p value = 0.24). These data established the importance of M-CSF and CTGF in the pathogenesis of pulmonary fibrosis.
CCL2−/− Mice Are Protected from Bleomycin-induced Pulmonary Fibrosis
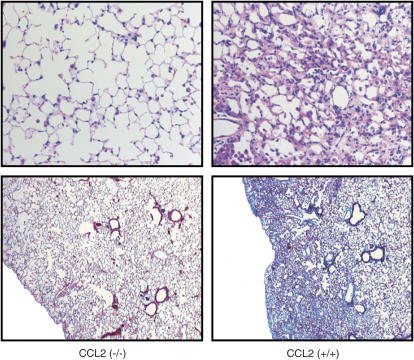
As mentioned, CCL2 and CCR2 are implicated in bleomycin-induced pulmonary fibrosis. However, mice genetically deficient in CCL2 have not been analyzed in our model of bleomycin-induced lung injury. Therefore, we performed intraperitoneal injections of bleomycin on CCL2−/− and CCL2+/+ mice. As shown in Figure 3, CCL2−/− mice were protected from bleomycin-induced pulmonary fibrosis when compared with CCL2+/+ mice, as demonstrated by decreased cellular influx to the lungs and reduced interstitial thickening (top panels), and decreased subpleural collagen deposition (bottom panels).
Figure 3.
CC chemokine ligand 2 (CCL2)−/− mice are protected from bleomycin-induced pulmonary fibrosis. CCL2−/− (left panels) and CCL2+/+ (right panels) mice were treated with intraperitoneal bleomycin. Lungs were harvested, sectioned, and stained with hematoxylin and eosin (H&E) (top panels) or trichrome (bottom panels). Photomicrographs are representative of at least two mice per condition. Original magnification: ×20 (H&E) and ×10 (trichrome).
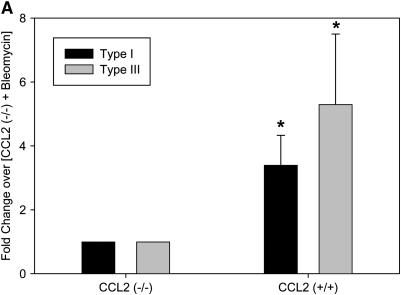
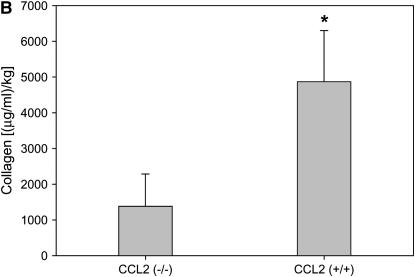
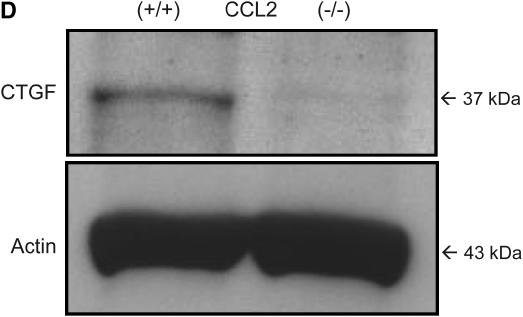
Similar to M-CSF−/− mice, CCL2−/− had decreased mRNA levels of type I and type III collagen (Figure 4A), and reduced total collagen in the lungs as determined by the Sircol collagen assay (Figure 4B). Furthermore, CCL2−/− mice also had reduced CTGF expression (Figures 4C and 4D), but no significant difference in the amount of active TGF-β (23.9 ± 13.9 pg/ml for CCL2−/− mice vs. 47.4 ± 21.9 pg/ml for CCL2+/+ mice; p value = 0.20), which is consistent with the observations in the M-CSF−/− and M-CSF+/+ mice. Of note, CCL2−/− mice also had reduced mRNA expression of CCL12 in the lungs after bleomycin treatment when compared with CCL2+/+ (see Figure E1 in the online supplement), consistent with previous findings published by Moore and colleagues (46). These findings implicate M-CSF, CCL2, CTGF, and CCL12 in the pathogenesis of pulmonary fibrosis, and support an in vivo relationship between M-CSF, CCL2, and CCL12 production.
Figure 4.
Loss of CC chemokine ligand 2 (CCL2) results in reduced collagen and connective tissue growth factor (CTGF) expression in response to bleomycin. Real-time polymerase chain reaction for type I and type II collagen (A) and CTGF (C) was performed on lung tissue from CCL2−/− and CCL2+/+ mice treated with bleomycin. (B) Sircol collagen assay on lungs from CCL2−/− and CCL2+/+ mice treated with bleomycin. Data represent mean and SD of two CCL2+/+ and five CCL2−/− mice. *p < 0.05 versus CCL2−/−. (D) Protein was isolated from CCL2−/− and CCL2+/+ mice treated with bleomycin, separated via SDS-PAGE, and Western blotted for CTGF (top) and actin (bottom) to ensure equal loading.
After Bleomycin Treatment, M-CSF−/− and CCL2−/− Have Reduced Numbers of Alveolar Macrophages in the Lungs
To investigate the effects of intraperitoneal bleomycin administration on inflammatory cell recruitment to the lungs, we performed two analyses. First, BAL was performed on mice treated with bleomycin, and assessment of the total number and type of cells isolated from M-CSF−/− and M-CSF+/+ mice is shown in Table 1. Although M-CSF−/− and M-CSF+/+ mice did not exhibit a significant difference in the total number of cells recruited to the lung after bleomycin injury, M-CSF−/− mice had a marked reduction in the number of alveolar macrophages when compared with M-CSF+/+ mice (mean value of 35,471 ± 8,417 macrophages for M-CSF−/− mice vs. 59,028 ± 8,039 for M-CSF+/+ mice; p < 0.05). Similarly, CCL2−/− mice displayed a significant reduction in the total number of cells recruited to the lung after bleomycin treatment when compared with CCL2+/+ mice, with a marked decrease in the number of alveolar macrophages (62,787 ± 6,942 macrophages for CCL2−/− mice vs. 126,600 ± 28,419 macrophages for CCL2+/+ mice; p < 0.05) and lymphocytes (Table 2).
TABLE 1.
ANALYSIS OF BRONCHOALVEOLAR LAVAGE CELL DIFFERENTIALS OF MACROPHAGE COLONY-STIMULATING FACTOR−/− AND MACROPHAGE COLONY-STIMULATING FACTOR+/+ MICE
| Parameter | M-CSF−/− PBS (n = 1) | M-CSF−/− Bleomycin (n = 3) | M-CSF+/+ PBS (n = 2) | M-CSF+/+ Bleomycin (n = 3) |
|---|---|---|---|---|
| BAL cells | ||||
| Total | 5,500 | 54,333 ± 9,244 | 12,500 ± 3,536 | 62,333 ± 7,535 |
| Macrophages | 3,476 | 35,471 ± 8,417* | 12,350 ± 3,748 | 59,028 ± 8,039 |
| Neutrophils | 578 | 18,393 ± 12,266 | 0 | 1,339 ± 697 |
| Lymphocytes | 1,447 | 469 ± 469 | 150 ± 212 | 1,967 ± 754 |
Definition of abbreviations: BAL = bronchoalveolar lavage; M-CSF = macrophage colony-stimulating factor; PBS = phosphate-buffered saline.
Data represent mean ± SD.
p < 0.05 compared with M-CSF+/+ after bleomycin treatment.
TABLE 2.
ANALYSIS OF BRONCHOALVEOLAR LAVAGE CELL DIFFERENTIALS OF CC CHEMOKINE LIGAND 2−/− AND CC CHEMOKINE LIGAND 2+/+ MICE
| Parameter | CCL2−/− PBS (n = 2) | CCL2−/− Bleomycin (n = 4) | CCL2+/+ PBS (n = 2) | CCL2+/+ Bleomycin (n = 4) |
|---|---|---|---|---|
| BAL cells | ||||
| Total | 14,400 ± 7,920 | 68,000 ± 6,916* | 29,500 ± 26,163 | 143,333 ± 27,285 |
| Macrophages | 14,100 ± 7,495 | 62,787 ± 6,942† | 26,620 ± 22,090 | 126,600 ± 28,419 |
| Neutrophils | 300 ± 424 | 1,801 ± 448 | 2,400 ± 3,394 | 1,860 ± 1.092 |
| Lymphocytes | 0 | 3,412 ± 1,287‡ | 480 ± 679 | 14,873 ± 1,412 |
Definition of abbreviations: BAL = bronchoalveolar lavage; CCL2 = CC chemokine ligand 2; PBS = phosphate-buffered saline.
Data represent mean ± SD.
p < 0.05 compared with CCL2+/+ after bleomycin treatment.
p < 0.05 compared with CCL2+/+ after bleomycin treatment.
p < 0.05 compared with CCL2+/+ after bleomycin treatment.
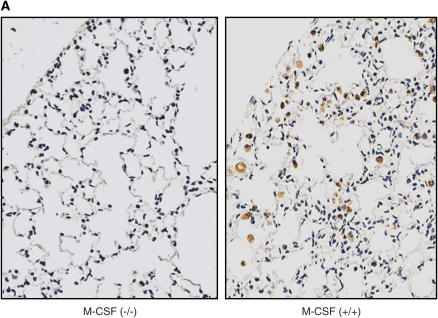
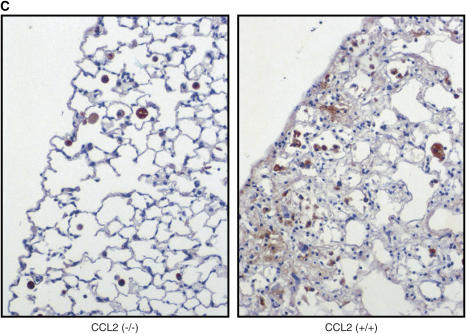
Second, lung sections of mice treated with bleomycin were stained with CD68, a marker of monocytes and macrophages. As shown in Figures 5A and 5B, M-CSF−/− mice had reduced numbers of CD68-positive cells in the lungs compared with M-CSF+/+ animals, which can be attributed to the reduced number of macrophages in the M-CSF−/− mice. Similarly, CCL2−/− also had less CD68-positive cells in the lung after bleomycin treatment (Figure 5C). These data support an important role for alveolar macrophages in the pathogenesis of pulmonary fibrosis.
Figure 5.
Loss of macrophage colony-stimulating factor (M-CSF) or CC chemokine ligand 2 (CCL2) results in reduced mononuclear phagocyte recruitment to the lung after bleomycin treatment. Immunohistochemical staining for CD68 in lung sections from M-CSF−/− and M-CSF+/+ mice (A) and CCL2−/− and CCL2+/+ mice (C) after bleomycin treatment. The data for the M-CSF−/− and M-CSF+/+ mice were quantitated and are shown in graphical form in (B). *p < 0.05 versus M-CSF−/−, bleomycin treated. Data represent mean and SEM of at least three mice per condition for the M-CSF−/− and M-CSF+/+ mice, and at least two mice per condition for the CCL2−/− and CCL2+/+ mice. Original magnification: ×20. PBS = phosphate-buffered saline.
M-CSF Stimulation Causes the Secretion of CCL2 In Vitro and In Vivo
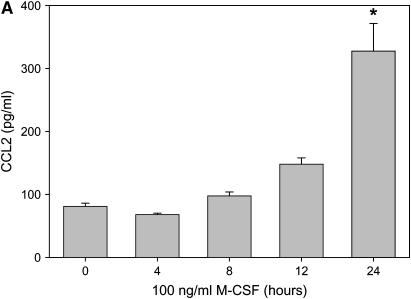
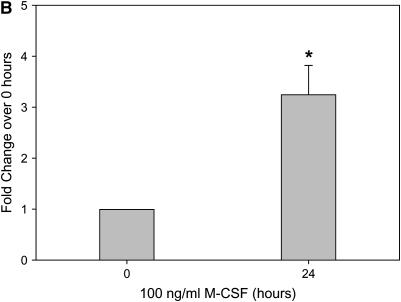
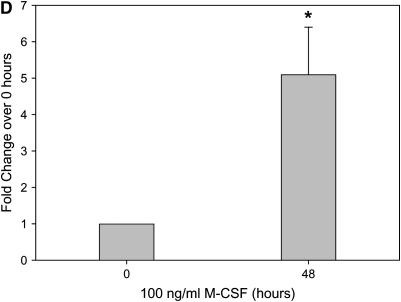
In our model of bleomycin-induced lung injury, the accumulation of macrophages in the lungs is correlated with more severe pulmonary fibrosis. Similarly, the decrease in the number of alveolar macrophages in M-CSF−/− and CCL2−/− mice treated with bleomycin led us to examine the relationship between M-CSF and CCL2. Therefore, we next sought to determine if M-CSF stimulation of macrophages induced CCL2 production. In an in vitro model, stimulation of mouse BMM and monocyte–derived macrophages with M-CSF increased CCL2 production and secretion, as shown via ELISA analysis (Figures 6A and 6C) and real-time PCR (Figures 6B and 6D). Macrophages were also stimulated with CCL2 to determine if M-CSF was produced or secreted. In contrast to the ability of M-CSF to induce CCL2, CCL2 stimulation of macrophages did not induce M-CSF production (data not shown). Of note, M-CSF stimulation of mouse BMMs also caused an increase in the production of CCL12 (Figure E2), suggesting that M-CSF may also support the development of pulmonary fibrosis via the induction of CCL12 and the subsequent recruitment of fibrocytes to the lungs.
Figure 6.
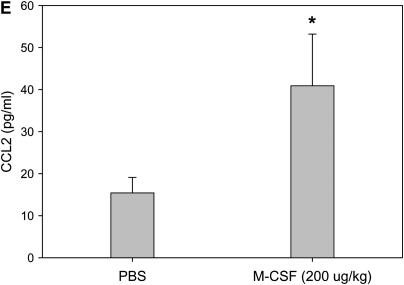
Macrophage colony-stimulating factor (M-CSF) induces CC chemokine ligand 2 (CCL2) secretion in vitro and in vivo. Mouse bone marrow–derived macrophages (A and B) or human monocyte–derived macrophages (C and D) were stimulated with 100 ng/ml of M-CSF for the indicated time points, and cell supernatants were collected and analyzed for CCL2 via ELISA (A and C). In addition, cells were lysed, RNA isolated, and real-time polymerase chain reaction performed for CCL2 (B and D). Graphs represent mean and SEM of at least three independent experiments. *p < 0.05 versus 0 hour. (E) Mice were anesthetized and intratracheal installation of M-CSF (200 μg/kg) was performed. Mice were killed, a bronchoalveolar lavage (BAL) was performed, and the BAL fluid collected and analyzed for CCL2 via ELISA. Graph represents mean and SEM of three mice per condition. *p < 0.05 versus phosphate-buffered saline (PBS) control.
We next sought to test if this relationship was maintained in vivo. We instilled M-CSF intratracheally into mice and measured CCL2 levels in the BALF. As shown in Figure 6E, CCL2 was increased in BALF after M-CSF installation when compared with the vehicle control animals. These data confirm that M-CSF mediated the secretion of CCL2 in vitro and in vivo.
Patients with IPF Exhibit Elevated Levels of M-CSF in BALF
Human lung diseases like IPF are accompanied by elevated levels of inflammatory cytokines and chemokines in the lung (13, 16). Because BALF from patients with IPF have elevated levels of CCL2 and CCL3 and striking increases in the number of mononuclear phagocytes recruited to the lung (13, 14, 16, 47), we next sought to determine if M-CSF levels were elevated in BALF from patients with this disease.
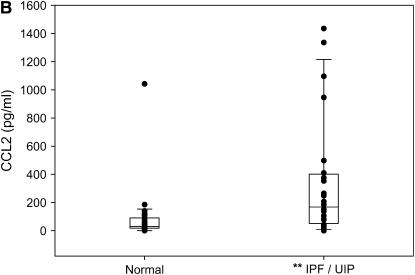
As shown in Figure 7A, patients with IPF/UIP had higher levels of M-CSF in BALF than did normal volunteers (median values, 154.6 versus 49.2 pg/ml; p = 0.0126). Consistent with previous findings (16, 47), CCL2 levels were also elevated in BALF from patients with IPF/UIP compared with normal subjects (median values, 168.2 vs. 30.1 pg/ml; p = 0.0011) (Figure 7B). Based on disease status, the correlation of M-CSF to CCL2 was greater among the patients with IPF/UIP than among the normal subjects (ρ = 0.53 vs. 0.43, respectively). There was a linear association between BALF M-CSF levels and the odds that a sample was from a patient with IPF/UIP (odds ratio, 1.005; 95% confidence interval, 1.001–1.010; p = 0.0194). After adjusting for the M-CSF level, CCL2 levels were not associated with the odds that the sample was obtained from a patient with IPF/UIP rather than a normal volunteer.
Figure 7.
Bronchoalveolar lavage fluid from patients with idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonitis (UIP) has elevated macrophage colony-stimulating factor (M-CSF) and CC chemokine ligand 2 (CCL2) when compared with normal volunteers. Blinded ELISA analysis of normal volunteers (n = 26) and patients with IPF/UIP (n = 24) for M-CSF (A) and CCL2 (B). Presented are box and whisker plots with the median value (interquartile range) of each dataset. Median values for M-CSF ELISA were 154.6 pg/ml for IPF/UIP versus 49.2 pg/ml for normal subjects. Median values for CCL2 ELISA were 168.2 pg/ml for IPF/UIP versus 30.1 pg/ml for normal subjects. *p = 0.0126 versus normal for M-CSF; **p = 0.0011 versus normal for CCL2 (p values obtained from Kruskal-Wallis test).
Interestingly, serum levels of M-CSF in patients with IPF/UIP were not elevated when compared with patients without IPF/UIP (data not shown), suggesting that elevated M-CSF levels in these patients may be compartmentalized to the lung.
DISCUSSION
Data in this article clarify a causal relationship between mononuclear phagocytes, M-CSF, and CCL2 in the pathogenesis of pulmonary fibrosis. In addition to functioning as a chemoattractant in vitro and in vivo (48, 49), we found that M-CSF induced the production and secretion of CCL2 in vitro and in vivo. We also found that M-CSF−/− and CCL2−/− animals were protected from developing pulmonary fibrosis after intraperitoneal bleomycin injection. Although M-CSF−/− mice treated with bleomycin had reduced lung mRNA levels of CCL2 when compared with M-CSF+/+ mice, the M-CSF levels in CCL2−/− and CCL2+/+ mice treated with bleomycin were not different (data not shown). These data further implicate M-CSF as a mediator of CCL2 expression, and places M-CSF upstream of CCL2 production in this model.
To address the potential mechanism underlying the protection afforded to M-CSF−/− and CCL2−/− mice, we showed that production of CTGF, an important growth factor involved in the development of fibrosis, is reduced in both of these bleomycin-treated mouse models. In addition, because we found a statistical relationship between M-CSF and CCL2 in BALF from patients with IPF/UIP, M-CSF can be identified as a potential molecular target and/or biomarker in IPF. Of note, Avivi-Green and colleagues recently demonstrated that discoidin domain receptor 1–deficient mice were protected from bleomycin-induced lung injury, and displayed decreased numbers of F4/80-positive macrophages in the lung and a trend toward decreased CCL2 levels in BALF when compared with discoidin domain receptor 1+/+ mice (50). We also found that CCL12 production related to M-CSF, and tracked that of CCL2 production in our models, suggesting that fibrocyte recruitment to the lungs may also be related to M-CSF concentrations. Taken together, these data suggest that M-CSF, CCL2, CCL12, and mononuclear phagocytes were key factors in the pathogenesis in our model of bleomycin-induced pulmonary fibrosis.
The production of CCL2 after M-CSF stimulation of mononuclear phagocytes has many implications. Support for an in vivo role for CCL2 in pulmonary fibrosis is evident in studies using mice deficient in CCR2, the receptor for CCL2. These mice have less fibrosis in response to bleomycin challenge than wild-type mice (29–31), complementing previous reports of increased levels of CCL2 in BALF from patients with IPF/UIP (13–16). Similarly, in animal models of wound healing, CCL2−/− mice have decreased collagen synthesis and delayed wound reepithelialization compared with CCL2+/+ mice (51). In our mouse model of bleomycin-induced lung injury, the amounts of active TGF-β and CTGF were increased after bleomycin treatment, and TGF-β has been shown to stimulate CTGF expression (52). In fibroblasts, CCL2 induces TGF-β and collagen production (53), and topical administration of M-CSF in an animal model of wound healing increases TGF-β1 mRNA levels via the activation of tissue macrophages (54). Although we did not find differences in the levels of active TGF-β in BALF from bleomycin-treated M-CSF−/− or CCL2−/− mice compared with wild-type background-control mice, this may be explained by several mechanisms. Several studies suggest that up-regulation of CTGF expression may occur independent of TGF-β activation or signaling (55–58). Alternatively, the lack of differences may also be due to the timing of the samples and/or small numbers of mice evaluated in this study.
Dysfunctional interactions between alveolar epithelial cells and fibroblasts also play a role in pulmonary fibrosis. Recent evidence suggests that TGF-β induces epithelial–mesenchymal transition, which may augment myofibroblast production in IPF (59). Moore and colleagues demonstrated that CCL2 inhibits prostaglandin E2 synthesis, and that decreased prostaglandin E2 synthesis results in enhanced fibroproliferation (60), another hallmark of pulmonary fibrosis. Therefore, we hypothesize that M-CSF–induced CCL2 secretion may result in mononuclear phagocyte recruitment, fibroproliferation, TGF-β–mediated CTGF expression, and eventual fibrosis.
Observations presented in this article are significant in several aspects. First, to our understanding, M-CSF has not been linked to the pathogenesis of pulmonary fibrosis in animals or humans. Second, M-CSF statistically correlated to CCL2 levels in BALF in patients with IPF/UIP, identifying M-CSF as a potential new target for the treatment or diagnosis of pulmonary fibrosis. With the data presented supporting the involvement of mononuclear phagocytes in the pathogenesis of pulmonary fibrosis, the importance of M-CSF becomes more apparent, especially considering its role in mononuclear phagocyte homeostasis. One study demonstrated that patients with IPF that were treated with the immunosuppressant, prednisone, had a greater percentage of alveolar macrophages in BALF (33). Another study showed that dexamethasone enhanced the M-CSF–induced proliferation of murine BMMs (61). This is significant, because patients with IPF generally do not respond favorably to corticosteroid therapy, revealing that the targeting of alveolar macrophages may be an important new direction in treating IPF. Furthermore, these data suggest a novel mechanism by which fibrosis may progress in the presence of corticosteroid treatment.
Lastly, this article begins to uncover a potential mechanism for mononuclear phagocytes in the pathogenesis of this disease, presumably due to the production and release of cytokines and profibrotic factors. In summary, although a role for mononuclear phagocytes in the pathogenesis of pulmonary fibrosis is supported in various studies (27, 30, 32, 33), the potential role for M-CSF as a primary mediator of mononuclear phagocyte recruitment and CCL2 secretion has not been established.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the assistance and expertise of personnel from Ohio State University, including Cheryl Schneider in the care and treatment of all mice used for this study, Susie Jones for her help with immunohistochemical staining, Dr. Christine Montague for her help with the connective tissue growth factor expression experiments, and Dr. Naeem Ali for his guidance in the evaluation of all patient samples. Additionally, the authors are grateful to Linda Powers (University of Iowa) for her efforts in coordinating the transfer of all patient samples.
Supported by the National Institutes of Health, National Heart, Lung, and Blood Institute grants T32 HL 07946–01 (C.P.B.), K23 HL 075076–01 (J.M.O.), RO-1 HL 66108, RO-1 HL 67176, RO-1 HL 63800, and PO-1 HL 70294-02 (C.B.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200609-1279OC on April 12, 2007
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Horiguchi J, Warren MK, Ralph P, Kufe D. Expression of the macrophage specific colony-stimulating factor (CSF-1) during human monocytic differentiation. Biochem Biophys Res Commun 1986;141:924–930. [DOI] [PubMed] [Google Scholar]
- 2.Rajavashisth TB, Andalibi A, Territo MC, Berliner JA, Navab M, Fogelman AM, Lusis AJ. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature 1990;344:254–257. [DOI] [PubMed] [Google Scholar]
- 3.Oster W, Lindemann A, Horn S, Mertelsmann R, Herrmann F. Tumor necrosis factor (TNF)-α but not TNF-β induces secretion of colony stimulating factor for macrophages (CSF-1) by human monocytes. Blood 1987;70:1700–1703. [PubMed] [Google Scholar]
- 4.Inaba T, Yamada N, Gotoda T, Shimano H, Shimada M, Momomura K, Kadowaki T, Motoyoshi K, Tsukada T, Morisaki N. Expression of M-CSF receptor encoded by c-fms on smooth muscle cells derived from arteriosclerotic lesion. J Biol Chem 1992;267:5693–5699. [PubMed] [Google Scholar]
- 5.Arceci RJ, Shanahan F, Stanley ER, Pollard JW. Temporal expression and location of colony-stimulating factor 1 (CSF-1) and its receptor in the female reproductive tract are consistent with CSF-1–regulated placental development. Proc Natl Acad Sci USA 1989;86:8818–8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shyy YJ, Wickham LL, Hagan JP, Hsieh HJ, Hu YL, Telian SH, Valente AJ, Sung KL, Chien S. Human monocyte colony-stimulating factor stimulates the gene expression of monocyte chemotactic protein-1 and increases the adhesion of monocytes to endothelial monolayers. J Clin Invest 1993;92:1745–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci USA 1995;92:8264–8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiao JH, Tripathi J, Mishra NK, Cai Y, Tripathi S, Wang XP, Imes S, Fishbein MC, Clinton SK, Libby P, et al. Role of macrophage colony-stimulating factor in atherosclerosis: studies of osteopetrotic mice. Am J Pathol 1997;150:1687–1699. [PMC free article] [PubMed] [Google Scholar]
- 9.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 1998;394:894–897. [DOI] [PubMed] [Google Scholar]
- 10.Gosling J, Slaymaker S, Gu L, Tseng S, Zlot CH, Young SG, Rollins BJ, Charo IF. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest 1999;103:773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med 2001;193:727–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood 2000;96:34–40. [PubMed] [Google Scholar]
- 13.Smith RE, Strieter RM, Zhang K, Phan SH, Standiford TJ, Lukacs NW, Kunkel SL. A role for C-C chemokines in fibrotic lung disease. J Leukoc Biol 1995;57:782–787. [DOI] [PubMed] [Google Scholar]
- 14.Rose CE Jr, Sung SS, Fu SM. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation 2003;10:273–288. [DOI] [PubMed] [Google Scholar]
- 15.Driscoll KE. Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res 1994;20:473–490. [DOI] [PubMed] [Google Scholar]
- 16.Capelli A, Di Stefano A, Gnemmi I, Donner CF. CCR5 expression and CC chemokine levels in idiopathic pulmonary fibrosis. Eur Respir J 2005;25:701–707. [DOI] [PubMed] [Google Scholar]
- 17.Traves SL, Smith SJ, Barnes PJ, Donnelly LE. Specific CXC but not CC chemokines cause elevated monocyte migration in COPD: a role for CXCR2. J Leukoc Biol 2004;76:441–450. [DOI] [PubMed] [Google Scholar]
- 18.Saetta M, Baraldo S, Zuin R. Neutrophil chemokines in severe exacerbations of chronic obstructive pulmonary disease: fatal chemo-attraction? Am J Respir Crit Care Med 2003;168:911–913. [DOI] [PubMed] [Google Scholar]
- 19.Fillion I, Ouellet N, Simard M, Bergeron Y, Sato S, Bergeron MG. Role of chemokines and formyl peptides in pneumococcal pneumonia–induced monocyte/macrophage recruitment. J Immunol 2001;166:7353–7361. [DOI] [PubMed] [Google Scholar]
- 20.Huffnagle GB, Strieter RM, Standiford TJ, McDonald RA, Burdick MD, Kunkel SL, Toews GB. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J Immunol 1995;155:4790–4797. [PubMed] [Google Scholar]
- 21.Standiford TJ, Kunkel SL, Lukacs NW, Greenberger MJ, Danforth JM, Kunkel RG, Strieter RM. Macrophage inflammatory protein-1 α mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol 1995;155:1515–1524. [PubMed] [Google Scholar]
- 22.Denis M. Proinflammatory cytokines in hypersensitivity pneumonitis. Am J Respir Crit Care Med 1995;151:164–169. [DOI] [PubMed] [Google Scholar]
- 23.Brieland JK, Jones ML, Flory CM, Miller GR, Warren JS, Phan SH, Fantone JC. Expression of monocyte chemoattractant protein-1 (MCP-1) by rat alveolar macrophages during chronic lung injury. Am J Respir Cell Mol Biol 1993;9:300–305. [DOI] [PubMed] [Google Scholar]
- 24.Riches DW, Henson PM. Functional aspects of mononuclear phagocyte involvement in lung inflammation. Ann N Y Acad Sci 1986;465:6–14. [DOI] [PubMed] [Google Scholar]
- 25.Lee TH. Interactions between alveolar macrophages, monocytes, and granulocytes: implications for airway inflammation. Am Rev Respir Dis 1987;135:S14–S17. [DOI] [PubMed] [Google Scholar]
- 26.Corrigan CJ, Kay AB. The roles of inflammatory cells in the pathogenesis of asthma and of chronic obstructive pulmonary disease. Am Rev Respir Dis 1991;143:1165–1168. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds HY. Lung inflammation and fibrosis: an alveolar macrophage-centered perspective from the 1970s to 1980s. Am J Respir Crit Care Med 2005;171:98–102. [DOI] [PubMed] [Google Scholar]
- 28.Inoshima I, Kuwano K, Hamada N, Hagimoto N, Yoshimi M, Maeyama T, Takeshita A, Kitamoto S, Egashira K, Hara N. Anti-monocyte chemoattractant protein-1 gene therapy attenuates pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 2004;286:L1038–L1044. [DOI] [PubMed] [Google Scholar]
- 29.Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine 2003;24:266–276. [DOI] [PubMed] [Google Scholar]
- 30.Okuma T, Terasaki Y, Kaikita K, Kobayashi H, Kuziel WA, Kawasuji M, Takeya M. C-C chemokine receptor 2 (CCR2) deficiency improves bleomycin-induced pulmonary fibrosis by attenuation of both macrophage infiltration and production of macrophage-derived matrix metalloproteinases. J Pathol 2004;204:594–604. [DOI] [PubMed] [Google Scholar]
- 31.Moore BB, Paine R III, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol 2001;167:4368–4377. [DOI] [PubMed] [Google Scholar]
- 32.Martinez FJ, Keane MP. Update in diffuse parenchymal lung diseases 2005. Am J Respir Crit Care Med 2006;173:1066–1071. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds HY, Fulmer JD, Kazmierowski JA, Roberts WC, Frank MM, Crystal RG. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest 1977;59:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehnert BE, Valdez YE, Lehnert NM, Park MS, Englen MD. Stimulation of rat and murine alveolar macrophage proliferation by lung fibroblasts. Am J Respir Cell Mol Biol 1994;11:375–385. [DOI] [PubMed] [Google Scholar]
- 35.Baran CP, Tridandapani S, Helgason CD, Humphries RK, Krystal G, Marsh CB. The inositol 5′-phosphatase SHIP-1 and the Src kinase Lyn negatively regulate macrophage colony-stimulating factor–induced Akt activity. J Biol Chem 2003;278:38628–38636. [DOI] [PubMed] [Google Scholar]
- 36.Eubank TD, Roberts R, Galloway M, Wang Y, Cohn DE, Marsh CB. GM-CSF induces expression of soluble VEGF receptor-1 from human monocytes and inhibits angiogenesis in mice. Immunity 2004;21:831–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Thoracic Society (ATS) and the European Respiratory Society (ERS). Idiopathic pulmonary fibrosis: diagnosis and treatment: international consensus statement. Am J Respir Crit Care Med 2000;161:646–664. [DOI] [PubMed] [Google Scholar]
- 38.Hunninghake GW, Lynch DA, Galvin JR, Gross BH, Muller N, Schwartz DA, King TE Jr, Lynch JP III, Hegele R, Waldron J, et al. Radiologic findings are strongly associated with a pathologic diagnosis of usual interstitial pneumonia. Chest 2003;124:1215–1223. [DOI] [PubMed] [Google Scholar]
- 39.Coxson HO, Hogg JC, Mayo JR, Behzad H, Whittall KP, Schwartz DA, Hartley PG, Galvin JR, Wilson JS, Hunninghake GW. Quantification of idiopathic pulmonary fibrosis using computed tomography and histology. Am J Respir Crit Care Med 1997;155:1649–1656. [DOI] [PubMed] [Google Scholar]
- 40.Wiktor-Jedrzejczak W, Ratajczak MZ, Ptasznik A, Sell KW, Ahmed-Ansari A, Ostertag W. CSF-1 deficiency in the op/op mouse has differential effects on macrophage populations and differentiation stages. Exp Hematol 1992;20:1004–1010. [PubMed] [Google Scholar]
- 41.Parvinen LM, Kilkku P, Makinen E, Liukko P, Gronroos M. Factors affecting the pulmonary toxicity of bleomycin. Acta Radiol Oncol 1983;22:417–421. [DOI] [PubMed] [Google Scholar]
- 42.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol 2005;33:9–13. [DOI] [PubMed] [Google Scholar]
- 43.Duncan MR, Frazier KS, Abramson S, Williams S, Klapper H, Huang X, Grotendorst GR. Connective tissue growth factor mediates transforming growth factor β–induced collagen synthesis: down-regulation by cAMP. FASEB J 1999;13:1774–1786. [PubMed] [Google Scholar]
- 44.Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol 1998;275:L365–L371. [DOI] [PubMed] [Google Scholar]
- 45.Pan LH, Yamauchi K, Uzuki M, Nakanishi T, Takigawa M, Inoue H, Sawai T. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J 2001;17:1220–1227. [DOI] [PubMed] [Google Scholar]
- 46.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 2006;35:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Standiford TJ, Rolfe MW, Kunkel SL, Lynch JP III, Burdick MD, Gilbert AR, Orringer MB, Whyte RI, Strieter RM. Macrophage inflammatory protein-1 α expression in interstitial lung disease. J Immunol 1993;151:2852–2863. [PubMed] [Google Scholar]
- 48.Vedham V, Phee H, Coggeshall KM. Vav activation and function as a rac guanine nucleotide exchange factor in macrophage colony-stimulating factor–induced macrophage chemotaxis. Mol Cell Biol 2005;25:4211–4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierce JH, Di Marco E, Cox GW, Lombardi D, Ruggiero M, Varesio L, Wang LM, Choudhury GG, Sakaguchi AY, Di Fiore PP. Macrophage-colony-stimulating factor (CSF-1) induces proliferation, chemotaxis, and reversible monocytic differentiation in myeloid progenitor cells transfected with the human c-fms/CSF-1 receptor cDNA. Proc Natl Acad Sci USA 1990;87:5613–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avivi-Green C, Singal M, Vogel WF. Discoidin domain receptor 1–deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med 2006;174:420–427. [DOI] [PubMed] [Google Scholar]
- 51.Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA. Wound healing in MIP-1α(−/−) and MCP-1(−/−) mice. Am J Pathol 2001;159:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor β response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ 1996;7:469–480. [PubMed] [Google Scholar]
- 53.Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor β1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem 1996;271:17779–17784. [DOI] [PubMed] [Google Scholar]
- 54.Wu L, Yu YL, Galiano RD, Roth SI, Mustoe TA. Macrophage colony-stimulating factor accelerates wound healing and upregulates TGF-β1 mRNA levels through tissue macrophages. J Surg Res 1997;72:162–169. [DOI] [PubMed] [Google Scholar]
- 55.Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem 2001;276:10594–10601. [DOI] [PubMed] [Google Scholar]
- 56.Leask A, Sa S, Holmes A, Shiwen X, Black CM, Abraham DJ. The control of ccn2 (ctgf) gene expression in normal and scleroderma fibroblasts. Mol Pathol 2001;54:180–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holmes A, Abraham DJ, Chen Y, Denton C, Shi-wen X, Black CM, Leask A. Constitutive connective tissue growth factor expression in scleroderma fibroblasts is dependent on Sp1. J Biol Chem 2003;278:41728–41733. [DOI] [PubMed] [Google Scholar]
- 58.Zhou G, Li C, Cai L. Advanced glycation end-products induce connective tissue growth factor–mediated renal fibrosis predominantly through transforming growth factor β–independent pathway. Am J Pathol 2004;165:2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial–mesenchymal transition in alveolar epithelial cells by transforming growth factor-β1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 2005;166:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore BB, Peters-Golden M, Christensen PJ, Lama V, Kuziel WA, Paine R III, Toews GB. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol 2003;284:L342–L349. [DOI] [PubMed] [Google Scholar]
- 61.Lloberas J, Soler C, Celada A. Dexamethasone enhances macrophage colony stimulating factor– and granulocyte macrophage colony stimulating factor–stimulated proliferation of bone marrow–derived macrophages. Int Immunol 1998;10:593–599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.