Abstract
Rationale: Computed tomography (CT) scanning of the lung may reduce phenotypic heterogeneity in defining subjects with chronic obstructive pulmonary disease (COPD), and allow identification of genetic determinants of emphysema severity and distribution.
Objectives: We sought to identify genes associated with CT scan distribution of emphysema in individuals without α1-antitrypsin deficiency but with severe COPD.
Methods: We evaluated baseline CT densitometry phenotypes in 282 individuals with emphysema enrolled in the Genetics Ancillary Study of the National Emphysema Treatment Trial, and used regression models to identify genetic variants associated with emphysema distribution.
Measurements and Main Results: Emphysema distribution was assessed by two methods—assessment by radiologists and by computerized density mask quantitation, using a threshold of −950 Hounsfield units. A total of 77 polymorphisms in 20 candidate genes were analyzed for association with distribution of emphysema. GSTP1, EPHX1, and MMP1 polymorphisms were associated with the densitometric, apical-predominant distribution of emphysema (p value range = 0.001–0.050). When an apical-predominant phenotype was defined by the radiologist scoring method, GSTP1 and EPHX1 single-nucleotide polymorphisms were found to be significantly associated. In a case–control analysis of COPD susceptibility limited to cases with densitometric upper-lobe–predominant cases, the EPHX1 His139Arg single-nucleotide polymorphism was associated with COPD (p = 0.005).
Conclusions: Apical and basal emphysematous destruction appears to be influenced by different genes. Polymorphisms in the xenobiotic enzymes, GSTP1 and EPHX1, are associated with apical-predominant emphysema. Altered detoxification of cigarette smoke metabolites may contribute to emphysema distribution, and these findings may lead to further insight into genetic determinants of emphysema.
Keywords: COPD, genetics, association analysis, computed tomography, emphysema
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
There are few studies regarding the genetic factors that may determine emphysema distribution. Computed tomography scanning may reduce phenotypic heterogeneity in chronic obstructive pulmonary disease genetic studies, and allow identification of genetic determinants of emphysema severity and distribution.
What This Study Adds to the Field
This study identifies genes encoding xenobiotic enzymes as differentially associated with distributional features of emphysema. Altered detoxification of cigarette smoke metabolites may contribute to emphysema distribution.
The main etiologic factor contributing to the development of chronic obstructive pulmonary disease (COPD) is cigarette smoking. Only a minority of individuals who smoke cigarettes develop COPD, suggesting that genetic variability may be an important determinant of disease. Clinically, COPD is usually diagnosed on the basis of airflow obstruction that is not fully reversible after the administration of inhaled bronchodilators. However, using spirometric measures to define case versus control status in genetic association studies of COPD ignores the inherent heterogeneity of the disorder. The clinical diagnosis of COPD includes chronic bronchitis, emphysema, and small airway disease, and each of these processes may have different genetic determinants. Genetic association studies of COPD have been plagued by frequent nonreplication (1). Although there are many reasons for nonreplication, such as small sample sizes, population stratification, and genetic heterogeneity (2), phenotypic heterogeneity may be an important reason for nonreplication. Identification of reproducible COPD phenotypes that capture specific features about COPD subtypes, such as radiologic quantification of emphysema, may facilitate the identification of COPD susceptibility genes. These observations may also provide insight into the pathways that contribute to the development of chronic bronchitis, small airway disease, or emphysema. Such insights may have future diagnostic and therapeutic implications.
Chest computed tomography (CT) scans in patients with COPD can lead to more accurate and earlier diagnosis of the extent of lung destruction, because individuals can have emphysema as an early manifestation of smoking-related lung injury before they develop airflow obstruction (3). Radiologists can visually score regional low-attenuation areas of the lung, but visual scoring may vary between readers. Objective quantification of emphysema can be automated with computer algorithms that measure the lung area occupied by pixels with attenuation below a threshold value (4–6); in turn, these CT phenotypes can be used in genetic association studies of COPD (7–9).
A proven genetic cause of emphysema is severe α1-antitrypsin deficiency (AATD). In deficient individuals, emphysema is often (but not always) observed to be predominant in the lung bases (10), suggesting that there may be genetic influences on the distribution of emphysema, and not just a genetic influence on overall COPD susceptibility. We hypothesized that the distribution of emphysema may be associated with genetic factors other than AATD in individuals with COPD. Understanding the genetic influences on emphysema distribution may provide further insight into the variable development of COPD in smokers.
METHODS
Populations
The National Emphysema Treatment Trial (NETT) was a multicenter treatment trial for the investigation of health-related outcomes in people with severe COPD randomized to medical versus surgical (lung volume reduction) treatment (11). Emphysema was an inclusion criterion. All participants had severe airflow limitation (FEV1 ⩽ 45% predicted) and evidence of emphysema on chest CT scan at the time of randomization. A subset of participants was enrolled in the NETT Genetics Ancillary Study; 282 of the 304 white individuals who provided blood samples for DNA extraction in the initial phase of the NETT Genetics Ancillary Study were included in this study of CT emphysema phenotypes.
The Normative Aging Study (NAS) is a longitudinal study of aging, performed by the Veterans Administration; the use of this cohort as a control group has been recently described (12–14). In brief, we selected 441 white individuals with no evidence of airflow obstruction (FEV1 > 80% predicted and FEV1/FVC ratio > 90% predicted) and at least a 10 pack-year smoking history; chest CT scans were not available on the control subjects.
Candidate Gene and Single-Nucleotide Polymorphism Selection and Genotyping
A total of 20 genes (77 single-nucleotide polymorphisms [SNPs]) were considered in the present study, including positional candidate genes in regions linked to COPD phenotypes (G protein–coupled receptor family C, group 5, member A; IL-8 receptors α and β; latent transforming growth factor β binding protein-4; matrix Gla protein; serine protease inhibitor E2; transforming growth factor β-1; microsomal glutathione S-transferase-1), biologically plausible genes based on COPD pathophysiology (elastin, matrix metalloproteinase-12), and genes selected on the basis of previously published significant associations with COPD (α1-antichymotrypsin, microsomal epoxide hydrolase, vitamin D binding protein, glutathione S-transferase M1 and P1, matrix metalloproteinase 1, surfactant protein B and D, tissue inhibitor of metalloproteinase-2, and tumor necrosis factor-α); see Table E1 in the online supplement for a summary of Human Genome Organization symbols and the number of SNPs genotyped. The rationale for the selection of these genes and SNPs, details about genotyping methods, as well as the results from a case–control analysis for COPD susceptibility have been published recently (1, 13). Unlike Hersh and colleagues, we did not include short tandem repeat markers in our analysis (1, 13).
CT Scan Densitometric Analysis
From October 1997 until July 2002, individuals enrolled in NETT underwent chest CT scanning. CT scans were performed on one of three types of scanners (General Electric, Fairfield, CT; Siemens, Malvern, PA; or Picker International, Toronto, Canada) during a breath hold at full inflation and without intravenous contrast enhancement. The subset of individuals included in our analyses had CT scans that were performed at a range of 2- to 8-mm slice thicknesses, with 75% of the scan data from 4 to 5 mm, and only one CT scan with 8-mm slice thickness. Densitometric measures of the lungs at −950 Hounsfield units were performed using the Pulmonary Analysis Software Suite (PASS, Iowa City, IA). This software segmented the lungs into thirds based on lung height, followed by the density mask analysis. Calculations were performed for each third of each lung as well as for the whole lung.
Apical and basal percent emphysema metrics from densitometric analysis were calculated by dividing the computer-generated voxel measures in the region by the total lung voxels in the region. The difference between the percent emphysema in the apical and basal one-third of the lung, respectively, was calculated for each individual to represent the densitometry distribution of emphysema.
Manual radiologist scoring methods in NETT have been described previously (15). Each CT scan was scored for emphysema by a radiologist who underwent training with a set of CT scan standards. Each CT scan was evaluated by a single reader, and the reader was blinded to the results of densitometric CT analysis. Each lung was divided into three zones, and scores between 0 and 4 were given for each subdivision, with 4 corresponding to very severe emphysema. To calculate NETT radiologist emphysema distribution based on the radiologist scores (with positive values for the difference corresponding to upper zone–predominant disease), the mean of the radiologist's scores for the upper third of the lung was subtracted from the mean of the lower third.
Statistical Analysis
General linear regression models were used to assess polymorphism–phenotype associations for each of the three CT densitometry phenotypes (apical percent emphysema, basal percent emphysema, and emphysema distribution) and the NETT radiologist scores. Models were adjusted for pack-years of smoking, age, sex, and post-bronchodilator FEV1% predicted. Haplotype analyses were performed using the expectation–maximization (EM) algorithm and score tests as implemented in the haplo.stats program (16). SAS/Genetics (SAS Institute, Cary, NC) was used for the overall case-control analysis, which allows for assessing additive allelic effects and tests for trends. Hardy-Weinberg equilibrium was verified in the controls using the exact test as implemented in SAS. Odds ratios (ORs) were calculated from a 2 × 2 table of allele counts.
RESULTS
The demographics, pulmonary function, and chest CT lung density characteristics are presented in Table 1. This table also includes the features of those individuals randomized into the medical and surgical treatment arms (which were used as a test–replication verification of the overall results). Of the 282 individuals with CT scan data, none had severe AATD (PI ZZ); 17 MZ, 19 MS, and 3 SZ individuals were included in all analyses, followed by a sensitivity analysis after exclusion of these individuals. Characteristics of the individuals in the genetics ancillary study are comparable to the 1,218 individuals randomized in the overall NETT (17). In our cohort, there was no correlation between post-bronchodilator FEV1 and densitometric measures of emphysema, and only modest correlation between diffusing capacity of carbon monoxide (DlCO) and distributional features of emphysema (Pearson correlation coefficient −0.21 between DlCO and upper lobe–predominant densitometric measure; p = 0.0003).
TABLE 1.
DEMOGRAPHICS AND CHARACTERISTICS OF NATIONAL EMPHYSEMA TREATMENT TRIAL SUBJECTS WITH BASELINE COMPUTED TOMOGRAPHY SCAN PHENOTYPES
| Characteristics | Overall Cohort (n = 282) | Medical Treatment (n = 138) | Surgical Treatment (n = 144) |
|---|---|---|---|
| Male, n (%) | 178 (63) | 89 (64) | 89 (62) |
| Age (SD), yr | 67.3 (6.0) | 67.4 (5.4) | 67.1 (6.5) |
| Pack-years of cigarettes (SD) | 66.9 (31.7) | 65.6 (32.6) | 68.1 (30.9) |
| FEV1% predicted (SD), post-bronchodilator | 28.1 (7.3) | 28.3 (6.9) | 28.0 (7.6) |
| DlCO % predicted (SD) | 30.0 (10.2) | 29.8 (10.6) | 30.2 (9.9) |
| Radiologist upper lobe–predominant (%) | 187 (66.3) | 89 (64) | 98 (68) |
| Radiologist score: mean upper lobes (SD) | 1.2 (0.3) | 1.2 (0.3) | 1.2 (0.3) |
| Radiologist score: mean upper lobes (SD) | 0.8 (0.3) | 0.8 (0.3) | 0.8 (0.2) |
| Radiologist score: upper − lower lobe (SD) | 0.4 (0.5) | 0.4 (0.5) | 0.4 (0.5) |
| Densitometry apical percent emphysema (SD) | 24 (18) | 24 (17) | 24 (18) |
| Densitometry basal percent emphysema (SD) | 10 (10) | 11 (10) | 10 (10) |
| Densitometry emphysema distribution (SD) | 14 (19) | 14 (18) | 14 (19) |
The distributional characteristics of emphysema (apical %, basal %) were associated with variants in different sets of genes (p ⩽ 0.05; Table 2). When the apical minus basal distribution of emphysema was considered, this set of eight genes was reduced to GSTP1, MMP1, and EPHX1 (Table 2). The inclusion of a variable for study center effects did not change the outcome. A sensitivity analysis performed after the exclusion of the 17 MZ, 19 MS, and 3 SZ individuals had no influence on the results.
TABLE 2.
OVERALL COHORT ASSOCIATIONS WITH QUANTITATIVE DENSITOMETRIC EMPHYSEMA DISTRIBUTION PHENOTYPES
| Effect Estimate for Genotype | ||||||
|---|---|---|---|---|---|---|
| Percent Emphysema | Gene | No. of SNPs Genotyped | Significantly Associated SNPs* | Location | B (SE) | p Value |
| Apical 1/3 (upper lobe) | GSTP1 | 2 | rs947894 (Ile105Val) | Exon | −3.8 (1.4) | 0.008 |
| SERPINE2 | 17 | rs729631 | Intron | −5.4 (1.9) | 0.005 | |
| rs975278 | Intron | −5.0 (1.9) | 0.009 | |||
| TGFB1 | 5 | rs1982073 | Exon | 3.5 (1.6) | 0.025 | |
| rs1800469 | Promoter | 3.4 (1.6) | 0.036 | |||
| TIMP2 | 1 | rs2277698 | Exon | −4.7 (2.3) | 0.038 | |
| MMP1 | 1 | rs1799750 | Promoter | 3.2 (1.6) | 0.044 | |
| Basal 1/3 (lower lobe) | EPHX1 | 8 | rs2234922 (His139Arg) | Exon | 2.3 (1.1) | 0.034 |
| GPRC5A | 1 | rs850937 | Intron | 1.8 (0.8) | 0.033 | |
| LTBP4 | 4 | rs2077407 | Exon | −3.8 (1.4) | 0.010 | |
| Distribution of emphysema, % apical − % basal | GSTP1 | 2 | rs947894 (Ile105Val) | Exon | −5.0 (1.5) | 0.001 |
| EPHX1 | 8 | rs2234922 (His139Arg) | Exon | −5.9 (2.1) | 0.005 | |
| rs1051741 | Exon | −5.5 (2.6) | 0.034 | |||
| rs2260863 | Intron | 4.2 (1.8) | 0.016 | |||
| rs360063 | 3′ UTR | 3.2 (1.6) | 0.049 | |||
| MMP1 | 1 | rs1799750 | 4.1 (1.7) | 0.016 |
Definition of abbreviation: SNP = single-nucleotide polymorphism.
Only regression with p < 0.05 are shown; all regression models include covariates for genotype, age, sex, pack-years of smoking, and FEV1.
p ⩽ 0.05.
As a confirmatory strategy, we performed a test–replication analysis by dividing the data using randomization groups into baseline medical and baseline (preoperative) surgical subsets. For the distribution of emphysema, the main phenotype of interest, the GSTP1 SNP, rs947894 (Ile105Val), replicated in both the medical (p = 0.04) and surgical (p = 0.01) subsets (Table 3), despite the loss of power associated with dividing the cohort.
TABLE 3.
TEST–REPLICATION ANALYSIS OF SINGLE-NUCLEOTIDE POLYMORPHISM ASSOCIATIONS FOR COMPUTED TOMOGRAPHY DENSITOMETRY DISTRIBUTION OF EMPHYSEMA PARSING THE COHORT INTO THE RANDOMIZED MEDICAL AND SURGICAL ARMS OF THE NATIONAL EMPHYSEMA TREATMENT TRIAL
| Gene | SNP | p Value (Medical) | p Value (Surgical) |
|---|---|---|---|
| GSTP1 | Ile105Val | 0.04 | 0.01 |
| EPHX1 | rs1051741 | 0.04 | NS |
| His139Arg | 0.004 | NS | |
| MMP1 | rs1799750 | NS | 0.03 |
Definition of abbreviations: NS = nonsignificant; SNP = single-nucleotide polymorphism.
Only regression with p ⩽ 0.05 are shown; all regression models include covariates for genotype, age, sex, pack-years of smoking and FEV1.
We also analyzed the radiologist emphysema score distribution phenotype, which revealed associations with variants in GSTP1, EPHX1, SERPINE2, TGFB1, and SFTPD (p ⩽ 0.05; Table 4). The inclusion of a variable for study center effects in multivariable models did not change the results, nor did the exclusion of the data from individuals with the PI MZ, MS, and SZ allele combinations. Of note, the correlation between radiologist score–determined upper lobe–predominant emphysema and CT densitometric measures of upper lobe–predominant emphysema was 0.58 (p < 0.0001), suggesting strong but not complete correlation between these two quantitative phenotypes. Because only GSTP1 and EPHX1 were significant for both CT densitometric and radiologist score emphysema distribution phenotypes, we focused on these two genes in the haplotype analysis.
TABLE 4.
COMPARISON OF GENES WITH SIGNIFICANT SINGLE-NUCLEOTIDE POLYMORPHISM ASSOCIATION FOR COMPUTED TOMOGRAPHY DENSITOMETRY VERSUS RADIOLOGIST SCORING FOR EMPHYSEMA DISTRIBUTION
| Densitometry Distribution of Emphysema*
|
NETT Radiologist Score Emphysema Distribution*
|
||||
|---|---|---|---|---|---|
| Gene | SNP | B (SE)† | p Value | B (SE)† | p Value |
| GSTP1 | rs947894 | −5.0 (1.5) | 0.001 | −0.08 (0.04) | 0.034 |
| EPHX1 | rs2234922 | −5.9 (2.1) | 0.005 | −0.14 (0.05) | 0.010 |
| rs1051741 | −5.5 (2.6) | 0.034 | — | — | |
| rs2260863 | 4.2 (1.8) | 0.016 | — | — | |
| rs360063 | 3.2 (1.6) | 0.049 | — | — | |
| MMP1 | rs1799750 | 4.1 (1.7) | 0.016 | — | — |
| TGFB1 | rs1800469 | — | — | 0.11 (0.04) | 0.013 |
| rs1982073 | — | — | 0.10 (0.04) | 0.020 | |
| rs2241712 | — | — | 0.09 (0.04) | 0.032 | |
| SERPINE2 | rs1371028 | — | — | 0.14 (0.07) | 0.046 |
| SFTPD | rs721917 | — | — | 0.08 (0.04) | 0.046 |
Definition of abbreviations: NETT = National Emphysema Treatment Trial; SNP = single-nucleotide polymorphism.
Dashes indicate p > 0.05. Significant results based on both densitometry and radiologist scores are in bold.
Mean (SD) and [range] of densitometry distribution of emphysema percent = 14 (± 19) [−33 to 64] and of radiologist score emphysema distribution = 0.38 (± 0.50) [−1.2 to 2.4].
Only regressions with p ⩽ 0.05 are shown; all regression models include covariates for genotype, age, sex, pack-years of smoking and FEV1.
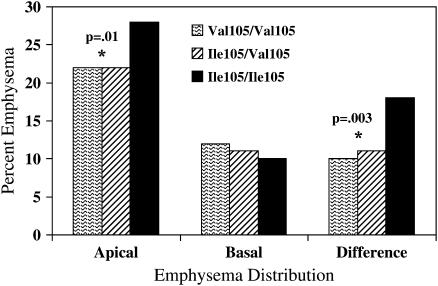
Association between haplotypes and emphysema phenotypes was evaluated for both GSTP1 and EPHX1. Global haplotype p values are presented in Table 5. Only GSTP1 had a significant global p value for both CT densitometric and radiologist score emphysema phenotypes. The most frequent GSTP1 haplotype was the Ile105 Ala114 variant (64%), with a score test result of +3.2 and a simulated haplotype p value of 0.001. The next most common haplotype, Val105Ala114 (frequency of 27%) had a score test result of −3.3 and a simulated p value of 0.0009. Because the haplotype association appeared to be driven by the GSTP1 SNP, rs947894 (Ile105Val), we evaluated the emphysema distribution characteristics by genotype for the Ile105Val polymorphism (Figure 1).The wild-type Ile105 homozygous genotype was associated with higher apical percent emphysema (p = 0.01) as well as apical-predominant distribution of disease (p = 0.003).
TABLE 5.
HAPLOTYPE ANALYSIS OF GSTP1 AND EPHX1 SINGLE-NUCLEOTIDE POLYMORPHISMS WITH COMPUTED TOMOGRAPHY DENSITOMETRY EMPHYSEMA PHENOTYPES
| GSTP1 Global p Value | EPHX1 Global p Value | |
|---|---|---|
| CT densitometric phenotypes | ||
| Apical % emphysema | NS | NS |
| Basal % emphysema | NS | NS |
| Emphysema distribution | 0.007 | NS |
| Radiologist score | ||
| Upper lobe score | 0.02 | NS |
| Lower lobe score | 0.04 | NS |
| Emphysema distribution | 0.02 | NS |
Definition of abbreviations: CT = computed tomography; NS = nonsignificant (p > 0.05).
Figure 1.
Histogram for percent emphysema by computed tomography densitometry in the apical and basal lung regions, and in the difference between apical and basal percent emphysema for each genotypic category of the GSTP1 SNP Ile105Val.
We performed a case–control association analysis of the significantly associated SNPs in GSTP1 and EPHX1 with COPD susceptibility by comparing NETT cases to NAS control subjects. A previous case-control association analysis has been performed including all NETT cases (1), which showed modest association to an SNP in EPHX1 and marginal evidence for association to GSTP1. The present analysis includes only those individuals with apical-predominant disease defined using densitometry, to assess whether using a more homogeneous COPD phenotype results in increased evidence for association. After limiting the case definition to 171 individuals with densitometric apical-predominant emphysema, EPHX1 rs2234922 demonstrated a change in OR for association from 0.73 for the Arg variant (95% confidence interval [CI], 0.56–0.96; p = 0.02) (1) to 0.60 (95% CI , 0.43–0.85; p = 0.005), despite the reduction in the number of COPD cases. No improvement in the strength of association, as determined by OR or significance, was observed for GSTP1 or other EPHX1 SNPs.
DISCUSSION
Progress in understanding the genetic contributions to COPD susceptibility and severity has been limited by lack of reproducibility of genetic association studies (1). COPD is an inherently heterogeneous condition, and more exact phenotypic specification of the emphysema component of COPD through CT scanning has the potential to provide an important advance in phenotyping individuals for genetic association studies. More homogeneous COPD phenotypes may allow us to identify genes for emphysema and emphysema distribution; these genes can then be evaluated in a broader context as COPD susceptibility genes. Presently, genetic and nongenetic determinants of emphysema distribution are poorly understood. Given the implication for therapy associated with distributional features of emphysema, we sought to identify genes that are associated with emphysema distribution, especially upper lobe–predominant disease.
A well documented genetic cause of emphysema is severe AATD, and, classically, the distribution of emphysema in AAT-deficient individuals is predominantly in the basilar aspect of the lungs. Development of emphysema in this disease likely has a strong contribution from gene × smoking interactions (18). We hypothesized that genetic susceptibility may be an important contributor to the distribution of emphysema in individuals not deficient in AAT.
In our study, we observed association of polymorphisms in the xenobiotic metabolizing enzymes, GSTP1 and EPHX1, with emphysema distribution, even after the exclusion of individuals with the PI MZ, MS, and SZ genotypes at the SERPINA1 locus. Oxidative stress, such as that induced by tobacco smoke, leads to the formation of reactive oxygen species and free radicals in the lung, which may lead to tissue and cellular damage that starts and perpetuates the lung injury process in COPD (19–21). The ability to decrease oxidative stress is mediated in part through these pathway enzymes. Glutathione S-transferases are expressed in the lung and serve as antioxidants and hydroperoxidases. Microsomal epoxide hydrolase has high activity in the lung and is involved in the initial metabolism of reactive epoxide intermediates.
GSTP1 has been investigated by multiple groups as a potential COPD susceptibility gene. The majority of glutathione S-transferase activity in the lung is likely provided by GSTP1 (22). Of the two common polymorphic variants—Ile105Val (exon 5) and Ala114Val (exon 6)—the wild-type Ile105 variant was associated with densitometric apical percent emphysema and upper lobe–predominant disease by radiologist scores in our study. From a functional standpoint, enzymatic activity is altered by the variant at the 105 position (an adenine-to-guanine change at nucleotide 315, resulting in the isoleucine-to-valine substitution), with higher efficiency of metabolism of aromatic epoxides in the presence of the 105Val polymorphism (23, 24). Polymorphisms in GSTP1 at this functional site may influence regional detoxification of xenobiotic/oxidant stressors. The Ile105 variant has been demonstrated to be associated with COPD in a Japanese cohort (25), and has also been associated with lung function decline in individuals with a family history of COPD in the Lung Health Study (26). In asthma cohorts, children with the Val105/Val105 had higher FEV1 and FVC % predicted values (27), and the presence of the Val105 genotype has been associated with an overall lower risk for asthma (28). In individuals challenged with intranasal diesel particles and allergens, the Ile105 genotype was associated with increase in IgE and histamine, thus enhancing the allergic response (29).
Despite the number of association studies that have demonstrated significant association of the Ile105 variant with obstructive lung disease phenotypes, the association of the Ile105Val variant has not been consistent across COPD case-control studies, with nonreplication in Boston Early-Onset COPD Study pedigrees (1) and in a case-control study in Korea (30). One contributor to nonreplication may be ethnic diversity between the case–control studies, but phenotypic heterogeneity may also contribute.
EPHX1 has also been investigated as a COPD susceptibility gene, and, in our current analysis, there is some evidence that the fast variant (His139Arg) is protective against upper lobe–predominant emphysema. Previous work by our group has demonstrated the protective effect of the fast allele (His139Arg, rs2234922; OR, 0.73; 95% CI, 0.56–0.96) in a case–control study of COPD using NETT cases (1), along with association of other SNPs in the EPHX1 gene with DlCO and exercise capacity phenotypes in NETT participants (13). Similar to GSTP1, microsomal epoxide hydrolase is important for the metabolism of by-products of cigarette smoke. The Tyr113His (rs1051740) “slow” variant in exon 3 has been associated with COPD (31, 32), and a haplotype that included the Tyr113His variant has been associated with rapid lung function decline in the Lung Health Study (33). Associations with COPD of polymorphic variants in this gene have not been replicated in Japanese or Korean populations (34, 35).
The association of GSTP1 and EPHX1 polymorphisms with emphysema distribution suggests a potential role for xenobiotic metabolism in contributing to and/or directing upper lobe–predominant emphysema. From this observation, we hypothesize that there are COPD genes relevant to disease susceptibility and others genes relevant to disease severity and distribution. In our study, this would be consistent with EPHX1 being associated with COPD susceptibility (with a lower OR in the case-control study even with fewer cases when the narrower definition of COPD cases is based on those with upper lobe–predominant emphysema) and GSTP1 being more relevant as a determinant for emphysema distribution and severity.
There are several limitations of the present study. First is the concern that three different models of CT scanners were used during the NETT, and the results from different scanners may bias our results. However, all of the densitometry measures were performed by a single center, and we did include a variable for each study center to capture systematic differences in CT scanners and CT scan interpretation. We have not replicated these findings in an independent cohort given the paucity of CT phenotyping of patients in large-scale COPD genetics studies. In addition, and as mentioned in previous studies in this cohort (13), a variable number of SNPs were tested for association in the genes of interest, so false-negative results for some genes might be observed due to inadequately capturing the linkage disequilibrium information across all of the genes. However, we used this initial SNP selection strategy to be able to compare our results—based on a more homogeneous emphysema phenotype—with results that have used only a spirometric definition of COPD. Given the number of comparisons performed, false-positive associations may be observed because of multiple testing. Many of the methods for controlling type I error in multiple testing are too conservative for correlated data (multiple SNPs in genes, or multiple CT scan phenotypes). Similar to Hersh and colleagues (13), we used a test–replication approach within the NETT cohort to address the issue of false-positive results; despite the loss of power associated with limiting the size in each group, we still observed association of GSTP1 Ile105Val with emphysema distribution.
Genetic association studies with chest CT phenotypes have not been performed to date in as large a cohort as that presented here. Genetic contributions to emphysema distribution have been suggested by prior research. In addition to severe AATD, which is classically (but not always) associated with lower lobe emphysema, the MMP-9 (C-1562T) polymorphism was associated with upper lobe–dominant emphysema in a case–control study of 84 Japanese patients with COPD, but was not associated with COPD in a case–control analysis (9). To date, no published studies have investigated a role for xenobiotic pathway enzymes and emphysema distribution. One possible explanation for our finding is that there are gradients of smoke metabolites and antioxidant protein expression in the lung from apex to base; if an imbalance of detoxification of smoke metabolites occurs in the upper part of the lung, then the major destructive effect of cigarette smoke in those with polymorphic variants would be in the upper lobes. Given the number of statistical comparisons performed in the current study, we recognize that replication is necessary in independent family-based and/or case-control studies.
In conclusion, we have demonstrated consistent association of the Ile105Val variant in GSTP1 with CT scan distribution of emphysema; there are no data to support an association with COPD susceptibility. Polymorphic variants in EPHX1 are associated with both emphysema distribution and COPD susceptibility. The role of the xenobiotic pathways and enzyme gradients in the lung should be investigated to elucidate the contribution to the development of specific distributional features of emphysema. Identification of genetic variants associated with distributional features of emphysema may have implications for selection for and outcome of lung volume reduction surgery.
Supplementary Material
Acknowledgments
The authors thank Ms. Jody Senter-Sylvia for her technical assistance with sample management and genotyping; she has reviewed the final manuscript and has provided permission for this acknowledgment.
Supported by National Institutes of Health grant K08 HL072918 and an American Lung Association Research award (D.L.D.), National Institutes of Health grants R01 HL71393 and R01 HL075478 (E.K.S.) and an American Lung Association Career Investigator award (E.K.S.). The National Emphysema Treatment Trial was supported by the U.S. National Heart, Lung, and Blood Institute (contracts N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services, and the Agency for Healthcare Research and Quality. The Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs, and is a component of the Massachusetts Veterans Epidemiology Research and Information Center.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200612-1797OC on March 15, 2007
Conflict of Interest Statement: D.L.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.P.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.A.H. has shares in VIDA Diagnostics, which is commercializing the software used in this study. A.A.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.O.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. F.J.M. is a coinvestigator in the GIPF-007 study, and has received total compensation from Interimmune of less than $10,000. His interaction with Actelion has been as principal investigator of the BUILD 1 at the University of Michigan, with personal total compensation less than $10,000. He has been a member of the Encysive Steering Committee for an inhaled vasodilator (Iloprost) in idiopathic pulmonary fibrosis–related pulmonary hypertension. He has been a member of several Pfizer-sponsored advisory boards and CME committees as well as the speaker's bureau for Pfizer relating exclusively to chronic obstructive pulmonary disease (COPD); total compensation is greater than $10,000 but less than $20,000. He has been a member of several advisory boards for Boehringer Ingelheim, as well as CME committees and the speaker's bureau for this company relating exclusively to COPD. Total compensation is greater than $10,000 but less than $20,000. P.D.S. has been an investigator for phase II, III, and IV clinical trials sponsored by GlaxoSmithKline (and Glaxo Wellcome, Inc., and SmithKline Beecham), Boehringer Ingelheim, Pfizer, Dey L.P. Pharmaceutical, Hoffman-LaRoche AG, Merck & Co., Inc., Altana Pharma, and Ono Pharma USA, Inc. He has served as an ad hoc consultant for Inflazyme Pharmaceuticals. F.C.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.P.U. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.J.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.K.S. has received grant support and consultant fees from GlaxoSmithKline.
References
- 1.Hersh CP, Demeo DL, Lange C, Litonjua AA, Reilly JJ, Kwiatkowski D, Laird N, Sylvia JS, Sparrow D, Speizer FE, et al. Attempted replication of reported chronic obstructive pulmonary disease candidate gene associations. Am J Respir Cell Mol Biol 2005;33:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman EK, Palmer LJ. Case-control association studies for the genetics of complex respiratory diseases. Am J Respir Cell Mol Biol 2000;22:645–648. [DOI] [PubMed] [Google Scholar]
- 3.Sanders C, Nath PH, Bailey WC. Detection of emphysema with computed tomography: correlation with pulmonary function tests and chest radiography. Invest Radiol 1988;23:262–266. [DOI] [PubMed] [Google Scholar]
- 4.Sakai N, Mishima M, Nishimura K, Itoh H, Kuno K. An automated method to assess the distribution of low attenuation areas on chest CT scans in chronic pulmonary emphysema patients. Chest 1994;106:1319–1325. [DOI] [PubMed] [Google Scholar]
- 5.Mishima M, Hirai T, Itoh H, Nakano Y, Sakai H, Muro S, Nishimura K, Oku Y, Chin K, Ohi M, et al. Complexity of terminal airspace geometry assessed by lung computed tomography in normal subjects and patients with chronic obstructive pulmonary disease. Proc Natl Acad Sci USA 1999;96:8829–8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishima M, Oku Y, Kawakami K, Sakai N, Fukui M, Hirai T, Chin K, Ohi M, Nishimura K, Itoh H, et al. Quantitative assessment of the spatial distribution of low attenuation areas on X-ray CT using texture analysis in patients with chronic pulmonary emphysema. Front Med Biol Eng 1997;8:19–34. [PubMed] [Google Scholar]
- 7.Ito I, Nagai S, Hoshino Y, Muro S, Hirai T, Tsukino M, Mishima M. Risk and severity of COPD is associated with the group-specific component of serum globulin 1F allele. Chest 2004;125:63–70. [DOI] [PubMed] [Google Scholar]
- 8.Sakao S, Tatsumi K, Igari H, Watanabe R, Shino Y, Shirasawa H, Kuriyama T. Association of tumor necrosis factor-α gene promoter polymorphism with low attenuation areas on high-resolution CT in patients with COPD. Chest 2002;122:416–420. [DOI] [PubMed] [Google Scholar]
- 9.Ito I, Nagai S, Handa T, Muro S, Hirai T, Tsukino M, Mishima M. Matrix metalloproteinase-9 promoter polymorphism associated with upper lung dominant emphysema. Am J Respir Crit Care Med 2005;172:1378–1382. [DOI] [PubMed] [Google Scholar]
- 10.Parr DG, Stoel BC, Stolk J, Stockley RA. Pattern of emphysema distribution in α1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med 2004;170:1172–1178. [DOI] [PubMed] [Google Scholar]
- 11.The National Emphysema Treatment Trial Research Group. Rationale and design of The National Emphysema Treatment Trial: a prospective randomized trial of lung volume reduction surgery. Chest 1999;116:1750–1761. [DOI] [PubMed] [Google Scholar]
- 12.Demeo DL, Mariani TJ, Lange C, Srisuma S, Litonjua AA, Celedon JC, Lake SL, Reilly JJ, Chapman HA, Mecham BH, et al. The SERPINE2 gene is associated with chronic obstructive pulmonary disease. Am J Hum Genet 2006;78:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hersh CP, Demeo DL, Lazarus R, Celedon JC, Raby BA, Benditt JO, Criner G, Make B, Martinez FJ, Scanlon PD, et al. Genetic association analysis of functional impairment in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Celedon JC, Lange C, Raby BA, Litonjua AA, Palmer LJ, DeMeo DL, Reilly JJ, Kwiatkowski DJ, Chapman HA, Laird N, et al. The transforming growth factor-β1 (TGFB1) gene is associated with chronic obstructive pulmonary disease (COPD). Hum Mol Genet 2004;13:1649–1656. [DOI] [PubMed] [Google Scholar]
- 15.National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001;345:1075–1083. [DOI] [PubMed] [Google Scholar]
- 16.Schaid DJ, Rowland CM, Tines DE, Jacobson RM, Poland GA. Score tests for association between traits and haplotypes when linkage phase is ambiguous. Am J Hum Genet 2002;70:425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood DE. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003;348:2059–2073. [DOI] [PubMed] [Google Scholar]
- 18.Silverman EK, Province MA, Campbell EJ, Pierce JA, Rao DC. Family study of α1-antitrypsin deficiency: effects of cigarette smoking, measured genotype, and their interaction on pulmonary function and biochemical traits. Genet Epidemiol 1992;9:317–331. [DOI] [PubMed] [Google Scholar]
- 19.Church DF, Pryor WA. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect 1985;64:111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnes PJ. Reactive oxygen species and airway inflammation. Free Radic Biol Med 1990;9:235–243. [DOI] [PubMed] [Google Scholar]
- 21.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev 2004;56:515–548. [DOI] [PubMed] [Google Scholar]
- 22.Fryer AA, Hume R, Strange RC. The development of glutathione S-transferase and glutathione peroxidase activities in human lung. Biochim Biophys Acta 1986;883:448–453. [DOI] [PubMed] [Google Scholar]
- 23.Sundberg K, Johansson AS, Stenberg G, Widersten M, Seidel A, Mannervik B, Jernstrom B. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1-1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis 1998;19:433–436. [DOI] [PubMed] [Google Scholar]
- 24.Hu X, O'Donnell R, Srivastava SK, Xia H, Zimniak P, Nanduri B, Bleicher RJ, Awasthi S, Awasthi YC, Ji X, et al. Active site architecture of polymorphic forms of human glutathione S-transferase P1-1 accounts for their enantioselectivity and disparate activity in the glutathione conjugation of 7β,8α-dihydroxy-9α,10α-ox y-7,8,9,10-tetrahydrobenzo(a)pyrene. Biochem Biophys Res Commun 1997;235:424–428. [DOI] [PubMed] [Google Scholar]
- 25.Ishii T, Matsuse T, Teramoto S, Matsui H, Miyao M, Hosoi T, Takahashi H, Fukuchi Y, Ouchi Y. Glutathione S-transferase P1 (GSTP1) polymorphism in patients with chronic obstructive pulmonary disease. Thorax 1999;54:693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He JQ, Ruan J, Connett JE, Anthonisen NR, Pare PD, Sandford AJ. Antioxidant gene polymorphisms and susceptibility to a rapid decline in lung function in smokers. Am J Respir Crit Care Med 2002;166:323–328. [DOI] [PubMed] [Google Scholar]
- 27.Carroll WD, Lenney W, Jones PW, Strange RC, Child F, Whyte MK, Primhak RA, Fryer AA. Effects of glutathione S-transferase M1, T1 and P1 on lung function in asthmatic families. Clin Exp Allergy 2005;35:1155–1161. [DOI] [PubMed] [Google Scholar]
- 28.Fryer AA, Bianco A, Hepple M, Jones PW, Strange RC, Spiteri MA. Polymorphism at the glutathione S-transferase GSTP1 locus: a new marker for bronchial hyperresponsiveness and asthma. Am J Respir Crit Care Med 2000;161:1437–1442. [DOI] [PubMed] [Google Scholar]
- 29.Gilliland FD, Li YF, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet 2004;363:119–125. [DOI] [PubMed] [Google Scholar]
- 30.Yim JJ, Yoo CG, Lee CT, Kim YW, Han SK, Shim YS. Lack of association between glutathione S-transferase P1 polymorphism and COPD in Koreans. Lung 2002;180:119–125. [DOI] [PubMed] [Google Scholar]
- 31.Xiao D, Wang C, Du MJ, Pang BS, Zhang HY, Xiao B, Liu JZ, Weng XZ, Su L, Christiani DC. Relationship between polymorphisms of genes encoding microsomal epoxide hydrolase and glutathione S-transferase P1 and chronic obstructive pulmonary disease. Chin Med J (Engl) 2004;117:661–667. [PubMed] [Google Scholar]
- 32.Smith CA, Harrison DJ. Association between polymorphism in gene for microsomal epoxide hydrolase and susceptibility to emphysema. Lancet 1997;350:630–633. [DOI] [PubMed] [Google Scholar]
- 33.Sandford AJ, Chagani T, Weir TD, Connett JE, Anthonisen NR, Pare PD. Susceptibility genes for rapid decline of lung function in the Lung Health Study. Am J Respir Crit Care Med 2001;163:469–473. [DOI] [PubMed] [Google Scholar]
- 34.Takeyabu K, Yamaguchi E, Suzuki I, Nishimura M, Hizawa N, Kamakami Y. Gene polymorphism for microsomal epoxide hydrolase and susceptibility to emphysema in a Japanese population. Eur Respir J 2000;15:891–894. [DOI] [PubMed] [Google Scholar]
- 35.Yim JJ, Park GY, Lee CT, Kim YW, Han SK, Shim YS, Yoo CG. Genetic susceptibility to chronic obstructive pulmonary disease in Koreans: combined analysis of polymorphic genotypes for microsomal epoxide hydrolase and glutathione S-transferase M1 and T1. Thorax 2000;55:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.