Abstract
Rationale: The technique used to provide continuous positive airway pressure (CPAP) to the newborn may influence lung function and breathing efficiency.
Objectives: To compare differences in gas exchange physiology and lung injury resulting from treatment of respiratory distress with either bubble or constant pressure CPAP and to determine if the applied flow influences short-term outcomes.
Methods: Lambs (133 d gestation; term is 150 d) born via cesarean section were weighed, intubated, and treated with CPAP for 3 hours. Two groups were treated with 8 L/minute applied flow using the bubble (n = 12) or the constant pressure (n = 12) technique. A third group (n = 10) received the bubble method with 12 L/minute bias flow. Measurements at study completion included arterial blood gases, oxygraphy, capnography, tidal flow, multiple breath washout, lung mechanics, static pressure–volume curves, and bronchoalveolar lavage fluid protein.
Measurements and Main Results: Birth weight and arterial gas variables at 15 minutes were comparable. Flow (8 or 12 L/min) did not influence the 3-hour outcomes in the bubble group. Bubble technique was associated with a higher pH, PaO2, oxygen uptake, and area under the flow–volume curve, and a decreased alveolar protein, respiratory quotient, PaCO2, and ventilation inhomogeneity compared with the constant pressure group.
Conclusions: Compared with constant pressure technique, bubble CPAP promotes enhanced airway patency during treatment of acute postnatal respiratory disease in preterm lambs and may offer protection against lung injury.
Keywords: lung compliance, stochastic processes, noninvasive ventilation, respiratory distress syndrome, continuous positive airway pressure
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Bubble continuous positive airway pressure (CPAP) has been used to treat neonatal lung disease for over 30 years, but the mechanistic basis of differences in physiological effects compared with other CPAP systems are not well understood.
What This Study Adds to the Field
In an ovine model of preterm lung disease, treatment with bubble CPAP immediately after birth enhances gas exchange, lung mechanics, gas mixing efficiency, and lung volume compared with constant pressure CPAP.
Bubble continuous positive airway pressure (B-CPAP) has been used for the treatment of respiratory distress syndrome in newborn infants for over 30 years (1). It has attracted the most clinical interest over the 20 years following the reports of Avery and colleagues (2) and, more recently, Van Marter and associates (3), highlighting the low incidence of chronic lung disease in preterm infants at the Columbia Presbyterian Medical Center in New York, where B-CPAP has been a cornerstone of clinical treatment practice since the 1970s. Despite the lengthy period over which B-CPAP has been used, surprisingly little is known about the importance or relevance of the bubble component of the CPAP treatment.
During B-CPAP, the expiratory limb of the CPAP circuit vents through an underwater seal. The resulting bubbles create pressure oscillations that are transmitted back to the airway opening. The noisy nature of the pressure waveform delivered to the airway opening generates broadband frequency composition and oscillatory pressure amplitudes that are in the order of 4 cm H2O around the mean pressure. Although this noisy component may contribute to gas mixing in a similar fashion to mechanisms present during high-frequency oscillatory ventilation (4), only two previous studies have examined the effect of B-CPAP on gas exchange. Lee and colleagues (4) randomized a group of infants ready for extubation to receive either B-CPAP or ventilator-derived (constant pressure) CPAP (CP-CPAP) for a period of 15 minutes before crossover to the alternative treatment. Although there were no differences in PaCO2 between B-CPAP and CP-CPAP groups, the babies treated with B-CPAP had lower respiratory rates and lower minute volumes, suggesting more efficient ventilation. More recently, Morley and colleagues reported a crossover study of clinically stable infants treated with CPAP in the neonatal intensive care unit (5). They found no differences in transcutaneous measurements of arterial CO2 or in oxygen saturation between CPAP with and without bubbles over the brief 30-minute study epochs. Both studies tested the effect of B-CPAP on clinically stable infants in the recovery stage of neonatal respiratory illness, and focused predominantly on the concept that the bubble component was likely to influence CO2 clearance.
In addition to provision of support during the weaning period, B-CPAP is used as a means of providing positive-pressure support in preterm infants from birth and during the acute stages of respiratory distress. Recent studies in an in vitro lung model suggested that a higher percentage of pressure oscillations at the airway opening would be transmitted to the lung in the presence of low compliance (6). We hypothesized that, in the surfactant-deficient newborn lung, B-CPAP would improve lung volume recruitment compared with CPAP delivering a constant pressure to the airway opening. The objectives of this study were to obtain detailed lung function assessments in preterm lambs allocated to receive either B-CPAP or CP-CPAP to determine the nature of physiological differences resulting from the different treatments, and the mechanisms by which such differences arise (7).
METHODS
Investigations were approved by the Animal Ethics Committee of the Western Australia Department of Agriculture and Cincinnati Children's Hospital Medical Centre. Further methodological detail can be obtained in the online supplement.
Animals, Delivery and Postnatal Care
We used a previously described ovine model of lung disease in moderately preterm lambs treated with CPAP via tracheal tube (8). Labor was induced with 20 mg intravenous epostane (Sanofi-Aventis, Bridgewater, NJ) and 0.5 mg/kg intramuscular betamethasone (Celestone Chronodose; Schering Plough, Kenilworth, NJ) at 131 to 132 days' gestation in date-mated Merino ewes carrying twins. Lambs were delivered about 40 hours later by cesarean section. Lambs (n = 5) randomized to no ventilation were killed (50 mg/kg intravenous pentobarbital) immediately after delivery. The remaining lambs were dried, intubated with a 4.5-mm internal diameter, cuffed tracheal tube, and treated with CPAP using heated, humidified 100% oxygen. Treated lambs were quasi-randomized to three groups: CP-CPAP (Bournes BP200; Bear Medical Systems, Riverside, CA) with a bias flow of 8 L/minute (n = 12), or B-CPAP using the same disposable circuit (Fisher and Paykel Healthcare, Auckland, NZ) using a bias flow of 8 L/minute (n = 12) or 12 L/minute (n = 10). The depth of underwater seal in the B-CPAP circuits was adjusted to achieve similar mean pressures at the airway opening to those obtained with the CP-CPAP system. The unsedated, spontaneously breathing lambs were positioned prone, covered with transparent occlusive wrap (NeoWrap; Fisher and Paykel Healthcare) and kept warm on an infant radiant warmer (Fisher and Paykel Healthcare). Blood from an umbilical arterial catheter was used for blood gas and pH measurements.
Physiological Measurements
Physiological differences between the two treatments were assessed at 150 minutes using an infant lung function system (Exhalyser; EcoMedics, Duernten, Switzerland). The system incorporated an ultrasonic flowmeter to measure flow and molar mass, a mainstream infrared CO2 analyzer (Duet EtCO2; Welch-Allyn OEM Technologies, Beaverton, OR) and a sidestream laser diode O2 sensor (Oxygraf; Oxygraf, Inc., Mountain View, CA). Tidal flow variables were determined from 30-second epochs recorded during regular quiet breathing using a sampling frequency of 200 Hz (see Figure E1 in the online supplement). Peak and trough CO2 and O2 were manually determined for each breath during the 30-second interval to determine average volume of CO2 removal (ΔCO2) and O2 (ΔO2) extraction. Respiratory quotient was determined as ΔCO2/ΔO2. Pressure was measured at the tracheal tube connector.
Multiple breath washout was performed using an adaptation of existing methodology (9). A sideport, positioned approximately 2 cm proximal to the tracheal tube connector, was used to blend a low-flow sulfurhexafluoride/oxygen mixture (79% SF6/21% O2) with the bias flow, to achieve a concentration of approximately 4% SF6 at the airway opening during wash-in. The supplemental SF6 flow was replaced by medical air during the washout to maintain constant flow and inspired oxygen concentrations throughout the study. Functional residual capacity (FRC) and the lung clearance index (LCI) were calculated from the washout trace (see Figure E2).
The mechanical impedance of the lower respiratory system was measured with customized measuring equipment using the low-frequency forced oscillation technique adapted for measurements in the ventilated preterm lamb (10, 11). Airway resistance (Raw), tissue damping (G), and tissue elastance (H) were obtained by fitting an empirical model to the averaged impedance spectra (12) (see Figure E3). Tissue compliance was determined as 1/H.
Lung Processing
Lambs were killed with an overdose of pentobarbital at the age of 3 hours and the tracheal tube was clamped for 3 minutes to facilitate oxygen absorption and lung collapse. The lamb thorax was opened and the pressure–volume relationship was determined (13). Volumes were corrected for the system compliance.
Tissue from the right lower lobe was immediately frozen in liquid nitrogen for wet-to-dry ratio and saturated phosphatidylcholine (Sat PC) measurements. Three repeated saline lavages of the left lung were combined for the bronchoalveolar lavage fluid (BALF) (8) and aliquots were saved for measurement of Sat PC (14, 15) and total protein (16).
Data Analysis and Statistics
Results are shown as mean (± SEM) unless specified otherwise. Statistics were analyzed using SPSS, version 14.0 (SPSS, Inc., Chicago, IL). Two-tailed unpaired t tests were used to assess significance of differences between two groups. Two-way analysis of variance (ANOVA) was used for all other statistical comparisons, with CPAP level and applied bias flow as the independent factors. Significance was accepted as p < 0.05.
RESULTS
Postnatal Characteristics and Outcome
Table 1 lists a summary of the animals in each treatment group. There were no differences in cord blood pH or birth weight for the four groups of lambs. Mean CPAP pressure was not different between treatments.
TABLE 1.
BASELINE VARIABLES
| Unventilated Controls* | Constant Pressure, 8 L/min | Bubble, 8 L/min | Bubble, 12 L/min | p | |
|---|---|---|---|---|---|
| Number | 5 | 12 | 12 | 10 | |
| Male/female | 3/2 | 6/6 | 8/4 | 5/5 | 0.57 |
| Body weight, kg | 3.0 (0.2) | 3.2 (0.1) | 3.3 (0.2) | 3.0 (0.1) | 0.17 |
| Cord blood pH | 7.43 (0.02) | 7.41 (0.02) | 7.41 (0.02) | 7.41 (0.01) | 0.93 |
| CPAP pressure, cm H2O† | 6.8 | 6.5 | 6.4 | 0.56 |
Definition of abbreviations: Bubble 8 L/min = bubble CPAP, 8 L/min bias flow; Bubble 12 L/min = bubble CPAP, 12 L/min bias flow; Constant Pressure 8 L/min = constant pressure CPAP, 8 L/min bias flow; CPAP = continuous positive airway pressure.
Controls are unventilated lambs at same gestation.
CPAP pressure is the mean pressure measured at the airway opening; SEM < 0.03 cm H2O for all groups.
Physiological Measurements
Varying bias flow between 8 and 12 L/minute did not have a significant impact on any of the physiological variables measured (see Table E1 and Figure E4).
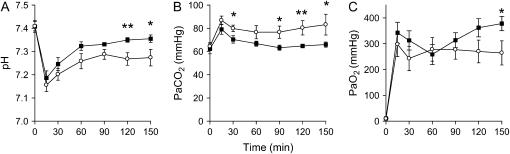
Measurements of pH, PaCO2 and PaO2 from birth to the start of the physiological measurements at 150 minutes are shown for the CP-CPAP and 8-L/minute B-CPAP groups in Figure 1. Combined data and comparisons of 8- and 12-L/minute B-CPAP groups are presented in the online supplement, and the detailed statistical comparison is given in Table E2. Despite similar blood gases at the commencement of the study, B-CPAP lambs maintained a lower PaCO2 than the constant pressure group from as early as 30 minutes after birth, and a significantly higher pH from 120 (p = 0.008) to 150 minutes. The initial rapid rise in PaO2 after birth can be partly attributed to the use of an FiO2 of 1.0. Although the PaO2 stabilized in the constant pressure group after 60 minutes, it continued to increase in the B-CPAP group and was significantly higher at 150 minutes compared with the CP-CPAP group (p = 0.023). Comparison of the mean differences in blood gas variables over the course of the study showed a significant difference between the groups for pH and PaO2 (see Table 2).
Figure 1.
Time course of changes in arterial blood gas values. The values for umbilical arterial pH (A), partial pressure of carbon dioxide (PaCO2, B), and partial pressure of oxygen (PaO2, C) are shown for the first 150 minutes of the study, up to the point of pulmonary physiological assessments. There were significant differences between the constant pressure (open symbols) and 8-L/minute bias flow bubble (closed symbols) continuous positive airway pressure group for pH, PaCO2, and PaO2. *p < 0.05; **p < 0.01. Specific p values are not included in the graph, for clarity, but are provided in Table E2 of the online supplement.
TABLE 2.
TREND ANALYSIS FOR ARTERIAL BLOOD GAS PARAMETERS
| Mean Difference (150 min – 0 min) | Constant Pressure, 8 L/min | Bubble, 8 L/min | p |
|---|---|---|---|
| Number | 12 | 12 | |
| pH | −0.13 (0.11) | −0.05 (0.07) | 0.042 |
| PaCO2, mm Hg | 18.9 (31.7) | 3.6 (14.3) | 0.144 |
| PaO2, mm Hg | 255 (160) | 371 (94.6) | 0.042 |
Values indicate mean difference (SD) between measurements obtained from the cord blood and at 150 minutes.
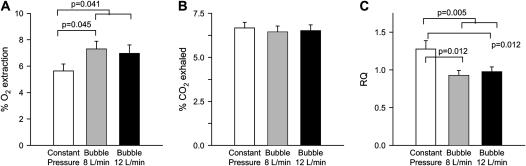
By indirect calorimetry, B-CPAP lambs extracted a greater amount of the inspired oxygen (p = 0.041), whereas there was no difference in the amount of CO2 removed with each breath (p = 0.22). This resulted in a lower respiratory quotient for B-CPAP lambs compared with the CP-CPAP group (p = 0.005) (Figure 2).
Figure 2.
Indirect calorimetry. Oxygen (O2) extraction and carbon dioxide (CO2) removal rates were calculated from the product of the breath-to-breath changes in inspired and expired O2 and CO2 and the minute volume (corrected for dry weight). Bubble continuous positive airway pressure (CPAP) animals extracted O2 more efficiently than constant pressure CPAP animals (p = 0.041). The significantly higher respiratory quotient (RQ) of the constant pressure CPAP group is suggestive of respiratory failure (p = 0.005).
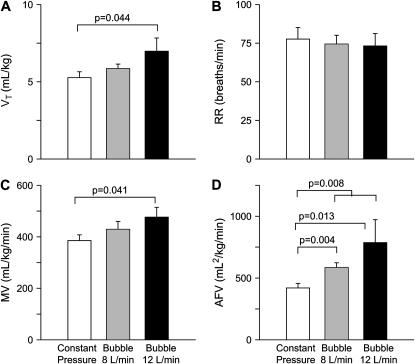
Measurements of tidal breathing demonstrated no differences in respiratory rate or in tidal volume (Vt) and minute volume after correction for body weight (Figure 3) between CP-CPAP and 8-L/minute B-CPAP, although these variables were significantly higher in the B-CPAP lambs treated with 12 L/minute bias flow. The area under the flow volume curve was greater in both 8- (p = 0.004) and 12-L/minute (p = 0.013) B-CPAP lambs compared with the CP-CPAP group.
Figure 3.
Tidal breathing. Measurements of (A) tidal volume (Vt), (B) respiratory rate (RR), (C) minute volume (MV), and (D) area under the flow–volume curve (AFV) obtained from 30-second recordings of regular breathing in quiet sleep. There was a significant increase in AFV (p = 0.008) for the bubble CPAP group as a whole, and increased Vt (p = 0.044) and MV (p = 0.041) in the 12-L/minute bubble CPAP group.
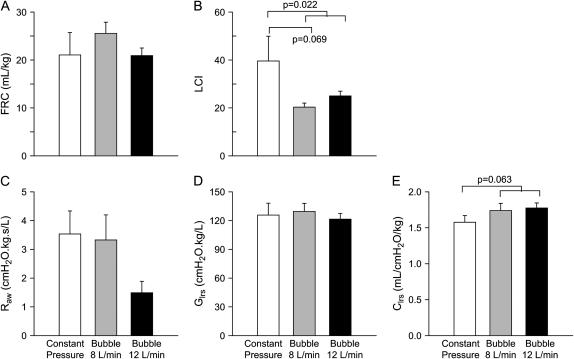
Results of multiple breath washout and lung mechanics measurements are shown in Figure 4. Measurements of FRC were highly variable. No significant difference in FRC between CP-CPAP and B-CPAP was found using this technique. The decrease in LCI in the B-CPAP lambs (p = 0.022) indicated less ventilation inhomogeneity than with CP-CPAP. Forced oscillatory mechanics measurements showed a strong trend (p = 0.063) toward improved tissue compliance in the B-CPAP group compared with CP-CPAP after correction for body size. This difference was not present after correction of measurements to lung volume measured at the distending pressure (p = 0.64), suggesting that the increased compliance was due to altered lung volume rather than any other factor contributing to altered compliance in vivo. Significant differences were not observed between the groups in airway resistance or tissue damping (tissue resistance) (p = 0.96), although a trend toward lower airway resistance was noted in the 12-L/minute B-CPAP group.
Figure 4.
Multiple breath washout and forced oscillatory mechanics. Measurements of (A) functional residual capacity (FRC) and (B) lung clearance index (LCI) were derived from multiple breath washouts using approximately 4% SF6 as the tracer gas. The higher LCI in the constant pressure group (p = 0.022) implies more inhomogeneous ventilation. The low-frequency forced oscillation technique was used to obtain partitioned measurements of the airway and tissue mechanics, including (C) airway resistance (Raw), (D) lower respiratory system tissue damping (tissue resistance, Glrs), and (E) lower respiratory system tissue compliance (Clrs). There was a trend to increased compliance in the bubble CPAP group.
Post Mortem Processing
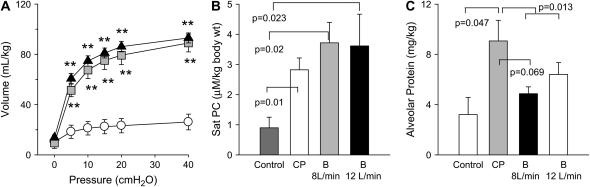
Although there was no difference in the deflation pressure–volume curve (Figure 5A) between CP-CPAP and 8-L/minute B-CPAP, there was a trend toward higher lung volume at low pressures in B-CPAP lambs on two-way ANOVA (p = 0.088 at 5 cm H2O and p = 0.061 at 0 cm H2O). CPAP groups had increased Sat PC levels in the BALF compared with nonventilated controls, but there was no significant difference in Sat PC levels between the CP-CPAP and B-CPAP groups (Figure 5B; p = 0.64). Likewise, similar wet:dry lung weights were observed for B-CPAP and CP-CPAP lambs (p = 0.13). CP-CPAP lambs had significantly higher alveolar protein levels compared with nonventilated control animals. The alveolar protein in the B-CPAP groups was lower than for the CP-CPAP group (p = 0.013) (Figure 5C). A similar trend was evident between CP-CPAP animals and B-CPAP animals treated with a bias flow of 8 L/minute (p = 0.069).
Figure 5.
Deflation pressure–volume curves, saturated phosphatidylcholine (Sat PC), and alveolar protein. (A) The deflation limb of the static pressure–volume relationship obtained post mortem in unventilated (open circles), constant pressure CPAP (gray squares), and 8-L/minute bubble CPAP (black triangles) lamb groups. Sat PC levels (B) and differences in alveolar protein levels (C) isolated from alveolar wash samples are shown for unventilated controls (dark gray bars), constant pressure CPAP (CP, white bars), and 8-L/minute (light gray bars) and 12-L/min (black bars) bubble (B) CPAP groups. **p < 0.0001 for constant pressure and bubble CPAP versus unventilated control animals.
DISCUSSION
Summary of Findings
We found that B-CPAP has physiological and biological advantages compared with the use of a CP-CPAP waveform in intubated, newborn, preterm lambs. We have confirmed findings of previous studies that showed B-CPAP can improve CO2 clearance. This is the first study to show that B-CPAP improves arterial oxygen levels, and potentially reduces atelectatic lung injury compared with CP-CPAP. The mechanisms promoting improved gas exchange include improved airway patency, and likely also lung volume recruitment. Increasing the intensity of the bubbling by increasing bias flow from 8 to 12 L/minute did not result in clear additional physiological or biological advantages.
Enhanced lung volume recruitment, due to noise-induced promotion of airway-opening events occurring at any given mean pressure, may explain part of the improved short-term respiratory physiology. The strong trends toward improved compliance after correction for body size and higher lung volumes on the post mortem pressure–volume curve support the presence of higher distending lung volumes in the B-CPAP group, although this was not able to be confirmed with the multiple breath washout lung volume measurements. The initial deterioration in PaO2 levels seen in both groups after the first 15 minutes could result from several different processes, including the following: delayed establishment of a stable FRC and transport of fresh gas to the alveolar compartment during initial clearance of the fetal lung fluid; increased pulmonary artery pressure with shunting; and impaired V̇/Q̇ matching with overinflation of more compliant lung regions before recruitment of less compliant zones, which would also contribute to the observed increase in PaCO2. The reversal in the arterial oxygen trend in the B-CPAP group toward the latter part of the study is consistent with active recruitment of the lung and enhanced perfusion. The improved lung volumes and mechanics were not due to an effect of the type of CPAP on total lung water because wet:dry lung weights were similar.
Lung volume recruitment is influenced by distending pressure (17). Pressure at the airway opening during B-CPAP is different from the immersion depth of the expiratory tubing in B-CPAP (18), and is influenced by the amount of applied bias flow (6). Differences in mean pressure cannot be used to explain the improved arterial oxygen levels seen in the B-CPAP group because we set the depth of the expiratory limb of the B-CPAP to achieve the same measured mean pressures at the airway opening as that achieved with CP-CPAP. There were no differences in mean pressure at the airway opening (Table 1).
Although the morphometry of the flow–volume loops appeared similar between study groups, the increased area under the flow–volume loop and the reduced inhomogeneity evidenced by a decrease in LCI with B-CPAP are suggestive of increased peripheral airway patency. Because mean pressure at the airway opening was the same for both groups (Table 1), it is unlikely that the increased peripheral airway patency is a consequence of differences in mean intrapulmonary pressure between the two groups.
There was a clear advantage of B-CPAP for lowering PaCO2. Although the increase in CO2 exhalation rate was not significant (p = 0.21), the trend was consistent with ventilation that is more efficient, given that there was no difference in the end-tidal CO2 concentration for each breath between groups (p = 0.98). We observed an increased oxygen extraction in the B-CPAP lambs. Given that this was coupled with a trend toward increased PaO2 in the B-CPAP group, it is likely that the more efficient use of inspired O2 and removal of CO2 was due to enhanced patency of the peripheral airways, and the greater surface area available for gas exchange due to increased lung volume. The relatively high respiratory quotient of the CP-CPAP group suggests respiratory failure in that group.
Other authors have suggested that the oscillatory component of the bubble waveform may augment gas exchange in a manner similar to that observed with high-frequency oscillatory ventilation (HFOV) (4, 19). Our findings could certainly be used to support this proposal, although it is unlikely to be the only mechanism involved, and the amplitude of the bubble oscillations is likely to be lower than those applied during HFOV. Airway patency via application of moderately high continuous distending pressures is essential to the effective improvement of gas exchange during HFOV. In our current study, the mean airway pressures did not differ between groups; hence, to benefit from the oscillatory component of the waveform, B-CPAP needed to also improve the airway patency, as is supported by our findings.
Our novel observation that B-CPAP may be associated with increased lung volumes compared with CP-CPAP warrants further consideration. Alveolar recruitment can be likened to the expansion of a complex network. Scale-free complex networks, as described by Barabasi (20), are found throughout biological systems and usually exhibit power-law behavior. An intriguing feature of power laws is that they often describe systems in equilibrium, but close to a critical point. As the lung hovers around the critical closing or opening pressure, it represents a complex network system in equilibrium on the verge of a significant change in volume (21).
Recruitment was previously considered to occur at the level of the alveoli. Recent studies have confirmed that the recruitment of terminal airspaces is governed by power-law distributions, arising from avalanches of airspace-opening events, as the applied pressure rises above airway pressure thresholds and propagates down the branching airway tree (22). Suki and colleagues suggested that this recruitment process may benefit from the superimposition of noise on the applied driving pressure, exploiting a phenomenon known as stochastic resonance (22). Pressure fluctuations above the mean pressure will promote greater lung volume recruitment than those pressure fluctuations falling below the mean pressure, provided the upper limit of the pressure fluctuations lies above the point of the lower inflexure of collapsed lung units on the pressure–volume curve (22).
The oscillatory component of the B-CPAP pressure waveform is superimposed on the desired mean CPAP and the low-frequency pressure fluctuations imposed by the subject's own spontaneous respiratory efforts. Although the net volume output of the superimposed oscillations is essentially zero, because these are applied to a nonlinear dynamic system, the noisy nature of the pressure waveform generated from the bubbling in the expiratory line may actually promote airway-opening events and consequently lung volume recruitment if superimposed on subthreshold respiratory effort.
In a recent in vitro study (6), we demonstrated that lung compliance is a determinant of the frequency and amplitude of the noisy component of B-CPAP that is transmitted from the airway opening to the lung. Because lung compliance is low when significant atelectasis is present, we suggested that the resultant increased amplitude of the noisy oscillations transmitted to the lung would promote reopening of atelectatic airspaces. Using this logic, it is reasonable to suggest that the physiological advantages of B-CPAP are most likely to be evident during neonatal transition when fluid must be cleared from the lung and FRC is established, and in the presence of acute neonatal respiratory distress syndrome when lung volume and compliance are low, rather than in the recovery stages of respiratory distress when compliance has improved and there is lower transmission of the pressure fluctuations. This hypothesis is supported by the findings of the current study, which demonstrated physiological benefits of B-CPAP compared with CP-CPAP in self-ventilating preterm lambs that developed moderately severe respiratory distress immediately after birth.
The principles of stochastic resonance suggest that the amplitude and frequency of the superimposed noise can be optimized, to achieve the most favorable amplification (i.e., volume recruitment events). Although we showed previously that the amplitude and frequency composition of the transmitted pressure waveform during B-CPAP will be determined by the mechanical characteristics of the lung (6), the amplitude could also be influenced by the amount of bias flow used. In the present in vivo study, we found no evidence of significant short-term physiological consequences of 8 versus 12 L/minute when using B-CPAP (see Table E1), although there was an apparent increase in alveolar Sat PC in the high-flow group. Given that increased work of breathing was observed previously with high flows (23), use of just sufficient flow to achieve consistent bubbling appears warranted until further evidence supporting the use of higher flows is established.
Examples of increased PaO2 have been observed after the application of biologically variable respiratory rates and Vts in animal models of adult lung disease (24–28). Previous research has only considered this concept in relation to cyclic mechanical ventilation and the relevance of noise for recruiting lung volume recruitment in B-CPAP has not been considered. To our knowledge, this is the first study demonstrating improved volume recruitment due to superimposition of noise in a model of preterm lung disease. Although there is some evidence that the superimposition of noise on the distending pressure waveform may also promote increased surfactant secretion (29), we were not able to demonstrate this in the current study.
A tantalizing observation was the apparent decrease in the alveolar protein content observed in the B-CPAP group, suggesting reduced lung injury. This finding is supported by recent evidence of a reduction in the fractional expired nitric oxide concentration in rabbits treated with endotracheal B-CPAP compared with ventilator-derived CPAP (19). Given that lung injury is associated with atelectasis (30), this observation is consistent with improved airspace stability at low lung volumes observed in the B-CPAP groups.
Clinical Relevance
To achieve the leak-free seal required for lung function testing, we delivered CPAP via a cuffed tracheal tube, which differs from the normal route via nasal prongs and the associated leak. Nonetheless, we believe that these findings remain relevant to treatment of the neonate with CPAP. The effect of variable leak on the oscillations has not been rigorously tested, but the oscillatory nature of the pressure waveform is palpable through the chest wall of the preterm infant treated with B-CPAP, suggesting that significant oscillations are transmitted to the lungs. This is especially likely in the acute phases of respiratory distress, when low compliance causes less damping of the pressure waveform as it is transmitted to the lung (6).
Our model was the relatively mature preterm lamb at 89% of full gestation. Although CPAP is frequently applied to similarly mature newborn infants, it is also often used for the primary treatment of acute respiratory distress in human infants of around 60 to 70% of full-term development. Because there are differences in the lung development of our lamb model to the more immature human infants, the relevance of our findings to extremely preterm babies requires demonstration of stochastic recruitment in less mature lungs, which may or may not be fluid-filled.
Our comparison was between CP-CPAP (ventilator-derived) and B-CPAP because our focus was to determine if and how the nature of the pressure waveform altered lung function. Variable-flow CPAP is frequently used in the neonatal clinical setting. Although it delivers a relatively constant pressure waveform at the airway opening, the variable-flow CPAP assembly offers lower extrinsic resistance compared with B-CPAP. The relevance of this benefit against potentially improved volume recruitment with B-CPAP is yet to be rigorously tested in infants with acute respiratory distress syndrome.
Conclusions
In the moderately preterm lamb breathing spontaneously with CPAP support, we found that B-CPAP promoted improved arterial oxygen levels, increased oxygen extraction, tended to stabilize lung volume at low pressures, and reduced alveolar protein levels compared with CP-CPAP delivered from a ventilator. These findings are consistent with improved volume recruitment, stabilization, and patency of peripheral airways. We suggest that the mechanism leading to these effects may be a consequence of stochastic resonance resulting from the superimposition of noise on the applied pressure signal. The difference between the B-CPAP and CP-CPAP were not large and may have been evident because of our experimental design. We used a CP-CPAP of 8 cm H2O (measured as ∼ 6.5 cm H2O at the airway) because we showed previously that gas exchange was better at 8 cm H2O than at 5 cm H2O with B-CPAP. We could detect the difference because we studied the animals at 150 minutes of age, after arterial blood gases had stabilized. The relevance of stochastic resonance and variability in the applied pressure waveform to ventilatory modalities used for more severe neonatal lung disease warrants further investigation.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the technical assistance of Dr. David Baldwin, Mr. Martin Leckie, Mr. Andrew Niccol, Dr. Ilias Nitsos, Mr. Neil Prime, Mr. Christian Saville, Dr. Sanjay Sinhal, and Ms. Kerry Williams, and for their assistance in the delivery and care of the animals used in the study.
Supported by HD12714 from the National Institutes of Health (A.H.J.), National Health and Medical Research Council (NHMRC) 10210 (J.J.P.), NHMRC 303261 (T.J.M.M.), and the Women and Infants Research Foundation. Unrestricted financial grant and equipment support from Fisher and Paykel Healthcare (NZ).
This research was presented at the Perinatal Society of Australia and New Zealand in April 2006 and at the Society for Pediatric Research, San Francisco, 2006.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200609-1368OC on April 12, 2007
Conflict of Interest Statement: J.J.P.'s department has received research grants totaling approximately $75,000 over the previous 3 years from Fisher and Paykel Healthcare. N.H. has no industry sponsorship. T.J.M.M. has received research grants totaling approximately $75,000 over the previous 3 years from Fisher and Paykel Healthcare. G.P.'s department has received research grants totaling approximately $75,000 over the previous 3 years from Fisher and Paykel Healthcare. G.B. is currently, and has been for 2 years, a full-time employee of Fisher and Paykel Healthcare, and is participating in the company employee share purchase scheme. C.B. is currently and has been an employee of Fisher and Paykel Healthcare since 2001. He owns shares in and is a participant in the Fisher and Paykel Healthcare share option plan. M.I. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.H.J., in collaboration with T.J.M.M. and J.J.P., has received research grants and equipment donation from Fisher and Paykel, Auckland, NZ to support in part this research, about $75,000 over 3 years.
References
- 1.Wung JT, Driscoll JM Jr, Epstein RA, Hyman AI. A new device for CPAP by nasal route. Crit Care Med 1975;3:76–78. [DOI] [PubMed] [Google Scholar]
- 2.Avery ME, Tooley WH, Keller JB, Hurd SS, Bryan MH, Cotton RB, Epstein MF, Fitzhardinge PM, Hansen CB, Hansen TN. Is chronic lung disease in low birth weight infants preventable? A survey of eight centers. Pediatrics 1987;79:26–30. [PubMed] [Google Scholar]
- 3.Van Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, Susser M, Paneth N, Leviton A. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics 2000;105:1194–1201. [DOI] [PubMed] [Google Scholar]
- 4.Lee KS, Dunn MS, Fenwick M, Shennan AT. A comparison of underwater bubble continuous positive airway pressure with ventilator-derived continuous positive airway pressure in premature neonates ready for extubation. Biol Neonate 1998;73:69–75. [DOI] [PubMed] [Google Scholar]
- 5.Morley CJ, Lau R, De Paoli A, Davis PG. Nasal continuous positive airway pressure: does bubbling improve gas exchange? Arch Dis Child Fetal Neonatal Ed 2005;90:F343–F344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillow JJ, Travadi JN. Bubble CPAP: is the noise important? An in vitro study. Pediatr Res 2005;57:826–830. [DOI] [PubMed] [Google Scholar]
- 7.Pillow JJ, Hillman N, Moss TJM, Polglase GR, Bold G, Prime N, Beaumont C, Ikegami M, Jobe AH. Physiological advantage of bubble vs ventilator-derived CPAP. Presented at the Pediatric Academic Societies Meeting, San Francisco, California; 2006.
- 8.Jobe AH, Kramer BW, Moss TJ, Newnham JP, Ikegami M. Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatr Res 2002;52:387–392. [DOI] [PubMed] [Google Scholar]
- 9.Schibler A, Henning R. Measurement of functional residual capacity in rabbits and children using an ultrasonic flow meter. Pediatr Res 2001;49:581–588. [DOI] [PubMed] [Google Scholar]
- 10.Pillow JJ, Sly PD, Hantos Z. Monitoring of lung volume recruitment and derecruitment using oscillatory mechanics during high-frequency oscillatory ventilation in the preterm lamb. Pediatr Crit Care Med 2004;5:172–180. [DOI] [PubMed] [Google Scholar]
- 11.Pillow JJ, Jobe AH, Collins RA, Hantos Z, Ikegami M, Moss TJM, Newnham JP, Willet KE, Sly PD. Variability in preterm lamb lung mechanics after intra-amniotic endotoxin is associated with changes in surfactant pool size and morphometry. Am J Physiol Lung Cell Mol Physiol 2004;287:L992–L998. [DOI] [PubMed] [Google Scholar]
- 12.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 1992;72:168–178. [DOI] [PubMed] [Google Scholar]
- 13.Jobe AH, Polk D, Ikegami M, Newnham J, Sly P, Kohen R, Kelly R. Lung responses to ultrasound-guided fetal treatments with corticosteroids in preterm lambs. J Appl Physiol 1993;75:2099–2105. [DOI] [PubMed] [Google Scholar]
- 14.Mason RJ, Nellenbogen J, Clements JA. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res 1976;17:281–284. [PubMed] [Google Scholar]
- 15.Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem 1959;234:466–468. [PubMed] [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Mulrooney N, Champion Z, Moss TJ, Nitsos I, Ikegami M, Jobe AH. Surfactant and physiological responses of preterm lambs to continuous positive airway pressure. Am J Respir Crit Care Med 2004;171:488–493. [DOI] [PubMed] [Google Scholar]
- 18.Kahn D, Habib RH, Weisner M, Steele A, Singh R, Courtney SE. The unpredictability of delivered bubble nasal continuous positive airway pressure (BNCPAP): do we know what we are doing? Presented at the Pediatric Academic Societies Meeting, San Francisco, California; 2006.
- 19.Hua YM, Yuh YS, Lee CM, Lien SH, Hung CH. Bubble CPAP elicits decreases in exhaled nitric oxide in rabbits. Pediatr Pulmonol 2006;41:779–786. [DOI] [PubMed] [Google Scholar]
- 20.Barabasi AL. Linked: the new science of networks. Cambridge, MA: Perseus; 2002.
- 21.Barabasi AL, Albert R. Emergence of scaling in random networks. Science 1999;286:509–512. [DOI] [PubMed] [Google Scholar]
- 22.Suki B, Alencar AM, Sujeer MK, Lutchen KR, Collins JJ, Andrade JS Jr, Ingenito EP, Zapperi S, Stanley HE. Life-support system benefits from noise. Nature 1998;393:127–128. [DOI] [PubMed] [Google Scholar]
- 23.Liptsen E, Aghai ZH, Pyon KH, Saslow JG, Nakhla T, Long J, Steele AM, Habib RH, Courtney SE. Work of breathing during nasal continuous positive airway pressure in preterm infants: a comparison of bubble vs variable-flow devices. J Perinatol 2005;25:453–458. [DOI] [PubMed] [Google Scholar]
- 24.Lefevre GR, Kowalski SE, Girling LG, Thiessen DB, Mutch WA. Improved arterial oxygenation after oleic acid lung injury in the pig using a computer-controlled mechanical ventilator. Am J Respir Crit Care Med 1996;154:1567–1572. [DOI] [PubMed] [Google Scholar]
- 25.Mutch WA, Harms S, Ruth Graham M, Kowalski SE, Girling LG, Lefevre GR. Biologically variable or naturally noisy mechanical ventilation recruits atelectatic lung. Am J Respir Crit Care Med 2000;162:319–323. [DOI] [PubMed] [Google Scholar]
- 26.Mutch WA, Harms S, Lefevre GR, Graham MR, Girling LG, Kowalski SE. Biologically variable ventilation increases arterial oxygenation over that seen with positive end-expiratory pressure alone in a porcine model of acute respiratory distress syndrome. Crit Care Med 2000;28:2457–2464. [DOI] [PubMed] [Google Scholar]
- 27.Mutch WA, Eschun GM, Kowalski SE, Graham MR, Girling LG, Lefevre GR. Biologically variable ventilation prevents deterioration of gas exchange during prolonged anaesthesia. Br J Anaesth 2000;84:197–203. [DOI] [PubMed] [Google Scholar]
- 28.Arold SP, Mora R, Lutchen KR, Ingenito EP, Suki B. Variable tidal volume ventilation improves lung mechanics and gas exchange in a rodent model of acute lung injury. Am J Respir Crit Care Med 2002;165:366–371. [DOI] [PubMed] [Google Scholar]
- 29.Arold SP, Suki B, Alencar AM, Lutchen KR, Ingenito EP. Variable ventilation induces endogenous surfactant release in normal guinea pigs. Am J Physiol Lung Cell Mol Physiol 2003;285:L370–L375. [DOI] [PubMed] [Google Scholar]
- 30.Mols G, Priebe HJ, Guttmann J. Alveolar recruitment in acute lung injury. Br J Anaesth 2006;96:156–166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.