Abstract
Bupropion, a norepinephrine and dopamine reuptake inhibitor and nicotinic acetylcholine receptor antagonist, facilitates smoking cessation and reduces some symptoms of nicotine withdrawal. However, the effects of bupropion on nicotine withdrawal-associated deficits in learning remain unclear. The present study investigated whether bupropion has effects on contextual and cued fear conditioning following withdrawal from chronic nicotine or when administered alone. Bupropion was administered alone for a range of doses (2.5, 5, 10, 20 or 40 mg/kg), and dose-dependent impairments in contextual and cued fear conditioning were observed (20 or 40 mg/kg). Follow-up studies investigated if bupropion disrupted acquisition or expression of fear conditioning. Bupropion (40 mg/kg) administration on training day only produced deficits in contextual fear conditioning. Alternatively, bupropion (20 or 40 mg/kg) administration during testing dose-dependently produced deficits in contextual and cued fear conditioning. To test the effect of bupropion on nicotine withdrawal, mice were withdrawn from 12 days of chronic nicotine (6.3 mg/kg/day) or saline treatment. Withdrawal from chronic nicotine disrupted contextual fear conditioning; however, 5 mg/kg bupropion reversed this deficit. Overall, these results indicate that a low dose of bupropion can reverse nicotine withdrawal deficits in contextual fear conditioning, but that high doses of bupropion produce deficits in fear conditioning.
Keywords: Bupropion, Nicotine, Withdrawal, Addiction, Learning, Mice
Introduction
Tobacco use is a major worldwide health problem. The World Health Organization has reported that tobacco use produces a major risk for cancer in developed nations, and that by the year 2020, an estimated 8.4 million people will die annually from tobacco-related diseases (Twombly, 2002, 2003). Once dependence on tobacco has been established, it is very difficult to quit: 42% of cigarette smokers attempt to quit each year, but less than 6% are successful (McIlvain et al., 1995). One treatment option for smoking cessation is bupropion, a drug that acts as a dopamine and norepinephrine reuptake inhibitor (Li et al., 2002), and is also a nicotinic acetylcholine receptor (nAChR) antagonist (Fryer and Lukas, 1999; Slemmer et al., 2000). Research has demonstrated that bupropion produced greater long-term rates of smoking cessation when compared to placebo or nicotine patch treatment (Hurt et al., 1997; Jorenby et al., 1999). Furthermore, bupropion decreased some nicotine withdrawal symptoms, such as increased irritability and impaired concentration (Shiffman et al., 2000) and also reduced cravings for nicotine (Brody et al., 2004; Durcan et al., 2002).
Although bupropion is effective in promoting smoking cessation and reducing nicotine withdrawal symptoms, the behavioral mechanisms by which it produces these effects are only beginning to be understood. Animal models have demonstrated that bupropion altered nicotine-associated changes in reward, nicotine self-administration, nicotine withdrawal-associated conditioned place aversion, and somatic signs of withdrawal. Acute nicotine enhanced brain reward functioning as measured by intercranial self-administration (ICSS), whereas withdrawal from chronic nicotine produced a decrease in brain reward functioning (Epping-Jordan et al., 1998; Harrison et al., 2002; Skjei and Markou, 2003). Cryan and colleagues (2003) reported that a low dose of bupropion blocked the acute nicotine enhancement of ICSS and also reversed the decrease in ICSS when rats were withdrawn from chronic nicotine. In addition, rats withdrawn from chronic nicotine exhibited somatic signs of nicotine withdrawal such as writhing or abdominal constrictions, and bupropion dose-dependently reversed these symptoms (Cryan et al., 2003; Malin et al., 2006). Malin and colleagues (2006) have also found that bupropion can reverse nicotine withdrawal-related conditioned place aversion. Furthermore, bupropion modulates nicotine self-administration. Both high doses (Rauhut et al., 2003; Rauhut et al., 2005) and intermediate doses (Bruijnzeel and Markou, 2003; Glick et al., 2002) of bupropion reduced nicotine self-administration; whereas some studies report that bupropion increased nicotine self administration (Rauhut et al., 2003; Shoaib et al., 2003), but these results appear to depend on the dose and strain tested. Taken together, these studies suggest that bupropion may facilitate smoking cessation by limiting the rewarding effects of nicotine use, as well as the somatic signs of nicotine withdrawal.
In humans, nicotine withdrawal is associated with disrupted cognition, such as difficulty in concentrating or impairment in working memory (Hughes et al., 1994; Jacobsen et al., 2005; Mendrek et al., 2006), and bupropion improved the ability to concentrate in smokers withdrawn from nicotine (Shiffman et al., 2000). However, few animal studies have examined the effects of nicotine withdrawal on processes involved in cognition, such as learning and memory, and no animal studies to date have investigated the effects of bupropion on learning and memory deficits that occur during nicotine withdrawal. One ideal animal model for examining the effects of nicotine withdrawal on learning and memory is Pavlovian fear conditioning, in which subjects form an association between a context and a footshock (contextual fear conditioning), and an association between an auditory stimulus and a footshock (cued fear conditioning). Previous studies have shown that acute nicotine enhanced contextual fear conditioning but had no effect on cued fear conditioning in mice (Davis et al., 2005; Davis et al., 2006; Gould and Higgins, 2003; Gould and Wehner, 1999; Wehner et al., 2004). In contrast, withdrawal from chronic nicotine produced impairments in contextual fear conditioning but not cued fear conditioning (Davis and Gould, 2007; Davis et al., 2005).
An important application of this animal model for nicotine withdrawal-associated deficits in learning and memory is to test compounds that may reverse the effects of nicotine withdrawal. Currently, nicotine replacement has been shown to reverse deficits in contextual fear conditioning following withdrawal from chronic nicotine (Davis et al., 2005). Furthermore, atomoxetine (a norepinephrine reuptake inhibitor) is also effective in ameliorating nicotine withdrawal-associated deficits in contextual fear conditioning (Davis and Gould, 2007). The effects of bupropion on other symptoms of nicotine withdrawal, such as somatic symptoms or alterations of reward and reinforcement (Bruijnzeel and Markou, 2003; Cryan et al., 2003), suggest that it may be effective in reversing nicotine withdrawal-associated deficits in learning. Therefore, the present study tested this hypothesis by determining whether bupropion can reverse nicotine withdrawal-associated deficits in contextual fear conditioning.
Methods
Subjects
Subjects were male C57BL/6J mice (ages 8-12 weeks). Mice were maintained on a 12 hour light-dark cycle (lights on at 7:00 am) and housed in groups of four with ad libitum access to food and water. Behavioral procedures occurred during the light phase of the cycle. The Temple University Institutional Animal Care and Use Committee approved all behavioral and surgical procedures. For all experiments, groups consisted of 7 to 12 mice.
Apparatus
Mice were trained and tested for contextual fear conditioning in four identical chambers (17.78 cm × 19.05 cm × 38.10 cm) that were housed in sound attenuating boxes (Med-Associates, St. Albans, VT). The front, back, and top chamber walls were Plexiglas, and side walls were stainless steel. The floors of the chambers were composed of metal rods that were connected to a shock generator and scrambler. Speakers were attached to the right wall of each chamber, and were used to administer the white noise conditioned stimulus (CS). Ventilation fans provided air exchange and background noise (69 dB), and were mounted on the right wall of each sound attenuating box. A computer connected to the chambers used Med-PC software to control stimulus administration. Testing for cued fear conditioning occurred in four identical altered chambers (20.32 × 22.86 × 17.78 cm) that were housed in sound attenuating boxes and located in a different room from the training chambers. The side walls of the chamber were made of aluminum, and all other walls were composed of Plexiglas. The chamber floors were covered in white plastic. Speakers for delivering the CS were mounted on the left wall of each chamber. The background of the sound attenuating chambers differed in color from training chambers, and a vanilla extract olfactory cue was added to further distinguish these chambers from the training chambers.
Behavioral Procedures: Contextual and Cued Fear Conditioning
Freezing (defined as the absence of all movement except for respiration) was used as the assessment of conditioned fear and was measured with a time-sampling procedure that has been described in detail elsewhere (Gould and Wehner, 1999). Briefly, mice were observed for one second during 10 second bins and were scored as freezing or active. During training, mice were placed in the chamber and baseline activity was scored for 120 seconds. Mice were then exposed to two coterminating CS (85 dB white noise) - US (0.57 mA footshock) pairings separated by a 120 second inter-trial interval. The CS was presented for 30 seconds, and the footshock was activated during the final two seconds of CS administration. After the first CS - US pairing, immediate freezing was scored during the 120 second inter-trial interval before the second presentation of the CS and US. The training session ended with a 30 second period during which freezing behavior was not recorded. Twenty-four hours after training, mice were tested for contextual fear conditioning by returning them to the training chambers and scoring freezing for 5 minutes. One hour later, mice were tested for both generalized freezing and for cued fear conditioning in an altered context. Once mice were placed in the altered context chambers, generalized freezing was scored for the first 3 minutes. Next, the CS was continuously activated and cued fear conditioning was scored for the next 3 minutes.
Drug Administration
Bupropion hydrochloride (Sigma, St. Louis, MO) was dissolved in saline and administered subcutaneously for all experiments. For all experiments investigating the effects of acute bupropion on fear conditioning, bupropion was dissolved in saline at 2.5, 5, 10, 20, or 40 mg/kg (injection volume of 10 mg/mL), and was administered subcutaneously twenty minutes before training and/or testing. The doses of bupropion were based on prior research that used a similar range of doses to investigate the effects of bupropion on locomotor activity (Mitchell et al., 2006; Redolat et al., 2005). Additionally, a control group in each experiment was administered saline subcutaneously 20 minutes before training and testing.
In all experiments involving nicotine withdrawal, nicotine hydrogen tartrate salt (Sigma, St. Louis, MO) was dissolved in saline and administered via mini-osmotic pumps (model 1002; Alzet, Cupertino, CA). Mice were anesthetized with 5% isoflurane and implanted subcutaneously with mini-osmotic pumps containing either 6.3 mg/kg/day nicotine (reported in freebase nicotine weight) or saline. This dose of nicotine was selected because previous research has demonstrated that withdrawal from chronic nicotine administration at this dose produces impairments in contextual fear conditioning in C57BL/6 mice, and also produces plasma nicotine levels comparable to what is observed in smokers (Benowitz et al., 1989; Davis and Gould, 2007; Davis et al., 2005; Henningfield and Keenan, 1993). Chronic nicotine or saline administration continued for 12 days following pump implantation, with all pumps removed on day 12. Training and testing took place on days 13 and 14, respectively.
Experimental Design
High doses of bupropion produce deficits in contextual and cued fear conditioning
The first experiment investigated the dose-dependent effects of bupropion on fear conditioning when bupropion was administered alone on both days. Twenty minutes before training, C57BL/6 mice received an injection of saline or bupropion. On testing day, mice were given an injection of saline or bupropion before testing contextual fear conditioning. One hour later, mice were tested for cued fear conditioning and mice in all groups received an additional injection of saline or bupropion. In this experiment, bupropion was administered on both days because the drug is typically administered over several days when used as a smoking cessation aid (Hurt et al., 1997; Jorenby et al., 1999), and would not be administered to patients just during the acquisition or recall of a task that assesses learning and memory.
The effects of bupropion on the acquisition of fear conditioning
Given that high doses of bupropion disrupted fear conditioning in the first experiment, additional experiments were conducted to determine whether high doses of bupropion impair the acquisition or expression of fear conditioning. To test for the effect of bupropion on acquisition of fear conditioning, saline or bupropion was administered 20 minutes before training. Twenty-four hours later, mice in all groups received an injection of saline 20 minutes before testing contextual and cued fear conditioning.
The effects of bupropion on the recall or expression of fear conditioning
In order to test for the effect of bupropion on the recall or expression of fear conditioning, mice in all groups were administered saline 20 minutes before training. On testing day, mice were given an injection of saline or bupropion 20 minutes before testing contextual fear conditioning, and an additional injection of saline or bupropion 20 minutes before testing cued fear conditioning.
Bupropion ameliorates nicotine withdrawal deficits in contextual fear conditioning in a dose-dependent manner
To investigate the effects of bupropion on nicotine withdrawal-related deficits in contextual fear conditioning, C57BL/6 mice were chronically treated with nicotine or saline for 12 days via mini-osmotic pumps, with all pumps removed on day 12. On training day (day 13), mice withdrawn from chronic nicotine or saline received an injection of saline or bupropion 20 minutes before training. The doses of bupropion were selected because previous experiments with bupropion revealed that high doses of bupropion (20 and 40 mg/kg) produced deficits in the expression of fear conditioning. Twenty-four hours later (day 14), mice in all groups were administered the same drug dose they had received on the previous day (i.e. saline, 2.5, 5, or 10 mg/kg bupropion) 20 minutes before testing contextual and cued fear conditioning.
Bupropion reverses nicotine withdrawal deficits in contextual fear conditioning by enhancing memory recall
The final experiment investigated whether bupropion administration before training alone or testing alone would reverse nicotine withdrawal-related deficits in contextual fear conditioning. C57BL/6 mice were chronically treated with nicotine or saline for 12 days via mini-osmotic pumps, with all pumps removed on day 12. Mice withdrawn from chronic nicotine were split into three groups: one which received saline on both training and testing day (days 13 and 14, respectively), mice that received 5 mg/kg bupropion on training day and saline on testing day, and mice that were administered saline on training day and 5 mg/kg bupropion on testing day. Mice withdrawn from chronic saline received an injection of saline 20 minutes before training and testing. As with previous experiments, mice in all groups received two injections on testing day: 20 minutes before testing contextual fear conditioning, and 20 minutes before testing cued fear conditioning.
Statistical Analyses
Data from the experiments involving acute bupropion were analyzed with one-way ANOVAs. Tukey post-hoc comparisons were used to test pair-wise comparisons between specific levels of acute and chronic drug treatment. For the withdrawal from chronic nicotine experiment, data were analyzed using a 2 (chronic drug treatment: nicotine, saline) × 4 (acute drug treatment: 2.5, 5, or 10 mg/kg bupropion, saline) ANOVA. A one-way ANOVA was used to determine whether bupropion administered on training or testing day reversed nicotine withdrawal-related deficits in contextual fear conditioning. For all ANOVAs, a Levene statistic was used to test the assumption of homogeneity of variance. If the assumption of homogeneity of variance was not met, a Games-Howell post-hoc test was used; otherwise a Tukey post-hoc test was used.
Results
High doses of bupropion produce deficits in contextual and cued fear conditioning
In order to determine whether the acute administration of bupropion alters contextual or cued fear conditioning, bupropion (2.5, 5, 10, 20, or 40 mg/kg) or saline was administered to C57BL/6 mice just prior to the training and testing of fear conditioning (Figure 1). A one-way ANOVA revealed a significant main effect for drug treatment when mice were tested for contextual fear conditioning [F(5, 54) = 17.53, p < 0.05] and cued fear conditioning [F(5, 54) = 12.26, p < 0.05]. Games-Howell post-hoc comparisons determined that mice treated with the 20 and 40 mg/kg doses of bupropion exhibited deficits in contextual and cued fear conditioning when compared to all other groups (saline and 2.5, 5, and 10 mg/kg bupropion groups; p < 0.05). In contrast, mice treated with the 2.5, 5, and 10 mg/kg doses of bupropion did not differ from saline treated mice in contextual and cued fear conditioning (p > 0.05). No significant effects of bupropion were observed for baseline freezing, immediate freezing, or pre-CS freezing (p > 0.05). Whereas it could be proposed that the impairments to cued fear conditioning could be due to the fact that mice received two injections of bupropion on testing day (one before the context test and one before the cue test), this possibility is unlikely given that the half-life of bupropion in the mouse brain is 28 minutes (Wang et al., 2006) and the second injection was administered one hour after the first injection.
Figure 1.
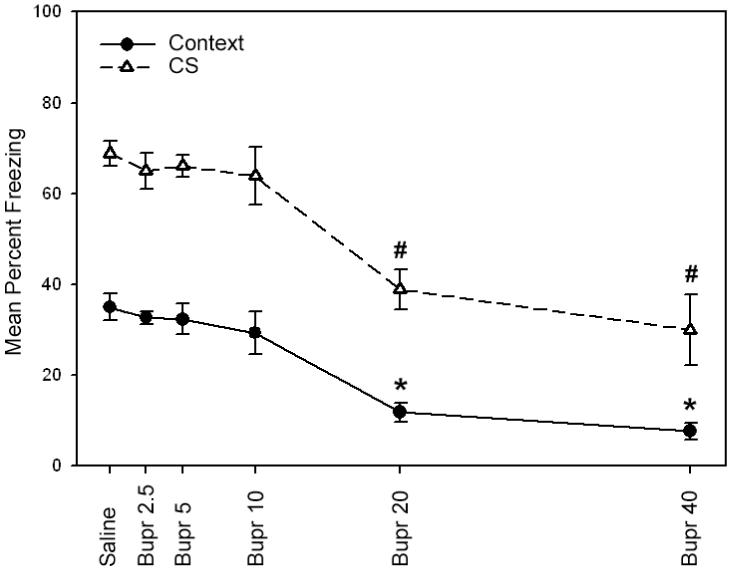
The effects of bupropion on contextual and cued fear conditioning in C57BL/6 mice when administered at training and testing. High doses of bupropion (20 and 40 mg/kg) impaired contextual and cued fear conditioning, suggesting that bupropion may dose-dependently disrupt the acquisition and/or expression of fear conditioning. Error bars indicate SEM. (*) indicates p < 0.05 compared to all other groups for contextual fear conditioning data, and (#) indicates p < 0.05 compared to all other groups for cued fear conditioning data.
The effects of bupropion on the acquisition of fear conditioning
Given that the higher doses of bupropion produced deficits in fear conditioning, additional experiments were conducted to test whether bupropion selectively impairs the acquisition or the expression of contextual and cued fear conditioning. Bupropion (2.5, 5, 10, 20 or 40 mg/kg) or saline was administered on training day only to determine whether bupropion impairs the acquisition of fear conditioning (Figure 2). A one-way ANOVA revealed a significant main effect for drug treatment when mice were tested for contextual fear conditioning [F(5, 45) = 7.68, p < 0.05]. Subsequent Tukey post-hoc comparisons revealed that mice that were administered the 40 mg/kg dose of bupropion were impaired in contextual fear conditioning compared to all other groups (p < 0.05), while mice treated with the lower doses of bupropion (2.5, 5, 10 or 20 mg/kg) were not significantly different from saline treated mice (p > 0.05). No significant differences were observed during baseline freezing, immediate freezing, pre-CS freezing, or during cued fear conditioning (p > 0.05). The fact that the highest dose of bupropion produced deficits in contextual fear conditioning but not cued fear conditioning suggests that this dose may disrupt memory acquisition of contextual fear conditioning.
Figure 2.
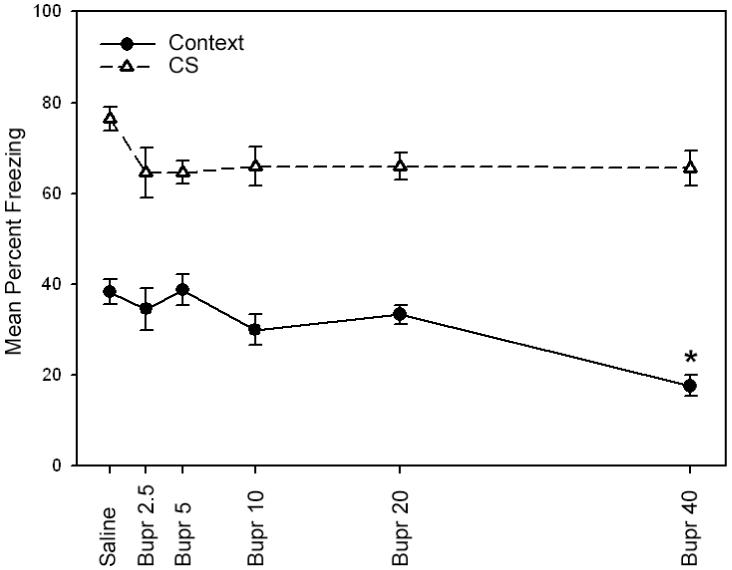
The effects of bupropion on fear conditioning in C57BL/6 mice when administered on training day alone. A high dose of bupropion (40 mg/kg) administered on training day to chronic nicotine-treated mice produced deficits in contextual fear conditioning but not cued fear conditioning. These results suggest that bupropion dose-dependently disrupts the acquisition of contextual fear conditioning. Error bars indicate SEM, and (*) indicates p < 0.05 compared to all other groups for contextual fear conditioning data.
The effects of bupropion on the recall or expression of fear conditioning
In order to test whether bupropion impairs the expression of fear conditioning, bupropion (2.5, 5, 10, 20 or 40 mg/kg) or saline was administered on testing day only (Figure 3). A one-way ANOVA revealed a significant main effect for drug treatment when mice were tested for contextual fear conditioning [F(5, 52) = 20.14, p < 0.05] and cued fear conditioning [F(5, 52) = 43.05, p < 0.05]. Subsequent Tukey post-hoc comparisons demonstrated that mice treated with the higher doses of bupropion (20 and 40 mg/kg) were significantly impaired in contextual and cued fear conditioning when compared to all other groups (saline and 2.5, 5, and 10 mg/kg bupropion groups; p < 0.05). Mice treated with the 2.5, 5, and 10 mg/kg doses of bupropion were not significantly different from saline treated mice in contextual and cued fear conditioning (p > 0.05). No significant differences were observed during baseline freezing, immediate freezing, or pre-CS freezing (p > 0.05). Thus, the results from the following experiment suggest that the 20 and 40 mg/kg doses of bupropion produce deficits in the expression of contextual and cued fear conditioning.
Figure 3.
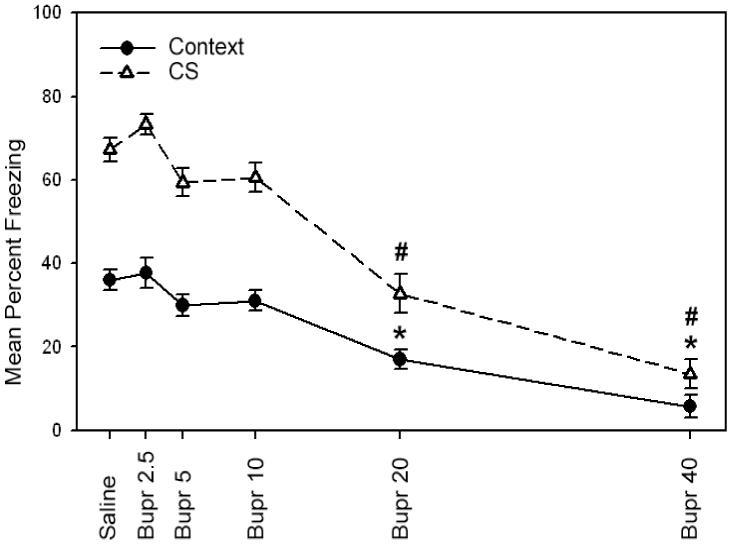
The effects of bupropion on fear conditioning in C57BL/6 mice when administered on testing day alone. Higher doses of bupropion (20 and 40 mg/kg) produced deficits in contextual and cued fear conditioning when administered on testing day. These results suggest that bupropion dose-dependently disrupts the expression of fear conditioning. Error bars indicate SEM. (*) indicates p < 0.05 compared to all other groups for contextual fear conditioning data, and (#) indicates p < 0.05 compared to all other groups for cued fear conditioning data.
Bupropion ameliorates nicotine withdrawal deficits in contextual fear conditioning in a dose-specific manner
The next experiment investigated whether bupropion could reverse nicotine withdrawal deficits in contextual fear conditioning. Following withdrawal from chronic nicotine administration, mice were treated with saline or bupropion (2.5, 5, or 10 mg/kg) prior to the training and testing of fear conditioning (Figure 4). A 2 (chronic drug treatment) × 4 (acute drug treatment) ANOVA revealed significant main effects for chronic drug treatment [F(1, 85) = 53.59, p < 0.05] and acute drug treatment [F(3, 85) = 5.14, p < 0.05] when mice were tested for contextual fear conditioning. Furthermore, a significant interaction between acute and chronic drug treatment was found [F(3, 85) = 5.85, p < 0.05]. Tukey post-hoc comparisons demonstrated that mice withdrawn from chronic nicotine who were treated with saline, 2.5, or 10 mg/kg doses of bupropion exhibited significantly lower levels of contextual fear conditioning when compared to mice withdrawn from chronic saline (p < 0.05). In contrast, nicotine withdrawn mice treated with the 5 mg/kg dose of bupropion exhibited levels of contextual fear conditioning that were similar to the saline treated group (p > 0.05), but were significantly greater than all other groups that were withdrawn from nicotine (p < 0.05). Tukey adjusted comparisons yielded no other significant differences between groups (p > 0.05). No significant differences were observed in baseline freezing, immediate freezing, or altered context freezing (pre-CS). Furthermore, there were no differences between groups in cued fear conditioning (p > 0.05), replicating studies that found that the effects of withdrawal from chronic nicotine are selective for contextual fear conditioning (Davis and Gould, 2007; Davis et al., 2005). Overall, these data suggest that bupropion dose-dependently reverses nicotine withdrawal-associated deficits in contextual fear conditioning.
Figure 4.
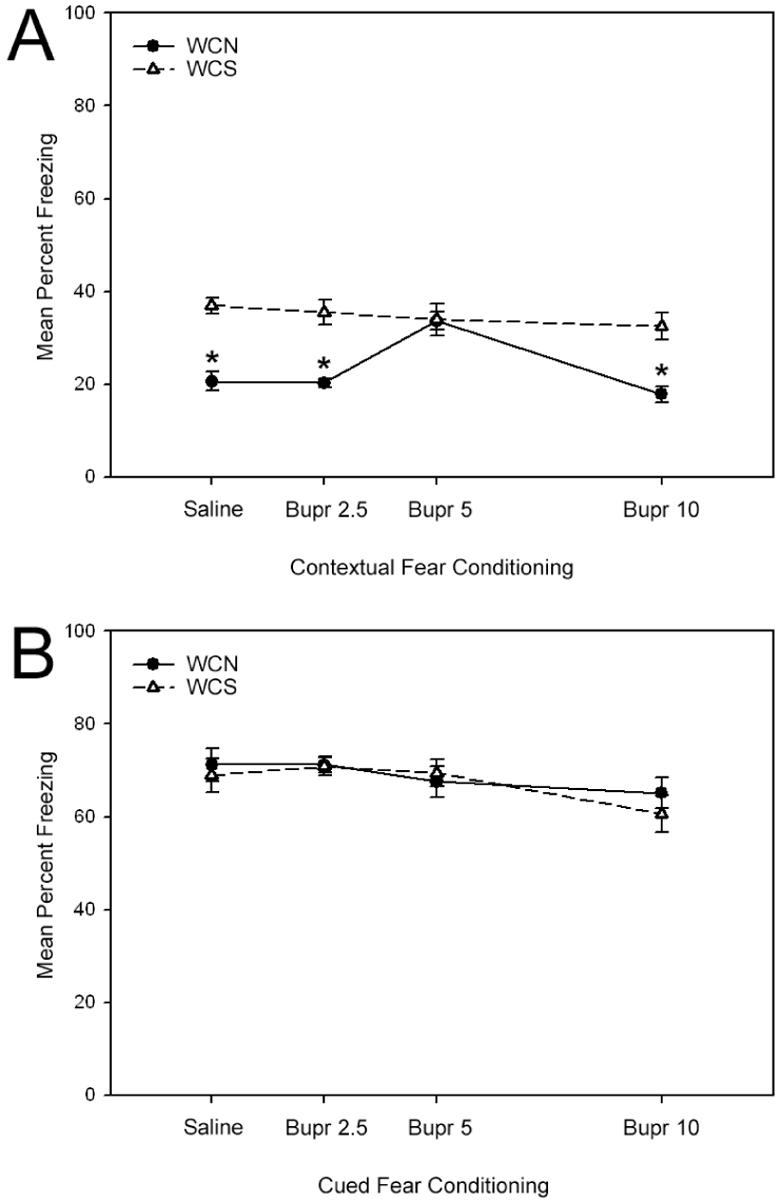
The effects of bupropion on fear conditioning during withdrawal from chronic nicotine (WCN) or withdrawal from chronic saline (WCS) in C57BL/6 mice. The administration of bupropion on both training and testing days dose-dependently reversed nicotine withdrawal-related deficits in contextual fear conditioning (Figure 4a). In contrast, no differences in cued fear conditioning were observed between groups (Figure 4b). Error bars indicate SEM, and (*) indicates p < 0.05 compared to all other chronic saline-treated groups.
Bupropion reverses nicotine withdrawal deficits in contextual fear conditioning by enhancing memory recall
Given that the previous experiment demonstrated that 5 mg/kg bupropion administered on both training and testing days reverses nicotine withdrawal deficits in contextual fear conditioning, the final experiment examined whether bupropion administered on training day alone or testing day alone could ameliorate nicotine withdrawal deficits in contextual fear conditioning (Figure 5). Mice withdrawn from chronic nicotine were administered 5 mg/kg bupropion on training or testing day only, or were given saline on both days. A one-way ANOVA revealed a significant main effect for drug treatment when mice were tested for contextual fear conditioning [F(3, 30) = 8.34, p < 0.05]. Games-Howell post-hoc comparisons revealed that mice withdrawn from chronic nicotine that were administered 5 mg/kg bupropion on testing day were not significantly different from mice withdrawn from saline (p > 0.05), but exhibited greater levels of contextual fear conditioning when compared to all other groups (i.e. mice withdrawn from chronic nicotine and administered saline on both days, or mice withdrawn from chronic nicotine and administered bupropion on training day; p < 0.05). In addition, mice withdrawn from chronic saline displayed greater levels of contextual fear conditioning when compared to chronic nicotine withdrawn mice that received either bupropion on training day or saline on both days (p < 0.05). No significant differences were observed during baseline freezing, immediate freezing, pre-CS freezing, or during cued fear conditioning (p > 0.05). Together, these data suggest that bupropion reverses nicotine withdrawal-related deficits in contextual fear conditioning by enhancing memory recall.
Figure 5.
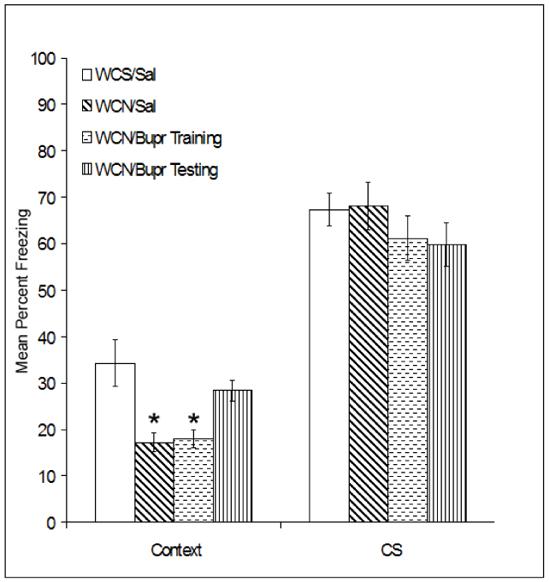
The effects of bupropion on fear conditioning during withdrawal from chronic nicotine (WCN) or withdrawal from chronic saline (WCS) when administered on training day alone or testing day alone. Bupropion administered on testing day alone reversed nicotine withdrawal-related deficits in contextual fear conditioning. In contrast, bupropion administered on training day alone had no effect. No differences in cued fear conditioning were observed between groups. Error bars indicate SEM, and (*) indicates p < 0.05 compared to all other chronic saline-treated groups.
Discussion
Research has shown that bupropion may facilitate smoking cessation by reducing some symptoms of nicotine withdrawal, such as somatic symptoms or alterations of reinforcement and reward (Bruijnzeel and Markou, 2003; Cryan et al., 2003). However, the effects of bupropion on deficits in learning and memory that occur during nicotine withdrawal remain unknown. The results of the present study are the first to demonstrate that 5 mg/kg bupropion reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Furthermore, bupropion ameliorates nicotine withdrawal-associated deficits in contextual fear conditioning when administered before testing but not before training. These data may suggest that nicotine withdrawal-related deficits in contextual fear conditioning result from an impairment in recall of the association, which can be reversed by bupropion. However, an alternative explanation is that withdrawal from chronic nicotine disrupts acquisition, and bupropion reverses deficits in contextual fear conditioning by enhancing retrieval processes. This latter position is supported by research indicating that norepinephrine plays a critical role in the retrieval of contextual and spatial memory (Murchison et al., 2004). Additionally, research has demonstrated that withdrawal from chronic nicotine produces deficits in acquisition and not recall (Portugal et al., 2007).
In addition to ameliorating nicotine withdrawal-associated deficits in contextual fear conditioning, data from the present study indicate that high doses of bupropion (20 and 40 mg/kg) disrupt contextual and cued fear conditioning. In order to determine whether bupropion impairs the acquisition or expression of fear conditioning, bupropion was administered on training or testing day only. When bupropion was administered on testing day, mice exhibited deficits in contextual and cued fear conditioning, suggesting that high doses of bupropion disrupt the expression of contextual and cued fear conditioning. When bupropion was administered on training day, deficits in contextual fear conditioning were observed in mice treated with 40 mg/kg bupropion, suggesting that high doses of bupropion can disrupt acquisition of contextual fear conditioning. It is also important to note that 40 mg/kg bupropion at training did not impair cued fear conditioning; and though studies have shown that higher doses of bupropion can alter locomotor activity in mice (Mitchell et al., 2006; Redolat et al., 2005), the lack of effect on cued fear conditioning suggests that the deficits in the acquisition of contextual fear conditioning are not due to other factors, such as changes in sensory or motor processes. Thus, the data from the present study suggest that bupropion can effectively counteract nicotine withdrawal-associated deficits in learning-related processes, but higher doses of bupropion can produce impairment in the acquisition and expression of contextual memories.
Given that bupropion functions as a dopamine (DA) and norepinephrine (NE) reuptake inhibitor (Li et al., 2002) and also as a nAChR antagonist (Fryer and Lukas, 1999; Slemmer et al., 2000), the exact mechanisms by which bupropion reverses nicotine withdrawal-associated deficits in contextual fear conditioning are unknown. One possibility is that bupropion may ameliorate nicotine withdrawal-associated deficits in contextual fear conditioning through its effects on NE activity. Previous research has demonstrated that nicotine enhances NE functioning in the hippocampus, a brain area that is required for contextual fear conditioning (Logue et al., 1997; Phillips and LeDoux, 1992). Specifically, Azam and McIntosh (2006) found that nAChR mediated NE release in both rat and mouse synaptosomes; it is noteworthy that this effect was greater in rats than mice and that different nAChR subtypes may mediate the effects across species. Barik and Wonnacott (2006) also found nAChR-mediated NE release in rat hippocampal slices, and Singer and colleagues (2004) observed increased hippocampal NE release and turnover in freely moving rats. Furthermore, bupropion administration in the absence of nicotine will increase hippocampal NE overflow in rats (Piacentini et al., 2003).
Alterations in hippocampal NE activity have also been reported during chronic nicotine administration in rats (Fu et al., 1998; Grilli et al., 2005) and following withdrawal from chronic nicotine (Barik and Wonnacott, 2006; Jacobs et al., 2002); however, both increases (Barik and Wonnacott, 2006; Grilli et al., 2005) and decreases (Fu et al., 1998; Jacobs et al., 2002) in NE release have been reported during chronic nicotine and withdrawal from chronic nicotine. These contrasting results across studies may be due to differences in the dose of nicotine used, the manner of nicotine administration, and differences in the time of testing following nicotine withdrawal. However, depletions of NE can produce deficits in conditioned place preference and conditioned place aversion (Ventura et al., 2007), which is consistent with the hypothesis that decreases in NE activity disrupt contextual conditioning. Additionally, previous research has demonstrated that atomoxetine (a NE reuptake inhibitor) reversed nicotine withdrawal-related deficits in contextual fear conditioning (Davis and Gould, 2007). These data, along with the present study, suggest that decreases in NE functioning during nicotine withdrawal may impair contextual learning, and that these deficits can be prevented by increasing levels of NE.
The effects of bupropion on DA and nAChR processes are alternative mechanisms by which bupropion could alleviate nicotine withdrawal-associated deficits in contextual fear conditioning. Both acute nicotine (Cao et al., 2005; Rada et al., 2001; Rahman et al., 2003; Rossi et al., 2005) and bupropion (Piacentini et al., 2003) increase DA release. In contrast, withdrawal from chronic nicotine decreases dopamine activity in brain areas related to nicotine addiction (Jacobs et al., 2002; Rada et al., 2001). If nicotine withdrawal results in decreased DA levels, bupropion may reverse withdrawal deficits by increasing DA activity. In addition, bupropion could alter nicotine withdrawal through reversing nicotine withdrawal-related changes in nAChR function. nAChRs are desensitized in response to chronic nicotine (Olale et al., 1997; Peng et al., 1997; Peng et al., 1994; Wooltorton et al., 2003), and increases in the number of nAChRs have also been reported during chronic nicotine administration (Marks et al., 1992; Nguyen et al., 2003; Perry et al., 1999; Whiteaker et al., 1998). Furthermore, withdrawal from chronic nicotine may result in the resensitization and recovery of function of nAChRs (Arnold et al., 2003; Balfour et al., 1998; Gentry et al., 2003). If hypersensitivity of nAChRs develops during nicotine withdrawal, this could contribute to nicotine withdrawal-related deficits of contextual fear conditioning, and bupropion could ameliorate these deficits by blocking nAChRs. In support, bupropion inhibits α3β4, α4β2, and α7 nAChRs in a noncompetitive manner (Fryer and Lukas, 1999; Slemmer et al., 2000). Overall, the results of these studies suggest that the effects of bupropion on DA and nAChR function may counteract the effects of nicotine withdrawal on contextual fear conditioning but further research is needed to better understand the involvement of these substrates in the effects of bupropion on nicotine withdrawal.
In addition to the diverse pharmacological targets of bupropion that may counteract the effects of nicotine withdrawal, the metabolites of bupropion may also have therapeutic properties. Bupropion is metabolized into (2S, 3R)- and (2S, 3S)-hydroxybupropion, (R, -R)- and (S, -S)-threohydrobupropion, and (R, -S)-, and (S, -R)-erythrohydrobupropion (Cooper et al., 1994), and previous studies suggest that the metabolites of bupropion may be responsible for some of the effects of bupropion as an antidepressant (Martin et al., 1990; Rotzinger et al., 1999; Young, 1991). Furthermore, (2S, 3S)-hydroxybupropion blocked the effects of acute nicotine on pain sensitivity, locomotor activity, and body temperature, whereas the (2S, 3R)- hydroxybupropion metabolite had no effect, suggesting that the metabolites of bupropion may be responsible for some of the effects of bupropion on acute nicotine administration (Damaj et al., 2004). These data suggest that a better understanding of the effects of bupropion metabolites may reveal the mechanisms by which bupropion facilitates smoking cessation, and may lead to the development of more effective smoking cessation therapeutics. Therefore, future research on the effects of bupropion on cognition should investigate whether the metabolites of bupropion can ameliorate the effects of nicotine withdrawal on cognition.
Acknowledgements
The authors would like to acknowledge grant support from the National Institute on Drug Abuse (DA017949 TG), and the National Cancer Institute/National Institute on Drug Transdisciplinary Tobacco Research Center Grant (P5084718 PI: Caryn Lerman Ph.D).
Footnotes
Financial Disclosures: Each author of this manuscript reports no conflicts of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold HM, Nelson CL, Sarter M, Bruno JP. Sensitization of cortical acetylcholine release by repeated administration of nicotine in rats. Psychopharmacology. 2003;165:346–58. doi: 10.1007/s00213-002-1260-6. [DOI] [PubMed] [Google Scholar]
- Azam L, McIntosh JM. Characterization of nicotinic acetylcholine receptors that modulate nicotine-evoked [3H]norepinephrine release from mouse hippocampal synaptosomes. Mol Pharmacol. 2006;70:967–76. doi: 10.1124/mol.106.024513. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Benwell ME, Birrell CE, Kelly RJ, Al-Aloul M. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Behav. 1998;59:1021–30. doi: 10.1016/s0091-3057(97)00537-6. [DOI] [PubMed] [Google Scholar]
- Barik J, Wonnacott S. Indirect modulation by alpha7 nicotinic acetylcholine receptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol. 2006;69:618–28. doi: 10.1124/mol.105.018184. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Porchet H, Jacob P., 3rd. Nicotine dependence and tolerance in man: pharmacokinetic and pharmacodynamic investigations. Prog Brain Res. 1989;79:279–87. doi: 10.1016/s0079-6123(08)62487-5. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Lee G, Smith E, Sadeghi M, Saxena S, et al. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res. 2004;130:269–81. doi: 10.1016/j.pscychresns.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel AW, Markou A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse. 2003;50:20–8. doi: 10.1002/syn.10242. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Surowy CS, Puttfarcken PS. Nicotinic acetylcholine receptor-mediated [3H]dopamine release from hippocampus. J Pharmacol Exp Ther. 2005;312:1298–04. doi: 10.1124/jpet.104.076794. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Wang CM, Cox RF, Norton R, Shea V, Ferris RM. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11:133–41. doi: 10.1038/npp.1994.43. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology. 2003;168:347–58. doi: 10.1007/s00213-003-1445-7. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, et al. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66:675–82. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–34. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ.Atomoxetine Reverses Nicotine Withdrawal-Associated Deficits in Contextual Fear Conditioning Neuropsychopharmacology 2007. E-pub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J Neurosci. 2005;25:8708–13. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Porter J, Gould TJ. Nicotine enhances both foreground and background contextual fear conditioning. Neurosci Lett. 2006;394:202–5. doi: 10.1016/j.neulet.2005.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan MJ, Deener G, White J, Johnston JA, Gonzales D, Niaura R, et al. The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clin Ther. 2002;24:540–51. doi: 10.1016/s0149-2918(02)85130-x. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–9. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther. 1999;288:88–92. [PubMed] [Google Scholar]
- Fu Y, Matta SG, Valentine JD, Sharp BM. Desensitization and resensitization of norepinephrine release in the rat hippocampus with repeated nicotine administration. Neurosci Lett. 1998;241:147–50. doi: 10.1016/s0304-3940(98)00018-4. [DOI] [PubMed] [Google Scholar]
- Gentry CL, Wilkins LH, Jr., Lukas RJ. Effects of prolonged nicotinic ligand exposure on function of heterologously expressed, human alpha4beta2- and alpha4beta4-nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2003;304:206–16. doi: 10.1124/jpet.102.041756. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur J Pharmacol. 2002;448:185–91. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Higgins SJ. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–57. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav Brain Res. 1999;102:31–9. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Grilli M, Parodi M, Raiteri M, Marchi M. Chronic nicotine differentially affects the function of nicotinic receptor subtypes regulating neurotransmitter release. J Neurochem. 2005;93:1353–60. doi: 10.1111/j.1471-4159.2005.03126.x. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology. 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Keenan RM. Nicotine delivery kinetics and abuse liability. J Consult Clin Psychol. 1993;61:743–50. doi: 10.1037//0022-006x.61.5.743. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–70. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. New Engl J Med. 1997;337:1195–02. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jacobs I, Anderson DJ, Surowy CS, Puttfarcken PS. Differential regulation of nicotinic receptor-mediated neurotransmitter release following chronic (-)-nicotine administration. Neuropharmacology. 2002;43:847–56. doi: 10.1016/s0028-3908(02)00166-1. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New Engl J Med. 1999;340:685–91. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Li SX, Perry KW, Wong DT. Influence of fluoxetine on the ability of bupropion to modulate extracellular dopamine and norepinephrine concentrations in three mesocorticolimbic areas of rats. Neuropharmacology. 2002;42:181–90. doi: 10.1016/s0028-3908(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–13. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Smith TD, Khambati HN, Meyers-Paal RL, Montellano AL, et al. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology. 2006;184:494–03. doi: 10.1007/s00213-005-0135-z. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, et al. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–84. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, Massol J, Colin JN, Lacomblez L, Puech AJ. Antidepressant profile of bupropion and three metabolites in mice. Pharmacopsychiatry. 1990;23:187–94. doi: 10.1055/s-2007-1014505. [DOI] [PubMed] [Google Scholar]
- McIlvain H, Susman JL, Davis C, Gilbert C. Physician counseling for smoking cessation: is the glass half empty? J Fam Pract. 1995;40:148–52. [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, et al. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–44. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell HA, Ahern TH, Liles LC, Javors MA, Weinshenker D. The effects of norepinephrine transporter inactivation on locomotor activity in mice. Biol Psychiatry. 2006;60:1046–52. doi: 10.1016/j.biopsych.2006.03.057. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–143. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–7. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Olale F, Gerzanich V, Kuryatov A, Wang F, Lindstrom J. Chronic nicotine exposure differentially affects the function of human alpha3, alpha4, and alpha7 neuronal nicotinic receptor subtypes. J Pharmacol Exp Ther. 1997;283:675–83. [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Wang F, Lindstrom J. Chronic nicotine treatment up-regulates alpha3 and alpha7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Mol Pharmacol. 1997;51:776–84. doi: 10.1124/mol.51.5.776. [DOI] [PubMed] [Google Scholar]
- Peng X, Katz M, Gerzanich V, Anand R, Lindstrom J. Human alpha 7 acetylcholine receptor: cloning of the alpha 7 subunit from the SH-SY5Y cell line and determination of pharmacological properties of native receptors and functional alpha 7 homomers expressed in Xenopus oocytes. Mol Pharmacol. 1994;45:546–54. [PubMed] [Google Scholar]
- Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ. Increased nicotinic receptors in brains from smokers: membrane binding and autoradiography studies. J Pharmacol Exp Ther. 1999;289:1545–52. [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–85. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Piacentini MF, Clinckers R, Meeusen R, Sarre S, Ebinger G, Michotte Y. Effect of bupropion on hippocampal neurotransmitters and on peripheral hormonal concentrations in the rat. J Appl Physiol. 2003;95:652–6. doi: 10.1152/japplphysiol.01058.2002. [DOI] [PubMed] [Google Scholar]
- Portugal GS, Kenney JW, Gould TJ. β2 subunit containing acetylcholine receptors mediate nicotine withdrawal deficits in the acquisition of contextual fear conditioning. Neurobiol Learn Mem. 2007 doi: 10.1016/j.nlm.2007.05.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology. 2001;157:105–10. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Effects of acute and chronic nicotine on somatodendritic dopamine release of the rat ventral tegmental area: in vivo microdialysis study. Neurosci Lett. 2003;348:61–4. doi: 10.1016/s0304-3940(03)00723-7. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Dwoskin LP, Bardo MT. Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration. Nicotine Tob Res. 2005;7:901–7. doi: 10.1080/14622200500381384. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology. 2003;169:1–9. doi: 10.1007/s00213-003-1450-x. [DOI] [PubMed] [Google Scholar]
- Redolat R, Vidal J, Gomez MC, Carrasco MC. Effects of acute bupropion administration on locomotor activity in adolescent and adult mice. Behav Pharmacol. 2005;16:59–62. doi: 10.1097/00008877-200502000-00007. [DOI] [PubMed] [Google Scholar]
- Rossi S, Singer S, Shearman E, Sershen H, Lajtha A. The effects of cholinergic and dopaminergic antagonists on nicotine-induced cerebral neurotransmitter changes. Neurochem Res. 2005;30:541–58. doi: 10.1007/s11064-005-2689-x. [DOI] [PubMed] [Google Scholar]
- Rotzinger S, Bourin M, Akimoto Y, Coutts RT, Baker GB. Metabolism of some “second”- and “fourth”-generation antidepressants: iprindole, viloxazine, bupropion, mianserin, maprotiline, trazodone, nefazodone, and venlafaxine. Cell Mol Neurobiol. 1999;19:427–42. doi: 10.1023/A:1006953923305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Sidhpura N, Shafait S. Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology. 2003;165:405–12. doi: 10.1007/s00213-002-1277-x. [DOI] [PubMed] [Google Scholar]
- Singer S, Rossi S, Verzosa S, Hashim A, Lonow R, Cooper T, et al. Nicotine-induced changes in neurotransmitter levels in brain areas associated with cognitive function. Neurochem Res. 2004;29:1779–92. doi: 10.1023/b:nere.0000035814.45494.15. [DOI] [PubMed] [Google Scholar]
- Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology. 2003;168:280–92. doi: 10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Slemmer JE, Martin BR, Damaj MI. Bupropion is a nicotinic antagonist. J Pharmacol Exp Ther. 2000;295:321–7. [PubMed] [Google Scholar]
- Twombly R. Tobacco use a leading global cancer risk, report says. J Natl Cancer Inst. 2003;95:11–12. doi: 10.1093/jnci/95.1.11. [DOI] [PubMed] [Google Scholar]
- Twombly R. World Health Organization takes on ‘tobacco epidemic’. J Natl Cancer Inst. 2002;94:644–6. doi: 10.1093/jnci/94.9.644. [DOI] [PubMed] [Google Scholar]
- Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci U S A. 2007;104:5181–5186. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JS, DeVane CL, Gibson BB, Donovan JL, Markowitz JS, Zhu HJ. Population pharmacokinetic analysis of drug-drug interactions among risperidone, bupropion, and sertraline in CF1 mice. Psychopharmacology. 2006;183:490–9. doi: 10.1007/s00213-005-0209-y. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, et al. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, Sharples CG, Wonnacott S. Agonist-induced up-regulation of alpha4beta2 nicotinic acetylcholine receptors in M10 cells: pharmacological and spatial definition. Mol Pharmacol. 1998;53:950–62. [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–85. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC. Hydroxylated metabolites of antidepressants. Psychopharmacol Bull. 1991;27:521–32. [PubMed] [Google Scholar]


