Abstract
Destabilization of the tetrameric fold of TTR (transthyretin) is important for aggregation of the protein which culminates in amyloid fibril formation. Many TTR mutations interfere with tetramer stability, increasing the amyloidogenic potential of the protein. The vast majority of proposed TTR fibrillogenesis inhibitors are based on in vitro assays with isolated protein, limiting their future use in clinical assays. In the present study we investigated TTR fibrillogenesis inhibitors using a cellular system that produces TTR intermediates/aggregates in the medium. Plasmids carrying wild-type TTR, V30M or L55P cDNA were transfected into a rat Schwannoma cell line and TTR aggregates were investigated in the medium using a dot-blot filter assay followed by immunodetection. Results showed that, in 24 h, TTR L55P forms aggregates in the medium, whereas, up to 72 h, wild-type TTR and V30M do not. A series of 12 different compounds, described in the literature as in vitro TTR fibrillogenesis inhibitors, were tested for their ability to inhibit L55P aggregate formation; in this system, 2-[(3,5-dichlorophenyl) amino] benzoic acid, benzoxazole, 4-(3,5-difluorophenyl) benzoic acid and tri-iodophenol were the most effective inhibitors, as compared with the reference iododiflunisal, previously shown by ex vivo and in vitro procedures to stabilize TTR and inhibit fibrillogenesis. Among these drugs, 2-[(3,5-dichlorophenyl) amino] benzoic acid and tri-iodophenol stabilized TTR from heterozygotic carriers of V30M in the same ex vivo conditions as those used previously for iododiflunisal. The novel cellular-based test herein proposed for TTR fibrillogenesis inhibitor screens avoids not only lengthy and cumbersome large-scale protein isolation steps but also artefacts associated with most current in vitro first-line screening methods, such as those associated with acidic conditions and the absence of serum proteins.
Keywords: aggregation, amyloid, anti-amyloidogenic drug, iododiflunisal, transthyretin
Abbreviations: AA, amyloid A associated amyloidosis; DCPA, 2-(3,5-dichlorophenyl) amino benzoic acid; DES, diethylstilbestrol; DFPB, 4-(3, 5-difluorophenyl) benzoic acid; DNP, dinitrophenol; FAP, familial amyloidotic polyneuropathy; FCS, foetal calf serum; IEF, isoelectric focusing; PS2, presenilin 2; TEM, transmission electron microscopy; TIP, tri-iodophenol; TTR, transthyretin; WT, wild-type
INTRODUCTION
FAP (familial amyloidotic polyneuropathy) is an autosomal dominant disorder characterized by the extracellular deposition of TTR (transthyretin) amyloid fibrils. This systemic disease has particular deposition in peripheral nerve. Over 80 TTR mutations have been related to amyloid deposition and disease [1], the most frequent being a substitution of a methionine residue for valine at position 30 (V30M). TTR is a homotetrameric protein produced in the liver and in the choroid plexus of the brain and functions as a transporter of T4 and vitamin A.
The knowledge acquired previously on structural and biochemical features characteristic of amyloidogenic intermediates, has paved the way for the investigation of drugs capable of interfering early in the amyloidogenic pathway. For TTR-related amyloidosis, the first step of a rational intervention is to obstruct tetramer dissociation and thus prevent potential aggregation-prone species from being generated. A second target is to inhibit the association between conformational intermediate species that lead to fibril elongation. Following these principles, several authors have reported the identification of compounds capable of inhibiting TTR fibril formation [2–6]. However, most of these fibril inhibition studies were performed under non-physiological acidic conditions believed to induce rapid conformational changes and no specific distinction was made regarding the molecular species affected by the drugs studied. Furthermore, these in vitro tests were performed in the test tube using prokaryotic rather than eukaryotic systems for the synthesis of human TTR. Cellular systems represent a more physiological in vitro approach to study inhibitors of amyloidogenesis which avoids isolation of the target protein. In the present study we describe, for the first time, a TTR-based cellular system for the screening of amyloid inhibitors. Using this system we also report comparative results of the activity of a series of drugs referred to in the literature as inhibitors of TTR fibril formation.
MATERIALS AND METHODS
Vector constructions
Vectors p169ZT carrying human WT (wild-type) TTR (p169ZT-hTTRwt) and p169ZT carrying human TTR L55P (p169ZT-hTTR55) were generated as previously described [7]. We further produced a construct carrying the V30M TTR mutation by site-directed mutagenesis using the QuikChange® mutagenesis kit (Stratagene) following the manufacturer's protocol and the appropriate primers: 5′-AATGTGGCCATGCATGTGTTC-3′ and 5′-GAACACATGCATGGCCACATT-3′.
Transfection and TTR expression in cell culture
Rat Schwannomas (RN22) (American type Cell Collection) were stably co-transfected with each of the TTR cDNA constructs (WT, V30M or L55P) plus a plasmid with resistance to neomycin (pLNeo, provided by Dr Paulo Vieira, Pasteur Institute, Paris, France) following the CaPO4–DNA precipitate method [8]. Briefly, cells were grown to approx. 30–50% confluence in 10 cm dishes (Costar). The appropriate purified cDNA TTR plasmid (25 μg) plus 5 μg of purified pLNeo plasmid were resuspended in 500 μl of 250 mM CaCl2 and diluted with 500 μl 2× Hepes-buffered saline (2X HEBS; 280 mM NaCl, 50 mM Hepes, 1.5 mM Na2HPO4, pH 7.05). The CaPO4–DNA precipitate was allowed to form by standing the mixture for 20 min at room temperature (22 °C) and was subsequently added to the cells. After 5 h incubation, cells were washed twice with PBS and fed with 10 ml of DMEM (Dulbecco's modified Eagle's medium; Gibco BRL) supplemented with 10% FCS (foetal calf serum) and 1% penicillin/streptomycin (complete medium). At 24 h later, transfection medium (250 mM CaCl2 and 2XHEBS) was replaced by complete medium supplemented with G418 (1 mg/ml). Resistant colonies arose after approx. 7 days of selection and were isolated and separated over the course of the following weeks.
TTR secretion was tested by quantitative ELISA [9]: 96-well-plates (Maxisorp-Nunc) were coated overnight at 4 °C with a polyclonal rabbit anti-human TTR antibody (1:500 dilution; DAKO) and blocked with 5% (w/v) non-fat skimmed milk in PBS; conditioned medium was applied to the wells for 1 h at room temperature; a secondary peroxidase-conjugated anti-human TTR antibody [The Binding Site; 1:500 dilution in PBS-T (PBS-0.05% Tween 20)] was used. Development was performed with ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid; Sigma]/H2O2. The TTR concentration in cell medium was calculated from a standard curve ranging from 5 to 340 ng/ml.
Since the vector used is under the control of the metallothionein promoter, experiments were performed in the presence of 100 μM ZnSO4 in the cell culture medium in order to induce TTR synthesis.
Immunoprecipitation and MS analysis
Zinc-treated cells expressing TTR were grown to approx. 100% confluence in complete medium supplemented with G418 which was then replaced by [14C]leucine-containing medium, and collected 4 h later. Cells were harvested and then lysed with 1 ml of lysis solution [30 mM Tris/HCl (pH 7.5), 300 mM NaCl, 1% Triton X-100 and 10 μM PMSF], centrifuged for 30 min at 16000 g, the pellet was discarded and the supernatant collected.
Both medium and cell supernatant were incubated with a rabbit anti-human TTR antibody (1:20 dilution; DAKO) and kept overnight at 4 °C with stirring. Next, 150 μl of protein A Sepharose beads [62.5 μg/μl in 30 mM Tris/HCl (pH 7.5)] (CL-4B, Pharmacia) were added and allowed to mix for 1 h at room temperature with agitation. The beads were washed three times with washing solution [30 mM Tris/HCl (pH 7.5), 300 mM NaCl and 1% Triton X-100] and the complex was eluted with SDS-loading buffer [62.5 mM Tris/HCl (pH 6.8), 2% SDS, 10% glycerol and 5% 2-mercaptoethanol]. Finally, samples were fractioned on SDS/PAGE (15% gels) under denaturing conditions and the gel was processed for autoradiography.
In some experiments, cells were grown in medium supplemented with 1% FCS and G418, for 24 h; the medium was collected and processed for immunoprecipitation as described above. After separation on an SDS/PAGE gel, proteins were stained following a silver stain protocol. The TTR band was excized from the gel and digested with trypsin. MALDI (matrix-assisted laser-desorption ionization) MS analysis was performed on a PerSeptive Voyager mass spectrometer in the linear mode [10].
Dot-blot filter assay for aggregate detection and drug assessment
Transfected and non-transfected cells were treated with ZnSO4 in complete medium supplemented with G418 for 3 days, grown to approx. 80% confluence and then incubated with serum-free medium supplemented with ZnSO4 for different periods of time. For the screening of drugs, cells were incubated with different concentrations of each compound for 48 h, prior to the addition of ZnSO4, and then for a further 3 days simultaneously with ZnSO4. At that point, cells reached approx. 80% confluence and were incubated with serum-free medium supplemented with ZnSO4 and the desired drug for 24 h. TTR in the medium was quantified by ELISA as described above, and 500 ng was applied on to a 0.2 μm pore cellulose acetate membrane filter (Schleicher and Schuell) using a manifold system (Gibco BRL) under vacuum to separate soluble proteins from aggregates. The filter was washed three times with PBS-T and processed for TTR immunodetection. Briefly, the membrane was blocked with 5% (w/v) non-fat skimmed milk in PBS for 1 h at room temperature, and then incubated with a 1:500 dilution of a rabbit anti-human TTR anti-serum (DAKO) for a further 1 h; an anti-rabbit HRP (horseradish peroxidase) conjugate (1:500) was used as the secondary antibody. Detection was performed with ECL® (enhanced chemiluminescence; GE Healthcare). Dot-blot quantification was performed using the ImageQuant program. Experiments were repeated at least three times in duplicate and the data shown are representative of the results obtained.
Chemical compounds studied for interaction with TTR
TIP (tri-iodophenol), flufenamic acid, diflunisal, diclofenac, resveratrol, DCPA {[2-(3,5-dichlorophenyl) amino] benzoic acid}, DNP (dinitrophenol), DES (diethylstilbestrol) and genistein were from Sigma. The diflunisal derivative, iododiflunisal (Mr 376.1), was synthesized as part of a screening programme for TTR polymerization inhibitors carried out at IIQAB-CSIC, Barcelona, and at the University of Oviedo, Oviedo, Spain. After synthesis, the compounds were purified by HPLC (99.9% purity) and characterized fully by NMR, MS and elemental analysis. The synthetic procedure will be reported elsewhere; details are available from G. Valencia on request. Benzoxazole, diclonisal and DFPB [4-(3, 5-difluorophenyl) benzoic acid] were also synthesized at IIQAB-CSIC and have been previously described (Table 1). The compounds were weighed and dissolved at approx. 10 mg/ml in DMSO and used at a final concentration of 1 μM.
Table 1. Compounds tested for the inhibition of aggregate formation by TTR L55P-expressing cells.
Recombinant protein
Recombinant TTR Y78F was produced in a bacterial expression system using Escherichia coli BL21 [11] and purified as previously described [12]. The protein concentration was determined using the Lowry method [13].
Western blot analysis
In some experiments, after application of the medium on the membrane filter, each dot was cut and boiled in SDS-loading buffer. The removed material was run on a 15% SDS denaturing acrylamide gel and then transferred on to a nitrocellulose membrane (Amersham). The membrane was blocked with 5% (w/v) non-fat skimmed milk in PBS for 1 h at room temperature and then incubated with a 1:500 dilution of a rabbit anti-human TTR anti-serum (DAKO) for a further 1 h at room temperature; for the secondary antibody (1:5000 dilution) a peroxidase-conjugated anti-rabbit IgG (Amersham) was used. Development was performed with DAB (3′,3′-diaminobenzidine; Sigma).
TEM (transmission electron microscopy)
Inhibition of fibrillogenesis by selected drugs was evaluated using TTR Y78F which aggregates under physiological conditions (PBS, pH 7.4) after 2 days at 37 °C. For negative staining, 5 μl of these preparations were adsorbed to glow-discharged carbon collodion film on 200-square mesh copper grids for 1 min. The grids were washed with deionized water and stained with 1% uranyl acetate (Fluka). Specimens were visualized with a Zeiss electron microscope operating at 60 kV and grids were exhaustively examined.
Assessment of tetrameric TTR stability by IEF (isoelectric focusing) in semi-denaturing conditions
The conditions used for the IEF of plasma TTR have been described previously by Altland et al. [14] and Almeida et al. [15]. Briefly, 30 μl of plasma from a TTR V30M heterozygotic carrier were incubated at 37 °C for 1 h, with 5 μl of a 10 mM solution of the compound to be tested. The preparations were then subjected to native PAGE to isolate TTR. The gel band containing TTR was excised and subjected to IEF in a gel with 4 M urea and 5% ampholytes (pH 4–6.5) at 1200 V for 6 h. Proteins in the gel were stained with Coomassie Blue. These semi-denaturing conditions allow the visualization of bands corresponding to the TTR monomer and tetramer and also to an oxidized form of the monomer. Gels were scanned (HP Scanjet 4470c; Hewlett Packard) and subjected to densitometry using the ImageQuant program. Experiments were repeated at least three times and data shown are representative of the results obtained.
Statistical analyses
All values are expressed as means±S.E.M. Comparison between groups was made using the Student's t test. A P value of less than 0.05 was considered statistically significant.
RESULTS
TTR expression
To set up a cellular system for the production of TTR aggregates, cells were transfected with different human TTR constructs (p169ZT-hTTRWT, p169ZT-hTTRV30M and p169ZT-hTTRL55P). Demonstration of TTR secretion and quantification of TTR levels in conditioned medium was performed by quantitative ELISA after 24 h in serum-free conditions. Cells were first treated with 100 μM ZnSO4 for 3 days to produce TTR. The medium was then replaced by serum-free medium for 24 h and TTR in the medium was quantified by ELISA as detailed in the Materials and methods section. The interval ranges of TTR expression differed; for TTR WT this varied from 100–300, was 350–1000 for TTR V30M and 300–900 for TTR L55P (expressed in ng/ml/106cells/24 h).
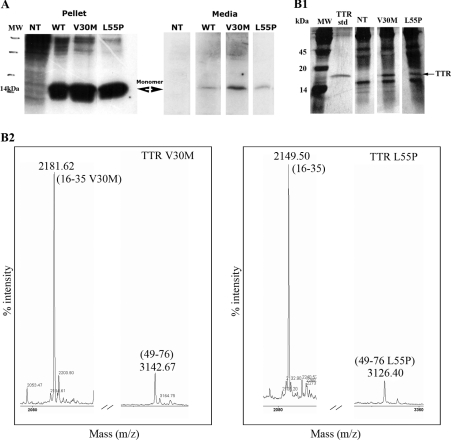
Protein expression and degradation was also assessed by immunoprecipitation on both medium and cell lysates. Figure 1(A) shows TTR from medium and cell lysates (pellet), separated under denaturing conditions (SDS/PAGE) after immunoprecipitation with an anti-human TTR antibody, and revealed that most of the protein produced was secreted into the medium as soluble non-degraded material. To further confirm the identity of each TTR variant expressed and secreted by the cells, we performed MS analysis of the protein in the conditioned medium of transfected cells. The results are displayed in Figure 1(B) which highlights the particular peaks corresponding to each mutation, thus confirming the expected TTR variant in each transfection. As shown in the left-hand panel of Figure 1(B2), a peptide of Mr 2181, representing residues 16–35 of TTR but with a methionine residue replacing valine, was detected in the preparation derived from TTR V30M transfected cells, not present in the preparation of the right-hand panel (derived from TTR L55P transfected cells), which had the corresponding non-mutated, normal Mr 2149 peptide. Conversely, in the preparation derived from TTR L55P transfected cells (right-hand panel), a mutated peptide of Mr 3126, representing residues 49–76 of TTR but with a proline residue replacing leucine, was detected, which was absent from the V30M preparation; instead, a normal, non-mutated peptide of Mr 3142 was observed.
Figure 1. TTR expression in transfected cells.
(A) Immunoprecipitation analysis of RN22 cell medium and pellet from non-transfected cells (NT) and from cells transfected with plasmids for TTR WT, V30M and L55P. (B) MS analysis of immunoprecipitated TTR from conditioned medium of TTR V30M and L55P transfected cells. After immunoprecipitation, proteins were separated on a SDS/PAGE gel which was silver stained (B1). MW, molecular mass standards; TTR std, standard TTR; NT, non-transfected cells. The bands corresponding to the TTR protein (indicated with an arrow) were cut, digested with trypsin and analysed by MS (B2), revealing specific peptides for TTR V30M (left-hand panel) and for L55P (right-hand panel).
Production of amyloidogenic TTR species
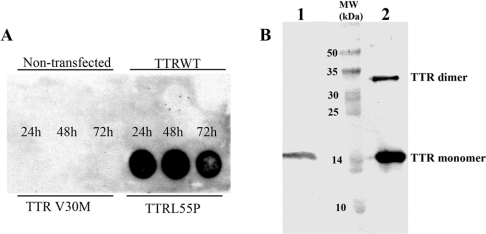
We next investigated the ability of the different constructs to induce the production of amyloidogenic species. Cells were grown in serum-free medium for 24, 48 or 72 h and then the medium was analysed for the presence of aggregated material retained by a 0.2 μm filter in a dot-blot assay. Although TTR aggregates were not detected in medium from cells transfected with constructs carrying the TTR WT or TTR V30M cDNA even after 72 h of cell culture, TTR L55P protein material was partially retained by the membrane filter as early as 24 h into culture (Figure 2A), suggesting the formation of oligomers or aggregates.
Figure 2. Aggregation in conditioned medium of RN22 cells.
(A) Dot-blot filter assays of medium from non-transfected RN22 cells or cells transfected with plasmid for WT and TTR mutants (V30M and L55P). TTR was quantified in the medium by ELISA and 500 ng was applied in the filter, followed by TTR immunodetection. (B) Western blot analysis of the material retained on the membrane filter revealed the presence of TTR (lane 1). Lane 2 shows the TTR standard. In both lanes the TTR monomer and dimer are observed. MW, molecular mass standard.
The dotted area was analysed by SDS/PAGE. The proteins were then transferred on to a nitrocellulose membrane and followed by immunodetection against TTR (Figure 2B), revealing the intact TTR monomer; under these conditions we did not have evidence for aggregate formation since SDS disrupted those aggregates. Even under denaturing conditions, TTR did not completely dissociate into monomers, originating a second band corresponding to the dimer with an apparent mass of approx. 32 kDa.
Comparative effects of diflunisal and iododiflunisal on TTR L55P aggregation
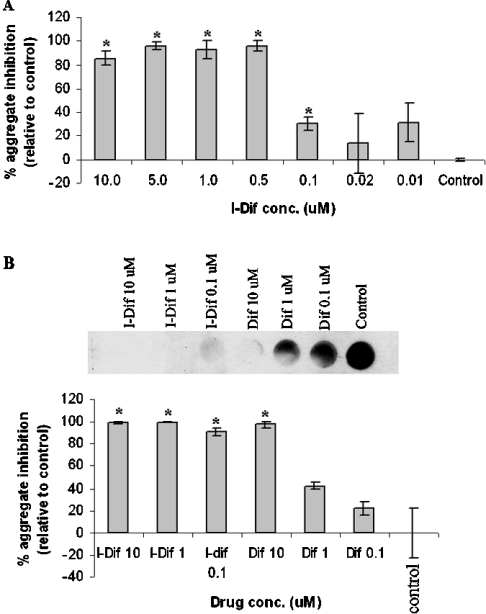
We next evaluated this system in the screening of drugs capable of interfering with TTR fibrillogenesis. Previously, it has been reported that an iodinated diflunisal derivative, iododiflunisal, was very potent as a TTR fibrillogenesis inhibitor, and as a stabilizer of the TTR tetrameric fold when compared with diflunisal [15], as assessed by in vitro and ex vivo approaches respectively. In the present study, we started by studying in our cellular system the effect of different iododiflunisal concentrations (0.01–10 μM) on TTR aggregation. As depicted in Figure 3(A), iododiflunisal inhibited L55P aggregate formation at concentrations between 0.1–10 μM.
Figure 3. Comparative effects of diflunisal and iododiflunisal on TTR L55P aggregation.
(A) Effect of different concentrations of iododiflunisal in L55P aggregate formation. Conditioned medium containing 500 ng of total TTR of cells expressing TTR L55P treated with different concentrations of iododiflunisal (0.01, 0.02, 0.1, 0.5, 1, 5 and 10 μM) or non-treated was applied on to an acetate cellulose membrane filter, followed by immunodetection of TTR aggregates with a specific anti-human TTR antibody, and quantified using the ImageQuant program. *P< 0.02. (B) Dot blot showing the effect of three different concentrations of iododiflunisal and diflunisal in L55P aggregate formation. The experiment was performed as in (A) and revealed that iododiflunisal has approx. 100-fold greater ability of preventing L55P aggregate formation than diflunisal. The histogram shows densitometric analysis of the dot blot showing the percentage of inhibition of aggregate formation by each compound, compared with the control (0% inhibition, no drug added). *P<0.03. I-Dif, iododiflunisal.
We next compared the inhibitory activity on TTR L55P fibrillogenesis of diflunisal and iododiflunisal at three different concentrations: 0.1, 1 and 10 μM. Results are shown in Figure 3(B) and show that iododiflunisal was able to inhibit aggregate formation at 0.1 μM, whereas for diflunisal, the same effect was only observed at a concentration of 10 μM. Thus iododiflunisal presented a 100-fold increase in inhibitory activity when compared with diflunisal. These observations confirmed the data referred to above indicating the much higher capacity of iododiflunisal over diflunisal to prevent TTR fibrillogenesis.
It is noteworthy that some variation occurred in these assays. However, the relative intra-assay activities between different drugs and between drugs and control remained significant, despite inter-assay fluctuations. For example, 0.1 μM of iododiflunisal inhibited L55P fibril formation by 25% as displayed in Figure 3(A), but by 90% as shown in Figure 3(B). Despite this variation, statistical significance was observed with the iododiflunisal in both experiments, when compared with the respective control.
Comparative aggregate inhibitory activities of selected compounds
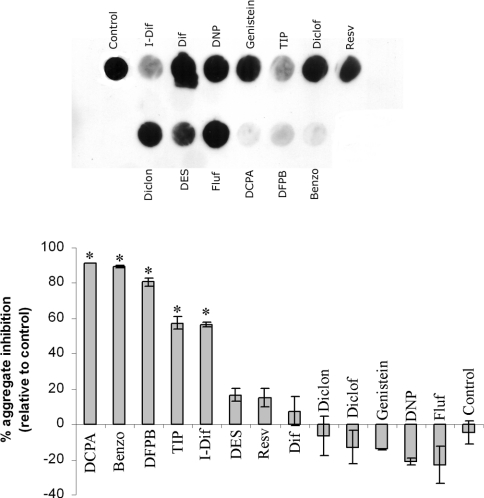
Following the results described above and to further assess the usefulness of the cellular model for screening purposes, we continued our studies on this cellular system with different drugs. We evaluated a series of compounds, selected based on their ability to inhibit amyloid fibril formation according to previously published literature (for references see Table 1), which in most cases used isolated recombinant TTR upon acidification to pH 4.4 and monitoring of turbidity at 400 nm. A further criterion for lead compound selection was individual products representative of different therapeutic categories such as, for instance, NSAIDs (non-steroidal anti-inflammatory drugs: diflunisal, diclofenac and flufenamic acid) or compounds sharing molecular structural similarities with those (DCPA, DFPB and diclonisal). Other small organic compounds also referred to as good amyloid inhibitors such as plant compounds (genistein and resveratrol), DES, benzoxazole and TIP were also selected for testing. Thus the compounds listed in Table 1 were tested at a 1 μM concentration and the results are shown in Figure 4. Different levels of inhibition were evident when compared with the iododiflunisal control (Figure 4, histogram). Among the compounds tested, TIP showed an inhibitory capacity similar to that of iododiflunisal (approx. 57% inhibition). Three of the compounds analysed revealed to be more efficient than iododiflunisal (DCPA, DFPB and benzoxazole), inhibiting aggregate formation in more than 80%. For the other assessed compounds including DES, resveratrol, diflunisal, DNP, genistein, diclofenac, diclonisal and flufenamic acid, no inhibitory effect was evident when compared with the control. Conditioned medium incubated with compounds just prior application on to the membrane showed a signal equivalent to conditioned medium alone (results not shown), ruling out a possible effect of the compounds on the immunoassay.
Figure 4. Screening for TTR anti-amyloidogenic drugs.
Filter assays of the conditioned medium from TTR L55P cells incubated with different drugs at a concentration of 1 μM. Conditioned medium of cells incubated with iododiflunisal and TIP, showed significantly less aggregates than control. DCPA, DFPB and benzoxazole showed the highest inhibitory effect whereas all of the other eight compounds tested produced no effect. The histogram shows densitometric analysis of the dot blot showing the percentage of inhibition of aggregate formation by each compound compared with the control (0% inhibition, no drug added). *P<0.006. Benzo, benzoxazole; Diclof, diclofenac; Diclon, diclonisal; Dif, diflunisal; Fluf, flufenamic acid; I-Dif, iododiflunisal; Resv, resveratrol.
TEM
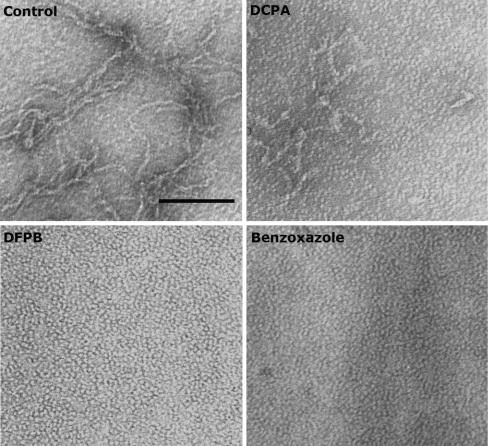
To compare the results obtained in the cellular model, we analysed the effect of six of the drugs under study (genistein, TIP, DES, DCPA, DFPB and benzoxazole) by TEM. This was performed with a different TTR variant, TTR Y78F, which is very prone to aggregation as compared with the L55P mutant. The soluble Y78F variant aggregates in about 48 h at pH 7.4, as reported by Almeida et al. [15] who observed that upon incubation with iododiflunisal no aggregates were visible and only round particles resembling the native TTR were present; in contrast, incubation with diflunisal or diclofenac did not prevent aggregate formation [15].
Figure 5 is representative of the results obtained in the present study and shows a complete inhibition of aggregate and fibril formation with DFPB and benzoxazole (bottom panels); DCPA was only able to partially inhibit aggregate and fibril formation (top right-hand panel). TIP inhibited aggregate and fibril formation whereas the addition of genistein and DES resulted in no visible effect by TEM (results not shown).
Figure 5. Morphological analysis of TTR Y78F by TEM.
TTR Y78F incubated for 48 h at 37 °C evolved from round particles to fibrils (control; top left-hand panel). When incubated with DFPB or benzoxazole, fibril formation was completely inhibited (bottom panels) whereas incubation with DCPA produced only a partial inhibition (top right-hand panel).
In vitro and ex vivo activities of selected compounds
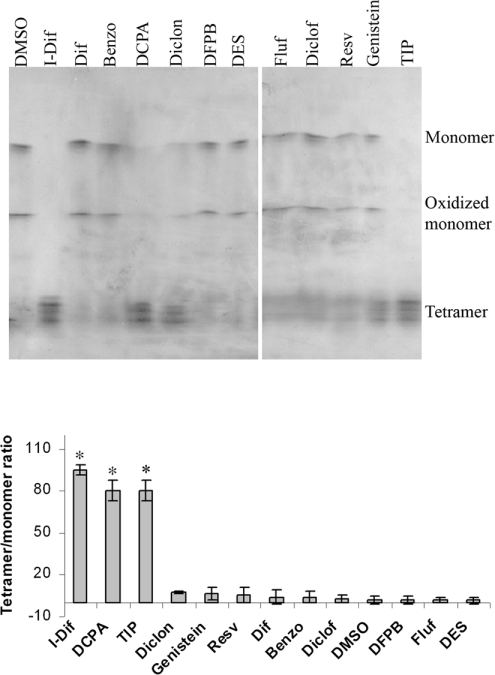
The same panel of compounds was further tested ex vivo for their effect on TTR stability by IEF analysis under semi-denaturing conditions, namely in the presence of 4 M urea. To perform the assays we basically used the same conditions as in previous studies [15]. Plasma from a heterozygotic carrier of TTR V30M was incubated with each of the compounds referred to in the legend of Figure 6 and, following IEF, the bands corresponding to the TTR tetramer and monomer were visualized, and the tetramer/monomer ratio was measured. Under the conditions used, TTR presented a characteristic pattern of two main bands, representing monomers (normal and oxidized form), and several bands of lower pI, representing different forms of tetramers.
Figure 6. Stabilization of tetrameric TTR V30M.
Plasma from a heterozygotic carrier of TTR V30M was incubated with different drugs. TTR was then separated by PAGE and the isolated protein was analysed by IEF. The different molecular species visualized in the IEF gel after Coomassie Blue staining are indicated. In the presence of iododiflunisal, DCPA and TIP, stronger bands corresponding to TTR tetramers were visualized and the monomer bands were strongly reduced or absent. The histogram shows densitometric analysis of IEF of isolated TTR V30M incubated with the different compounds and expressed as the tetramer/monomer ratio. *P<0.006. Benzo, benzoxazole; Diclof, diclofenac; Diclon, diclonisal; Dif, diflunesal; Fluf, flufenamic acid; I-Dif, iododiflunisal; Resv, resveratrol.
As compared with iododiflunisal, a similar stabilization effect was obtained with DCPA and with TIP, not evident for DFPB and benzoxazole under the conditions used. All of the other compounds in the cellular assay without inhibitory TTR fibrillogenesis activity failed to stabilize V30M ex vivo.
DISCUSSION
The process that converts a soluble protein into amyloid is complex and not fully understood. TTR amyloid deposits have been suggested as the causative agent of FAP. However, the finding that asymptomatic individuals carrying the TTR V30M mutation show a positive reaction for inflammatory markers and present non-fibrillar TTR deposits in the nerve raised the possibility that species besides mature fibrils could be involved in the disease [16]. In fact, in vitro studies have shown that TTR L55P aggregates are toxic to a Schwannoma cell line, inducing caspase 3 activation, whereas long fibrils do not produce significant caspase 3 activation. These observations suggest that a major target in FAP therapy is to inhibit the formation of intermediate species and that understanding of how native TTR converts into a toxic species is of major importance.
Initially most in vitro studies on TTR amyloidogenesis were performed by acidifying the protein [2,3,5,6], using an environment different from in vivo conditions. More recently, TTR variants such as Y78F, which are able to produce aggregates and fibrils at physiological pH have benefited research on anti-amyloidogenic drugs and disease mechanisms by avoiding pH-related artefacts. In both cases (with and without acidification), most studies are still conducted with purified recombinant proteins [2–6] and thus such approaches lack factors that occur in vivo. Thus a cellular system producing the amyloidogenic protein would represent an improved approach to study effects of modulating factors and drugs on fibrillogenesis. In fact, different cellular models have been generated in order to study amyloidogenesis. A cell culture system for generation of AA (amyloid A associated amyloidosis) was established using murine peritoneal macrophages after repeated injections of Hammersten casein [17]. Staining with Congo Red, thioflavin T and anti-AA revealed amyloid-like structures associated with macrophage clusters. Such systems can be useful in studies on the influence of chaperone molecules and other factors on the formation and degradation of amyloid fibrils. Similarly, cells transfected with the PS2 (presenilin 2) gene were used to examine the processing and degradation pathways of PS2 [18]. Furthermore, different cellular models have been created in order to study many different aspects of Alzheimer's disease [19–21]. In the present study we report on the establishment of a cellular system of transfected RN22 (rat Schwannoma) with WT TTR, V30M and L55P mutants. The latter was shown to form species that were retained by a filter, in contrast with the WT and V30M proteins.
Although being the most prevalent mutation responsible for the pathology, V30M does not show a very high amyloidogenic potential in vitro, since amyloid fibrils are only obtained after acidification. Furthermore, X-ray crystallography studies [22] revealed a structure similar to the WT counterpart and the differences found could not explain a significant instability of the protein. Thus, in vivo, this mutation plays a very important role in the pathology of FAP but, in vitro, the protein does not lend itself to study by the assays described in the present paper.
One of the advantages of this in vitro cellular system is that it measures aggregation under good physiological conditions because: (i) it uses a eukaryotic system for the production of TTR; (ii) the secreted protein is not isolated, avoiding modifications such as oxidations that might influence aggregation; and (iii) aggregation occurs in the cell medium, under physiological pH and in the presence of serum proteins. None of the previous in vitro available methodologies to screen for TTR inhibitors take into consideration these aspects, as TTR aggregation is measured on isolated recombinant TTR from bacteria and in the absence of serum proteins.
When the 12 compounds classified in the literature as good TTR fibrillogenesis inhibitors were tested on the cellular system, having iododiflunisal as the reference compound, only DCPA, benzoxazole, DFPB and TIP were able to prevent L55P aggregate formation in the conditioned medium, which reflects the above-mentioned limiting conditions, such as the aggressive environment imposed by acidification, the exclusive use in most instances of WT rather than mutant TTR and the absence of serum proteins [4,23,24]. With regard to the ultrastructural analysis, DCPA did not show an inhibitory effect as high as DFPB and benzoxazole, indicating that the Y78F mutant may not be as sensitive to this drug as TTR L55P and V30M are. Stabilization of tetrameric V30M TTR by iododiflunisal was previously shown in ex vivo tests using plasma from hetero- and homo-zygous carriers. The sample was directly analysed without previous treatment with cross-linkers and did not suffer further processing such as immunoprecipitation. Urea (4 M) allowed discrimination of susceptibility to tetramer dissociation into monomeric species, directly visualized on the IEF gel without the use of TTR antibodies for quantification of the tetramer/monomer ratio. We also did not observe a stabilizing effect of benzoxazole and DFPB by ex vivo testing under the conditions used; this fact is suggestive that these two drugs either inhibit TTR fibrillogenesis by mechanisms other than tetramer stabilization, such as inhibition of polymerization of soluble intermediary species generated after tetramer dissociation, or alternatively they might have a more effective anti-fibrillogenic effect in L55P and in Y78F.
At any rate, testing of the V30M variant is most pertinent in studies searching for TTR anti-fibrillogenic drugs as besides being the most common mutant found in FAP, further application of the drug under study encompasses in vivo testing in animal models which are available for this mutant [7].
The main conclusions of this screening work indicate that: (i) iododiflunisal, DCPA and TIP are the best stabilizers of V30M tetramers in plasma from carriers of this mutant, and clearly inhibit aggregation in the cellular system; also, DFPB displayed anti-aggregation properties for L55P and Y78F but did not stabilize V30M tetramers, possibly by acting through mechanisms other than tetrameric stabilization. Therefore iododiflunisal, DCPA and TIP are promising for the treatment of V30M-associated FAP but need to undergo further stages of drug development to overcome their toxicity. (ii) Compounds with virtually null stabilization of the tetrameric structure (as assessed ex vivo), such as flufenamic acid, diclofenac, resveratrol, genistein, diflunisal and DES, clearly had no effect on TTR aggregation in cell culture and thus further testing of them may not be worthwhile.
The cellular model described in the present study represents a step forward in the study of events occurring very early in TTR amyloidogenesis and of factors/drugs affecting such processes; moreover, our cellular model supplies a unique tool to test drugs independently of the mechanism behind their action, i.e. tetramer stabilization, inhibition of aggregation and others. We believe that this system will have important implications in future drug screening and design while constituting an important tool for the selection of compounds that merit preclinical testing.
Acknowledgments
We thank Paul Moreira for his assistance in the production of recombinant TTRs. This work was supported by the Portuguese Foundation for Science and Technology (FCT) through a fellowship to I. C. (SFRH/BPD/9416/2002), POCTI (Programa Operacional Ciěncia, Tecnologia, Inovação) and POCI (Programa Operacional Ciěncia e Inovação) grants, and by the Gulbenkian Foundation.
References
- 1.Saraiva M. J., Sousa M. M., Cardoso I., Fernandes R. Familial amyloidotic polyneuropathy: protein aggregation in the peripheral nervous system. J. Mol. Neurosci. 2004;23:35–40. doi: 10.1385/jmn:23:1-2:035. [DOI] [PubMed] [Google Scholar]
- 2.Klabunde T., Petrassi H. M., Oza V. B., Raman P., Kelly J. W., Sacchettini J. C. Rational design of potent human transthyretin amyloid disease inhibitors. Nat. Struct. Biol. 2000;7:312–321. doi: 10.1038/74082. [DOI] [PubMed] [Google Scholar]
- 3.Oza V. B., Petrassi H. M., Purkey H. E., Kelly J. W. Synthesis and evaluation of anthranilic acid-based transthyretin amyloid fibril inhibitors. Bioorg. Med. Chem. Lett. 1999;9:1–6. doi: 10.1016/s0960-894x(98)00696-9. [DOI] [PubMed] [Google Scholar]
- 4.Baures P. W., Peterson S. A., Kelly J. W. Discovering transthyretin amyloid fibril inhibitors by limited screening. Bioorg. Med. Chem. 1998;6:1389–1401. doi: 10.1016/s0968-0896(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 5.Baures P. W., Oza V. B., Peterson S. A., Kelly J. W. Synthesis and evaluation of inhibitors of transthyretin amyloid formation based on the non-steroidal anti-inflammatory drug, flufenamic acid. Bioorg. Med. Chem. 1999;7:1339–1347. doi: 10.1016/s0968-0896(99)00066-8. [DOI] [PubMed] [Google Scholar]
- 6.Razavi H., Palaninathan S. K., Powers E. T., Wiseman R. L., Purkey H. E., Mohamedmohaideen N. N., Deechongkit S., Chiang K. P., Dendle M. T., Sacchettini J. C., Kelly J. W. Benzoxazoles as transthyretin amyloid fibril inhibitors: synthesis, evaluation, and mechanism of action. Angew. Chem. Int. Ed. Engl. 2003;42:2758–2761. doi: 10.1002/anie.200351179. [DOI] [PubMed] [Google Scholar]
- 7.Sousa M. M., Fernandes R., Palha J. A., Taboada A., Vieira P., Saraiva M. J. Evidence for early cytotoxic aggregates in transgenic mice for human transthyretin. Am. J. Pathol. 2002;161:1935–1948. doi: 10.1016/S0002-9440(10)64469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sambrook J., Fritsch E. F., Maniatis T. 2nd edition. Cold Spring Harbor, New York, U.S.A.: Cold Spring Harbor Laboratory; 1989. Molecular Cloning: a Laboratory Manual. [Google Scholar]
- 9.Sousa M. M., Berglund L., Saraiva M. J. Transthyretin in high density lipoproteins: association with apolipoprotein A-I. J. Lipid Res. 2000;41:58–65. [PubMed] [Google Scholar]
- 10.Henzel W. J., Billeci T. M., Stults J. T., Wond S. C., Grimley C., Watanabe C. Identifying proteins from two-dimensional gels by molecular mass searching of peptide fragments in protein sequence databases. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5011–5015. doi: 10.1073/pnas.90.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuya H., Saraiva M. J. M., Gawinowicz M. A., Alves I. L., Costa P. P., Sasaki H., Goto I., Sakaki Y. Production of recombinant human transthyretin with biological activities toward the understanding of the molecular basis of familial amyloidotic polyneuropathy (FAP) Biochemistry. 1991;30:2415–2421. doi: 10.1021/bi00223a017. [DOI] [PubMed] [Google Scholar]
- 12.Almeida M. R., Damas A. M., Lans M. C., Brower A., Saraiva M. J. Thyroxine binding to transthyretin Met119. Comparative studies of different heterozygotic carriers and structural analysis. Endocrine. 1997;6:309–315. doi: 10.1007/BF02820508. [DOI] [PubMed] [Google Scholar]
- 13.Lowry O. H., Rosenbrough N. J., Farr A. L., Randall J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Altland K., Rauh S., Hacker R. Demonstration of human prealbumin by double one-dimensional slab gel electrophoresis. Electrophoresis. 1981;2:148–155. [Google Scholar]
- 15.Almeida M. R., Macedo B., Cardoso I., Alves I., Valencia G., Arsequell G., Planas A., Saraiva M. J. Selective binding to transthyretin and tetramer stabilization in serum from patients with familial amyloidotic polyneuropathy by an iodinated diflunisal derivative. Biochem. J. 2004;381:351–356. doi: 10.1042/BJ20040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sousa M. M., Cardoso I., Fernandes R., Guimaraes A., Saraiva M. J. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am. J. Pathol. 2001;159:1993–2000. doi: 10.1016/s0002-9440(10)63050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palm M., Nielsen E. H., Svehag S. E. An in vitro cellular system for generation of AA amyloid. APMIS. 1997;105:603–608. doi: 10.1111/j.1699-0463.1997.tb05059.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim T. W., Pettingell W. H., Hallmark O. G., Moir R. D., Wasco W., Tanzi R. E. Endoproteolytic cleavage and proteasomal degradation of presenilin 2 in transfected cells. J. Biol. Chem. 1997;272:11006–11010. doi: 10.1074/jbc.272.17.11006. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto K., Akao Y., Yi H., Shamoto-Nagai M., Maruyama W., Naoi M. Overexpression of amyloid precursor protein induces susceptibility to oxidative stress in human neuroblastoma SH-SY5Y cells. J. Neural Transm. 2006;113:125–135. doi: 10.1007/s00702-005-0318-0. [DOI] [PubMed] [Google Scholar]
- 20.Iuvone T., De Filippis D., Esposito G., D'Amico A., Izzo A. A. The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid-β peptide-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2006;317:1143–1149. doi: 10.1124/jpet.105.099317. [DOI] [PubMed] [Google Scholar]
- 21.Yang A. J., Knauer M., Burdick D. A., Glabe C. Intracellular Aβ1–42 aggregates stimulate the accumulation of stable, insoluble amyloidogenic fragments of the amyloid precursor protein in transfected cells. J. Biol. Chem. 1995;270:14786–14792. doi: 10.1074/jbc.270.24.14786. [DOI] [PubMed] [Google Scholar]
- 22.Terry C. J., Damas A. M., Oliveira P., Saraiva M. J., Alves I. L., Costa P. P., Matias P. M., Sakaki Y., Blake C. C. Structure of Met30 variant of transthyretin and its amyloidogenic implications. EMBO J. 1993;12:735–741. doi: 10.1002/j.1460-2075.1993.tb05707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamski-Werner S. L., Palaninathan S. K., Sacchettini J. C., Kelly J. W. Diflunisal analogues stabilize the native state of transthyretin. Potent inhibition of amyloidogenesis. J. Med. Chem. 2004;47:355–374. doi: 10.1021/jm030347n. [DOI] [PubMed] [Google Scholar]
- 24.Greeen N. S., Palaninathan S. K., Sacchettini J. C., Kelly J. W. Synthesis and characterization of potent bivalent inhibitors that bind prior to transthyretin tetramerization. J. Am. Chem. Soc. 2003;125:13404–13414. doi: 10.1021/ja030294z. [DOI] [PubMed] [Google Scholar]
- 25.Miroy G. J., Lai Z., Lashuel H. A., Peterson S. A., Strang C., Kelly J. W. Inhibiting transthyretin amyloid fibril formation via protein stabilization. Proc. Natl. Acad. Sci. U.S.A. 1996;93:15051–15056. doi: 10.1073/pnas.93.26.15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oza V. B., Smith C., Raman P., Koepf E. K., Lashuel H. A., Petrassi H. M., Chiang K. P., Powers E. T., Sachettinni J., Kelly J. W. Synthesis, structure, and activity of diclofenac analogues as transthyretin amyloid fibril formation inhibitors. J. Med. Chem. 2002;45:321–332. doi: 10.1021/jm010257n. [DOI] [PubMed] [Google Scholar]
- 27.Cardoso I., Merlini G., Saraiva M. J. 4′-Iodo-4′-deoxydoxorubicin and tetracyclines disrupt transthyretin amyloid fibrils in vitro producing non-cytotoxic species. Screening for TTR fibril disrupters. FASEB J. 2003;17:803–809. doi: 10.1096/fj.02-0764com. [DOI] [PubMed] [Google Scholar]
- 28.Morais-de-Sa E., Pereira P. J., Saraiva M. J., Damas A. M. The crystal structure of transthyretin in complex with diethylstilbestrol: a promising template for the design of amyloid inhibitors. J. Biol. Chem. 2004;279:53483–53490. doi: 10.1074/jbc.M408053200. [DOI] [PubMed] [Google Scholar]
- 29.Green N. S., Foss T. R., Kelly J. W. Genistein, a natural product from soy, is a potent inhibitor of transthyretin amyloidosis. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14545–14550. doi: 10.1073/pnas.0501609102. [DOI] [PMC free article] [PubMed] [Google Scholar]