Abstract
For a long period lactate was considered as a dead-end product of glycolysis in many cells and its accumulation correlated with acidosis and cellular and tissue damage. At present, the role of lactate in several physiological processes has been investigated based on its properties as an energy source, a signalling molecule and as essential for tissue repair. It is noteworthy that lactate accumulation alters glycolytic flux independently from medium acidification, thereby this compound can regulate glucose metabolism within cells. PFK (6-phosphofructo-1-kinase) is the key regulatory glycolytic enzyme which is regulated by diverse molecules and signals. PFK activity is directly correlated with cellular glucose consumption. The present study shows the property of lactate to down-regulate PFK activity in a specific manner which is not dependent on acidification of the medium. Lactate reduces the affinity of the enzyme for its substrates, ATP and fructose 6-phosphate, as well as reducing the affinity for ATP at its allosteric inhibitory site at the enzyme. Moreover, we demonstrated that lactate inhibits PFK favouring the dissociation of enzyme active tetramers into less active dimers. This effect can be prevented by tetramer-stabilizing conditions such as the presence of fructose 2,6-bisphosphate, the binding of PFK to f-actin and phosphorylation of the enzyme by protein kinase A. In conclusion, our results support evidence that lactate regulates the glycolytic flux through modulating PFK due to its effects on the enzyme quaternary structure.
Keywords: glycolysis, lactate, metabolism, phosphofructokinase, regulation, tetramer
Abbreviations: DMEM, Dulbecco's modified Eagle's medium; F1,6BP, fructose 1,6-bisphosphate; F2,6BP, fructose 2,6-bisphosphate; F6P, fructose 6-phosphate; FBS, fetal bovine serum; PEP, phosphoenolpyruvate; PFK, 6-phosphofructo-1-kinase; PKA, protein kinase A
INTRODUCTION
Lactate is the final product of anaerobic glycolysis in muscle cells and erythrocytes and is classically correlated with metabolic acidosis and muscle fatigue [1,2]. For this reason, during the 20th century, lactate was largely considered a dead-end waste product and a key factor in acidosis-induced tissue damage [1]. However, this view is changing owing to results where lactate appears as an important source of systemic energy, as a signalling molecule and as a co-ordinator of cell metabolism in many tissues and organs [1–8]. Lactate production is regulated within cells and thereby its accumulation alters the concentration of other glycolytic intermediates such as F6P (fructose 6-phosphate), F1,6BP (fructose 1,6-bisphosphate) and PEP (phosphoenolpyruvate) [9]. However, it it is not clear how lactate affects the concentration of these intermediates and the glycolytic flux.
PFK (6-phosphofructo-1-kinase; phosphofructokinase; ATP:D-fructose-6-phosphate-1-phosphotranferase; EC 2.7.1.11) is a key regulatory glycolytic enzyme characterized by allosteric kinetics, a complex oligomeric structure and multiple modes of regulation [10]. This enzyme is regulated by a variety of ligands including its substrates, the reaction products and various other cellular metabolites such as AMP, F2,6BP (fructose 2,6-bisphosphate), glucose 1,6-bisphosphate, citrate and others [10–12]. ATP, one of PFK's substrates, has a dual effect on the enzyme activating it up to 1 mM and inhibiting it at higher concentrations [10], whereas F2,6BP reverses the inhibitory effects of ATP [12].
Muscle PFK has various oligomeric states: the active tetrameric form dissociates into inactive dimers and monomers upon dilution. However, at high concentrations, it can aggregate into larger oligomeric conformations that have the same activity as tetramers [10]. Several regulatory factors alter the equilibrium between these forms, e.g. pH, ionic strength and temperature, where acidic medium, as well as high ionic strengths and temperature favour the formation of dimers, and alkaline medium, low ionic strengths and temperature favour the formation of larger complex conformations of the enzyme [13]. It has been suggested that the distinct oligomeric forms have different kinetic and regulatory properties, which were observed by studying PFK at low concentrations (where dimers are favoured) or at high concentrations (where tetramers are favoured) [14,15]. In addition, allosteric regulators of the enzyme also modulate its oligomeric equilibrium; it has been described that citrate stabilizes PFK dimers, which was related to the inhibitory effects of this compound on PFK, an effect that is counteracted by ADP, which stabilizes the tetrameric configuration of the enzyme [16].
Inhibition of PFK has been correlated with decelerated glycolytic flux and with alterations in cell glycolytic intermediate concentrations [17–22]. Accumulation of lactate has been correlated with the inhibition of PFK activity due to acidification of the intracellular milieu [23]. However, it is clear that the effects of lactate on cells occur even when the pH is not altered [8]. The aim of the present study was to investigate the effects of lactate directly on PFK activity and mechanism of action, correlating the observed effects with the physiological action of lactate on glycolytic metabolism.
EXPERIMENTAL
Materials
ATP, F6P and F2,6BP were purchased from Sigma. 32Pi was purchased from Instituto de Pesquisas Energéticas e Nucleares. [γ-32P]ATP was prepared according to Maia et al. [25]. Purified PFK was obtained from rabbit skeletal muscle according to the method developed by Kemp [26], with modifications introduced by Kuo et al. [27]. All protein content measurements were performed as described by Lowry et al. [28]. The C2C12 cell line was obtained from the Cell Bank of the Hospital Universitário Clementino Fraga Filho, UFRJ, Brazil, and maintained in DMEM (Dulbecco's modified Eagle's medium; Invitrogen) supplemented with 10% (v/v) FBS (fetal bovine serum; Invitrogen).
PFK activity for purified protein
PFK activity was measured using the method described by Sola-Penna et al. [29] with the modifications introduced by Zancan and Sola-Penna [20,21], in a reaction medium containing 50 mM Tris/HCl (pH 7.4), 5 mM MgCl2, 5 mM (NH4)2SO4, 1 mM [γ-32P]ATP (4 μCi/nmol), 1 mM F6P and 5 μg/ml PFK, except when indicated. In experiments investigating the pH-dependent effects of lactate, the Tris/HCl buffer was replaced by Mes/Tris buffered at pH 6.5, 6.7, 7.0, 7.4 or 8.2, and in PFK concentration-dependent experiments, the concentration of PFK is indicated for each experiment. The reaction was stopped by the addition of a suspension of activated charcoal in 0.1 M HCl and 0.5 M mannitol and, after centrifugation (2000 g at 4 °C for 15 min), the supernatant containing [1-32P]F1,6P formed was analysed in a liquid-scintillation counter. Appropriate blanks in the absence of F6P were performed and subtracted from all measurements to discount ATP hydrolysis. The enzyme activity was expressed as m-units of PFK activity, where 1 m-unit was considered as the formation of 1 nmol of F1,6P per min of reaction.
Intrinsic fluorescence measurements
Intrinsic fluorescence measurements were performed in a Jasco spectrofluorimeter in medium containing 100 mM Tris/HCl (pH 7.4), 5 mM (NH4)2SO4, 5 mM MgCl2, 1 mM F6P, 1 mM ATP and the indicated concentration of PFK in the absence or the presence of 10 mM lactate. Appropriate reference spectra were subtracted from the data to correct for background interference that were always less than 2% of the fluorescence signal. The excitation wavelength was set at 280 nm, and fluorescence emission was recorded at 90° scanned from 300 to 400 nm. The centre of mass (c.m.) of fluorescence spectra was calculated using the SigmaPlot 10.0 software (Systat) and the equation:
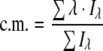 |
(1) |
where Σ is the sum over all wavelengths (λ) from 300 to 400 nm and Iλ is the fluorescence intensity at a given wavelength [30].
Glucose consumption experiments
Animal experimentation was conducted according to the Animal Care Procedures and the legal requirements of the Brazilian Government. Quadriceps were isolated from mice killed by cervical dislocation and were immediately used for glucose consumption experiments. Muscles were incubated in Krebs–Ringer–Henseleit buffer (6 mM KCl, 1 mM Na2HPO4, 0.2 mM NaH2PO4, 1.4 mM MgCl2, 1 mM CaCl2, 128 mM NaCl, 10 mM Hepes and 0.5% BSA, pH 7.4), containing 4.5 or 15 mM glucose in the absence and in the presence of 100 nM insulin. The rate of glucose consumption was measured as previously described [19].
PFK activity in mice skeletal muscle homogenates
Quadriceps were isolated from mice killed by cervical dislocation and immediately used for PFK activity experiments. Muscles were incubated for 2 h at 37 °C in Krebs–Ringer–Henseleit buffer with or without 10 mM lactate in the absence and in the presence of 100 nM insulin. After incubation, muscles were homogenized in the same buffer and used to determined PFK activity. PFK activity was determined in a buffer containing 50 mM Tris/HCl (pH 7.4), 5 mM MgCl2, 5 mM (NH4)2SO4, 1 mM ATP, 1 mM F6P, 0.5 mM NADH, 2 units/ml aldolase, 2 units/ml triosephosphate isomerase, 2 units/ml α-glycerophosphate dehydrogenase and 100 μg/ml of the muscle homogenates, as described previously [29].
Determination of F6P/F1,6BP ratio
C2C12 cells were grown at 37 °C in DMEM supplemented with 10% (v/v) FBS for 2 days when a confluent single layer of cells were observed. Cells (1×107) were treated for 2 h in the presence or not of 10 mM lactate. Afterwards, cells were washed with PBS and lysed with 10% TCA (trichloroacetate). The lysates were neutralized with 2 M Tris, centrifuged (3000 g at 4 °C for 10 min) and the supernatants were used to determine the content of F6P and F1,6BP, using standardized methods [31] as follows. F6P was determined by adding the neutralized supernatant of 1×107 cells to a reaction medium containing 50 mM Tris/HCl (pH 7.4), 5 mM MgCl2, 5 mM (NH4)2SO4, 1 mM ATP, 0.5 mM NADH, 10 units/ml PFK, 2 units/ml aldolase, 2 units/ml triosephosphate isomerase and 2 units/ml α-glycerophosphate dehydrogenase. The amount of F6P was assessed via the oxidation of NADH. A calibration curve of F6P was created in order to determine the F6P content of the cells. F1,6BP was determined by adding the neutralized supernatant of 1×107 cells to a reaction buffer containing 50 mM Tris/HCl (pH 7.4), 5 mM MgCl2, 5 mM (NH4)2SO4, 0.5 mM NADH, 2 units/ml aldolase, 2 units/ml triosephosphate isomerase and 2 units/ml α-glycerophosphate dehydrogenase. The amount of F1,6BP was assessed via the oxidation of NADH. A calibration curve of F1,6BP was created in order to determine the F1,6BP content of the cells.
Statistics and calculations
Statistical analyses and non-linear regression were performed using SigmaPlot 10.0 software integrated with SigmaStat 3.1 packages (Systat). Unless otherwise indicated, a Student's t test was used. P ≤ 0.05 were used to consider statistically different mean values.
Kinetic parameters for lactate inhibition were calculated through non-linear regression using the experimental data to fit the parameters of the equation:
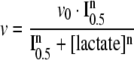 |
(2) |
where v is the PFK activity calculated for a given concentration of lactate ([lactate]), v0 is the PFK activity measured in the absence of lactate, I0.5 is inhibition constant that is equal to the concentration of lactate that induces 50% of maximal inhibition of PFK and n is the cooperativity index for lactate-induced inhibition of PFK.
Kinetic parameters for ATP effects on PFK were calculated through non-linear regression using the experimental data to fit the parameters of the equation:
 |
(3) |
where v is the PFK activity calculated for a given concentration of ATP ([ATP]), Vmax is the maximal velocity calculated, K0.5 is the affinity constant for the catalytic component of the ATP curve, which is equal to the concentration of F6P responsible for half-activation of the enzyme by ATP, n1 is the cooperativity index for this catalytic component, I0.5 is the affinity constant for the inhibitory component of the ATP curve, which is equal to the concentration of ATP responsible for 50% of the maximal inhibition of the enzyme by ATP and n2 is the cooperativity index for this inhibitory component.
Kinetic parameters for F6P effects on PFK were calculated through non-linear regression using the experimental data to fit the parameters of the equation:
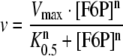 |
(4) |
where v is the PFK activity calculated for a given concentration of F6P ([F6P]), Vmax is the maximal velocity calculated at saturating concentrations of F6P, K0.5 is the affinity constant for F6P, which is equal to the concentration of F6P responsible for half-activation of the enzyme by F6P and n is the cooperativity index for this phenomenon.
RESULTS AND DISCUSSION
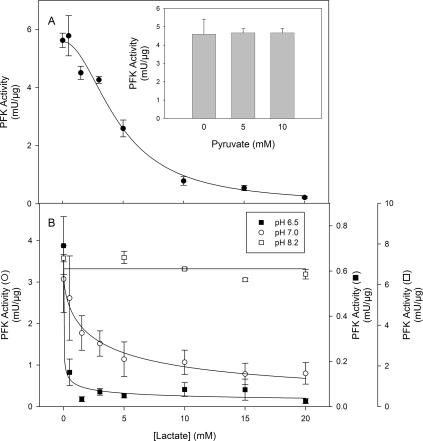
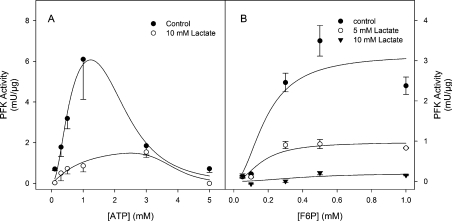
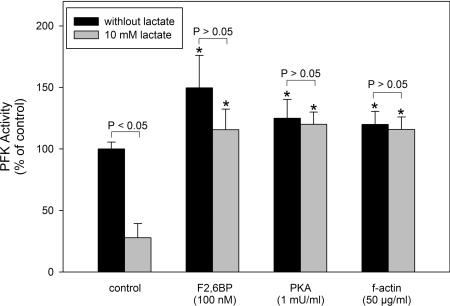
Lactate promotes a dose-dependent inhibition of PFK activity measured at pH 7.4 (Figure 1A) giving an I0.5 of 4.6±0.3 mM and a cooperativity index of 2.0±0.2 (Table 1). This inhibition is specific for lactate and is not due to possible effects on medium pH, since pyruvate, the oxidized form of lactate, does not promote any effects on PFK activity (Figure 1A, inset). The specificity of lactate effects are corroborated by the fact that oxalate and malate did not promote any inhibitory effects on PFK activity (Table 1). Moreover, citrate, a known allosteric inhibitor of PFK [10,15], was less potent than lactate in inhibiting PFK, highlighting the physiological relevance of the present findings (Table 1). It is important to note that the pKa for pyruvate (2.39) is lower than for lactate (3.08) [32], conferring to pyruvate the property of acidifing the medium more than lactate, supporting the notion that lactate effects are not due to any alteration in medium pH. However, the effects of lactate on PFK activity are modulated by alterations in medium pH, since the inhibition potency is increased when the medium is acidified and completely abolished at alkaline pH. Figure 1(B) shows the effects of lactate on PFK activity measured at pH 8.2, 7.0 or 6.5, where it is noticeable that the enzyme becomes more susceptible to the lactate effects at lower pH values, and is unaffected at pH 8.2. Indeed, both the I0.5 for lactate and the cooperativity index decrease when the medium pH is lowered (Table 2). This fact augments the relevance of the effects observed on PFK, since the formation of lactate occurs simultaneously with the acidification of the intracellular milieu [1–7], which turns lactate into a potent inhibitor of PFK and possibly of the glycolytic flux. PFK loses most of its regulatory properties at pH 8.2, when the tetrameric conformation of the enzyme is favoured and the enzyme works at its maximal velocity [10–17,33]. Our results show that at pH 8.2 PFK is unaffected by lactate, suggesting that the effects of the ligand occur through modulation of the enzyme's allosteric regulatory properties. Lactate alters the saturation curve pattern for both substrates of PFK, namely ATP and F6P (Figure 2). Figure 2(A) shows that the presence of 10 mM lactate decreases the catalytic activity of PFK and shifts the catalytic component for ATP to the right region of the curve. This is indicative of the reduction in the affinity of both sites for ATP on muscle PFK, the catalytic and the inhibitory allosteric sites. Indeed, 10 mM lactate induces an increase of 70% in the K0.5 for ATP and a reduction of 40% on the cooperativity index for the catalytic component (n1) of the ATP curve (Table 3). Moreover, the I0.5 for ATP on PFK activity and the cooperativity index for the inhibitory allosteric component (n2) are increased by 67 and 110% respectively. A similar picture is observed for the F6P saturation curve of PFK, where lactate promotes a dose-dependent reduction in the maximal velocity of the enzyme (Vmax), which is clearly observed in Figure 2(B) and is quantified in Table 3, as well as in the presence of 10 mM lactate when a significant reduction in the affinity for F6P is observed, without alteration in the cooperative index for this component (Table 3). The reductions on the affinity of PFK for both its substrates, and for the inhibitory effects of ATP, are indicative of an allosteric modulation of the enzyme promoted by lactate. In addition, this pattern of modulation of these kinetic components (affinity for both substrates, affinity for inhibitory ATP and the maximal velocity) is similar to the one observed when the fully active tetramers of PFK dissociate into less active dimers [10,13,14,33–35]. In addition, it is well described that PFK is more susceptible to dissociation in acidic medium (a condition that potentiates the effects of lactate) being stabilized in the tetrameric conformation at pH 8.2 [15,36], a condition that abolishes lactate-induced inhibition of the enzyme. These statements support the possibility that lactate effects on PFK are due to dissociation of the enzyme tetramers to dimers. In order to assess whether the lactate-induced inhibition of muscle PFK occurs through dissociation of the enzyme, we tested the effects of lactate on PFK activity under conditions where the tetrameric conformation of the enzyme is stabilized. The inhibitory effects of 10 mM lactate, which promotes 73% inhibition of PFK activity, were abolished when the experiments were performed in the presence of 100 nM F2,6BP, 1 m-unit/ml PKA (protein kinase A) or 50 μg/ml f-actin (Figure 3). Our results show a 50, 25 and 20% activation of PFK induced by F2,6BP, PKA and f-actin respectively (Figure 3, black bars), however lactate does not inhibit the enzyme under these conditions (Figure 3). F2,6BP is a potent activator of PFK, which reduces its K0.5 for F6P and counteracts the inhibitory effects of ATP [2], through the stabilization of the tetrameric structure of the enzyme [2]. PFK is phosphorylated by PKA upon adrenergic stimulation leading to a more active tetrameric-stable enzyme [35,37]. In the same way, PFK associates with f-actin, which promotes the same effects described above for F2,6BP, including the stabilization of the tetrameric conformation of the enzyme [38,39]. Therefore these three PFK modulators stabilize the enzyme tetrameric structure through different mechanisms; F2,6BP and f-actin present distinct binding sites and PKA promotes a covalent modification of the enzyme, counteracting the effects of lactate on PFK activity. These results corroborate that lactate is acting on PFK to dissociate the tetrameric structure of the enzyme, which inhibits its catalytic activity.
Figure 1. Effects of lactate on PFK activity at different pH values.
(A) Effects of lactate on PFK activity measured at pH 7.4. (A) Inset: effects of pyruvate on PFK activity at pH 7.4. (B) Effects of lactate on PFK activity measured at pH 6.5 (■), 7.0 (○) and 8.2 (□). Lines are the results of non-linear regression fitting eqn 1 to the experimental data. Values are mean±S.E.M. of at least four independent experiments.
Table 1. Inhibitory potency of mono-, di- and tri-carboxylic acids on PFK.
Experiments were performed as described for the inset to Figure 1(A). Values are means±S.E.M. of four independent experiments (n=4). *P<0.05 compared with control; †P<0.05 compared with 10 mM lactate; ‡P<0.05 compared with 5 mM lactate.
| Addition | Concentration | PFK activity (% of control) |
|---|---|---|
| Control | – | 100±11 |
| Lactate | 5 mM | 47±4*† |
| Lactate | 10 mM | 13±1*‡ |
| Pyruvate | 5 mM | 103±10‡ |
| Pyruvate | 10 mM | 102±11† |
| Oxalate | 10 mM | 85±12† |
| Malate | 10 mM | 82±14† |
| Citrate | 10 mM | 61±12*† |
Table 2. Effects of pH on the kinetic parameters for lactate-induced inhibition of skeletal muscle PFK.
The parameters were calculated fitting eqn (1) to the experimental data obtained under the same conditions shown in Figure 1. *P<0.05 compared with pH 6.7, 7.0 or 7.4; †P<0.05 compared with pH 7.0 or 7.4; ‡P<0.05 compared with pH 6.5, 7.0 or 7.4; §P<0.05 compared with pH 6.5, 6.7 or 7.4; n.d. not determined.
| pH | I0.5 (mM) | n |
|---|---|---|
| 6.5 | 0.60±0.08* | 0.4±0.1† |
| 6.7 | 1.2±0.1‡ | 0.5±0.1† |
| 7.0 | 3.0±0.3§ | 0.7±0.1§ |
| 7.4 | 4.6±0.3 | 2.0±0.2 |
| 8.2 | n.d. | n.d. |
Figure 2. Effects of lactate on substrate-dependent modulation of PFK.
(A) ATP-dependence on PFK activity in the absence (●) or the presence (○) of 10 mM lactate. Lines are the results of non-linear regression fitting eqn (3) to the experimental data. Values are means±S.E.M. for at least four independent experiments. (B) F6P-dependence on PFK activity in the absence (●) or the presence of 5 (○) or 10 mM (▼) lactate. Lines are the results of non-linear regression fitting eqn (4) to the experimental data. Values are means±S.E.M. for at least four independent experiments.
Table 3. Effects of lactate on the kinetic parameters for ATP and F6P on skeletal muscle PFK.
The parameters for ATP were calculated fitting eqn (3) to the experimental data presented in Figure 2(A) and for F6P were calculated fitting the parameters of eqn (4) to the experimental data presented in Figure 2(B). *P<0.05 compared with the control in the absence of lactate. †P<0.05 compared with the control in the absence of lactate and to the presence of 5 mM lactate.
| Kinetic parameters for ATP | |||||||
|---|---|---|---|---|---|---|---|
| Stimulatory component | Inhibitory component | Kinetic parameters for F6P | |||||
| Lactate | K0.5 (mM) | n1 | I0.5 (mM) | n2 | Vmax (m-units/μg) | K0.5 (mM) | n |
| None | 0.57±0.05 | 2.1±0.4 | 2.27±0.33 | 3.8±0.5 | 3.2±0.2 | 0.19±0.03 | 2.0±0.1 |
| 5 mM | – | – | – | – | 0.98±0.08* | 0.16±0.02 | 2.0±0.2 |
| 10 mM | 0.93±0.07* | 1.2±0.2* | 3.79±0.32* | 8.0±0.9* | 0.21±0.02† | 0.41±0.03† | 2.0±0.7 |
Figure 3. Prevention of lactate effects on PFK by tetramer-stabilizing agents.
PFK activity was assessed by adding the neutralized supernatant of 1×107 cells to a reaction medium containing 50 mM Tris/HCl (pH 7.4), 5 mM MgCl2, 5 mM (NH4)2SO4, 1 mM [γ-32P]ATP, 1 mM F6P and 5 μg/ml PFK, as indicated in the Experimental section, in the absence and the presence of 100 nM F2,6BP, 1 m-unit/ml PKA or 50 μg/ml f-actin. Plotted values were calculated relative to the control activity in the absence of additives, and are means±S.E.M. for at least four independent experiments. *P<0.05 compared with the respective control in the absence or presence of 10 mM lactate (as determined by a Student's t test).
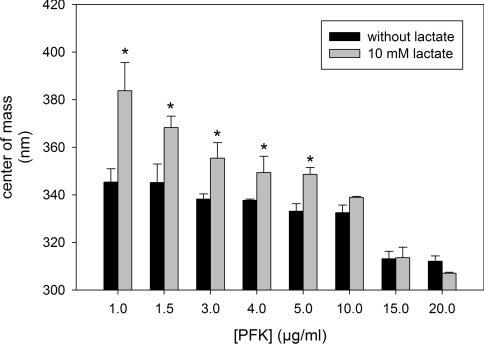
In order to evaluate the effects of lactate on the quaternary structure of PFK we measured the centre of mass of the intrinsic fluorescence spectra of the enzyme. This technique has been used to identify the transition between PFK tetramers, dimers and monomers [22,34]. It has been demonstrated that the smaller configurations of the enzyme had their tryptophan residues more exposed to the polar environment, decreasing the energy of its intrinsic fluorescence emission when excited at 280 nm [22,34]. This is a very accurate technique and is very useful to evaluate the transition between distinct PFK quaternary structures induced by ligands [22] and by the enzyme concentration [34]. The equilibrium between PFK monomers, dimers and tetramers is affected by the concentration of the enzyme, where dissociation is observed upon dilution [13–15,34]. This phenomenon can be observed in Figure 4 where it is clear that there is a decrease in the centre of mass of the intrinsic fluorescence spectra in a PFK concentration-dependent manner (Figure 4, black bars), confirming that at high concentrations of PFK, the tetrameric structure is favoured. Moreover, at PFK concentrations lower than 10 μg/ml, the presence of 10 mM lactate augmented the centre of mass of intrinsic fluorescence spectra of the enzyme (Figure 4), which is directly correlated with the property of lactate to dissociate PFK structures. However, this effect is prevented when the tetrameric structure is stabilized by high concentrations of the enzyme such as 10, 15 or 20 μg/ml (Figure 4).
Figure 4. Effects of lactate on the centre of mass of PFK intrinsic fluorescence spectra measured at different PFK concentrations.
Centre of mass was calculated using eqn (3). Values are means±S.E.M. for at least four independent experiments. *P<0.05 compared with the absence of lactate.
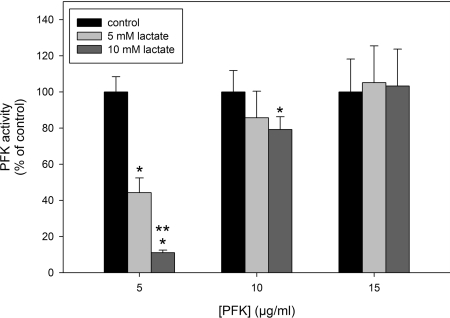
On the basis that lactate-induced dissociation of PFK is prevented at high concentrations of the enzyme, it would be expected that, at these concentrations, lactate will not inhibit PFK activity. Figure 5 shows the effects of lactate on PFK activity in the presence of 5, 10 and 15 μg/ml PFK, where it is clear that lactate-induced inhibition of the enzyme is strongly attenuated when the experiment is performed with 10 μg/ml PFK and completely abrogated with 15 μg/ml PFK. Together, these results show that the inhibitory and modulatory effects of lactate are due to the dissociation of PFK promoted by this metabolite within its physiological intracellular range of concentrations. This allows us to propose lactate as a physiological modulator of PFK activity and, consequently, as a feedback inhibitor of cell glycolytic flux.
Figure 5. Effects of lactate on PFK activity assessed at different concentrations of the enzyme.
Values are means±S.E.M. for at least four independent experiments. *P<0.05 compared with the control in the absence of lactate. **P<0.05 compared with the presence of 5 mM lactate.
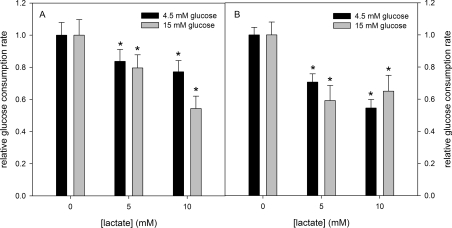
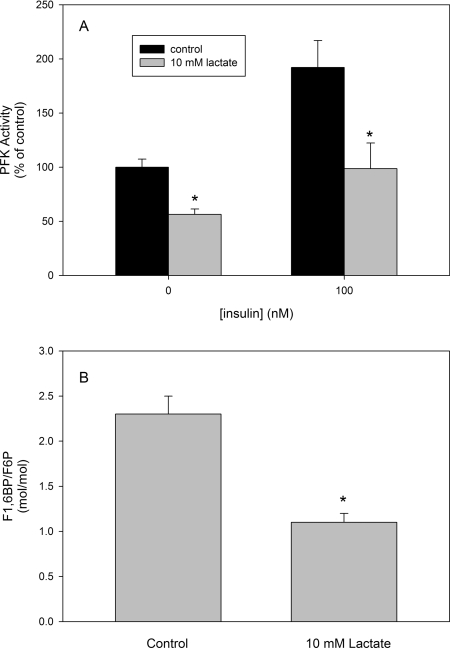
In order to address the ability of lactate in regulating glucose metabolism we measured the effects of lactate on the glucose consumption by skeletal muscle. For this purpose, isolated mice quadriceps were incubated in Krebs–Ringer–Henseleit buffer with 4.5 or 15 mM glucose added in the absence or the presence of 100 nM insulin, and the rate of medium glucose consumption was measured. Lactate decreased the rate of glucose consumption in conditions mimicking normo- (4.5 mM glucose) or hyper- (15 mM) glycaemia, independent of the presence or absence of insulin (Figure 6). Corroborating that this effect was due to PFK inhibition, we measured PFK activity from mice skeletal muscles incubated for 2 h with or without 10 mM lactate and in the absence or the presence of 100 nM insulin. Our results show that incubation with lactate resulted in a decrease in PFK activity of approx. 50%, independent of the presence or absence of insulin (Figure 7A). The degree of PFK inhibition by lactate is compatible with the decrease in glucose consumption shown in Figure 6, suggesting that the latter is dependent on the former. Moreover, in order to strengthen the statement that lactate physiologically inhibits PFK activity, we cultured C2C12 myoblasts and incubated these cells for 2 h in the absence or the presence of 10 mM lactate. After incubation, cells were disrupted and the F1,6BP/F6P ratio was measured. Figure 7(B) shows that lactate decreased the F1,6BP/F6P ratio, revealing the in situ inhibition of PFK in these cells. It is noteworthy that the glycolytic rate depends on the activity of lactate dehydrogenase, which reduces pyruvate forming lactate, so that the glycolytic flux is stimulated as much as lactate is formed [1]. Actually, what occurs is that cell lactate diffuses to the extracellular milieu, and the formation of lactate is useful for the oxidation of NADH guaranteeing glycolytic activity. However, it is not very clear whether the accumulation of lactate in the cell promotes deleterious effects on cell energy production, since it has been correlated with some muscle injuries and fatigue [1], as well as being involved in cell repair, signalling and can be used as substrate for energy production [1–5]. The present study does not resolve this problem, but makes it clear that lactate is a PFK modulator. The inhibition promoted by lactate of PFK activity is counteracted by several physiological signals such as an increase in F2,6BP, the action of PKA and the association of the enzyme with f-actin, which is mediated by many other signals [17,19–22,33–36,40–46]. Therefore lactate may act as an allosteric modulator of PFK, whose final effect depends on several other regulators of the enzyme.
Figure 6. Effects of lactate on mice skeletal muscle glucose consumption.
Glucose consumption was assessed in the absence (A) or the presence (B) of 100 nM insulin. Values are means±S.E.M. for at least four independent experiments. *P<0.05 compared with the respective control in the absence of lactate and in the presence of 4.5 or 15 mM glucose. The absolute rates for glucose consumption are 0.32±0.03 mmol·g−1·h−1 in the presence of 4.5 mM glucose and 0.36±0.03 mmol·g−1·h−1 in the presence of 15 mM glucose, in the absence of insulin, and 0.78±0.06 mmol·g−1·h−1 in the presence of 4.5 mM glucose and 0.86±0.08 mmol·g−1·h−1 in the presence of 15 mM glucose, in the presence of 100 nM insulin.
Figure 7. Effects of lactate on intact mice skeletal muscle PFK activity and on the F1,6BP/F6P ratio in C2C12 myoblasts.
(A) Muscle slices were incubated for 2 h in Krebs–Ringer–Henseleit buffer as described in the Experimental section, in the absence and presence of 100 nM insulin. After incubation, muscles were homogenized in the same buffer and PFK activity was measured. The absolute PFK activity in the absence of lactate and insulin was 5.9±0.4 m-units/mg of protein. *P<0.05 compared with the control in the same group. (B) Confluent C2C12 cells were incubated for 2 h in the absence and the presence of 10 mM lactate. After incubation cells were harvested, disrupted and the F1,6BP and F6P content were measured. Values are means±S.E.M. for three independent experiments. *P<0.05 compared with the control.
In conclusion, in the present study we corroborate the statement of many authors that lactate cannot any more be considered as a dead-end waste product of glycolysis, but rather an active metabolite acting as a fuel, a tissue-repair inducer and a signalling molecule co-ordinating cell and systemic function, as well as a regulator of glycolytic flux modulating PFK activity. Actually, this modulation can also serve to co-ordinate cell metabolism, since the PFK rate can direct glucose metabolism to energy production, to the pentose–phosphate shunt and to other biosynthetic pathways [20,21,47]. Hence the effects of lactate on PFK described in the present study can be correlated with all of the other lactate properties described above.
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação Ary Frausino/Fundação Educacional Chales Darwin (FAF/FECD Programa de Oncobiologia), Programa de Núcleos de Excelência (PRONEX), and Instituto do Milênio em Inovação de Fármacos e Medicamentos (IM-INOFAR).
References
- 1.Gladden L. B. Lactate metabolism: a new paradigm for the third millennium. J. Physiol. 2004;558:5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kristensen M., Albertsen J., Rentsch M., Juel C. Lactate and force production in skeletal muscle. J. Physiol. 2005;562:521–526. doi: 10.1113/jphysiol.2004.078014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philp A., Macdonald A. L., Watt P. W. Lactate: a signal coordinating cell and systemic function. J. Exp. Biol. 2005;208:4561–4575. doi: 10.1242/jeb.01961. [DOI] [PubMed] [Google Scholar]
- 4.Robinson B. H. Lactic acidemia and mitochondrial disease. Mol. Genet. Metab. 2006;89:3–13. doi: 10.1016/j.ymgme.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Boussouar F., Benahmed M. Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab. 2004;15:345–350. doi: 10.1016/j.tem.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 7.Kim J. W., Dang C. V. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–8930. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 8.Mathupala S. P., Colen C. B., Parajuli P., Sloan A. E. Lactate and malignant tumors: a therapeutic target at the end stage of glycolysis. J. Bioenerg. Biomembr. 2007;39:73–77. doi: 10.1007/s10863-006-9062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voit E., Neves A. R., Santos H. The intricate side of systems biology. Proc. Natl. Acad. Sci. U.S.A. 2006;103:9452–9457. doi: 10.1073/pnas.0603337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uyeda K. Phosphofructokinase. Adv. Enzymol. 1979;48:193–244. doi: 10.1002/9780470122938.ch4. [DOI] [PubMed] [Google Scholar]
- 11.Beitner R. The role of glucose-1,6-bisphosphate in the regulation of carbohydrate metabolism in muscle. Trends Biochem. Sci. 1979;4:228–230. [Google Scholar]
- 12.Hers H. G., van Schaftingen E. Fructose 2,6-bisphosphate 2 years after its discovery. Biochem. J. 1982;206:1–12. doi: 10.1042/bj2060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luther M. A., Cai G.-Z., Lee J. C. Thermodynamics of dimer and tetramer formations in rabbit muscle phosphofructokinase. Biochemistry. 1986;25:7931–7937. doi: 10.1021/bi00372a022. [DOI] [PubMed] [Google Scholar]
- 14.Aragón J. J., Sols A. Regulation of enzyme activity in the cell: effect of enzyme concentration. FASEB J. 1991;5:2945–2950. doi: 10.1096/fasebj.5.14.1752361. [DOI] [PubMed] [Google Scholar]
- 15.Lee J. C., Hesterberg L. K., Luther M. A., Cai G.-Z. Rabbit muscle phosphofructokinase. In: Herve G., editor. Allosteric Enzymes. Boca Raton, FL: CRC Press; 1989. pp. 231–254. [Google Scholar]
- 16.Hesterberg L. K., Lee J. C. Self-association of rabbit muscle phosphofructokinase at pH 7.0: stoichiometry. Biochemistry. 1981;20:2974–2980. doi: 10.1021/bi00513a040. [DOI] [PubMed] [Google Scholar]
- 17.El-Bacha T., de Freitas M. S., Sola-Penna M. Cellular distribution of phosphofructokinase activity and implications to metabolic regulation in human breast cancer. Mol. Genet. Metab. 2003;79:294–299. doi: 10.1016/s1096-7192(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 18.El-Bacha T., Menezes M. M., Azevedo e Silva M. C., Sola-Penna M., Da Poian A. T. Mayaro virus infection alters glucose metabolism in cultured cells through activation of the enzyme 6-phosphofructo 1-kinase. Mol. Cell Biochem. 2004;266:191–198. doi: 10.1023/b:mcbi.0000049154.17866.00. [DOI] [PubMed] [Google Scholar]
- 19.Meira D. D., Marinho-Carvalho M. M., Teixeira C. A., Veiga V. F., Da Poian A. T., Holandino C., de Freitas M. S., Sola-Penna M. Clotrimazole decreases human breast cancer cells viability through alterations in cytoskeleton-associated glycolytic enzymes. Mol. Genet. Metab. 2005;84:354–362. doi: 10.1016/j.ymgme.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Zancan P., Sola-Penna M. Regulation of human erythrocyte metabolism by insulin: cellular distribution of 6-phosphofructo-1-kinase and its implication for red blood cell function. Mol. Genet. Metab. 2005;86:401–411. doi: 10.1016/j.ymgme.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Zancan P., Sola-Penna M. Calcium influx: a possible role for insulin modulation of intracellular distribution and activity of 6-phosphofructo-1-kinase in human erythrocytes. Mol. Genet. Metab. 2005;86:392–400. doi: 10.1016/j.ymgme.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Zancan P., Rosas A. O., Marcondes M. C., Marinho-Carvalho M. M., Sola-Penna M. Clotrimazole inhibits and modulates heterologous association of the key glycolytic enzyme 6-phosphofructo-1-kinase. Biochem. Pharmacol. 2007;73:1520–1527. doi: 10.1016/j.bcp.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Marin-Hernandez A., Rodriguez-Enriquez S., Vital-Gonzalez P. A., Flores-Rodriguez F. L., Macias-Silva M., Sosa-Garrocho M., Moreno-Sanchez R. Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J. 2006;273:1975–1988. doi: 10.1111/j.1742-4658.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- 24.Reference deleted
- 25.Maia J. C. C., Gomes S. L., Juliani M. H. Preparation of [α-32P] and [γ-32P]-nucleoside triphosphate, with high specific activity. In: Morel C. M., editor. Genes and Antigenes of Parasites, a Laboratory Manual. Fundação Oswaldo Cruz: Rio de Janeiro; 1983. pp. 146–157. [Google Scholar]
- 26.Kemp R. G. Phosphofructokinase. Methods Enzymol. 1975;42:72–77. doi: 10.1016/0076-6879(75)42096-1. [DOI] [PubMed] [Google Scholar]
- 27.Kuo H.-J., Malencik D. A., Liou R.-S., Anderson S. R. Factors affecting the activation of rabbit muscle phosphofructokinase by actin. Biochemistry. 1986;25:1278–1286. doi: 10.1021/bi00354a013. [DOI] [PubMed] [Google Scholar]
- 28.Lowry O. H., Rosenbrough N. L., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 29.Sola-Penna M., Santos A. C., Serejo F. C., Alves G. G., El-Bacha T., Faber-Barata J., Pereira M. F., Da Poian A. T., Sorenson M. M. A radioassay for phosphofructokinase-1 activity in cell extracts and purified enzyme. J. Biochem. Biophys. Methods. 2002;50:129–140. doi: 10.1016/s0165-022x(01)00180-4. [DOI] [PubMed] [Google Scholar]
- 30.Lakowicz J. R. New York: Kluwer Academic/Plenum; 1999. Principles of Fluorescence Spectroscopy. [Google Scholar]
- 31.Lowry O. H., Passonneau J. V. New York: Academic Press; 1972. A flexible system of enzymatic analysis. [Google Scholar]
- 32.CRC. Handbook of Chemistry and Physics. In: Lide D. R., editor. 77th ed. New York: CRC; 1997. [Google Scholar]
- 33.Alves G. G., Marinho-Carvalho M. M., Atella G. C., Silva-Neto M. A., Sola-Penna M. Allosteric regulation of 6-phosphofructo-1-kinase activity of fat body and flight muscle from the bloodsucking bug Rhodnius prolixus. Ann. Acad. Bras. Cienc. 2007;79:53–62. doi: 10.1590/s0001-37652007000100008. [DOI] [PubMed] [Google Scholar]
- 34.Marinho-Carvalho M. M., Zancan P., Sola-Penna M. Modulation of 6-phosphofructo-1-kinase oligomeric equilibrium by calmodulin: formation of active dimers. Mol. Genet. Metab. 2006;87:253–261. doi: 10.1016/j.ymgme.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Silva A. P., Alves G. G., Araujo A. H., Sola-Penna M. Effects of insulin and actin on phosphofructokinase activity and cellular distribution in skeletal muscle. Ann. Acad. Bras. Cienc. 2004;76:541–548. doi: 10.1590/s0001-37652004000300008. [DOI] [PubMed] [Google Scholar]
- 36.Bock P. E., Frieden C. Phosphofructokinase. II. Role of ligands in pH-dependent structural changes of the rabbit muscle enzyme. J. Biol. Chem. 1976;251:5637–5643. [PubMed] [Google Scholar]
- 37.Alves G. G., Sola-Penna M. Epinephrine modulates cellular distribution of muscle phosphofructokinase. Mol. Genet. Metab. 2003;78:302–306. doi: 10.1016/s1096-7192(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 38.Liou R.-S., Anderson S. Activation of rabbit muscle phosphofructokinase by F-actin and reconstituted thin filaments. Biochemistry. 1980;19:2684–2688. doi: 10.1021/bi00553a022. [DOI] [PubMed] [Google Scholar]
- 39.Clarke F., Stephan P., Morton D. J., Wiedemann J. The role of actin and associated structural proteins in the organization of glycolytic enzymes. In: Barden J., Dos Remedios C., editors. Actin: Structure and Function in Muscle and Non-Muscle Cells. Sydney: Academic Press; 1983. pp. 249–257. [Google Scholar]
- 40.Ashkenazy-Shahar M., Beitner R. Serotonin decreases cytoskeletal and cytosolic glycolytic enzymes and the levels of ATP and glucose 1,6-bisphosphate in skin, which is prevented by the calmodulin antagonists thioridazine and clotrimazole. Biochem. Mol. Med. 1997;60:187–193. doi: 10.1006/bmme.1996.2562. [DOI] [PubMed] [Google Scholar]
- 41.Ashkenazy-Shahar M., Ben-Porat H., Beitner R. Insulin stimulates binding of phosphofructokinase to cytoskeleton and increases glucose 1,6-bisphosphate levels in NIH-3T3 fibroblasts, which is prevented by calmodulin antagonists. Mol. Genet. Metab. 1998;65:213–219. doi: 10.1006/mgme.1998.2759. [DOI] [PubMed] [Google Scholar]
- 42.Assouline-Cohen M., Ben-Porat H., Beitner R. Activation of membrane skeleton-bound phosphofructokinase in erythrocytes induced by serotonin. Mol. Genet. Metab. 1998;63:235–238. doi: 10.1006/mgme.1997.2673. [DOI] [PubMed] [Google Scholar]
- 43.Assouline-Cohen M., Beitner R. Effects of Ca2+ on erythrocyte membrane skeleton-bound phosphofructokinase, ATP levels, and hemolysis. Mol. Genet. Metab. 1999;66:56–61. doi: 10.1006/mgme.1998.2773. [DOI] [PubMed] [Google Scholar]
- 44.Glass-Marmor L., Beitner R. Detachment of glycolytic enzymes from cytoskeleton of melanoma cells induced by calmodulin antagonists. Eur. J. Pharmacol. 1997;328:241–248. doi: 10.1016/s0014-2999(97)83051-8. [DOI] [PubMed] [Google Scholar]
- 45.Glass-Marmor L., Beitner R. Taxol (paclitaxel) induces a detachment of phosphofructokinase from cytoskeleton of melanoma cells and decreases the levels of glucose 1,6-bisphosphate, fructose 1,6-bisphosphate and ATP. Eur. J. Pharmacol. 1999;370:195–199. doi: 10.1016/s0014-2999(99)00155-7. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz D., Beitner R. Detachment of the glycolytic enzymes, phosphofructokinase and aldolase, from cytoskeleton of melanoma cells, induced by local anesthetics. Mol. Genet. Metab. 2000;69:159–164. doi: 10.1006/mgme.2000.2960. [DOI] [PubMed] [Google Scholar]
- 47.Messana I., Orlando M., Cassiano L., Pennacchietti L., Zuppi C., Castagnola M., Giardina B. Human erythrocyte metabolism is modulated by the O2-linked transition of hemoglobin. FEBS Lett. 1996;390:25–28. doi: 10.1016/0014-5793(96)00624-2. [DOI] [PubMed] [Google Scholar]