Abstract
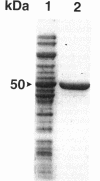
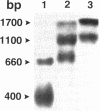
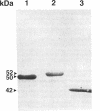
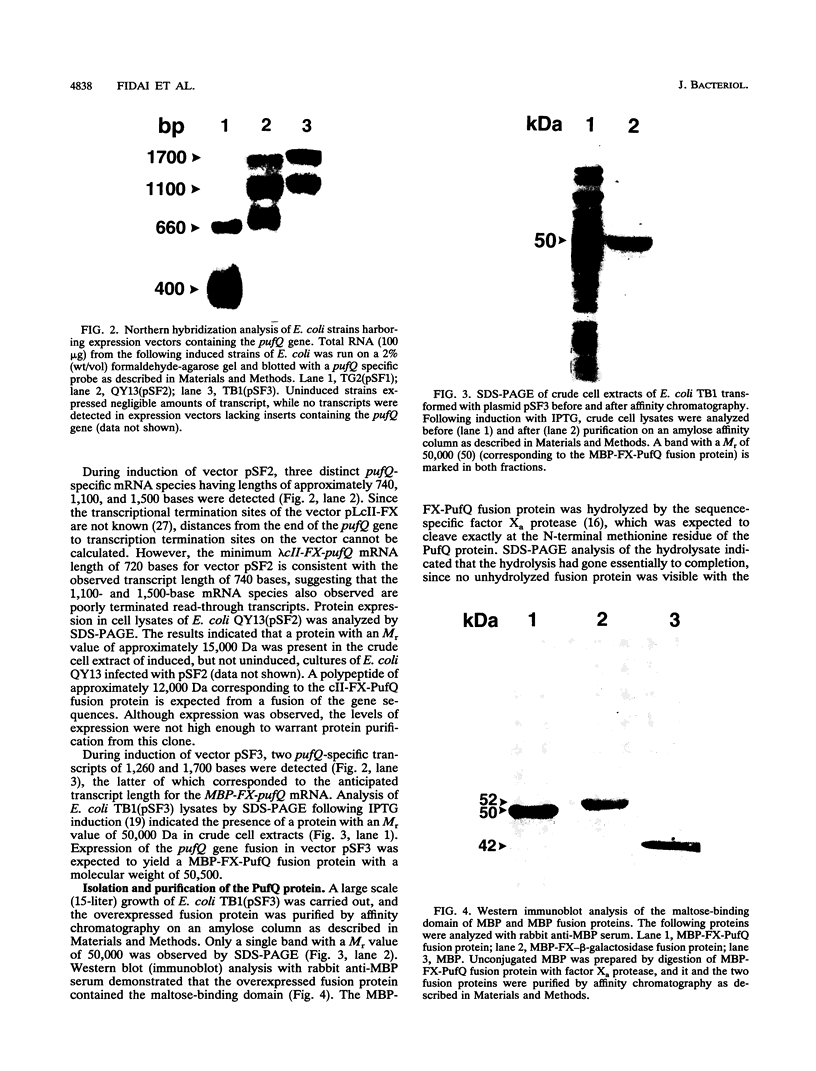
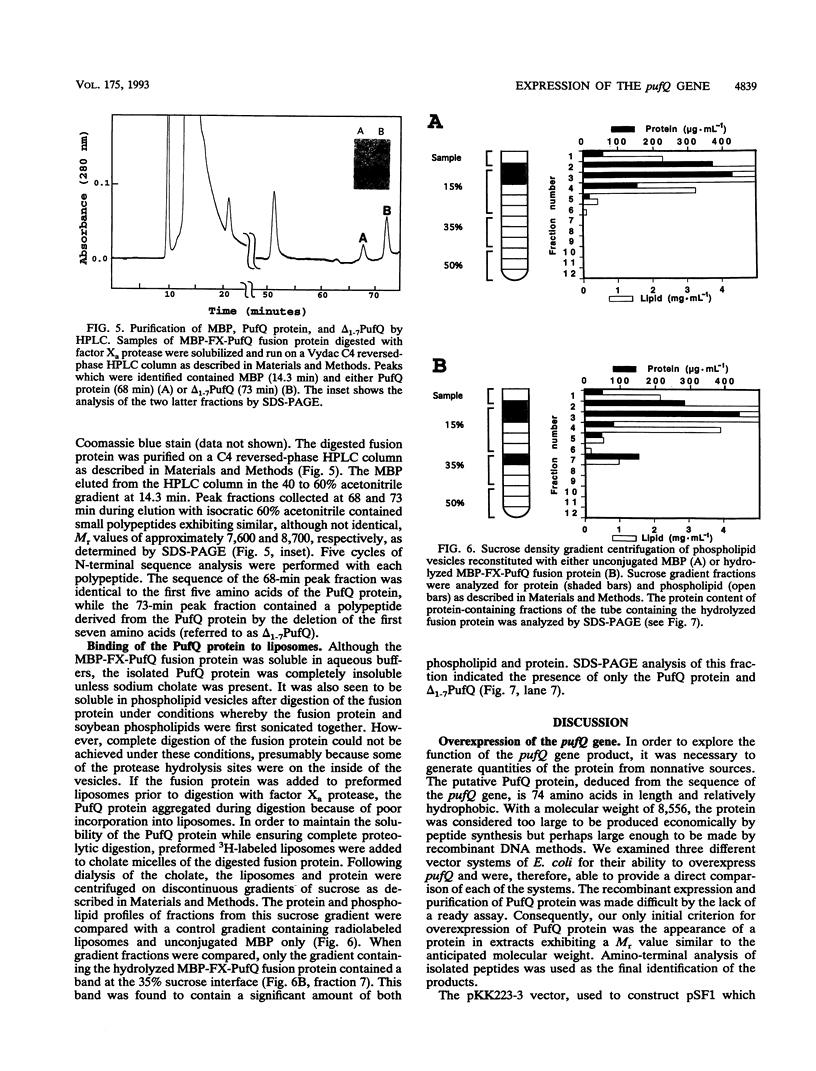
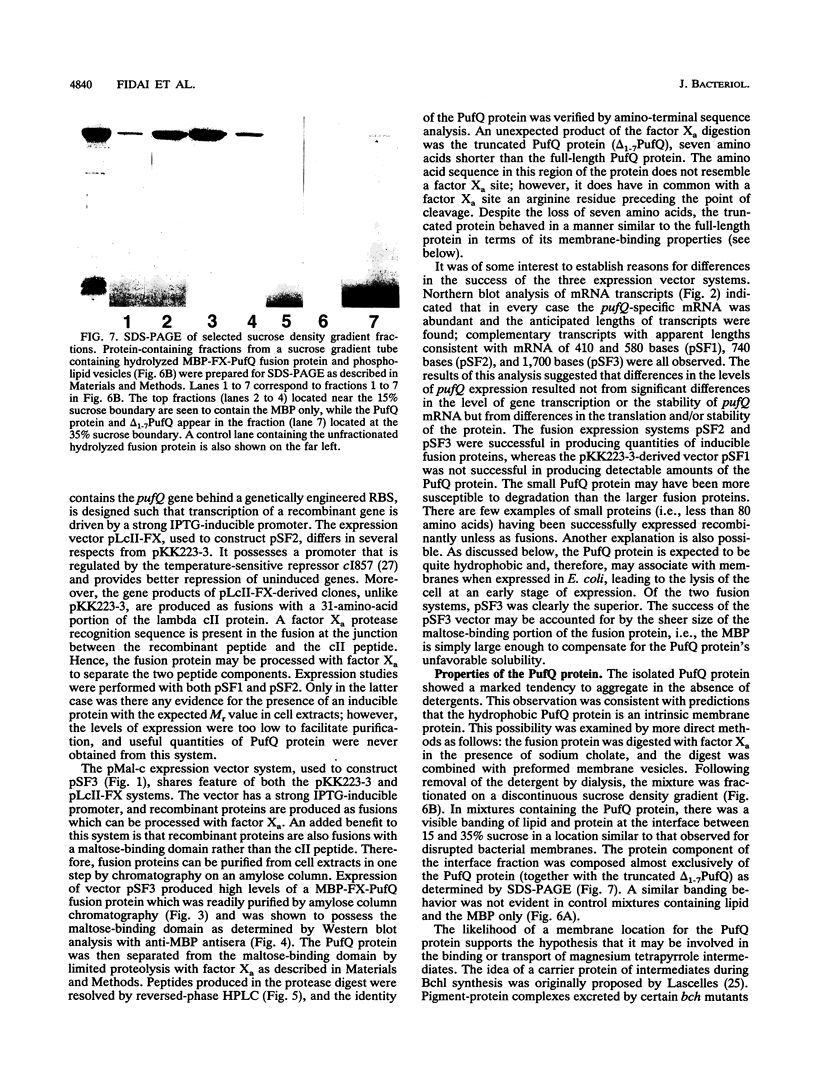
Genetic studies have shown that the expression of the pufQ gene is required for normal levels of bacteriochlorophyll biosynthesis in Rhodobacter capsulatus. Yet, the exact function of the pufQ gene is unknown, and a pufQ gene product has never been isolated. We describe the recombinant overexpression of pufQ in Escherichia coli, as well as the purification and characterization of its gene product, the 74-amino-acid PufQ protein. Site-directed mutagenesis was used to facilitate the cloning of the pufQ gene into various expression vector systems of E. coli, including pKK223-3, pLcII-FX, and pMal-c. Although high levels of pufQ transcription were evident from constructs of all three vectors, high levels of protein expression were apparent only in the pMal-c system. In vector pMal-c, the recombinant PufQ protein is expressed as a fusion with an amino-terminal maltose-binding domain. After affinity purification on an amylose column, full-length PufQ protein was released from the fusion protein by limited proteolysis with the enzyme factor Xa. The PufQ protein demonstrated a strong tendency to associate with phospholipid vesicles, consistent with the view that it is an integral membrane protein. The PufQ protein was subsequently purified by high-performance liquid chromatography and identified by amino-terminal sequence analysis. A possible role for the PufQ protein in the transport of bacteriochlorophyll biosynthetic intermediates is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Forrest M. E., Cohen S. N., Beatty J. T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989 Jan;171(1):473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Marrs B. L. Rhodobacter capsulatus puf operon encodes a regulatory protein (PufQ) for bacteriochlorophyll biosynthesis. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7074–7078. doi: 10.1073/pnas.85.19.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Young D. A., Marrs B. L. Analysis of the Rhodobacter capsulatus puf operon. Location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J Biol Chem. 1988 Apr 5;263(10):4820–4827. [PubMed] [Google Scholar]
- Biel A. J. Control of bacteriochlorophyll accumulation by light in Rhodobacter capsulatus. J Bacteriol. 1986 Nov;168(2):655–659. doi: 10.1128/jb.168.2.655-659.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biel A. J., Marrs B. L. Transcriptional regulation of several genes for bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata in response to oxygen. J Bacteriol. 1983 Nov;156(2):686–694. doi: 10.1128/jb.156.2.686-694.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollivar D. W., Bauer C. E. Association of tetrapyrrole intermediates in the bacteriochlorophyll a biosynthetic pathway with the major outer-membrane porin protein of Rhodobacter capsulatus. Biochem J. 1992 Mar 1;282(Pt 2):471–476. doi: 10.1042/bj2820471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollivar D. W., Bauer C. E. Nucleotide Sequence of S-Adenosyl-l-Methionine: Magnesium Protoporphyrin Methyltransferase from Rhodobacter capsulatus. Plant Physiol. 1992 Jan;98(1):408–410. doi: 10.1104/pp.98.1.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Holy A. Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6929–6933. doi: 10.1073/pnas.81.22.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger G., Gingras G. Structure and expression of the puf operon messenger RNA in rhodospirillum rubrum. J Biol Chem. 1988 Jun 5;263(16):7639–7645. [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Clark W. G., Davidson E., Marrs B. L. Variation of levels of mRNA coding for antenna and reaction center polypeptides in Rhodopseudomonas capsulata in response to changes in oxygen concentration. J Bacteriol. 1984 Mar;157(3):945–948. doi: 10.1128/jb.157.3.945-948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomber S. A., Chaudhri M., Connor A., Britton G., Hunter C. N. Localized transposon Tn5 mutagenesis of the photosynthetic gene cluster of Rhodobacter sphaeroides. Mol Microbiol. 1990 Jun;4(6):977–989. doi: 10.1111/j.1365-2958.1990.tb00670.x. [DOI] [PubMed] [Google Scholar]
- ESNOUF M. P., WILLIAMS W. J. The isolation and purification of a bovine-plasma protein which is a substrate for the coagulant fraction of Russell's-viper venom. Biochem J. 1962 Jul;84:62–71. doi: 10.1042/bj0840062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C. N., McGlynn P., Ashby M. K., Burgess J. G., Olsen J. D. DNA sequencing and complementation/deletion analysis of the bchA-puf operon region of Rhodobacter sphaeroides: in vivo mapping of the oxygen-regulated puf promoter. Mol Microbiol. 1991 Nov;5(11):2649–2661. doi: 10.1111/j.1365-2958.1991.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Klug G., Cohen S. N. Pleiotropic effects of localized Rhodobacter capsulatus puf operon deletions on production of light-absorbing pigment-protein complexes. J Bacteriol. 1988 Dec;170(12):5814–5821. doi: 10.1128/jb.170.12.5814-5821.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lascelles J. The accumulation of bacteriochlorophyll precursors by mutant and wild-type strains of Rhodopseudomonas spheroides. Biochem J. 1966 Jul;100(1):175–183. doi: 10.1042/bj1000175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. 1984 Jun 28-Jul 4Nature. 309(5971):810–812. doi: 10.1038/309810a0. [DOI] [PubMed] [Google Scholar]
- Oelze J. Regulation of tetrapyrrole synthesis by light in chemostat cultures of Rhodobacter sphaeroides. J Bacteriol. 1988 Oct;170(10):4652–4657. doi: 10.1128/jb.170.10.4652-4657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards W. R., Wallace R. B., Tsao M. S., Ho E. The nature of a pgiment-protein complex excreted from mutants of Rhodopseudomonas sphaeroides. Biochemistry. 1975 Dec 30;14(26):5554–5561. doi: 10.1021/bi00697a003. [DOI] [PubMed] [Google Scholar]
- Summers W. C. A simple method for extraction of RNA from E. coli utilizing diethyl pyrocarbonate. Anal Biochem. 1970 Feb;33(2):459–463. doi: 10.1016/0003-2697(70)90316-7. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellington C. L., Beatty J. T. Promoter mapping and nucleotide sequence of the bchC bacteriochlorophyll biosynthesis gene from Rhodobacter capsulatus. Gene. 1989 Nov 30;83(2):251–261. doi: 10.1016/0378-1119(89)90111-x. [DOI] [PubMed] [Google Scholar]
- Wiessner C., Dunger I., Michel H. Structure and transcription of the genes encoding the B1015 light-harvesting complex beta and alpha subunits and the photosynthetic reaction center L, M, and cytochrome c subunits from Rhodopseudomonas viridis. J Bacteriol. 1990 Jun;172(6):2877–2887. doi: 10.1128/jb.172.6.2877-2887.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. Q., MacGregor B. J., Donohue T. J., Kaplan S., Yen B. Genetic and physical mapping of the Rhodobacter sphaeroides photosynthetic gene cluster from R-prime pWS2. Plasmid. 1991 May;25(3):163–176. doi: 10.1016/0147-619x(91)90010-t. [DOI] [PubMed] [Google Scholar]
- Yang Z. M., Bauer C. E. Rhodobacter capsulatus genes involved in early steps of the bacteriochlorophyll biosynthetic pathway. J Bacteriol. 1990 Sep;172(9):5001–5010. doi: 10.1128/jb.172.9.5001-5010.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Map of genes for carotenoid and bacteriochlorophyll biosynthesis in Rhodopseudomonas capsulata. J Bacteriol. 1976 May;126(2):619–629. doi: 10.1128/jb.126.2.619-629.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Cook D. N., Leach F., Armstrong G. A., Alberti M., Hearst J. E. Oxygen-regulated mRNAs for light-harvesting and reaction center complexes and for bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus during the shift from anaerobic to aerobic growth. J Bacteriol. 1986 Dec;168(3):1180–1188. doi: 10.1128/jb.168.3.1180-1188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsebo K. M., Hearst J. E. Genetic-physical mapping of a photosynthetic gene cluster from R. capsulata. Cell. 1984 Jul;37(3):937–947. doi: 10.1016/0092-8674(84)90428-8. [DOI] [PubMed] [Google Scholar]
- di Guan C., Li P., Riggs P. D., Inouye H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coli by fusion to maltose-binding protein. Gene. 1988 Jul 15;67(1):21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]