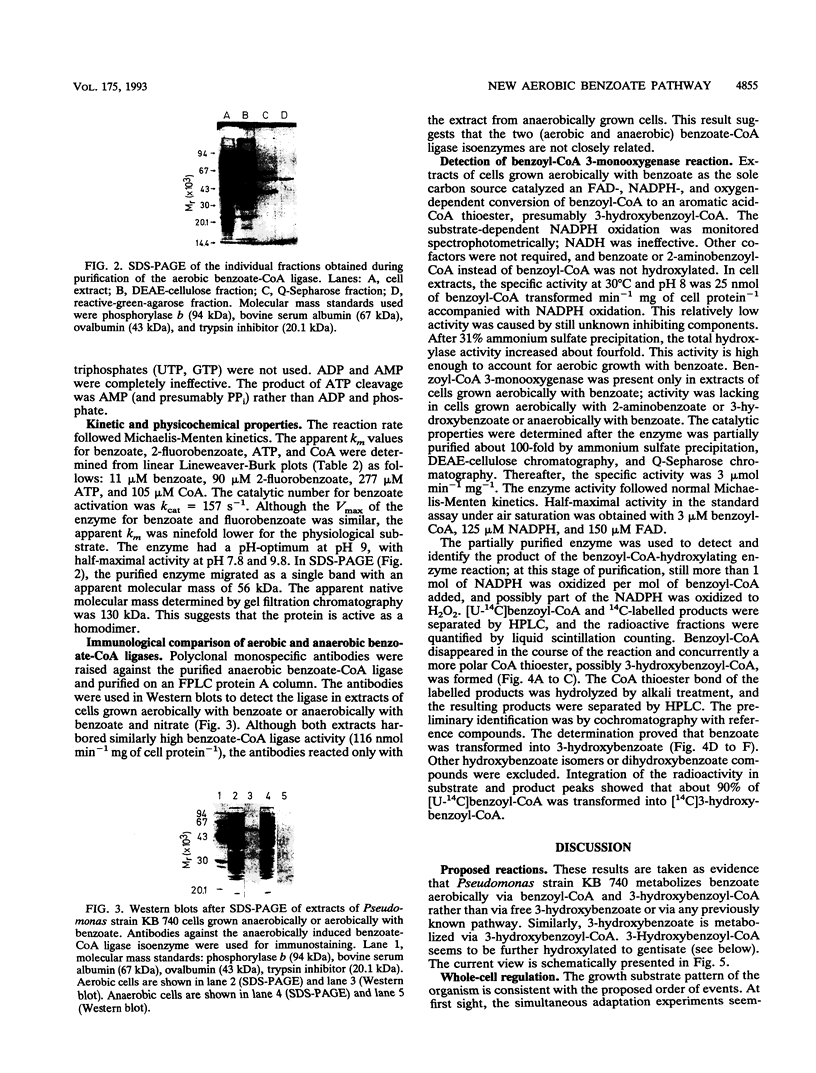
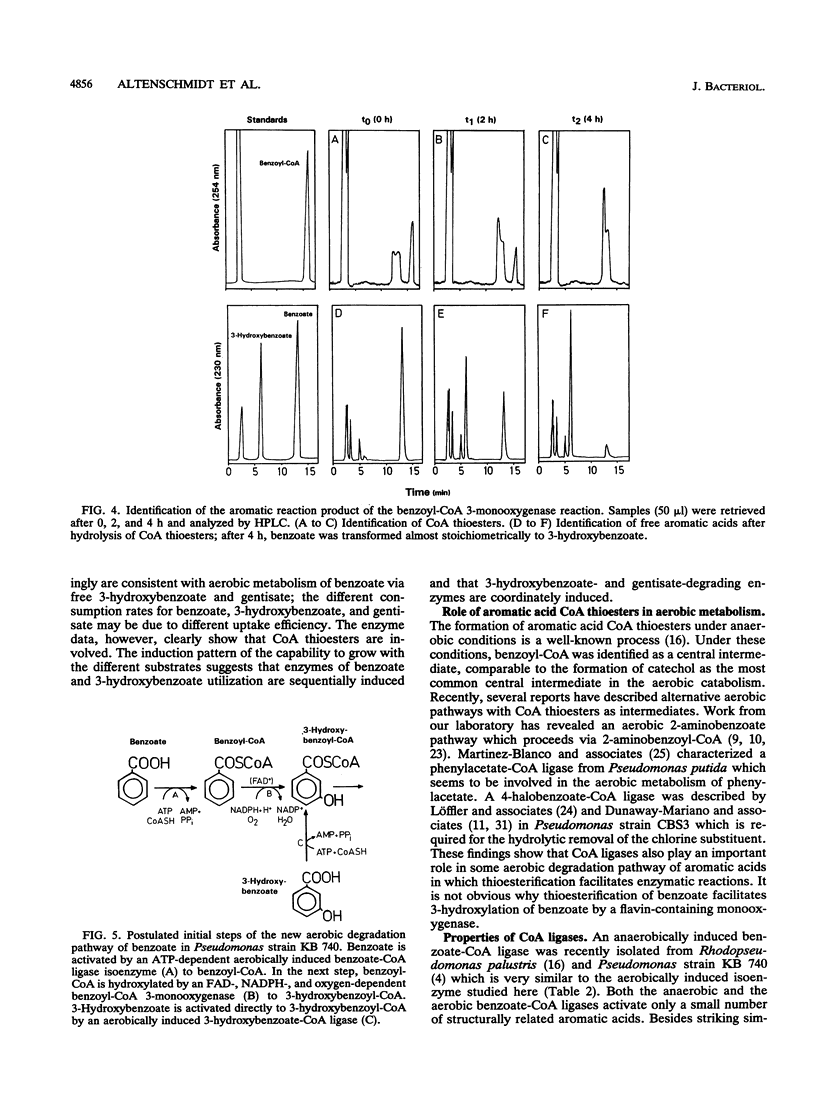
Abstract
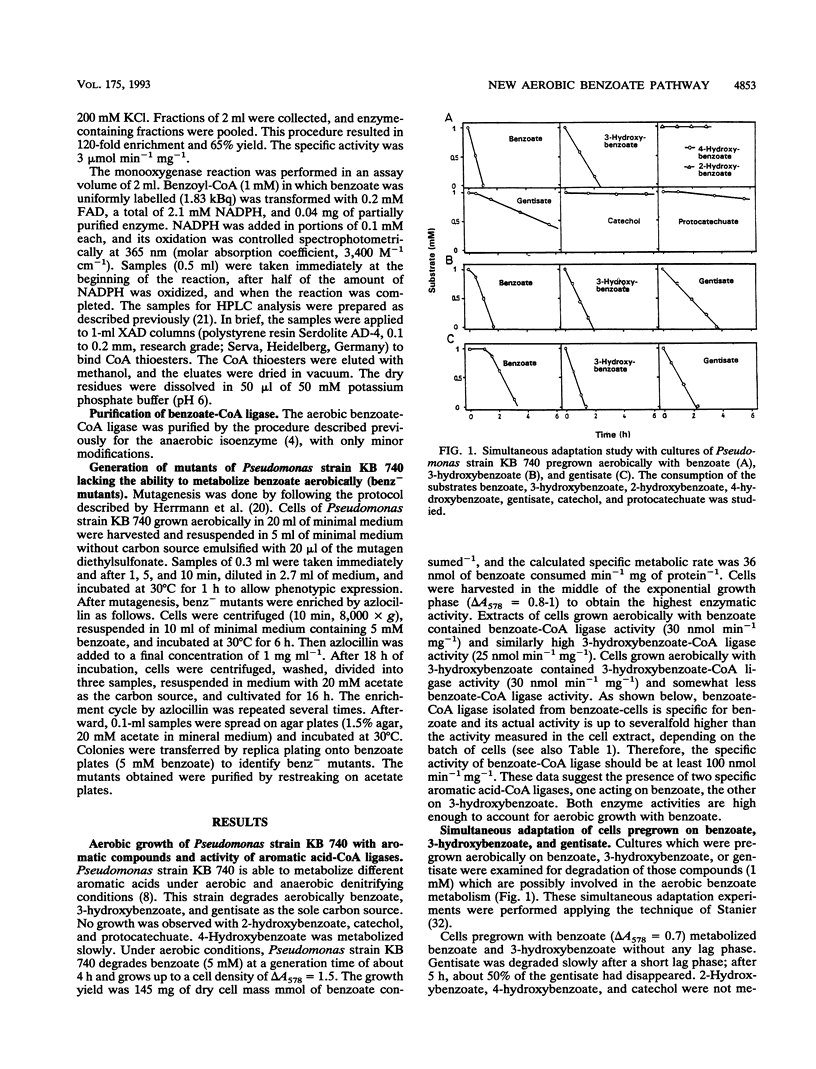
A denitrifying Pseudomonas sp. is able to oxidize aromatic compounds compounds completely to CO2, both aerobically and anaerobically. It is shown that benzoate is aerobically oxidized by a new degradation pathway via benzoyl-coenzyme A (CoA) and 3-hydroxybenzoyl-CoA. The organism grew aerobically with benzoate, 3-hydroxybenzoate, and gentisate; catechol, 2-hydroxybenzoate, and protocatechuate were not used, and 4-hydroxybenzoate was a poor substrate. Mutants were obtained which were not able to utilize benzoate as the sole carbon source aerobically but still used 3-hydroxybenzoate or gentisate. Simultaneous adaptation experiments with whole cells seemingly suggested a sequential induction of enzymes of a benzoate oxidation pathway via 3-hydroxybenzoate and gentisate. Cells grown aerobically with benzoate contained a benzoate-CoA ligase (AMP forming) (0.1 mumol min-1 mg-1) which converted benzoate but not 3-hydroxybenzoate into its CoA thioester. The enzyme of 130 kDa composed of two identical subunits of 56 kDa was purified and characterized. Cells grown aerobically with 3-hydroxybenzoate contained a similarly active CoA ligase for 3-hydroxybenzoate, 3-hydroxybenzoate-CoA ligase (AMP forming). Extracts from cells grown aerobically with benzoate catalyzed a benzoyl-CoA- and flavin adenine dinucleotide-dependent oxidation of NADPH with a specific activity of at least 25 nmol NADPH oxidized min-1 mg of protein-1; NADH and benzoate were not used. This new enzyme, benzoyl-CoA 3-monooxygenase, was specifically induced during aerobic growth with benzoate and converted [U-14C]benzoyl-CoA stoichiometrically to [14C]3-hydroxybenzoyl-CoA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenschmidt U., Bokranz M., Fuchs G. Novel aerobic 2-aminobenzoate metabolism. Nucleotide sequence of the plasmid carrying the gene for the flavoprotein 2-aminobenzoyl-CoA monooxygenase/reductase in a denitrifying Pseudomonas sp. Eur J Biochem. 1992 Jul 15;207(2):715–722. doi: 10.1111/j.1432-1033.1992.tb17100.x. [DOI] [PubMed] [Google Scholar]
- Altenschmidt U., Eckerskorn C., Fuchs G. Evidence that enzymes of a novel aerobic 2-amino-benzoate metabolism in denitrifying Pseudomonas are coded on a small plasmid. Eur J Biochem. 1990 Dec 12;194(2):647–653. doi: 10.1111/j.1432-1033.1990.tb15664.x. [DOI] [PubMed] [Google Scholar]
- Altenschmidt U., Fuchs G. Novel aerobic 2-aminobenzoate metabolism. Purification and characterization of 2-aminobenzoate-CoA ligase, localisation of the gene on a 8-kbp plasmid, and cloning and sequencing of the gene from a denitrifying Pseudomonas sp. Eur J Biochem. 1992 Apr 15;205(2):721–727. doi: 10.1111/j.1432-1033.1992.tb16835.x. [DOI] [PubMed] [Google Scholar]
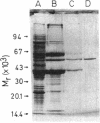
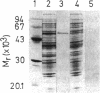
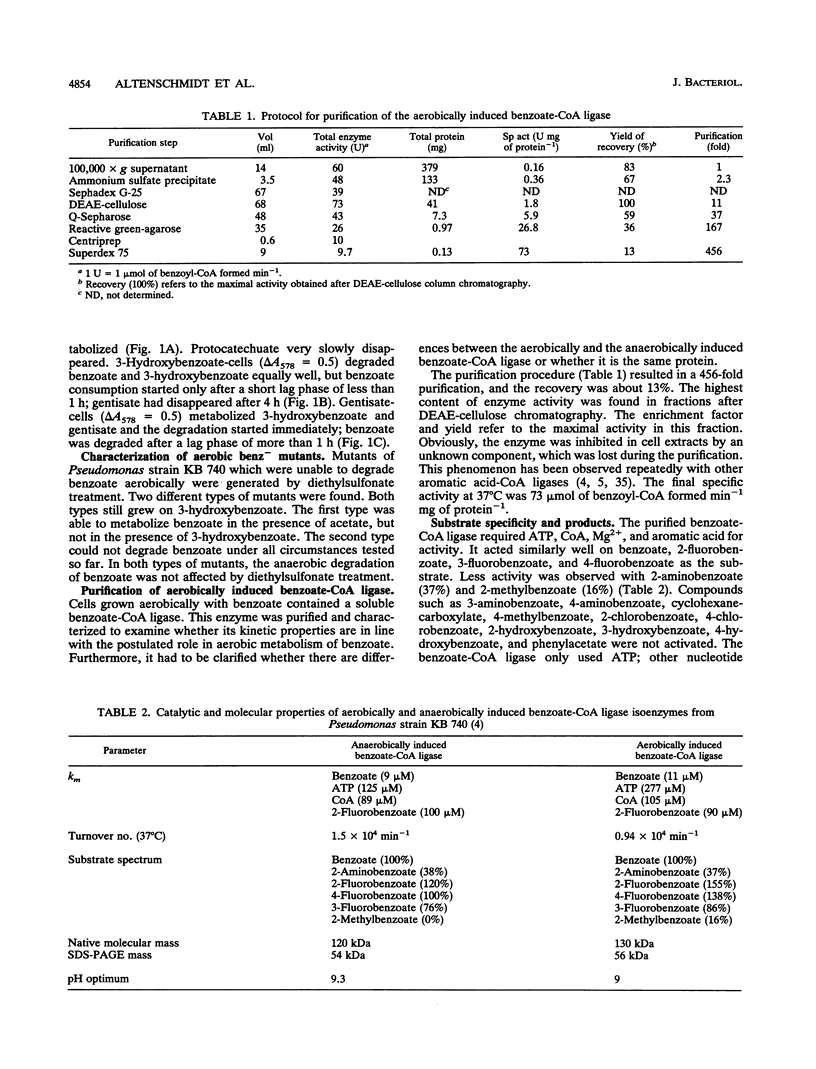
- Altenschmidt U., Oswald B., Fuchs G. Purification and characterization of benzoate-coenzyme A ligase and 2-aminobenzoate-coenzyme A ligases from a denitrifying Pseudomonas sp. J Bacteriol. 1991 Sep;173(17):5494–5501. doi: 10.1128/jb.173.17.5494-5501.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biegert T., Altenschmidt U., Eckerskorn C., Fuchs G. Enzymes of anaerobic metabolism of phenolic compounds. 4-Hydroxybenzoate-CoA ligase from a denitrifying Pseudomonas species. Eur J Biochem. 1993 Apr 1;213(1):555–561. doi: 10.1111/j.1432-1033.1993.tb17794.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun K., Gibson D. T. Anaerobic degradation of 2-aminobenzoate (anthranilic acid) by denitrifying bacteria. Appl Environ Microbiol. 1984 Jul;48(1):102–107. doi: 10.1128/aem.48.1.102-107.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buder R., Fuchs G. 2-Aminobenzoyl-CoA monooxygenase/reductase, a novel type of flavoenzyme. Purification and some properties of the enzyme. Eur J Biochem. 1989 Nov 20;185(3):629–635. doi: 10.1111/j.1432-1033.1989.tb15159.x. [DOI] [PubMed] [Google Scholar]
- Buder R., Ziegler K., Fuchs G., Langkau B., Ghisla S. 2-Aminobenzoyl-CoA monooxygenase/reductase, a novel type of flavoenzyme. Studies on the stoichiometry and the course of the reaction. Eur J Biochem. 1989 Nov 20;185(3):637–643. doi: 10.1111/j.1432-1033.1989.tb15160.x. [DOI] [PubMed] [Google Scholar]
- Chang K. H., Liang P. H., Beck W., Scholten J. D., Dunaway-Mariano D. Isolation and characterization of the three polypeptide components of 4-chlorobenzoate dehalogenase from Pseudomonas sp. strain CBS-3. Biochemistry. 1992 Jun 23;31(24):5605–5610. doi: 10.1021/bi00139a025. [DOI] [PubMed] [Google Scholar]
- Crawford R. L. Pathways of 4-hydroxybenzoate degradation among species of Bacillus. J Bacteriol. 1976 Jul;127(1):204–210. doi: 10.1128/jb.127.1.204-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Fuchs G. Anaerobic degradation of aromatic compounds. Annu Rev Microbiol. 1988;42:289–317. doi: 10.1146/annurev.mi.42.100188.001445. [DOI] [PubMed] [Google Scholar]
- Geissler J. F., Harwood C. S., Gibson J. Purification and properties of benzoate-coenzyme A ligase, a Rhodopseudomonas palustris enzyme involved in the anaerobic degradation of benzoate. J Bacteriol. 1988 Apr;170(4):1709–1714. doi: 10.1128/jb.170.4.1709-1714.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groseclose E. E., Ribbons D. W., Hughes H. 3-Hydroxybenzoate 6-hydroxylase from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1973 Dec 10;55(3):897–903. doi: 10.1016/0006-291x(73)91228-x. [DOI] [PubMed] [Google Scholar]
- Herrmann H., Klopotowski T., Günther E. The Hfr status of Pseudomonas aeruginosa is stabilized by integrative suppression. Mol Gen Genet. 1986 Sep;204(3):519–523. doi: 10.1007/BF00331034. [DOI] [PubMed] [Google Scholar]
- Koch J., Fuchs G. Enzymatic reduction of benzoyl-CoA to alicyclic compounds, a key reaction in anaerobic aromatic metabolism. Eur J Biochem. 1992 Apr 1;205(1):195–202. doi: 10.1111/j.1432-1033.1992.tb16768.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langkau B., Ghisla S., Buder R., Ziegler K., Fuchs G. 2-Aminobenzoyl-CoA monooxygenase/reductase, a novel type of flavoenzyme. Identification of the reaction products. Eur J Biochem. 1990 Jul 31;191(2):365–371. doi: 10.1111/j.1432-1033.1990.tb19131.x. [DOI] [PubMed] [Google Scholar]
- Löffler F., Müller R., Lingens F. Purification and properties of 4-halobenzoate-coenzyme A ligase from Pseudomonas sp. CBS3. Biol Chem Hoppe Seyler. 1992 Oct;373(10):1001–1007. doi: 10.1515/bchm3.1992.373.2.1001. [DOI] [PubMed] [Google Scholar]
- Martínez-Blanco H., Reglero A., Rodriguez-Aparicio L. B., Luengo J. M. Purification and biochemical characterization of phenylacetyl-CoA ligase from Pseudomonas putida. A specific enzyme for the catabolism of phenylacetic acid. J Biol Chem. 1990 Apr 25;265(12):7084–7090. [PubMed] [Google Scholar]
- Mohamed M el-S, Fuchs G. Purification and characterization of phenylacetate-coenzyme A ligase from a denitrifying Pseudomonas sp., an enzyme involved in the anaerobic degradation of phenylacetate. Arch Microbiol. 1993;159(6):554–562. doi: 10.1007/BF00249035. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nilsson B. O., Svalander P. C., Larsson A. Immunization of mice and rabbits by intrasplenic deposition of nanogram quantities of protein attached to Sepharose beads or nitrocellulose paper strips. J Immunol Methods. 1987 May 4;99(1):67–75. doi: 10.1016/0022-1759(87)90033-0. [DOI] [PubMed] [Google Scholar]
- Poh C. L., Bayly R. C. Evidence for isofunctional enzymes used in m-cresol and 2,5-xylenol degradation via the gentisate pathway in Pseudomonas alcaligenes. J Bacteriol. 1980 Jul;143(1):59–69. doi: 10.1128/jb.143.1.59-69.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten J. D., Chang K. H., Babbitt P. C., Charest H., Sylvestre M., Dunaway-Mariano D. Novel enzymic hydrolytic dehalogenation of a chlorinated aromatic. Science. 1991 Jul 12;253(5016):182–185. doi: 10.1126/science.1853203. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y. Simultaneous Adaptation: A New Technique for the Study of Metabolic Pathways. J Bacteriol. 1947 Sep;54(3):339–348. doi: 10.1128/jb.54.3.339-348.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelis M. L., Palleroni N. J., Stanier R. Y. The metabolism of aromatic acids by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol. 1967;59(1):302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]