Abstract
Parathyroid hormone (PTH) and PTH-related protein (PTHrP) are calciotropic hormones interacting with a shared seven-transmembrane domain G protein-coupled receptor, which is located predominantly in bone and kidney. To map the interface of the bimolecular interaction between hormone and receptor, we designed and radioiodinated a bioactive, photoreactive PTH agonist, 125I-[Nle8,18,Lys13(ɛ-p-(3-I-Bz)Bz),l-2-Nal23,Arg26,27,Tyr34]bPTH-(1–34)NH2 (125I-all-R-K13). This ligand contains a photoreactive benzophenone moiety attached to the side chain of Lys13. All other lysyl residues are substituted by argynyls. The analog photocrosslinks specifically to the recombinant hPTH/PTHrP receptor stably transfected into human embryonic kidney cells (HEK-293/C-21 cells, ≈400,000 receptors per cell), generating a diffuse ≈87-kDa band on SDS/PAGE autoradiography. To identify the “contact domain” within the hPTH/PTHrP receptor involved in binding of 125I-all-R-K13, the radiolabeled band containing the ligand–receptor conjugate was subjected to chemical and enzymatic cleavage. Two independent pathways of sequential digestion were used: Route A, lysyl endopeptidase C, then endo-N-glycosidase F, followed by cyanogen bromide; Route B, cyanogen bromide followed by endo-N-glycosydase F. The identified domain is in contact with position 13 in 125I-all-R-K13 and corresponds to amino acids 173–189 of the hPTH/PTHrP receptor, located at the C-terminal region of the N-terminal extracellular domain.
Keywords: contact domain, benzophenone
Parathyroid hormone (PTH) and PTH-related protein (PTHrP) exert their biological actions through interactions with a shared membrane-bound G protein-coupled heptahelical receptor (PTH/PTHrP receptor). The impetus for elucidating the nature of the ligand–receptor bimolecular interaction comes from a desire to understand the mechanism of action and expression of bioactivity for both PTH and PTHrP in normal physiology. Insights from such investigations might point to new directions for the design of novel hormone analogs to be used in treating disorders of calcium or bone metabolism, such as hyperparathyroidism, malignancy-associated hypercalcemia, and osteoporosis.
Two approaches, one “hormone-centered” and one “receptor-centered,” have been pursued to further understanding of the ligand–PTH/PTHrP receptor interaction. The hormone-centered approach was undertaken first and is based on study of structure–activity relations for PTH and PTHrP. Extensive investigations have been conducted by several laboratories, and the results reviewed recently (1, 2). This approach succeeded in mapping functional domains within the hormone for receptor binding and receptor activation and revealed, in detail, structural features responsible for biological properties to the level of a single amino acid. However, this approach alone cannot be used to deduce the domains of the receptor in contact with the hormone. Furthermore, in many cases, the consequences of modifying the primary structure of the hormone cannot be correlated unambiguously with effects on receptor interaction. Structural modifications in the hormone may alter directly the interaction with an important complementary structural feature present in the receptor. Alternatively, some structural modifications in the hormone may produce either local or global conformational changes that prevent adaptation of an optimal “bioactive conformation.” In essence, the hormone-centered approach is “blind” to the structure of receptor.
The “receptor-centered” approach has succeeded in providing novel insights. Point-mutated, truncated, and chimeric PTH/PTHrP receptors have been created, and information has been obtained regarding the importance of specific receptor domains and single amino acids necessary for receptor function (3–6). However, analysis of the consequences of modifications of the receptor structure cannot be used to predict interacting complementary structural elements in the hormone. Similarly, one cannot determine whether a modification in the receptor produces its biological consequences as a result of a local change at a “contact point,” which affects directly the interaction with the hormone, versus a global effect caused by a conformational change leading to a modified receptor topology. Hence, conclusions drawn from both the hormone-centered and the receptor-centered approaches regarding the nature of the “hormone–receptor” bimolecular interface are inferential at best.
Therefore, the method of choice for identifying hormone–receptor interacting domains is a direct one based on analyses of specifically formed crosslinking sites. We have applied photoaffinity crosslinking to identify a PTH agonist binding domain in the hPTH/PTHrP receptor (7, 8). This approach produces a covalent hormone–receptor complex, which is subjected to exhaustive enzymatic and chemical cleavage, until a restricted hormone–receptor domain conjugate is generated. Following this strategy, a hormone-binding or “contact domain” within the receptor can be directly and unambiguously identified.
The human embryonic kidney HEK-293/C-21 cell line stably expresses ≈400,000 hPTH/PTHrP receptors per cell, which are coupled equally to both the cAMP and intracellular calcium signaling pathways (9). In this study, we have used a specific, high-affinity radiolabeled photoreactive analog of PTH, 125I-[Nle8,18,Lys13(ɛ-pBz2),l-2-Nal23,Tyr34]bPTH-(1–34)NH2, to label hPTH/PTHrP receptors expressed in C-21 cells (10) with higher efficiency (≈70%) than has been achieved previously (≈5%) (11–15).
We now report the use of specific enzymatic and chemical cleavage of the ligand–receptor photoconjugate, followed by electrophoretic analysis to directly identify a heptadecapeptide domain within the hPTH/PTHrP receptor involved in the binding to the midregion of a PTH agonist ligand.
MATERIALS AND METHODS
Materials.
IodoGen was purchased from Pierce. DMEM, fetal bovine serum, trypsin, and PBS were all obtained from GIBCO/BRL. Na125I was obtained from Amersham. Lysyl endopeptidase (Lys-C) from Achromobacter lyticus (EC 3.4.21.50) was purchased from Wako Chemicals. Endoglycosidase F/N-glycosidase F (Endo F) was purchased from Boehringer Mannheim. Polylysine and all other reagents were purchased from Sigma.
Synthesis of [Nle8,18,Lys13(ɛ-p-(3-I-Bz)Bz),l-2-Nal23,Arg26,27,Tyr34]bPTH-(1–34)NH2 (all-R-K13).
(i) Peptide was synthesized on solid phase by an Applied Biosystem 430A peptide synthesizer using HOBt/NMP chemistry. The synthesis of Nα-Fmoc[Nle8,18,l-2-Nal23,Arg26,27,Tyr34]bPTH-(1–34)NH2 was carried out on p-methylbenzydrylamine·HCl resin (0.5 mmol, 0.57 mmol/g) using Nα-Boc-protected amino acid derivatives activated by HOBt/DCC before coupling. Ala1 was coupled as the Fmoc derivative. The Fmoc-peptidyl-resin was cleaved with liquid hydrogen fluoride (10% anisole at −5°C for 75 min). The crude Fmoc-peptide was purified on RP-HPLC equipped with a Vydac C18 PrePack cartridge (300 Å, 15–20 μm, 5.5 × 30 cm) using a linear gradient 0–25% B over 10 min followed by 25–70% B over 180 min at a flow rate of 70 ml/min [B = 0.1% (vol/vol) trifluoracetic acid in acetonitrile]. Fractions were analyzed on RP-HPLC equipped with a Vydac C18 column (300 Å, 5 μm, 4.6 × 150 mm) using a linear gradient of 30–80% B over 30 min at a flow rate of 1 ml/min. Fractions containing the pure product were pooled and lyophilized. (ii) Solution phase modifications: 30 mg (3.6 × 10−6 mol, peptide content 60%) of Nα-Fmoc[Nle8,18,l-2-Nal23,Arg26,27,Tyr34]bPTH-(1–34)NH2 was dissolved in 1 ml dimethylformamide in the presence of 3.68 mg of succinimidyl p-(3-iodobenzoyl)benzoate (8.5 × 10−6 mol, 2.4 eq) and 10 μl of N,N-diisopropylethylamine. Upon completion of reaction (1 h) the mixture was treated for 30 min with 300 μl of piperidine to remove the Nα-Fmoc protecting group. The reaction mixture then was diluted with 50 ml of H2O, and the solution lyophilized. Crude all-R-K13 was purified by semipreparative RP-HPLC using a Vydac C18 column (300 Å, 15 μm, 2.2 × 25 cm) using a linear gradient 0–10% B over 10 min followed by 10–50% B over 200 min. The pure product obtained after lyophilization was characterized by analytical RP-HPLC (30 min, 20–50% B, tr = 24.0 min, k′ = 9.91), amino acid analysis and fast atom bombardment mass spectrometry.
Radioiodination of 125I-[Nle8,18,Lys13(ɛ-p-(3-I-Bz)Bz),l-2-Nal23,Arg26,27,Tyr34]bPTH-(1–34)-NH2 (125I-all-R-K13).
Radioiodination was carried out following a previously described procedure (16) with the following modifications: The mixture was purified by RP-HPLC using a Vydac C18 analytical column. The separation used a linear gradient 30–40% B in A [A = 0.1% (vol/vol) trifluoracetic acid in H2O] over 30 min at a flow rate of 1.0 ml/min and was monitored in tandem by UV absorption (220 nm) and an in-line γ-radiation detector. Fractions of 0.5 ml were collected in Nunc tubes. The fractions containing the radiolabeled PTH agonist (125I-all-R-K13) were stored in the same tubes at −80°C until use.
Cell Culture.
“Parental” PTH/PTHrP receptor-lacking HEK-293 cells, HEK-293 cells stably transfected with hPTH/PTHrP receptor (clone C-21, ≈400,000 receptors per cell) (10) and human osteoblast-like Saos-2/B-10 cells (17) were maintained as previously described (7).
PTH/PTHrP Receptor Binding Assay.
HEK-293/C-21 and parental HEK-293 cells were subcultured (3 × 105 cells per well) in polylysine-coated 24-well plates and grown to near-confluency (≈2 days). Radioreceptor assays were carried out according to a previously published procedure (7, 10).
Adenylyl Cyclase Assay.
The activation of adenylyl cyclase by PTH analogs was carried out in Saos-2/B-10 cells as previously described (7).
Photoaffinity Labeling of HEK293/C-21 Cells with 125I-all-R-K13.
HEK-293/C-21 cells were harvested from ten 15-cm tissue culture plates (≈7 × 108 cells) 4 days after confluency. The cell pellet obtained by centrifugation at 800 × g for 4 min was washed twice by centrifugation at 800 × g for 4 min with 50% (vol/vol) DMEM/PBS. The washed cell pellet was resuspended in 10 ml of wash medium (DMEM supplemented with 5% fetal bovine serum) and mixed with 1 ml (0.5 mCi) of 125I-all-R-K13. Aliquots (700 μl) of cells plus radioligand were added to each well of 12-well tissue culture plates, which were preblocked with 1 ml of the wash medium for 30 min. After 30 min incubation at room temperature (rt), the culture dish (without the lid) was placed on ice at a distance of 10 cm from six 15-W, 365-nm UV lamps in a Stratalinker 2400 (Stratagene). The photoreaction was carried out for 60 min at 4°C. Cells were collected into a 15-ml plastic tube (Falcon), centrifuged at 800 × g for 5 min, and washed three times with 15 ml of cold PBS by centrifugation at 800 × g. The crosslinked, radiolabeled cell pellet was stored at −80°C for further analysis.
Membrane Protein Preparation.
The pellet of radiolabeled crosslinked cells was resuspended in 12 ml of Tris buffer (50 mM Tris·HCl, 0.01% (wt/vol) NaN3, pH 8.5). Cells were lysed by three cycles of freezing and thawing. The crude cell lysate was centrifuged at 60,000 × g for 60 min at 5°C. The membrane pellet was collected and redissolved in 12 ml of 25 mM Tris·HCl, 2.0% (vol/vol) Triton X-100, 0.01% (wt/vol) NaN3, pH 9.0 at rt and extracted for 2 h on a rotating platform. The extract was centrifuged at 3,000 × g for 1 h at 5°C, and the supernatant collected. The extracted membrane proteins were precipitated by the addition of 5 vol of cold acetone. This preparation was stored at −20°C until use.
Digestion of Membrane Proteins by Lys-C.
The acetone precipitate was centrifuged at 800 × g for 10 min, and the pellet dried at rt. The dried pellet was resuspended in 100 μl of 25 mM Tris·HCl buffer containing 0.01% (wt/vol) SDS, 0.05% (vol/vol) Triton X-100, pH 9. Exhaustive digestion with Lys-C (2 mg/ml final concentration) was carried out for 24 h at 37°C. This incubation mixture was subjected to SDS/PAGE analysis.
Endo F Treatment.
The gel-excised, partially purified radiolabeled hormone–receptor conjugate obtained from the Lys-C digestion (see the following electrophoresis section) was dissolved in 0.1% (vol/vol) Triton X-100, 0.2% (wt/vol) SDS, 5 mM EDTA, and 50 mM DTT in 100 mM sodium acetate (pH 6.0). The mixture was treated with Endo F (final concentration 0.5 unit per ml) at 37°C overnight. The resulting deglycosylated proteins were analyzed by electrophoresis on a 16.5% (wt/vol) Tricine (N-[tris(hydroxymethyl)methyl]glycine) gel, and fragments were identified by autoradiography.
Cyanogen Bromide (CNBr) Cleavage of the Crosslinked Receptor (i) or Lys-C-Derived Fragment (ii).
(i) The dried acetone-precipitated membrane protein (containing the crosslinked receptor and equal to 1 ml initial membrane extract) was resuspended in 100 μl of 70% (vol/vol) formic acid containing 1% (vol/vol) Triton-100 and 0.2% (vol/vol) SDS. CNBr (Aldrich) dissolved in acetonitrile (100 mg/ml) was added to the membrane sample at a final concentration of 30 mg/ml. The reaction mixture was flushed with N2 and incubated in a sealed vial overnight at rt in the dark. (ii) CNBr cleavage of the Lys-C-derived and glycosylated fragment eluted from SDS gels was performed under identical conditions.
Electrophoresis and Autoradiography.
The dried acetone-precipitated membrane proteins, including the conjugate 125I-all-R-K13–hPTH/PTHrP receptor, were separated by 7.5% (wt/vol) SDS/PAGE (18). The radiolabeled fragments derived from either protease or chemical digestion were separated by 16.5% (wt/vol) Tricine gel electrophoresis (19). After electrophoresis, the gels were dried and exposed to x-ray film with intensifying screens (Kodak XAR-5) overnight or for 2 days at −80°C. The apparent molecular masses of the radiolabeled bands on the gels were determined by comparison to the mobilities of high and low molecular mass standards (Bio-Rad and Amersham). After autoradiography, the radioactive fragments were excised from dried gels and rehydrated with extraction buffer (0.01% (wt/vol) SDS, 100 mM NH4HCO3, pH 7.8). The reswollen excised gels were cut into small pieces and passively eluted in extraction buffer for 2 days at rt on a rotating platform. The eluted samples were concentrated on Speed-Vac.
RESULTS
Characterization of Benzophenone-Containing Ligand, All-R-K13.
The purity of the peptide obtained exceeded 96% (as assessed by analytical RP-HPLC). Its structural integrity was confirmed by amino acid analysis and fast atom bombardment mass spectrometry.
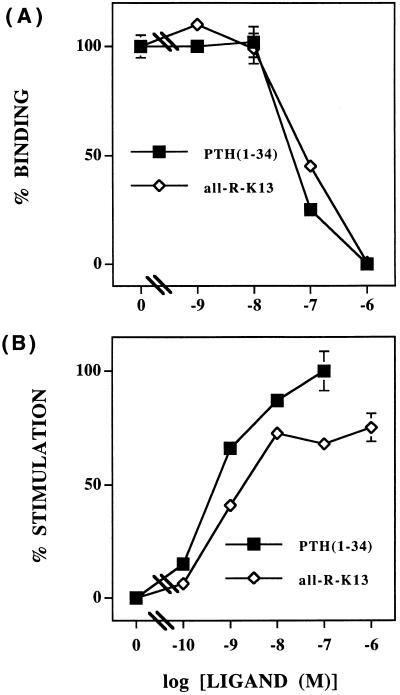
The affinity of all-R-K13 for the hPTH/PTHrP receptor stably expressed in HEK-293/C-21 cells, as assessed by binding competition with mono-125I-PTH, is approximately equal to the parent agonist, [Nle8,18,Tyr34]bPTH-(1–34)NH2 (IC50 ≈ 10−7 M) (Fig. 1A). It is a full agonist (EC50 ≈ 1 nM) for PTH-mediated activation of adenylyl cyclase in human osteoblast-like Saos-2/B-10 cells, and its potency is very similar to the parent agonist (Fig. 1B).
Figure 1.
In vitro characterization of all-R-K13 ligand. (A) Competition for 125I-PTH-(1–34)-binding in HEK-293/C-21 cells by all-R-K13 (line with open figures) and [Nle8,18,Tyr34]bPTH-(1–34)NH2 (line with filled figures). (B) Dose–response curves for the stimulation of adenylyl cyclase activity in Saos-2/B-10 cells by all-R-K13 (line with open figures) and [Nle8,18,Tyr34]bPTH-(1–34)NH2 (line with filled figures). Similar results were obtained in two additional experiments.
Photoaffinity Labeling of hPTH/PTHrP Receptor.
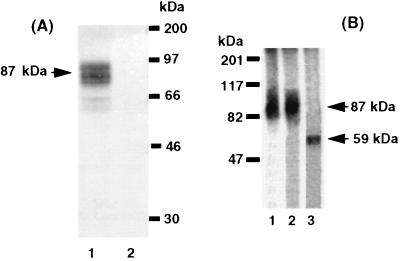
Photo-induced crosslinking of the RP-HPLC purified, benzophenone-containing radioligand, 125I-all-R-K13, to the hPTH/PTHrP receptor in HEK-293/C-21 cells yields a single, competable, broad ≈87-kDa (70- to 90-kDa) band on 7.5% (wt/vol) SDS/PAGE (Fig. 2A). The similar molecular sizes of the predominant bands obtained under reducing and nonreducing conditions suggests that the conjugated product lacks disulfide-linked chains (Fig. 2B, lanes 1 and 2). The apparent molecular mass of the band was similar to that observed for crosslinking of receptor by a structurally related photoreactive PTH analog, [Nle8,18,Lys13(ɛ-pBz2),2-l-Nal23,Tyr34]bPTH-(1–34)NH2 (K13) (8). Deglycosylation of the excised and eluted broad 87-kDa band with Endo F (20) shifted it to a narrow band of ≈59 kDa (Fig. 2B, lane 3). This molecular mass corresponds well with the molecular mass of the hPTH/PTHrP receptor, calculated from the cDNA sequence (8, 21), combined with 125I-all-R-K13.
Figure 2.
Autoradiographs of HEK-293/C-21 cells, expressing hPTH/PTHrP receptor, photolabeled by 125I-all-R-K13. (A) Crosslinking in the absence (lane 1) and presence (lane 2) of 10−5 M [Nle8,18,Tyr34]bPTH-(1–34)NH2. The arrow shows the position of the predominant ≈87-kDa crosslinked ligand–receptor conjugate. (B) SDS/PAGE in the absence (lane 1) and presence (lane 2) of 2-mercaptoethanol. Deglycosylation of the initial ligand–receptor conjugate was achieved by treatment with Endo F (lane 3). Arrows indicate the positions of the photocrosslinked receptor (≈87 kDa) and its deglycosylated form (≈59 kDa). Samples were loaded on 7.5% (wt/vol) SDS/PAGE. Molecular mass markers are also indicated.
Taken together, these data suggest that the putative radiolabeled ligand–receptor conjugate is a heteroglycosylated mixture of a single molecular entity that lacks interchain disulfide bridges.
Identification of the Ligand-Binding Domain.
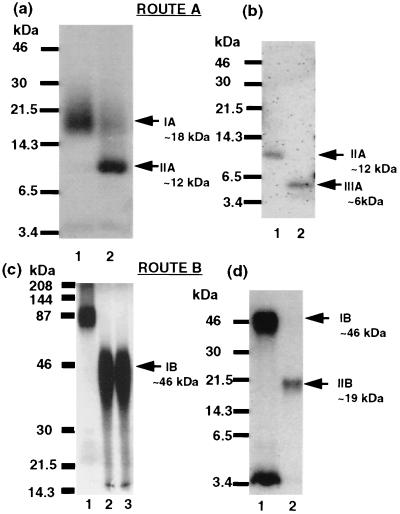
Two cleavage modalities were used to unambiguously identify a ligand-binding domain of the hPTH/PTHrP receptor. Route A consists of Lys-C digestion of the intact receptor–ligand conjugate (IA), Endo F-mediated deglycosylation (IIA), and CNBr cleavage (IIIA). For fragment IA, overnight incubation of the excised and eluted 87-kDa band (representing the 125I-all-R-K13–PTH/PTHrP receptor conjugate) with Lys-C (22) generated a broad radiolabeled band centered around ≈18 kDa (Fig. 3A, lane 1) on SDS/PAGE. For fragment IIA, deglycosylation of the excised and eluted Lys-C-generated 18-kDa band IA with Endo F produced a narrow band of ≈12 kDa (Fig. 3A, lane 2). Both the broad nature of the 18-kDa band IA and its conversion into a sharp 12-kDa band IIA upon deglycosylation suggests that the heterogeneity observed is due the presence of multiple forms of hPTH/PTHrP receptor glycosylated to different extents. For IIIA, treatment of the excised and eluted 12-kDa band IIA (Fig. 3B, lane 1) with CNBr in 70% formic acid with 1% (vol/vol) Triton and 0.2% (wt/vol) SDS converted it to a smaller radioactive fragment that migrates at ≈6 kDa (Fig. 3B, lane 2). Route B consists of CNBr cleavage of intact receptor conjugate (IB) and Endo F-mediated deglycosylation (IIB). For fragment IB, overnight incubation of the excised and eluted 87-kDa band, representing the 125I-all-R-K13–PTH/PTHrP receptor conjugate (Fig. 3C lane 1), with CNBr generated a broad band (≈35–50 kDa) centered at ≈46 kDa (Fig. 3C, lanes 2 and 3, nonreducing and reducing conditions) on SDS/PAGE. As in route A, the similar size of the predominant bands under nonreducing and reducing conditions suggests that no interchain disulfide bridges are included in the detected crosslinked fragments. For fragment IIB, treatment of the excised and eluted 46-kDa band IB (Fig. 3D, lane 1) with Endo F generated a smaller, radiolabeled narrow band of ≈19 kDa (Fig. 3D, lane 2). Route B differs from route A by changing the order of CNBr and Endo F treatments. Treatment of the eluted 125I-all-R-K13–hPTH/PTHrP receptor conjugate (≈87 kDa) with either Lys-C (≈18 kDa) in IA or with CNBr (≈46 kDa) in IB generated glycosylated bands that lack interchain disulfide bridges.
Figure 3.
Autoradiographs of enzymatic and chemical processing of the photoconjugated 125I-all-R-K13–hPTH/PTHrP receptor complex. Route A: (a) The intact radiolabeled 125I-all-R-K13–hPTH/PTHrP receptor photoconjugate was exhaustively digested by Lys-C (lane 1; IA ≈18 kDa) and analyzed by 16.5% (wt/vol) Tricine SDS/PAGE. The radiolabeled band was excised, eluted from the gel, and treated by Endo F (see Materials and Methods) (lane 2, IIA ≈12 kDa). Arrows indicates the positions of the radioligand crosslinked Lys-C-derived fragment (≈18 kDa) and its deglycosylated form (≈12 kDa). Molecular mass markers are indicated at the left. (b) Exhaustive CNBr digestion of Lys-C treated radiolabeled band. Deglycosylated Lys-C-derived band before treatment with CNBr (lane 1; IIA ≈12 kDa). The excised and eluted sample from lane 1 after exhaustive digestion by CNBr (see Materials and Methods) (lane 2; IIIA ≈6 kDa). Arrows indicates the positions of the deglycosylated radioligand crosslinked Lys-C-derived fragment (≈12 kDa) and its CNBr-generated fragment (≈6 kDa). Molecular mass markers are indicated at the left. Route B: (c) The intact radiolableled 125I-all-R-K13–hPTH/PTHrP receptor photoconjugate before (lane 1) and after an exhaustive CNBr digestion (fragment IB ≈46 kDa, position indicated by arrow on the right) resolved on a 7.5% (wt/vol) SDS/PAGE in the absence (lane 2) or presence of 2-mercaptoethanol (lane 3). (d) The 125I-all-R-K13–hPTH/PTHrP receptor photoconjugate CNBr-derived fragment before (lane 1; IB ≈46 kDa) and after exhaustive Endo F treatment (lane 2; IIB ≈19 kDa), resolved on a 16.5% (wt/vol) Tricine SDS/PAGE. The arrows indicate the position of the 46-kDa CNBr-derived fragment (IB) and its ≈19-kDa deglycosylated form (IIB). Molecular mass markers are indicated at the left.
DISCUSSION
Photoaffinity labeling has emerged as an attractive method for studying the interactions of biological macromolecules with their ligands (23–25). Within the last several years, it has become feasible to use a photocrosslinked conjugate as a starting point for mapping the “contact” domains, and even “amino acid contacts,” between a biologically active compound and its partner macromolecule (26–31).
Our approach to using photocrosslinking to identify directly a contact domain in the hPTH/PTHrP receptor responsible for hormone binding relied on three parallel efforts: (i) the design and synthesis of a bioactive PTH analog that is resistant to certain kinds of cleavages and incorporates a photoreactive moiety and a radiolabel; (ii) production of sufficiently high quantities of functional hPTH/PTHrP receptor to permit its crosslinking, exhaustive digestion, and subsequent electrophoretic analysis of the fragments generated; (iii) the devising of a scheme for a series of cleavages that identifies unambiguously a hormone-binding domain within the hPTH/PTHrP receptor.
The radiolabeled photoreactive ligand, 125I-all-R-K13, was designed specifically for this study. It incorporates a photoreactive moiety, the p-(3-I-Bz)Bz group, attached to the side chain of Lys13. The benzophenone moiety has several advantages over other photoreactive groups based on its reaction kinetics, specificity of insertion sites during photoactivation, and high crosslinking efficiency (8, 32). Position 13 was selected for incorporation of the benzophenone (7) based on a number of studies that indicated that this site, in the midregion of the molecule, is tolerant of a wide range of hydrophobic modifications (11, 16, 33–37), including substitution by a benzophenone moiety (7). Replacement of Trp23 with L-2-naphthylalanyl (Nal) is pharmacologically well tolerated and was performed to simplify the synthetic procedure and eliminate a potentially chemically labile residue from the photoaffinity ligand (7).
We also demonstrated that substitution by arginine of all the lysines in the 1–34 region of PTH (the all-R-K13 analog) could be achieved with full preservation of receptor binding affinity (EC50 ≈ 10−7 M) when tested in HEK-293/C-21 cells (data not shown). The absence of lysine residues in all-R-K13 eliminates any potential Lys-C cleavage sites from the hormone sequence. Similarly, substitution of Met8,18 by the isosteric nonnatural amino acid, Nle, is well tolerated in terms of bioactivity and makes the analog resistant to chemical cleavage by CNBr (38). The absence of both lysine and methionine in 125I-all-R-K13 permits exposure of 125I-all-R-K13 to enzymatic digestion by Lys-C and chemical cleavage by CNBr without loss of radiolabel (125I on Tyr34).
Increased quantities of receptor were available as a result of the cloning and stable expression of the hPTH/PTHrP receptor in a human cellular (HEK-293) background (8–10). These cells therefore were used for crosslinking and harvesting of hormone–receptor conjugate.
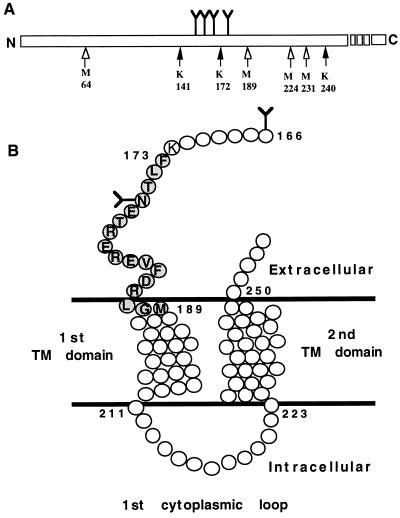
Two routes, A and B, were taken to generate a small domain within the hPTH/PTHrP receptor, which is crosslinked to the midregion of the radioiodinated agonist analog, all-R-K13. By using two different routes, it is possible to achieve a high level of confidence regarding the identity of an agonist-binding domain. Fig. 4A displays schematically all the potential Lys-C and CNBr cleavage sites within the hPTH/PTHrP receptor sequence that are glycosylated and within the molecular size range (hPTH/PTHrP receptor-[64–240]) of the CNBr-generated fragment IB (≈46 kDa). Exhaustive Lys-C-mediated cleavage of the band representing the intact photoaffinity-conjugated hormone–receptor complex produces a glycosylated fragment migrating as ≈18 kDa (IA). This band is reduced in size to 12 kDa (IIA) after Endo F treatment. This 12-kDa fragment (IIA) represents a combination of the crosslinked radioligand 125I-all-R-K13 (4616 Da) and an unspecified fragment of the hPTH/PTHrP receptor (≈7.5 kDa). Analysis of all the potential Lys-C-generated hPTH/PTHrP receptor-derived fragments included in the hPTH/PTHrP receptor sequence 64–240 predicts that only the hPTH/PTHrP receptor-[173–240] (8031 Da) fragment is of appropriate size to produce, in combination with the ligand, the observed 12-kDa band (IIA).
Figure 4.
(A) Schematic representation of all the potential CNBr (open arrows) and Lys-C (solid arrows) cleavage sites within the hPTH/PTHrP receptor sequence that are glycosylated and within the the molecular size range of the largest observed CNBr-generated fragment IB (≈46 kDa). Y represents the position of the four conserved N-linked glycosylation sites. (B) Schematic representation of a membrane-embedded portion of the hPTH/PTHrP receptor (amino-acids 166–254). The agonist-binding receptor domain Phe173–Met189 (shaded circles), identified in this report, is shown. Undetailed structures of the first and second transmembrane (TM) helical domains and the first cytoplasmic loop are included. Y represents putative N-linked glycosylation sites, and open circles represent amino acids.
This Phe173–Lys240 fragment, produced upon cleavages at Lys172 and Lys240, contains a single potential glycosylation site at Asn176. Therefore, Endo F treatment of the precursor band should shift the molecular size as observed (IA, ≈18 kDa to IIA, ≈12 kDa). In addition, cleavage of the deglycosylated conjugate fragment IIA with CNBr produces a band (IIIA) with ≈6 kDa. The radiolabeled conjugate represented by this band is composed of the 4.5-kDa radioligand and a residual ≈1.5-kDa fragment derived from the hPTH/PTHrP receptor. The candidate sequence of the receptor residing in the Endo F-treated fragment (IIA) corresponds to hPTH/PTHrP receptor-[173–240] (8031 Da). This fragment contains three methionine residues (positions 189, 224, and 231) (Fig. 4A). Upon exhaustive cleavage with CNBr, four fragments should be produced, corresponding to: Phe173–Hse189 (2113 Da), Ile190–Hse224 (4046 Da), His225–Hse231 (894 Da), and Leu232–Lys240 (1032 Da). The limited resolution of molecular mass by Tricine gels (Fig. 3B) does not permit us to make an unambiguous assignment among fragments that are within ≈1 kDa of the calculated molecular mass of the residual receptor-related sequence in band IIIA (≈1.5 kDa). Because the combined size of hPTH/PTHrP receptor-[190–224] (4046 Da) and the radiolabled ligand (4616 Da) exceeds the observed band size (IIIA, 6 kDa), we can exclude the fragment Ile190–Hse224 (4046 Da) as the crosslinked domain. Therefore, at the end of the analysis following route A, we conclude that the crosslinked domain is one of three possibilities: Phe173–Hse189, His225–Hse231, or Leu232–Lys240.
Following route B, exhaustive CNBr digestion of the initial radiolabeled ligand–receptor conjugate (≈87 kDa) yields a glycosylated band of 35–50 kDa (IB) (Fig. 3C, lanes 2 and 3, under nonreducing and reducing conditions). Endo F treatment of the broad ≈35- to 50-kDa radiolabeled band (IB) yields a narrow band (IIB) at ≈19 kDa (Fig. 3D, lane 2). The molecular size of this band (IIB) is composed of the 4.5-kDa radioligand and a residual ≈14.5-kDa unspecified fragment derived from the hPTH/PTHrP receptor. Analysis of the hPTH/PTHrP receptor sequence reveals that only one fragment corresponding to ≈14.5 kDa and containing a putative glycosylation site can be generated by CNBr treatment, hPTH/PTHrP receptor-[64–189] fragment (14,449 Da). This fragment contains all four putative glycosylation sites: Asn151, Asn161, Asn166, and Asn176 (39), which explains the large shift in molecular size after treatment with Endo F (47 kDa for IB to 19 kDa for IIB). From analysis of the results obtained from route B, we conclude that the photocrosslinking of 125I-all-R-K13 occurs within the Glu64–Hse189 domain (Fig. 4B).
Combining the results obtained from routes A and B, it is evident that only one of the three crosslinked domains potentially generated by route A, Phe173–Hse189, overlaps with the domain, Glu64–Hse189, identified by route B. This sequence corresponds to the C-terminal region of the N-terminal extracellular domain of the receptor and may include amino acid residues thought to insert into the membrane as part of the N-terminal first transmembrane domain (39, 40) (Fig. 4B).
If crosslinking to receptor with the photoreactive 125I-all-R-K13 analog were nonspecific, either multiple receptor domains or no unambiguous domain would be identified. Instead, we are able to conclude that 125I-all-R-K13 crosslinks through its midregion (position 13) to a single restricted binding domain within the hPTH/PTHrP receptor. This binding domain is the heptadecapeptide, F173LTNETREREVFDRLGM189, which contains a single potential N-glycosylation site on Asn176 (Fig. 4B). A third cleavage modality, which included deglycosylation as the last processing step, demonstrated unequivocally that the smallest crosslinked domain contains an N-linked glycosylation site and provided a direct validation for Phe173–Met189 being the contact domain (data not shown).
Our findings are consistent with a number of earlier characterizations of the PTH/PTHrP receptor. Mutagenesis studies in which the rat PTH/PTHrP receptor[171–189] domain was replaced by the corresponding sequence from the rat secretin receptor produced a dramatic loss of PTH binding affinity (4). Deletion of residues 142–181 from the rat PTH/PTHrP receptor resulted in a loss of more than 90% of PTH binding affinity (3).
The binding domain identified by this investigation, Phe173–Met189, may not be the only agonist binding domain within the receptor. Other regions of the hormone may interact with other domains of the receptor. PTH/PTHrP receptor mutagenesis studies suggest that the third extracellular loop, as well as residues 31–47 in the aminoterminal extracellular domain, are important for binding (3, 4). Interestingly, studies of receptors mutated in the aminoterminal extracellular domain reveal differing affinities for the agonist PTH-(1–34) compared with the antagonist PTH-(7–34) (41). Future photoaffinity crosslinking studies may be able to identify directly differences in the nature of agonist versus antagonist interaction with the receptor.
Whether the ligand we used (all-R-K13) interacts with the extracellular or membranous portion of the 17-amino acid receptor domain we identified cannot be determined from our studies. The boundaries of the domain we identified are set by the cleavage agents and not by the actual contact surface between hormone and receptor that lies within the domain we have delineated. Additional efforts, including microsequencing, will be necessary to identify with greater precision the actual amino acid-to-amino acid “contact point.” The delineation of a 17-amino acid “contact domain” is a major first step en route to a more detailed mapping of the hormone–receptor bimolecular interface.
Acknowledgments
This work was supported, in part, by Grant RO1-DK47940 from the National Institute of Diabetes, Digestive and Kidney Diseases to M.R.
ABBREVIATIONS
- pBz2
p-benzoyl-benzoyl
- p(3-I-Bz)Bz
p-(3-I-benzoyl)-benzoyl
- Endo-F
endoglycosidase F/N-glycosidase F
- Lys-C
lysyl endopeptidase
- Nal
naphthylalanyl
- PTH
parathyroid hormone
- PTHrP
PTH-related protein
- all-R-K13
[Nle8,18,Lys13(ɛ-p-(3-I-Bz)Bz, l-2-Nal23,Arg26,27,Tyr34)]bPTH-(1–34)NH2
- 125I-all-R-K13
125I-[Nle8,18,Lys13(ɛ-p-(3-I-Bz)Bz)
- l-2-Nal23
Arg26,27,Tyr34]bPTH-(1–34)NH2
- Tricine
N-[tris(hydroxymethyl)methyl]glycine
- rt
room temperature
References
- 1.Chorev M, Rosenblatt M. In: The Parathyroids. Bilezikian J P, Levine M A, Marcus R, editors. New York: Raven; 1994. pp. 139–156. [Google Scholar]
- 2.Chorev M, Rosenblatt M. In: Principles of Bone Biology. Bilezikian J P, Raisz L G, Rodan G A, editors. New York: Academic; 1996. pp. 305–323. [Google Scholar]
- 3.Lee C W, Gardella T J, Abou-Samra A B, Nussbaum S R, Segre G V, Potts J T, Jr, Kronenberg H M, Jüppner H. Endocrinology. 1994;135:1488–1495. doi: 10.1210/endo.135.4.7523099. [DOI] [PubMed] [Google Scholar]
- 4.Lee C W, Luck M D, Jüppner H, Potts J T, Jr, Kronenberg H M, Gardella T J. Mol Endocrinol. 1995;9:1269–1278. doi: 10.1210/mend.9.10.8544835. [DOI] [PubMed] [Google Scholar]
- 5.Gardella T J, Jüppner H, Wilson A K, Keutmann H T, Abou-Samra A-B, Segre G V, Bringhurst R, Potts J T, Jr, Nussbaum S R, Kronenberg H M. Endocrinology. 1994;135:1186–1194. doi: 10.1210/endo.135.3.8070362. [DOI] [PubMed] [Google Scholar]
- 6.Huang Z, Chen Y, Pratt S, Chen T-H, Bambino T, Shoback D M, Nissenson R A. Mol Endocrinol. 1995;9:1240–1249. doi: 10.1210/mend.9.9.7491116. [DOI] [PubMed] [Google Scholar]
- 7.Nakamoto C, Behar V, Chin K, Adams A E, Suva L J, Rosenblatt M, Chorev M. Biochemistry. 1995;34:10546–10552. doi: 10.1021/bi00033a029. [DOI] [PubMed] [Google Scholar]
- 8.Adams A E, Pines M, Nakamoto C, Behar V, Yang Q M, Bessalle R, Chorev M, Rosenblatt M, Levine M, Suva L J. Biochemistry. 1995;34:10553–10559. doi: 10.1021/bi00033a030. [DOI] [PubMed] [Google Scholar]
- 9.Pines M, Fukayama S, Costas K, Meurer E, Goldsmith P K, Xu X, Muallem S, Behar V, Chorev M, Rosenblatt M, Tashjian A H, Jr, Suva L J. Bone. 1996;18:381–389. doi: 10.1016/8756-3282(96)00008-7. [DOI] [PubMed] [Google Scholar]
- 10.Pines M, Adams A E, Stueckle S, Bessalle R, Rashti-Behar V, Chorev M, Rosenblatt M, Suva L J. Endocrinology. 1994;135:1713–1716. doi: 10.1210/endo.135.4.7925136. [DOI] [PubMed] [Google Scholar]
- 11.Coltrera M D, Potts J T, Jr, Rosenblatt M. J Biol Chem. 1981;256:10555–10559. [PubMed] [Google Scholar]
- 12.Wright B S, Tyler G A, O’Brien R, Caporale L H, Rosenblatt M. Proc Natl Acad Sci USA. 1987;84:26–30. doi: 10.1073/pnas.84.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nissenson R A, Karpf D, Bambino T, Winer J, Canga M, Nyiredy K, Arnaud C D. Biochemistry. 1987;26:1874–1878. doi: 10.1021/bi00381a013. [DOI] [PubMed] [Google Scholar]
- 14.Shigeno C, Hiraki Y, Westerberg D P, Potts J T, Jr, Segre G V. J Biol Chem. 1988;263:3864–3871. [PubMed] [Google Scholar]
- 15.Shigeno C, Hiraki Y, Westerberg D P, Potts J T, Jr, Segre G V. J Biol Chem. 1988;263:3872–3878. [PubMed] [Google Scholar]
- 16.Roubini E, Doung L T, Gibbons S W, Leu C-T, Caulfield M P, Chorev M, Rosenblatt M. Biochemistry. 1992;31:4026–4033. doi: 10.1021/bi00131a018. [DOI] [PubMed] [Google Scholar]
- 17.Rodan S B, Wesolowski G, Ianacone J, Thiede M A, Rodan G A. J Endocrinol. 1989;122:219–227. doi: 10.1677/joe.0.1220219. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 19.Schägger H, von Jagow G. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 20.Haselbeck, A. & Hösel, W. (1988) Topics in Biochemistry No. 8 (Boehringer Mannheim).
- 21.Schipani E, Karga H, Karaplis A C, Potts J T, Jr, Kronenberg H M, Segre G V, Abou-Samra A-B, Jüppner H. Endocrinology. 1993;132:2157–2165. doi: 10.1210/endo.132.5.8386612. [DOI] [PubMed] [Google Scholar]
- 22.Masaki T, Nakamura K, Isono M, Soejima M. Agric Biol Chem. 1978;42:1443–1445. [Google Scholar]
- 23.Chowdhry V, Westheimer F H. Annu Rev Biochem. 1979;48:293–325. doi: 10.1146/annurev.bi.48.070179.001453. [DOI] [PubMed] [Google Scholar]
- 24.Hazum E. Endocrinol Rev. 1983;4:352–362. doi: 10.1210/edrv-4-4-352. [DOI] [PubMed] [Google Scholar]
- 25.Kotzyba-Hilbert F, Kapfer I, Goeldner M. Angew Chem Int Ed Engl. 1995;34:1296–1312. [Google Scholar]
- 26.Miller T W, Kaiser R T. Proc Natl Acad Sci USA. 1988;85:5429–5433. doi: 10.1073/pnas.85.15.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y-M, Marnerakis M, Stimson E R, Maggio J E. J Biol Chem. 1995;270:1213–1220. doi: 10.1074/jbc.270.3.1213. [DOI] [PubMed] [Google Scholar]
- 28.Williams K P, Shoelson S E. J Biol Chem. 1993;268:5361–5364. [PubMed] [Google Scholar]
- 29.Keutmann H T, Rubin D A. Endocrinology. 1993;132:1305–1312. doi: 10.1210/endo.132.3.7679977. [DOI] [PubMed] [Google Scholar]
- 30.Boyd N D, Kage R, Dumas J J, Krause J E, Leeman S E. Proc Natl Acad Sci USA. 1996;93:433–437. doi: 10.1073/pnas.93.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanton M P, Li Y M, Stimson E R, Maggio J E, Cohen J B. Mol Pharmacol. 1994;46:1048–1055. [PubMed] [Google Scholar]
- 32.Dormán G, Prestwich G D. Biochemistry. 1994;33:5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 33.Abou-Samra A-B, Freeman M, Jüppner H, Uneno S, Segre G V. J Biol Chem. 1990;265:58–62. [PubMed] [Google Scholar]
- 34.Chorev M, Roubini E, McKee R L, Gibbons S W, Reagan J E, Goldman M E, Caulfield M P, Rosenblatt M. Peptides. 1991;12:57–62. doi: 10.1016/0196-9781(91)90167-n. [DOI] [PubMed] [Google Scholar]
- 35.Chorev M, Caulfield M P, Roubini E, McKee R L, Gibbons S W, Leu C-T, Levy J J, Rosenblatt M. Int J Pept Protein Res. 1992;40:445–455. [PubMed] [Google Scholar]
- 36.Newman W, Beall L D, Levine M A, Cone J L, Randhawa Z I, Bertolini D R. J Biol Chem. 1989;264:16359–16366. [PubMed] [Google Scholar]
- 37.Shigeno C, Hiraki Y, Keutmann H T, Sterm A M, Potts J T, Jr, Segre G V. Anal Biochem. 1989;179:268–273. doi: 10.1016/0003-2697(89)90126-7. [DOI] [PubMed] [Google Scholar]
- 38.Rosenblatt M, Goltzman D, Keutmann H T, Tregear G W, Potts J T., Jr J Biol Chem. 1976;251:159–164. [PubMed] [Google Scholar]
- 39.Jüppner H, Abou-Samra A B, Freeman M W, Kong X F, Schipani S, Richards J, Kolakowski L F, Jr, Hock J, Potts J T, Jr, Kronenberg H M, Segre G V. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 40.Abou-Samra A-B, Jüppner H, Force T, Freeman M W, Kong X F, Schipani E, Urena P, Richards J, Bonventre J V, Potts J T, Jr, Kronenberg H M, Segre G V. Proc Natl Acad Sci USA. 1992;89:2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jüppner H, Schipani E, Bringhurst F R, McClure I, Keutmann H T, Potts J T, Jr, Kronenberg H M, Abuo-Samra A B, Segre G V, Gardella T J. Endocrinology. 1994;134:879–884. doi: 10.1210/endo.134.2.8299582. [DOI] [PubMed] [Google Scholar]