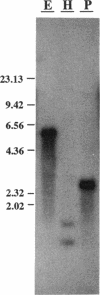
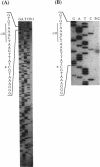
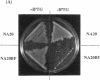
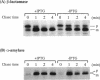
Abstract
By using a DNA fragment of Escherichia coli ffh as a probe, the Bacillus subtilis ffh gene was cloned. The complete nucleotide sequence of the cloned DNA revealed that it contained three open reading frames (ORFs). Their order in the region, given by the gene product, was suggested to be ORF1-Ffh-S16, according to their similarity to the gene products of E. coli, although ORF1 exhibited no significant identity with any other known proteins. The orf1 and ffh genes are organized into an operon. Genetic mapping of the ffh locus showed that the B. subtilis ffh gene is located near the pyr locus on the chromosome. The gene product of B. subtilis ffh shared 53.9 and 32.6% amino acid identity with E. coli Ffh and the canine 54-kDa subunit of signal recognition particle, respectively. Although there was low amino acid identity with the 54-kDa subunit of mammalian signal recognition particle, three GTP-binding motifs in the NH2-terminal half and amphipathic helical cores in the COOH-terminus were conserved. The depletion of ffh in B. subtilis led to growth arrest and drastic morphological changes. Furthermore, the translocation of beta-lactamase and alpha-amylase under the depleted condition was also defective.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaya Y., Nakano A., Ito K., Mori M. Isolation of a yeast gene, SRH1, that encodes a homologue of the 54K subunit of mammalian signal recognition particle. J Biochem. 1990 Mar;107(3):457–463. doi: 10.1093/oxfordjournals.jbchem.a123067. [DOI] [PubMed] [Google Scholar]
- Bernstein H. D., Poritz M. A., Strub K., Hoben P. J., Brenner S., Walter P. Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature. 1989 Aug 10;340(6233):482–486. doi: 10.1038/340482a0. [DOI] [PubMed] [Google Scholar]
- Byström A. S., Hjalmarsson K. J., Wikström P. M., Björk G. R. The nucleotide sequence of an Escherichia coli operon containing genes for the tRNA(m1G)methyltransferase, the ribosomal proteins S16 and L19 and a 21-K polypeptide. EMBO J. 1983;2(6):899–905. doi: 10.1002/j.1460-2075.1983.tb01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T., Gilmore R. The signal recognition particle receptor mediates the GTP-dependent displacement of SRP from the signal sequence of the nascent polypeptide. Cell. 1989 May 19;57(4):599–610. doi: 10.1016/0092-8674(89)90129-3. [DOI] [PubMed] [Google Scholar]
- Connolly T., Rapiejko P. J., Gilmore R. Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science. 1991 May 24;252(5009):1171–1173. doi: 10.1126/science.252.5009.1171. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R. Mapping of the genes for Bacillus subtilis ribosomal proteins S6 and S16: comparison of the chromosomal distribution of ribosomal protein genes in this bacterium with the distribution in Escherichia coli. Mol Gen Genet. 1983;192(3):386–390. doi: 10.1007/BF00392179. [DOI] [PubMed] [Google Scholar]
- Dever T. E., Glynias M. J., Merrick W. C. GTP-binding domain: three consensus sequence elements with distinct spacing. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1814–1818. doi: 10.1073/pnas.84.7.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Gill D. R., Hatfull G. F., Salmond G. P. A new cell division operon in Escherichia coli. Mol Gen Genet. 1986 Oct;205(1):134–145. doi: 10.1007/BF02428043. [DOI] [PubMed] [Google Scholar]
- Hann B. C., Poritz M. A., Walter P. Saccharomyces cerevisiae and Schizosaccharomyces pombe contain a homologue to the 54-kD subunit of the signal recognition particle that in S. cerevisiae is essential for growth. J Cell Biol. 1989 Dec;109(6 Pt 2):3223–3230. doi: 10.1083/jcb.109.6.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner D. J. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 1990;185:223–228. doi: 10.1016/0076-6879(90)85022-g. [DOI] [PubMed] [Google Scholar]
- High S., Dobberstein B. The signal sequence interacts with the methionine-rich domain of the 54-kD protein of signal recognition particle. J Cell Biol. 1991 Apr;113(2):229–233. doi: 10.1083/jcb.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K., Otaka E., Osawa S. Purification and characterization of 30S ribosomal proteins from Bacillus subtilis: correlation to Escherichia coli 30S proteins. Mol Gen Genet. 1982;185(2):239–244. doi: 10.1007/BF00330792. [DOI] [PubMed] [Google Scholar]
- Ishiwa H., Tsuchida N. New shuttle vectors for Escherichia coli and Bacillus subtilis. I. Construction and characterization of plasmid pHY460 with twelve unique cloning sites. Gene. 1984 Dec;32(1-2):129–134. doi: 10.1016/0378-1119(84)90040-4. [DOI] [PubMed] [Google Scholar]
- Krieg U. C., Walter P., Johnson A. E. Photocrosslinking of the signal sequence of nascent preprolactin to the 54-kilodalton polypeptide of the signal recognition particle. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8604–8608. doi: 10.1073/pnas.83.22.8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T. V., Wiedmann M., Girshovich A. S., Bochkareva E. S., Bielka H., Rapoport T. A. The signal sequence of nascent preprolactin interacts with the 54K polypeptide of the signal recognition particle. Nature. 1986 Apr 17;320(6063):634–636. doi: 10.1038/320634a0. [DOI] [PubMed] [Google Scholar]
- Lauffer L., Garcia P. D., Harkins R. N., Coussens L., Ullrich A., Walter P. Topology of signal recognition particle receptor in endoplasmic reticulum membrane. 1985 Nov 28-Dec 4Nature. 318(6044):334–338. doi: 10.1038/318334a0. [DOI] [PubMed] [Google Scholar]
- Luirink J., High S., Wood H., Giner A., Tollervey D., Dobberstein B. Signal-sequence recognition by an Escherichia coli ribonucleoprotein complex. Nature. 1992 Oct 22;359(6397):741–743. doi: 10.1038/359741a0. [DOI] [PubMed] [Google Scholar]
- Lütcke H., High S., Römisch K., Ashford A. J., Dobberstein B. The methionine-rich domain of the 54 kDa subunit of signal recognition particle is sufficient for the interaction with signal sequences. EMBO J. 1992 Apr;11(4):1543–1551. doi: 10.1002/j.1460-2075.1992.tb05199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Imai Y., Nakamura A., Yamane K. Small cytoplasmic RNA of Bacillus subtilis: functional relationship with human signal recognition particle 7S RNA and Escherichia coli 4.5S RNA. J Bacteriol. 1992 Apr;174(7):2185–2192. doi: 10.1128/jb.174.7.2185-2192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Nakamura A., Takamatsu H., Yoshikawa H., Yamane K. Cloning and characterization of a Bacillus subtilis gene homologous to E. coli secY. J Biochem. 1990 Apr;107(4):603–607. doi: 10.1093/oxfordjournals.jbchem.a123093. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Takamatsu H., Akiyama Y., Ito K., Yamane K. Complementation of the protein transport defect of an Escherichia coli secY mutant (secY24) by Bacillus subtilis secY homologue. FEBS Lett. 1990 Oct 29;273(1-2):75–78. doi: 10.1016/0014-5793(90)81054-r. [DOI] [PubMed] [Google Scholar]
- Ohmura K., Nakamura K., Yamazaki H., Shiroza T., Yamane K., Jigami Y., Tanaka H., Yoda K., Yamasaki M., Tamura G. Length and structural effect of signal peptides derived from Bacillus subtilis alpha-amylase on secretion of Escherichia coli beta-lactamase in B. subtilis cells. Nucleic Acids Res. 1984 Jul 11;12(13):5307–5319. doi: 10.1093/nar/12.13.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. J., Silhavy T. J. The E. coli ffh gene is necessary for viability and efficient protein export. Nature. 1992 Oct 22;359(6397):744–746. doi: 10.1038/359744a0. [DOI] [PubMed] [Google Scholar]
- Poritz M. A., Strub K., Walter P. Human SRP RNA and E. coli 4.5S RNA contain a highly homologous structural domain. Cell. 1988 Oct 7;55(1):4–6. doi: 10.1016/0092-8674(88)90003-7. [DOI] [PubMed] [Google Scholar]
- Römisch K., Webb J., Herz J., Prehn S., Frank R., Vingron M., Dobberstein B. Homology of 54K protein of signal-recognition particle, docking protein and two E. coli proteins with putative GTP-binding domains. Nature. 1989 Aug 10;340(6233):478–482. doi: 10.1038/340478a0. [DOI] [PubMed] [Google Scholar]
- Römisch K., Webb J., Lingelbach K., Gausepohl H., Dobberstein B. The 54-kD protein of signal recognition particle contains a methionine-rich RNA binding domain. J Cell Biol. 1990 Nov;111(5 Pt 1):1793–1802. doi: 10.1083/jcb.111.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y., Takamatsu H., Nakamura K., Yamane K. Sequencing reveals similarity of the wild-type div+ gene of Bacillus subtilis to the Escherichia coli secA gene. Gene. 1991 Feb 1;98(1):101–105. doi: 10.1016/0378-1119(91)90110-w. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V., Walter P. Binding sites of the 19-kDa and 68/72-kDa signal recognition particle (SRP) proteins on SRP RNA as determined in protein-RNA "footprinting". Proc Natl Acad Sci U S A. 1988 Mar;85(6):1801–1805. doi: 10.1073/pnas.85.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V., Walter P. Each of the activities of signal recognition particle (SRP) is contained within a distinct domain: analysis of biochemical mutants of SRP. Cell. 1988 Jan 15;52(1):39–49. doi: 10.1016/0092-8674(88)90529-6. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck J. C., Vogel D. W., Ulbrich N., Erdmann V. A. The Bacillus subtilis scRNA is related to the 4.5S RNA from Escherichia coli. Nucleic Acids Res. 1988 Mar 25;16(6):2719–2719. doi: 10.1093/nar/16.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers W. C. A simple method for extraction of RNA from E. coli utilizing diethyl pyrocarbonate. Anal Biochem. 1970 Feb;33(2):459–463. doi: 10.1016/0003-2697(70)90316-7. [DOI] [PubMed] [Google Scholar]
- Takamatsu H., Fuma S., Nakamura K., Sadaie Y., Shinkai A., Matsuyama S., Mizushima S., Yamane K. In vivo and in vitro characterization of the secA gene product of Bacillus subtilis. J Bacteriol. 1992 Jul;174(13):4308–4316. doi: 10.1128/jb.174.13.4308-4316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M., Kurzchalia T. V., Bielka H., Rapoport T. A. Direct probing of the interaction between the signal sequence of nascent preprolactin and the signal recognition particle by specific cross-linking. J Cell Biol. 1987 Feb;104(2):201–208. doi: 10.1083/jcb.104.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. A., Bott K. F. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J Bacteriol. 1968 Apr;95(4):1439–1449. doi: 10.1128/jb.95.4.1439-1449.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K., Hirata Y., Furusato T., Yamazaki H., Nakayama A. Changes in the properties and molecular weights of Bacillus subtilis M-type and N-type alpha-amylases resulting from a spontaneous deletion. J Biochem. 1984 Dec;96(6):1849–1858. doi: 10.1093/oxfordjournals.jbchem.a135019. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zopf D., Bernstein H. D., Johnson A. E., Walter P. The methionine-rich domain of the 54 kd protein subunit of the signal recognition particle contains an RNA binding site and can be crosslinked to a signal sequence. EMBO J. 1990 Dec;9(13):4511–4517. doi: 10.1002/j.1460-2075.1990.tb07902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]