Abstract
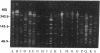
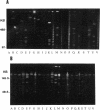
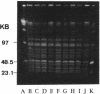
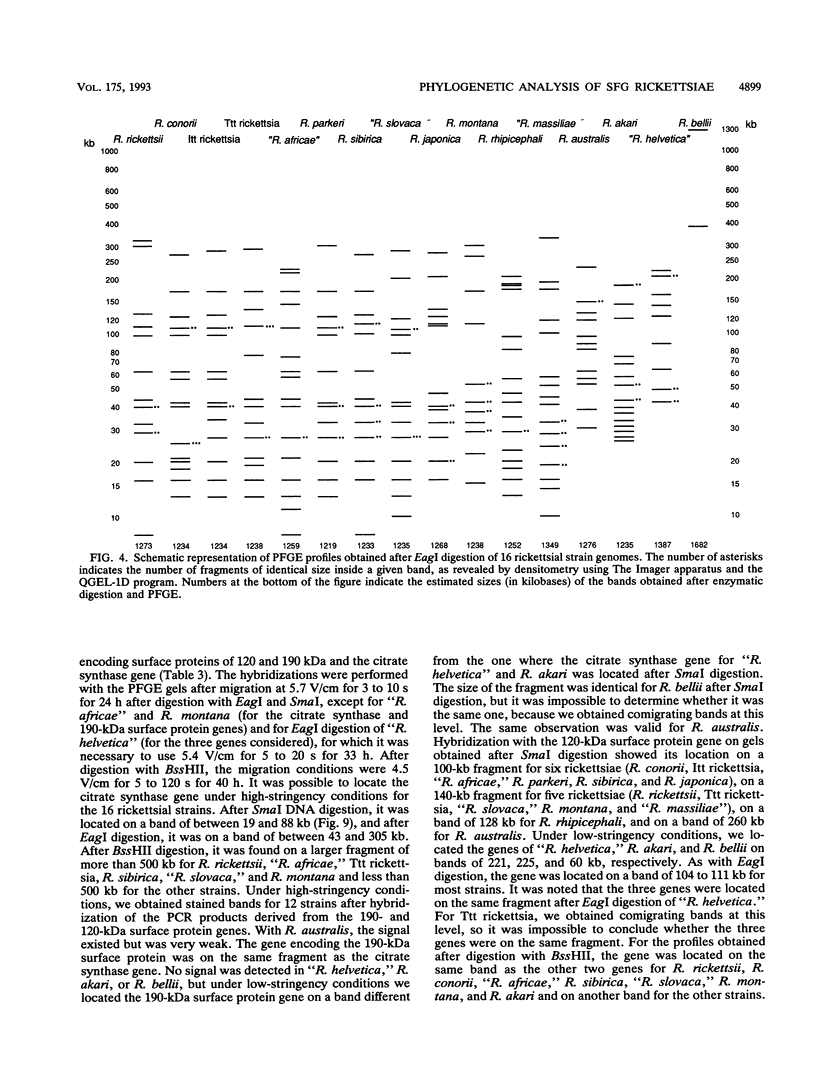
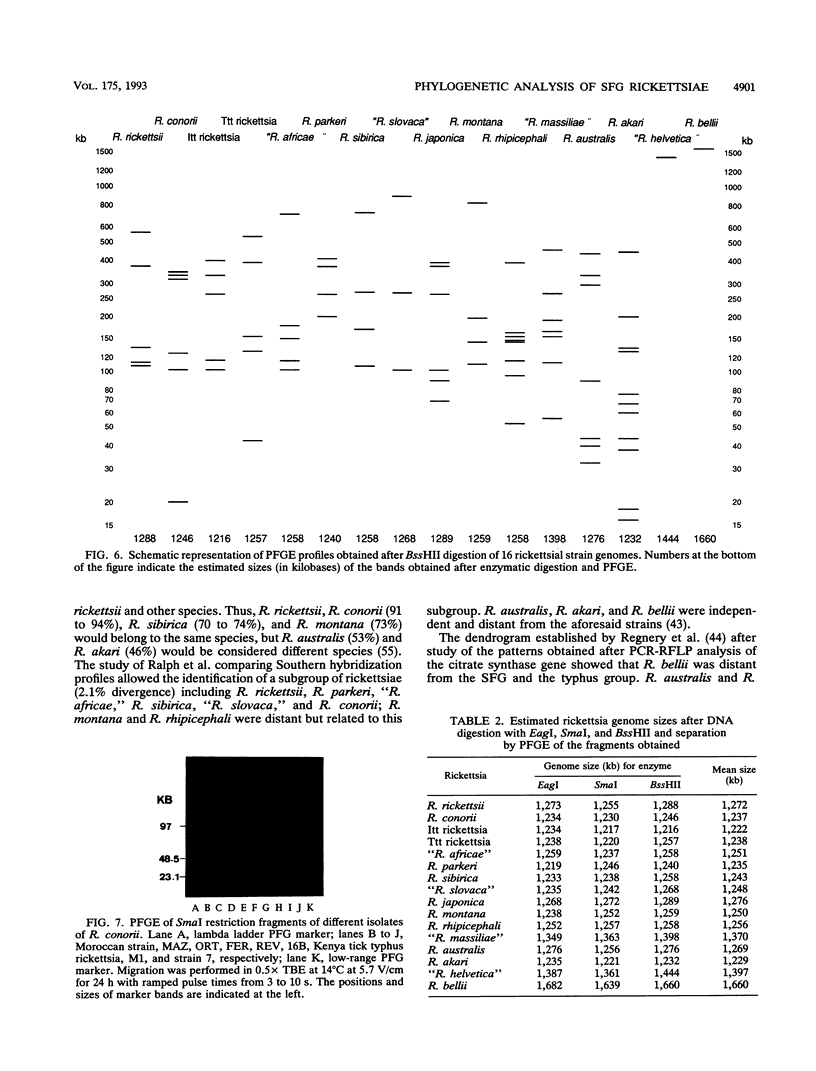
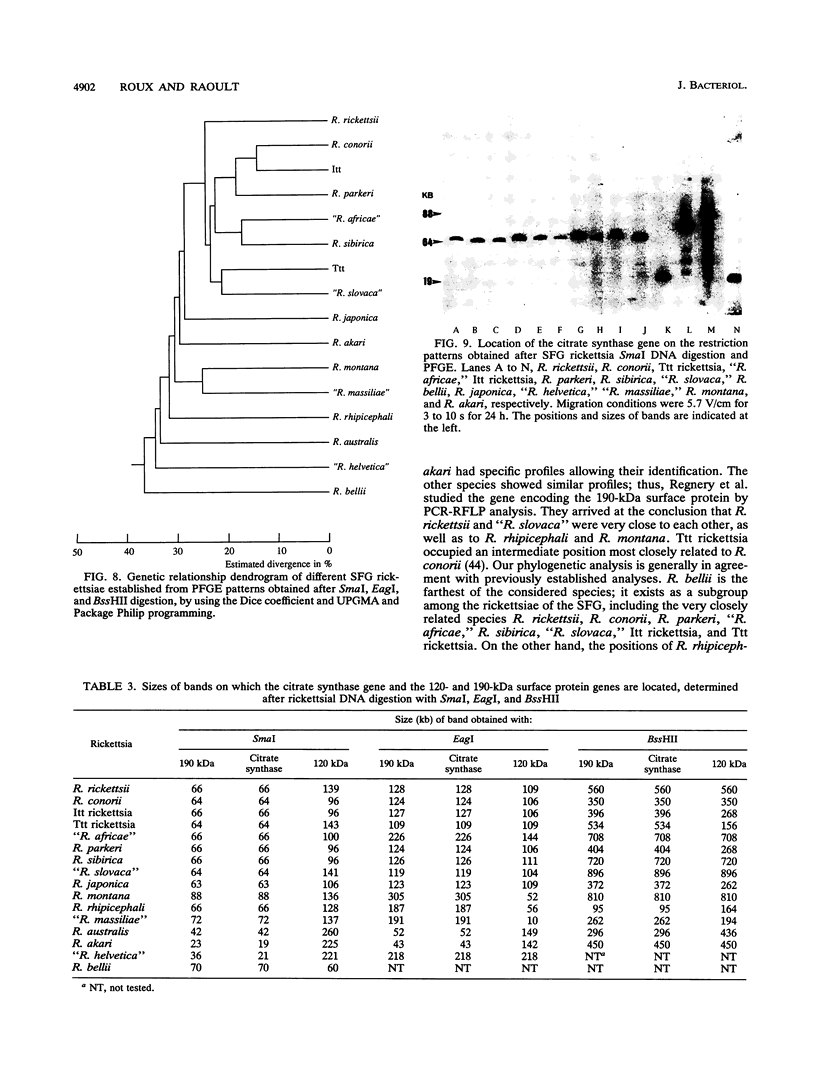
Using pulsed-field gel electrophoresis, we studied the chromosomes of spotted fever group rickettsiae. We digested the DNA of 16 species currently known to belong to this group with SmaI, EagI, and BssHII. The genome size of 13 rickettsiae was between 1,200 and 1,300 kb. "Rickettsia massiliae" and "R. helvetica" genome sizes were 1,370 and 1,397 kb, respectively, and that of R. bellii was 1,660 kb. It was possible to obtain distinctive patterns for each species, but in R. conorii, 10 isolates exhibited the same profiles, showing that pulsed-field gel electrophoresis is a good interspecies identification tool. We achieved a phylogenetic analysis of these bacteria by using the Dice coefficient and UPGMA and Package Philip programming. We established a dendrogram of the genetic relationships between the different species showing the existence of a cluster in the spotted fever group rickettsiae including R. conorii, R. rickettsii, R. parkeri, R. sibirica, "R. africae," "R. slovaca," Thai tick typhus rickettsia, and Israeli tick typhus rickettsia. We located three genes previously cloned and sequenced (genes encoding the R. rickettsii surface proteins of 120 and 190 kDa and the R. prowazekii citrate synthase gene), using Southern hybridization. The genes encoding citrate synthase and the surface protein of 190 kDa were usually located on the same band, and it is hypothesized that they are relatively close on the chromosome.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allardet-Servent A., Bourg G., Ramuz M., Pages M., Bellis M., Roizes G. DNA polymorphism in strains of the genus Brucella. J Bacteriol. 1988 Oct;170(10):4603–4607. doi: 10.1128/jb.170.10.4603-4607.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. E., McDonald G. A., Jones D. C., Regnery R. L. A protective protein antigen of Rickettsia rickettsii has tandemly repeated, near-identical sequences. Infect Immun. 1990 Sep;58(9):2760–2769. doi: 10.1128/iai.58.9.2760-2769.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B. E., Regnery R. L., Carlone G. M., Tzianabos T., McDade J. E., Fu Z. Y., Bellini W. J. Sequence analysis of the 17-kilodalton-antigen gene from Rickettsia rickettsii. J Bacteriol. 1987 Jun;169(6):2385–2390. doi: 10.1128/jb.169.6.2385-2390.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL E. J., KOHLS G. M., STOENNER H. G., LACKMAN D. B. NONPATHOGENIC RICKETTSIAS RELATED TO THE SPOTTED FEVER GROUP ISOLATED FROM TICKS, DERMACENTOR VARIABILIS AND DERMACENTOR ANDERSONI FROM EASTERN MONTANA. J Immunol. 1963 May;90:770–781. [PubMed] [Google Scholar]
- BELL E. J., PICKENS E. G. A toxic substance associated with the rickettsias of the spotted fever group. J Immunol. 1953 May;70(5):461–472. [PubMed] [Google Scholar]
- BELL E. J., STOENNER H. G. Immunologic relationships among the spotted fever group of rickettsias determined by toxin neutralization tests in mice with convalescent animal serums. J Immunol. 1960 Feb;84:171–182. [PubMed] [Google Scholar]
- Beati L., Finidori J. P., Gilot B., Raoult D. Comparison of serologic typing, sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein analysis, and genetic restriction fragment length polymorphism analysis for identification of rickettsiae: characterization of two new rickettsial strains. J Clin Microbiol. 1992 Aug;30(8):1922–1930. doi: 10.1128/jcm.30.8.1922-1930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkelund S., Stephens R. S. Construction of physical and genetic maps of Chlamydia trachomatis serovar L2 by pulsed-field gel electrophoresis. J Bacteriol. 1992 May;174(9):2742–2747. doi: 10.1128/jb.174.9.2742-2747.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birren B. W., Lai E., Clark S. M., Hood L., Simon M. I. Optimized conditions for pulsed field gel electrophoretic separations of DNA. Nucleic Acids Res. 1988 Aug 11;16(15):7563–7582. doi: 10.1093/nar/16.15.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch R., Buchrieser C., Rocourt J. Subtyping of Listeria monocytogenes serovar 4b by use of low-frequency-cleavage restriction endonucleases and pulsed-field gel electrophoresis. Res Microbiol. 1991 Jul-Aug;142(6):667–675. doi: 10.1016/0923-2508(91)90080-t. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W., Aeschlimann A., Peter O., Hayes S. F., Philip R. N. Ixodes ricinus: vector of a hitherto undescribed spotted fever group agent in Switzerland. Acta Trop. 1979 Dec;36(4):357–367. [PubMed] [Google Scholar]
- Bygraves J. A., Maiden M. C. Analysis of the clonal relationships between strains of Neisseria meningitidis by pulsed field gel electrophoresis. J Gen Microbiol. 1992 Mar;138(3):523–531. doi: 10.1099/00221287-138-3-523. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- GIMENEZ D. F. STAINING RICKETTSIAE IN YOLK-SAC CULTURES. Stain Technol. 1964 May;39:135–140. doi: 10.3109/10520296409061219. [DOI] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Gilmore R. D., Jr, Hackstadt T. DNA polymorphism in the conserved 190 kDa antigen gene repeat region among spotted fever group Rickettsiae. Biochim Biophys Acta. 1991 Jul 26;1097(1):77–80. doi: 10.1016/0925-4439(91)90027-7. [DOI] [PubMed] [Google Scholar]
- Gilmore R. D., Jr, Joste N., McDonald G. A. Cloning, expression and sequence analysis of the gene encoding the 120 kD surface-exposed protein of Rickettsia rickettsii. Mol Microbiol. 1989 Nov;3(11):1579–1586. doi: 10.1111/j.1365-2958.1989.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Goldwasser R. A., Steiman Y., Klingberg W., Swartz T. A., Klingberg M. A. The isolation of strains of rickettsiae of the spotted fever group in Israel and their differentiation from other members of the group by immunofluorescence methods. Scand J Infect Dis. 1974;6(1):53–62. doi: 10.3109/inf.1974.6.issue-1.10. [DOI] [PubMed] [Google Scholar]
- Heinzen R., Stiegler G. L., Whiting L. L., Schmitt S. A., Mallavia L. P., Frazier M. E. Use of pulsed field gel electrophoresis to differentiate Coxiella burnetii strains. Ann N Y Acad Sci. 1990;590:504–513. doi: 10.1111/j.1749-6632.1990.tb42260.x. [DOI] [PubMed] [Google Scholar]
- Kauc L., Mitchell M., Goodgal S. H. Size and physical map of the chromosome of Haemophilus influenzae. J Bacteriol. 1989 May;171(5):2474–2479. doi: 10.1128/jb.171.5.2474-2479.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallavia L. P. Genetics of rickettsiae. Eur J Epidemiol. 1991 May;7(3):213–221. doi: 10.1007/BF00145669. [DOI] [PubMed] [Google Scholar]
- Marrero M., Raoult D. Centrifugation-shell vial technique for rapid detection of Mediterranean spotted fever rickettsia in blood culture. Am J Trop Med Hyg. 1989 Feb;40(2):197–199. doi: 10.4269/ajtmh.1989.40.197. [DOI] [PubMed] [Google Scholar]
- Philip C. B., Hoogstraal H., Reiss-Gutfreund R., Clifford C. M. Evidence of rickettsial disease agents in ticks from Ethiopian cattle. Bull World Health Organ. 1966;35(2):127–131. [PMC free article] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Burgdorfer W., Gerloff R. K., Hughes L. E., Bell E. J. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [PubMed] [Google Scholar]
- Public Health Weekly Reports for NOVEMBER 8, 1946. Public Health Rep. 1946 Nov 8;61(45):1605–1640. [PMC free article] [PubMed] [Google Scholar]
- Ralph D., Pretzman C., Daugherty N., Poetter K. Genetic relationships among the members of the family rickettsiaceae as shown by DNA restriction fragment polymorphism analysis. Ann N Y Acad Sci. 1990;590:541–552. doi: 10.1111/j.1749-6632.1990.tb42264.x. [DOI] [PubMed] [Google Scholar]
- Regnery R. L., Spruill C. L., Plikaytis B. D. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol. 1991 Mar;173(5):1576–1589. doi: 10.1128/jb.173.5.1576-1589.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. G., Wisseman C. L., Jr Tick-borne rickettsiae of the spotted fever group in West Pakistan. II. Serological classification of isolates from West Pakistan and Thailand: evidence for two new species. Am J Epidemiol. 1973 Jan;97(1):55–64. doi: 10.1093/oxfordjournals.aje.a121485. [DOI] [PubMed] [Google Scholar]
- Roux V., Drancourt M., Raoult D. Determination of genome sizes of Rickettsia spp. within the spotted fever group, using pulsed-field gel electrophoresis. J Bacteriol. 1992 Nov;174(22):7455–7457. doi: 10.1128/jb.174.22.7455-7457.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römling U., Tümmler B. The impact of two-dimensional pulsed-field gel electrophoresis techniques for the consistent and complete mapping of bacterial genomes: refined physical map of Pseudomonas aeruginosa PAO. Nucleic Acids Res. 1991 Jun 25;19(12):3199–3206. doi: 10.1093/nar/19.12.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Econome J. G., Schutt A., Klco S., Cantor C. R. A physical map of the Escherichia coli K12 genome. Science. 1987 Jun 12;236(4807):1448–1453. doi: 10.1126/science.3296194. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Uchida T., Uchiyama T., Kumano K., Walker D. H. Rickettsia japonica sp. nov., the etiological agent of spotted fever group rickettsiosis in Japan. Int J Syst Bacteriol. 1992 Apr;42(2):303–305. doi: 10.1099/00207713-42-2-303. [DOI] [PubMed] [Google Scholar]
- Walker D. H. Rocky Mountain spotted fever: a disease in need of microbiological concern. Clin Microbiol Rev. 1989 Jul;2(3):227–240. doi: 10.1128/cmr.2.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil M. D., McClelland M. Enzymatic cleavage of a bacterial genome at a 10-base-pair recognition site. Proc Natl Acad Sci U S A. 1989 Jan;86(1):51–55. doi: 10.1073/pnas.86.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisburg W. G., Dobson M. E., Samuel J. E., Dasch G. A., Mallavia L. P., Baca O., Mandelco L., Sechrest J. E., Weiss E., Woese C. R. Phylogenetic diversity of the Rickettsiae. J Bacteriol. 1989 Aug;171(8):4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson L. R., Plano G. V., Winkler H. H., Krause D. C., Wood D. O. Nucleotide sequence of the Rickettsia prowazekii ATP/ADP translocase-encoding gene. Gene. 1989 Aug 15;80(2):269–278. doi: 10.1016/0378-1119(89)90291-6. [DOI] [PubMed] [Google Scholar]
- Wood D. O., Williamson L. R., Winkler H. H., Krause D. C. Nucleotide sequence of the Rickettsia prowazekii citrate synthase gene. J Bacteriol. 1987 Aug;169(8):3564–3572. doi: 10.1128/jb.169.8.3564-3572.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]