Abstract
Bcl-2, Bcl-XL, and Bax are members of the Bcl-2 family that play key roles in the regulation of apoptosis. These proteins are believed to be membrane bound and their ability to undergo both homodimerization and heterodimerization has been proposed to regulate apoptosis. Herein we report that in murine thymocytes, Bcl-2 is exclusively membrane-bound, whereas Bax is present predominantly in the cytosol and Bcl-XL is present in both soluble and membrane-bound forms. Induction of apoptosis in murine thymocytes by dexamethasone or γ-irradiation shifts the subcellular locations of Bax and Bcl-XL from soluble to membrane-bound forms. A similar shift in the localization of Bax from the cytosol to membranes was observed in HL-60 leukemia cells upon induction of apoptosis by staurosporine. Inhibition of apoptosis with cycloheximide inhibits the movement of Bax and Bcl-XL in thymocytes from the cytosol into membranes induced by dexamethasone treatment. These movements may represent an important step in the pathway by which members of this family regulate apoptosis.
Keywords: programmed cell death, Bcl-2, Bax, diphtheria toxin
Bcl-2, Bax, and Bcl-XL are members of the expanding Bcl-2 family that play key roles in the regulation of apoptosis. Overexpression of Bcl-2 and Bcl-XL enhances cell survival by suppressing apoptosis in a number of cells subject to a wide range of apoptosis-inducing stimuli, including growth factor withdrawal, radiation, and chemotherapeutic agents (for reviews, see refs. 1–4). Unlike Bcl-2 and Bcl-XL, overexpression of Bax accelerates cell death upon growth factor withdrawal (5). These proteins have been proposed to regulate apoptosis through both homo- and heterodimerization (5–7).
Bcl-2, Bax, and Bcl-XL contain a hydrophobic segment at their C-terminal ends (5, 8, 9) that is believed to serve as a membrane anchor. Subcellularly, Bcl-2 has been localized to the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes (10, 11). Similarly, Bcl-XL has been recently localized to outer membranes of mitochondria (12). Although the subcellular localization of Bax has not been documented, Bax has been suggested to colocalize within the same subcellular compartments as Bcl-2 due to the presence of its putative C-terminal transmembrane segment and its ability to dimerize with Bcl-2 (5).
In this article, we report unique subcellular localization of Bax and Bcl-XL in normal murine thymocytes. In addition, we describe the rapid intracellular redistribution of these proteins upon induction of apoptosis.
MATERIALS AND METHODS
Subcellular Localization of Bax, Bcl-2, and Bcl-XL.
Murine thymocytes were dispersed from murine thymus in the presence of Iscove’s medium (Biofluids, Rockville, MD). The cells were centrifuged, washed in Iscove’s medium, and resuspended at a cell density of 5 × 107 cells per ml in lysis buffer [10 mM Hepes, pH 7.4/38 mM NaCl/phenylmethylsulfonyl fluoride (25 μg/ml)/leupeptin (1 μg/ml)/aprotinin (1 μg/ml)]. The cell suspension was homogenized in a Dounce homogenizer and centrifuged at 900 × g to pellet the nuclei in a Sorvall SA-600 rotor. The postnuclear supernatant was then centrifuged at 130,000 × g in a Beckman Ti 80 rotor to pellet the membranes. Both the crude membranes and the nuclear pellet were resuspended in a volume of the lysis buffer equal to that of the supernatant. The relative levels of Bax, Bcl-2, and Bcl-XL in the cytosolic, crude membrane, and nuclear fractions were analyzed by Western blotting with the appropriate corresponding antibodies (Y.-T.H. and R.J.Y., unpublished results).
For the study of dexamethasone- and γ-irradiation-induced apoptosis, murine thymocytes were resuspended in Iscove’s medium containing 10% fetal bovine serum at a cell density of 7.5 × 107 cells per ml. Thymocytes were subjected to treatment with 2 μM dexamethasone or 8 Gy of γ-radiation from a 137Cs source. At 2 and 4 h after treatment, cells were collected, centrifuged, and washed once in Iscove’s medium. Subcellular fractionation was then carried out as described earlier. For the cycloheximide inhibition study, cycloheximide at 10 μg/ml was added either without or in conjunction with the addition of 2 μM dexamethasone. The cells were collected and subcellularly fractionated as described above.
For staurosporine treatment of HL-60 cells, the cells were treated either in the absence or presence of 1 μM staurosporine for 2–4 h. The cells were then harvested, washed one time in Iscove’s medium, lyzed, and homogenized in the lysis buffer (1 × 107 cells per ml). Subcellular fractionation was carried out as described above.
SDS/PAGE and Western Blot Analysis.
SDS/PAGE was carried out on 12% polyacrylamide gels by the method of Laemmli (13). Twenty-three microliters of each protein sample from subcellular fractionation was analyzed by SDS/PAGE. The gels were either stained with Coomassie blue or electroblotted onto Immobilon membranes (14). Immunoblot analysis with either anti-human (h) Bcl-2 124 (Dako), anti-hBax 2D2, anti-mouse (m) Bax 5B7, anti-universal (u) Bcl-XL 2H12, anti-mBcl-2 10C4 monoclonal antibodies was carried out as described (Y.-T.H. and R.J.Y., unpublished results).
RESULTS
Subcellular Localization of Bax, Bcl-2, and Bcl-XL.
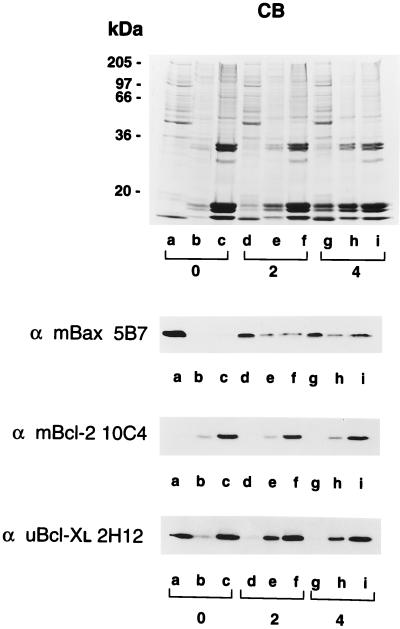
Soluble, crude membrane, and nuclear fractions of murine thymocytes were prepared by hypotonic lysis, Dounce homogenization, and differential centrifugation. The protein samples were analyzed by Coomassie blue-stained SDS/PAGE (Fig. 1 Top) and Western blot analyses with anti-mBax 5B7, anti-mBcl-2 10C4, and anti-uBcl-XL 2H12 monoclonal antibodies for the detection of murine Bax, Bcl-2, and Bcl-XL, respectively. Bax was found predominantly as a soluble protein in murine thymocytes (Fig. 1 Top, Western blot, lanes a–c). Bcl-2, on the other hand, was not detected in the soluble fraction and was present in both high speed membrane pellet and nuclear fractions (Fig. 1 Middle, Western blot, lanes a–c). Finally, Bcl-XL was detected in all three compartments of thymocytes (Fig. 1 Bottom, Western blot, lanes a–c).
Figure 1.
Differential localization of Bax and Bcl-XL and their association with membranes in response to dexamethasone-induced apoptosis. Apoptosis was induced in murine thymocytes by the addition of dexamethasone. Untreated cells (lanes a–c) and cells incubated 2 h (lanes d–f) and 4 h (lanes g–i) with dexamethasone were hypotonically lyzed, homogenized with a Dounce homogenizer, and separated into soluble (lanes a, d, and g), high-speed membrane pellet (lanes b, e, and h), and nuclear fractions (lanes c, f, and i) by differential centrifugation. The protein samples were analyzed by SDS/PAGE on gels stained with Coomassie blue (CB) or by Western blotting with anti-mBax 5B7, anti-mBcl-2 10C4, and anti-uBcl-XL 2H12 monoclonal antibodies.
Membrane Association of Bax and Bcl-XL upon Apoptosis.
The unexpected and distinct subcellular localization of Bax and Bcl-XL prompted us to investigate their subcellular distribution during apoptosis. When murine thymocytes were treated with 2 μM dexamethasone for 2 and 4 h, the overall protein level of either Bax, Bcl-2, or Bcl-XL did not change (data not shown). To examine the subcellular distribution of these proteins, thymocytes were hypotonically lyzed and homogenized with a Dounce homogenizer after 2 and 4 h of incubation with dexamethasone. The soluble protein, crude membrane, and nuclear fractions from untreated and dexamethasone-treated thymocytes were analyzed by SDS/PAGE and Western blotting. Coomassie blue staining of the SDS/polyacrylamide gel indicated that low molecular weight histones were released from nuclei into soluble and membrane fractions upon apoptosis induction (Fig. 1, Coomassie blue-stained gel, lanes d, e, g, and h). Western blot analyses with anti-mBax 5B7 antibody showed that at 2 and 4 h after dexamethasone treatment, a significant shift in the subcellular localization of Bax from the cytosol to either crude membrane or nuclear compartments was observed (Fig. 1 Top, Western blot, lanes e, f, h, and i). On the other hand, Bcl-2, remained compartmentalized to either crude membrane or nuclear fractions upon induction of apoptosis, as determined by anti-mBcl-2 10C4 labeling (Fig. 1 Middle, Western blot). Finally, Western blot analysis with anti-uBcl-XL 2H12 indicated that Bcl-XL, which initially displayed both soluble and membrane-associated forms (Fig. 1 Bottom, Western blot, lanes a–c), became predominantly membrane-associated within 2 h after induction of apoptosis (Fig. 1 Bottom, Western blot, lanes e, f, h, and i). Thus, both Bax and Bcl-XL migrated from the cytosol to the membrane fraction during apoptosis induction by dexamethasone.
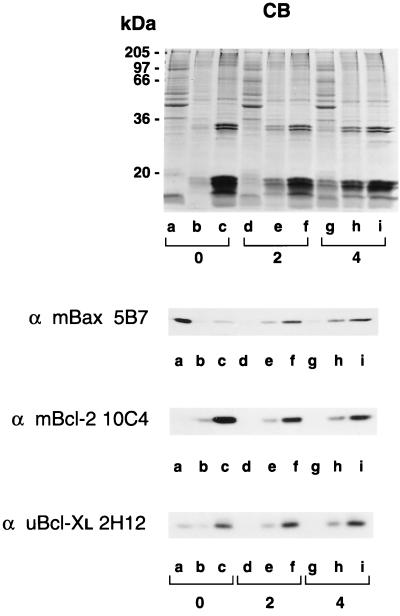
In addition to dexamethasone treatment, we examined γ-irradiation-induced apoptosis in murine thymocytes. At 2 and 4 h after irradiation, thymocytes were hypotonically lyzed, homogenized with a Dounce homogenizer, and subcellularly fractionated. The protein samples were again analyzed on both Coomassie blue-stained SDS/polyacrylamide gels and Western blots (Fig. 2). Upon induction of apoptosis, consistent with the dexamethasone results, Bax was found to shift its localization from cytosol to membranes (Fig. 2 Top, Western blot). The membrane localization of Bcl-2 was found to be unchanged in response to apoptosis (Fig. 2 Middle, Western blot). Finally, Bcl-XL shifted from its preapoptotic dual localization in soluble and membrane-bound forms to become exclusively membrane bound upon induction of apoptosis (Fig. 2 Bottom, Western blot).
Figure 2.
Association of Bax and Bcl-XL with membranes in response to γ-irradiation-induced apoptosis. Apoptosis was induced in murine thymocytes by γ-irradiation. Untreated cells (lanes a–c) and cells collected 2 h (lanes d–f) and 4 h (lanes g–i) after irradiation were hypotonically lyzed, homogenized in a Dounce homogenizer, and separated into soluble (lanes a, d, and g), high-speed membrane pellet (lanes b, e, and h), and nuclear fractions (lanes c, f, and i) by differential centrifugation. The protein samples were analyzed by SDS/PAGE on gels stained with Coomassie blue (CB) or by Western blotting with anti-mBax 5B7, anti-mBcl-2 10C4, and anti-uBcl-XL 2H12 monoclonal antibodies.
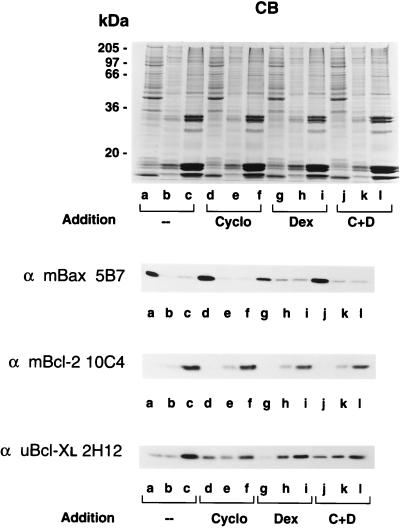
Inhibition of Apoptosis Blocks Dexamethasone-Induced Shift of Bax and Bcl-XL.
To determine whether inhibition of apoptosis blocks the apoptosis-related shift in the localization of Bax and Bcl-XL, murine thymocytes were incubated in growth medium for 2 h either in the absence or presence of cycloheximide (a known inhibitor of thymocyte apoptosis), dexamethasone, or a combination of cycloheximide and dexamethasone. The resulting samples from subcellular fractionation were analyzed by both SDS/PAGE and Western blotting. The relative percentages of soluble and membrane-bound Bax and Bcl-XL were determined by densitometric analysis. As shown in Fig. 3, the protein banding patterns of an individual set of samples stained with Coomassie blue were very similar. Western blot analysis with anti-mBax 5B7 antibody indicated that Bax was predominantly in its soluble form when incubated alone (80% soluble vs. 20% membrane bound; Fig. 3 Top, Western blot, lanes a–c) or in the presence of cycloheximide (80% soluble vs. 20% membrane bound; Fig. 3 Top, Western blot, lanes d–f). Treatment of thymocytes with dexamethasone shifted a significant portion of cytosolic Bax into membranes (55% soluble vs. 45% membrane bound; Fig. 3 Top, Western blot, lanes g–i) and this shift was partially blocked by the presence of cycloheximide (70% soluble vs 30% membrane bound; Fig. 3 Top, Western blot, lanes j–l). The subcellular localization of Bcl-2 was not affected by either dexamethasone or cycloheximide treatment (Fig. 3 Middle, Western blot). Bcl-XL has both cytosolic and membrane-bound forms in untreated (15% soluble vs. 85% membrane bound; Fig. 3 Bottom, Western blot, lanes a–c) and cycloheximide-treated cells (30% soluble vs. 70% membrane bound; Fig. 3 Bottom, Western blot, lanes d–f). Bcl-XL became exclusively membrane bound upon treatment with dexamethasone (3% soluble vs 97% membrane bound; Fig. 3 Bottom, Western blot, lanes g–i). This membrane localization was blocked by the presence of cycloheximide (25% soluble vs. 75% membrane bound; Fig. 3 Bottom, Western blot, lanes j–l). This suggests that the inhibition of dexamethasone-induced apoptosis blocks the shift of cytosolic Bcl-XL and, to some extent, Bax into membranes.
Figure 3.
Inhibition of dexamethasone-induced apoptosis by cycloheximide blocks migration of Bax and Bcl-XL into membranes. Murine thymocytes were incubated in the culture medium in the absence or in the presence of cycloheximide, dexamethasone, or a combination of cycloheximide and dexamethasone for 2 h. The cells were collected, hypotonically lyzed, homogenized in a Dounce homogenizer, and fractionated into soluble (lanes a, d, g, and j), high-speed membrane pellet (lanes b, e, h, and k), and nuclear fractions (lanes c, f, i, and l) by differential centrifugation. The samples were analyzed by SDS/PAGE on gels stained with Coomassie blue (CB) or by Western blotting with anti-mBax 5B7, anti-mBcl-2 10C4, and anti-uBcl-XL 2H12 monoclonal antibodies.
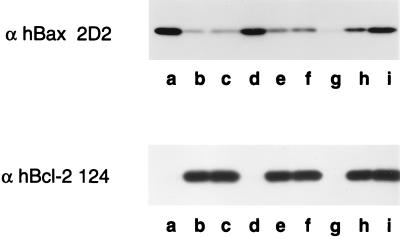
Staurosporine Treatment of HL-60 Cells Promotes Membrane Association of Bax.
To determine whether the apoptosis-related shift in the localization of Bax is a common phenomenon, we examined its redistribution in another established system of apoptosis that uses staurosporine to induce apoptosis of HL-60 promyelocytic leukemia cells. These cells lack detectable Bcl-XL but express Bax and Bcl-2. In these cells, most of the Bax is in the cytosol although a detectable amount of it appears membrane bound (Fig. 4 Upper, Western blot, lanes a–c). Treatment of the cells with staurosporine shifts the localization of Bax from preferentially cytosolic to membrane-bound forms within 4 h (Fig. 4 Upper, Western blot, lanes g–i). Like thymocyte and splenocyte studies, the membrane localization of Bcl-2 is unaffected by staurosporine (Fig. 4 Lower, Western blot). This suggests that the movement of Bax into membranes during apoptosis is independent of the presence of Bcl-XL. In staurosporine-treated HL-60 cells, there appeared to be proteolysis of proteins. In addition to membrane insertion, some of the soluble Bax may be degraded during apoptosis.
Figure 4.
Staurosporine treatment of HL-60 cells promotes membrane association of Bax. HL-60 cells were treated either in absence (lanes a–c) or in the presence of 1 μM staurosporine for 2 h (lanes d–f) and 4 h (lanes g–i). The cells were collected, hypotonically lyzed, homogenized with a Dounce homogenizer, and fractionated into soluble (lanes a, d, and g), high-speed membrane pellet (lanes b, e, and h), and nuclear fractions (lanes c, f, and i) by differential centrifugation. The samples were analyzed by Western blotting with anti-hBax 2D2 and anti-hBcl-2 124 monoclonal antibodies.
Unlike thymocytes where apoptosis is blocked by protein synthesis inhibition, HL-60 can execute the apoptotic pathway even in the presence of cycloheximide (15). We found that the addition of cycloheximide did not block staurosporine-induced Bax insertion into membranes (data not shown). This suggests that the membrane-bound Bax molecules previously resided in the cytosol and were not newly synthesized molecules translated during apoptosis.
DISCUSSION
Bcl-2, Bax, and Bcl-XL are believed to be a group of membrane proteins that regulate apoptosis through in vivo dimerizations. Herein we demonstrate that Bax, rather than being an integral membrane protein like Bcl-2, is soluble, on the basis of its presence in the supernatant of healthy murine thymocytes after high-speed ultracentrifugation. Similarly, a significant portion of Bcl-XL is found to be cytosolic. Hydropathy analyses of the sequences of Bax and Bcl-XL predict a transmembrane domain at their C-terminal regions (5, 9). In Bcl-2, this C-terminal domain is thought to mediate membrane attachment to various organelles. Removal of this hydrophobic tail from Bcl-2 caused a range of results from either complete abrogation of the survival function of this protein to no effect at all (16–18). On the other hand, truncation of the C-terminal hydrophobic tails of Bax or Bcl-XL appeared to have no effect on their roles in promoting either cell death or survival (J. Lee, Y.-T.H., R.J.Y., and K. A. Wood, unpublished data; ref. 19). The observation that Bax and a significant fraction of Bcl-XL are cytosolic suggests that their C-terminal hydrophobic domains may be hidden either within the interior of the proteins or may be involved in binding other cytosolic factors. Interestingly, glycophorin, a red blood cell protein, is regarded as a soluble protein, in spite of the fact that it has a predicted transmembrane segment (20).
Induction of apoptosis in murine thymocytes by either dexamethasone or γ-irradiation resulted in a shift in the localization of Bax and Bcl-XL from soluble to membrane-bound forms. The movement of these proteins into membranes during dexamethasone-induced apoptosis is blocked by cycloheximide. This suggests that the site along the apoptotic pathway requiring protein synthesis is upstream of the event leading to Bax and Bcl-XL insertion into membranes. In a different cellular system, the HL-60 promyelocytic leukemia, we observed Bax movement into membranes during staurosporine-induced apoptosis. This suggests that the movement of these proteins into membranes during apoptosis may be extensive among various cell types, with Bax promoting cell death and Bcl-XL promoting cell survival.
The mechanisms by which Bcl-2, Bcl-XL, and Bax regulate apoptosis are still elusive. Recently, crystal and solution structures of Bcl-XL indicated that the α-helical structure in Bcl-XL resembles that of the membrane translocation domain of diphtheria toxin (19). The fact that a significant amount of Bax became membrane-bound suggests that it too may have α-helical structure similar to that of Bcl-XL and diphtheria toxin. In diphtheria toxin, a shift in protein deposition from a soluble to a membrane-bound form is triggered by a change in pH (21–23). Our studies demonstrate a similar activity among members of the Bcl-2 family, where approximately half of the Bax and virtually all of Bcl-XL become membrane-associated during the induction of apoptosis. However, soluble Bax and Bcl-XL did not insert into membranes when exposed in vitro to low pH (pH 5.2; unpublished data). We therefore see no evidence that the acidification of the intracellular milieu that occurs during apoptosis (24, 25) is responsible for Bcl-XL or Bax insertion. The membrane-bound form of Bax resists alkali extraction, suggesting strong membrane association (unpublished data). This redistribution of Bax and Bcl-XL into membranes is illustrated in Fig. 5. In thymocytes undergoing apoptosis, the trigger for Bax and Bcl-XL insertion into membranes is not yet known. Phosphorylation of Bcl-2 at serine residues has been implicated in the inactivation of its function during apoptosis (26, 27). It would be interesting to determine whether Bax and Bcl-XL can be phosphorylated and whether such modification may affect their insertion into membranes. Furthermore, it would be critical to address whether the sites of action of Bax and Bcl-XL are in the cytosol or in membranes and whether the predicted transmembrane segments of Bax and Bcl-XL play a role in their insertions into membranes during apoptosis.
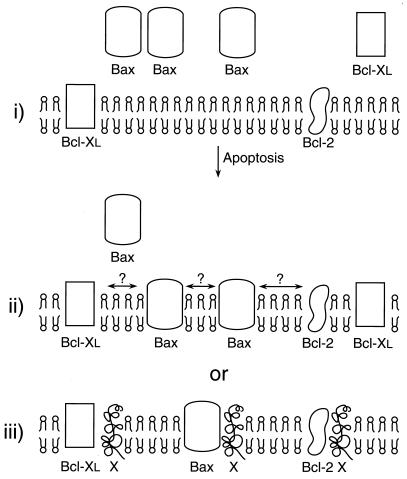
Figure 5.
Schematic representation of membrane insertion of Bax and Bcl-XL. In murine thymocytes, Bax is cytosolic, Bcl-2 is predominantly membrane bound, and Bcl-XL is present in both soluble and membrane-bound forms (i). Upon induction of apoptosis by dexamethasone or γ-irradiation, virtually all the cytosolic Bcl-XL and a significant amount of Bax becomes membrane-bound. It is not known whether Bax, in its membrane-associated state, undergoes hetero- or homodimerizations (ii). Alternatively, it is possible that Bax, Bcl-2, and Bcl-XL in their membrane-bound states may be competing for the binding of a yet unknown protein(s) in membranes to promote either cell death or survival (iii).
Acknowledgments
We thank Shu-Chan Hsu (Stanford University) for technical assistance and critical review of the manuscript, Steven Rafferty for helpful suggestions on the manuscript, and Katherine Wood and Pat Johnson for technical discussion. K.G.W. is a Howard Hughes Medical Institute–National Institutes of Health research scholar.
ABBREVIATIONS
- h
human
- m
mouse
- u
cross-reactive with human, mouse, and rat
References
- 1.Boise L H, Gottschalk A R, Quintáns J, Thompson C B. Curr Top Microbiol Immunol. 1995;200:107–121. doi: 10.1007/978-3-642-79437-7_8. [DOI] [PubMed] [Google Scholar]
- 2.Reed J C. Hematol Oncol Clin North Am. 1995;9:451–473. [PubMed] [Google Scholar]
- 3.White E. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Yang E, Korsmeyer S J. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 5.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 6.Sedlak T W, Oltvai Z N, Yang E, Wang K, Boise L H, Thompson C B, Korsmeyer S J. Proc Natl Acad Sci USA. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang E, Zha J, Jockel J, Boise L H, Thompson C B, Korsmeyer S J. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 8.Tsujimoto Y, Ikegaki N, Croce C M. Oncogene. 1987;2:3–7. [PubMed] [Google Scholar]
- 9.Boise L H, González-García M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nuñez G, Thompson C B. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 10.Hockenbery D M, Nuñez G, Milliman C, Schreiber R D, Korsmeyer S J. Nature (London) 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 11.Krajewski S, Tanaka S, Takayama S, Schibler M J, Fenton W, Reed J C. Cancer Res. 1993;53:4701–4714. [PubMed] [Google Scholar]
- 12.González-García M, Pérez-Ballestero R, Ding L, Duan L, Boise L H, Thompson C B, Nuñez G. Development (Cambridge, UK) 1994;120:3033–3042. doi: 10.1242/dev.120.10.3033. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Molday L L, Cook N J, Kaupp U B, Molday R S. J Biol Chem. 1990;265:18690–18695. [PubMed] [Google Scholar]
- 15.Martin S J, Lennon S V, Bonham A M, Cotter T G. J Immunol. 1990;145:1859–1867. [PubMed] [Google Scholar]
- 16.Alnemri E S, Robertson N M, Fernandes T F, Croce C M, Litwack G. Proc Natl Acad Sci USA. 1992;89:7295–7299. doi: 10.1073/pnas.89.16.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hockenbery D M, Oltvai Z N, Yin X-M, Milliman C L, Korsmeyer S J. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 18.Borner C, Martinou I, Mattmann C, Irmler M, Schaerer E, Martinou J-C, Tschopp J. J Cell Biol. 1994;126:1059–1068. doi: 10.1083/jcb.126.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muchmore S W, Sattler M, Liang H, Meadows R P, Harlan J E, Yoon H S, Nettesheim D, Chang B S, Thompson C B, Wong S-L, Ng S-C, Fesik S W. Nature (London) 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 20.Anderson R A, Marchesi V T. Nature (London) 1985;318:295–298. doi: 10.1038/318295a0. [DOI] [PubMed] [Google Scholar]
- 21.Kagan B L, Finkelstein A, Colombini M. Proc Natl Acad Sci USA. 1981;78:4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu V W, Holmes R K. J Biol Chem. 1984;259:12226–12233. [PubMed] [Google Scholar]
- 23.Zhan H, Oh K J, Shin Y-K, Hubbell W L, Collier R J. Biochemistry. 1995;34:4856–4863. doi: 10.1021/bi00014a043. [DOI] [PubMed] [Google Scholar]
- 24.Rebollo A, Gómez J, Aragón A M, Lastres P, Silva A, Pérez-Sala D. Exp Cell Res. 1995;218:581–585. doi: 10.1006/excr.1995.1195. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Eastman A. J Biol Chem. 1995;270:3203–3211. doi: 10.1074/jbc.270.7.3203. [DOI] [PubMed] [Google Scholar]
- 26.May W S, Tyler P G, Ito T, Armstrong D K, Qatsha K A, Davidson N E. J Biol Chem. 1994;269:26865–26870. [PubMed] [Google Scholar]
- 27.Haldar S, Jena N, Croce C M. Proc Natl Acad Sci USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]