Abstract
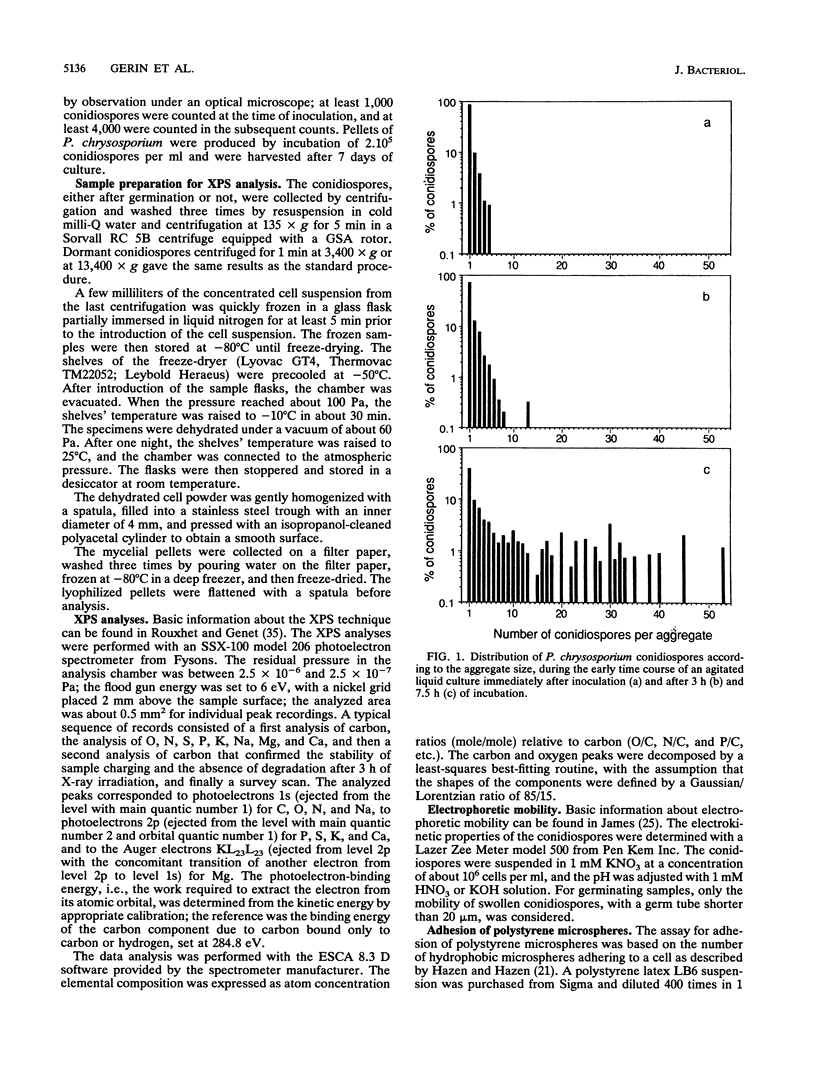
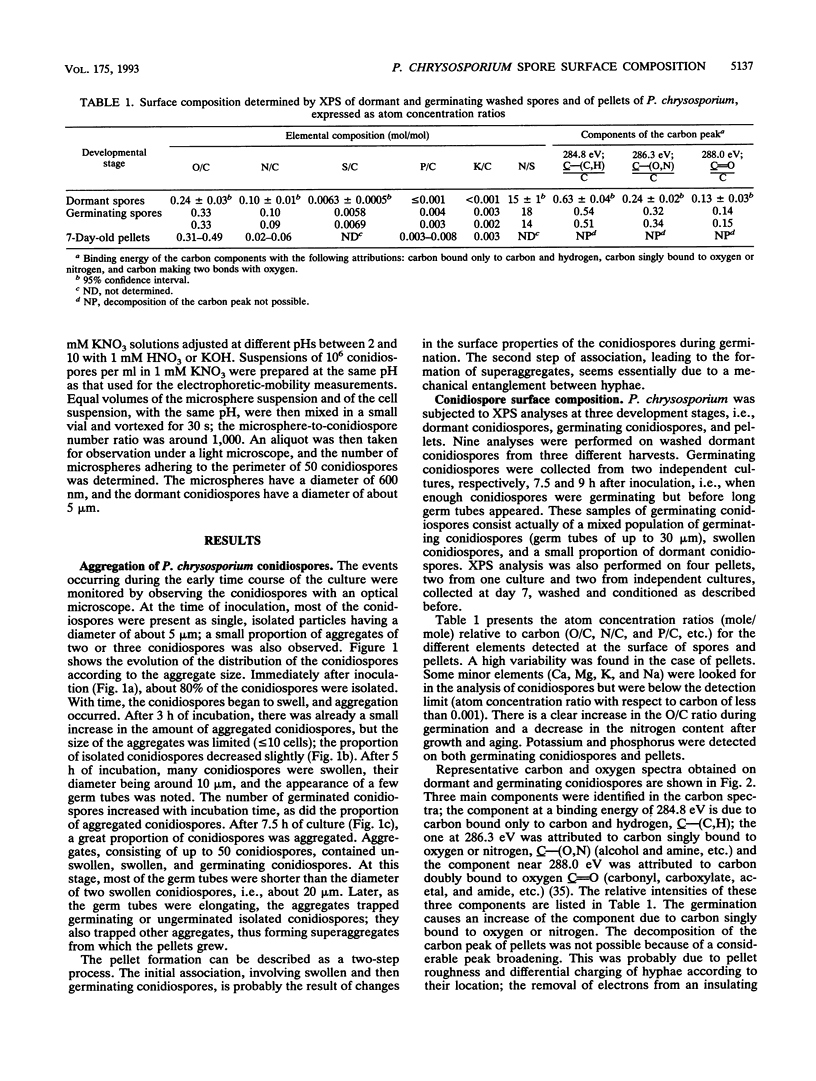
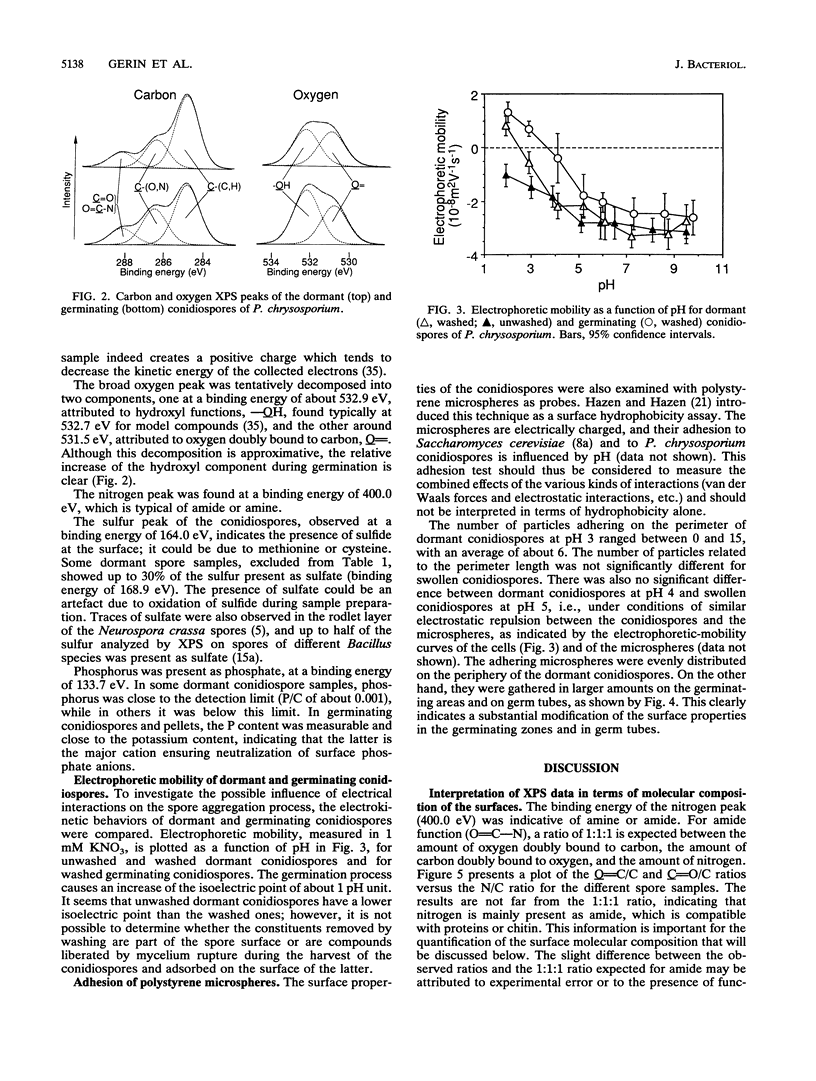
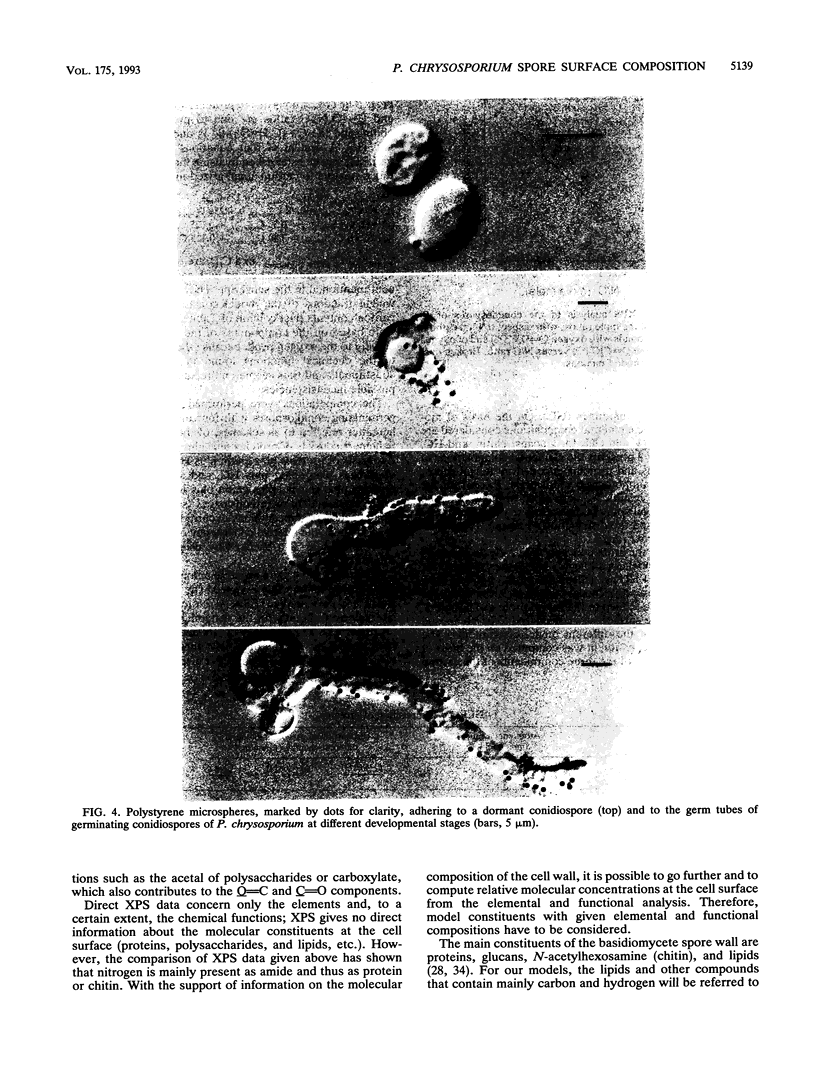
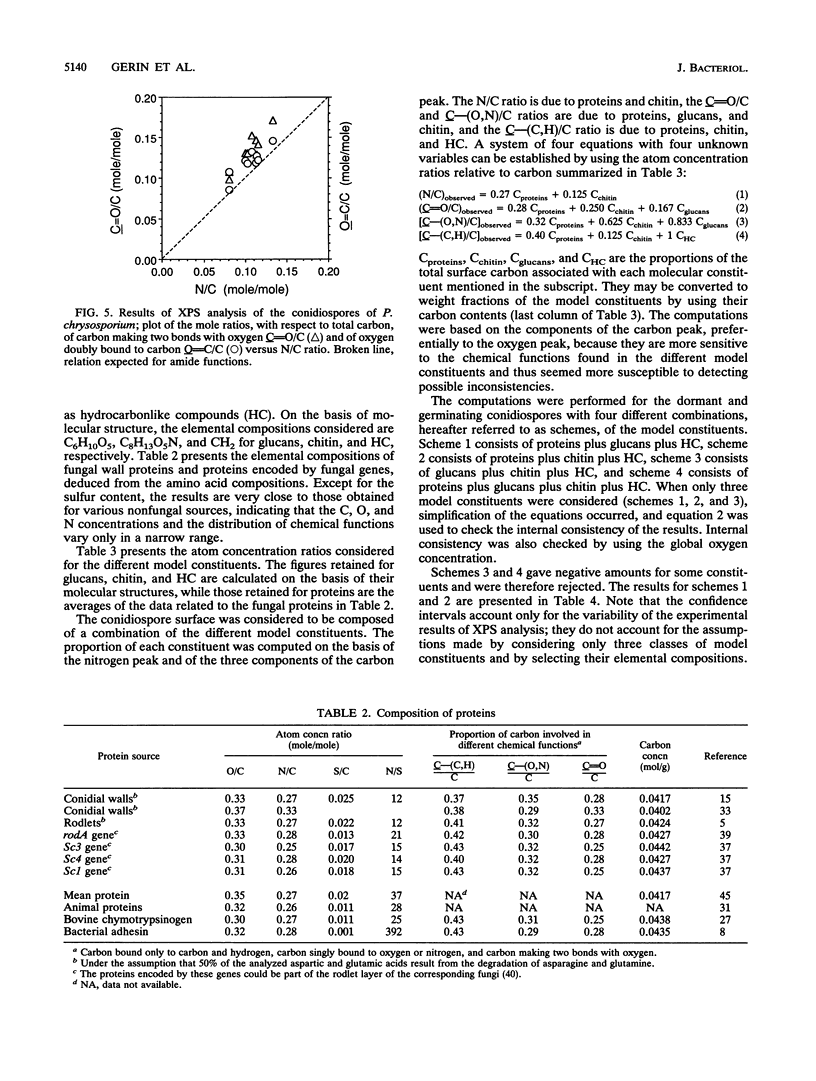
The conidiospores of the white rot basidiomycete Phanerochaete chrysosporium tend to aggregate during swelling and germination in agitated liquid medium; as time passes, the initial aggregates tend to associate together and to capture conidiospores that remain isolated. The surface chemical compositions of the conidiospores and of developed hyphae were analyzed by X-ray photoelectron spectroscopy. The data were interpreted by modelling the surface in terms of proteins, polysaccharides and hydrocarbonlike compounds. The surface molecular composition of the dormant conidiospores was estimated to be about 45% proteins, 20% carbohydrates, and 35% hydrocarbonlike compounds. There was an increase in the polysaccharide content during germination. Later, when the hyphae were developed, the polysaccharide content became still higher, and the protein content dropped. The initial step of aggregation is attributed to polysaccharide bridging; its occurrence cannot be explained by a change of the overall hydrophobicity or electrical properties of the conidiospores.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beever R. E., Dempsey G. P. Function of rodlets on the surface of fungal spores. Nature. 1978 Apr 13;272(5654):608–610. doi: 10.1038/272608a0. [DOI] [PubMed] [Google Scholar]
- Beever R. E., Redgwell R. J., Dempsey G. P. Purification and chemical characterization of the rodlet layer of Neurospora crassa conidia. J Bacteriol. 1979 Dec;140(3):1063–1070. doi: 10.1128/jb.140.3.1063-1070.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mot R., Proost P., Van Damme J., Vanderleyden J. Homology of the root adhesin of Pseudomonas fluorescens OE 28.3 with porin F of P. aeruginosa and P. syringae. Mol Gen Genet. 1992 Feb;231(3):489–493. doi: 10.1007/BF00292721. [DOI] [PubMed] [Google Scholar]
- Fisher D. J., Richmond D. V. The electrophoretic properties and some surface components of penicillium conidia. J Gen Microbiol. 1970 Dec;64(2):205–214. doi: 10.1099/00221287-64-2-205. [DOI] [PubMed] [Google Scholar]
- Hamer J. E., Howard R. J., Chumley F. G., Valent B. A mechanism for surface attachment in spores of a plant pathogenic fungus. Science. 1988 Jan 15;239(4837):288–290. doi: 10.1126/science.239.4837.288. [DOI] [PubMed] [Google Scholar]
- Mozes N., Léonard A. J., Rouxhet P. G. On the relations between the elemental surface composition of yeasts and bacteria and their charge and hydrophobicity. Biochim Biophys Acta. 1988 Nov 22;945(2):324–334. doi: 10.1016/0005-2736(88)90495-6. [DOI] [PubMed] [Google Scholar]
- Rizza V., Kornfeld J. M. Components of conidial and hyphal walls of Penicillium chrysogenum. J Gen Microbiol. 1969 Nov;58(3):307–315. doi: 10.1099/00221287-58-3-307. [DOI] [PubMed] [Google Scholar]
- Ruel K., Joseleau J. P. Involvement of an Extracellular Glucan Sheath during Degradation of Populus Wood by Phanerochaete chrysosporium. Appl Environ Microbiol. 1991 Feb;57(2):374–384. doi: 10.1128/aem.57.2.374-384.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sietsma J. H., Wessels J. G. Chemical analysis of the hyphal wall of Schizophyllum commune. Biochim Biophys Acta. 1977 Jan 24;496(1):225–239. doi: 10.1016/0304-4165(77)90131-3. [DOI] [PubMed] [Google Scholar]
- Stringer M. A., Dean R. A., Sewall T. C., Timberlake W. E. Rodletless, a new Aspergillus developmental mutant induced by directed gene inactivation. Genes Dev. 1991 Jul;5(7):1161–1171. doi: 10.1101/gad.5.7.1161. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Zehnder A. J. Influence of interfaces on microbial activity. Microbiol Rev. 1990 Mar;54(1):75–87. doi: 10.1128/mr.54.1.75-87.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Mei H. C., Léonard A. J., Weerkamp A. H., Rouxhet P. G., Busscher H. J. Surface properties of Streptococcus salivarius HB and nonfibrillar mutants: measurement of zeta potential and elemental composition with X-ray photoelectron spectroscopy. J Bacteriol. 1988 Jun;170(6):2462–2466. doi: 10.1128/jb.170.6.2462-2466.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




