Abstract
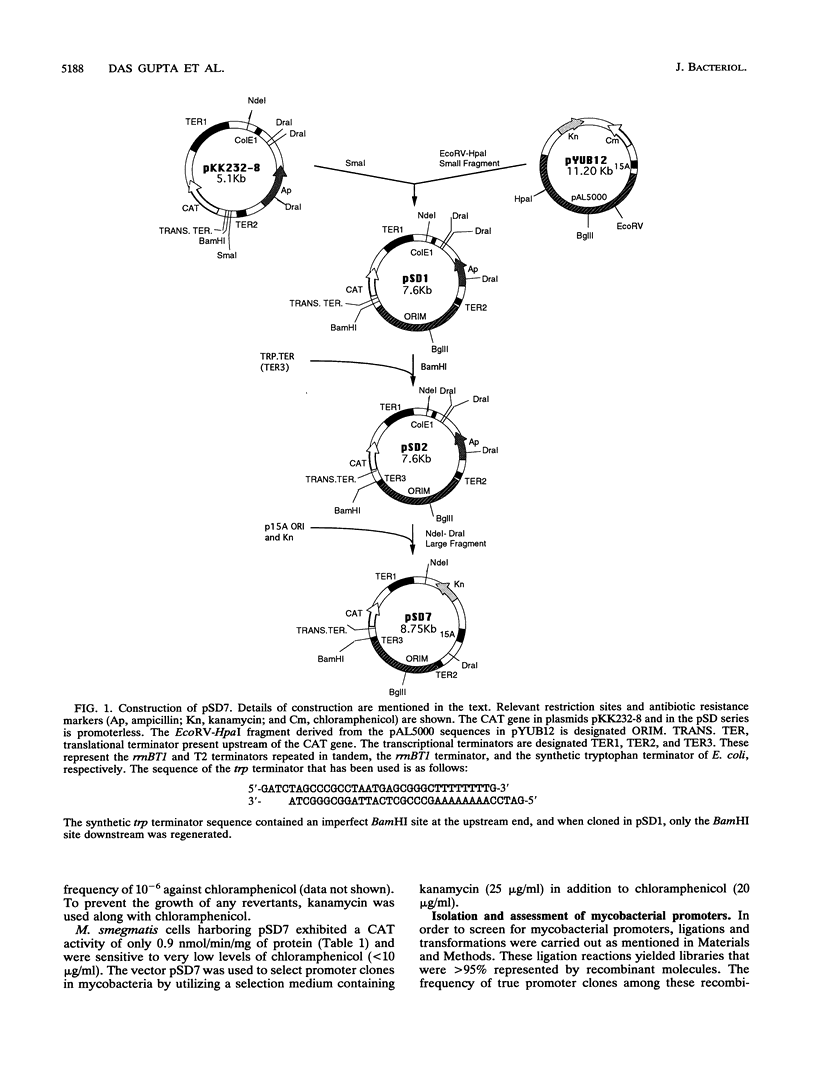
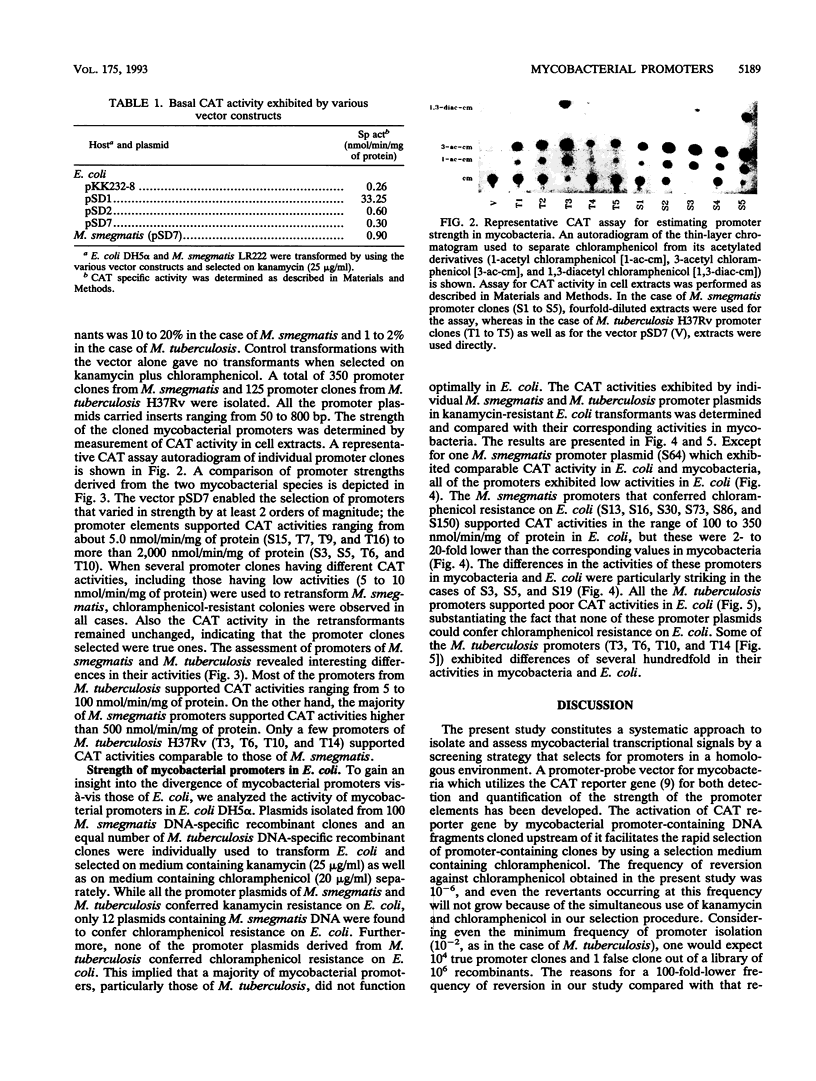
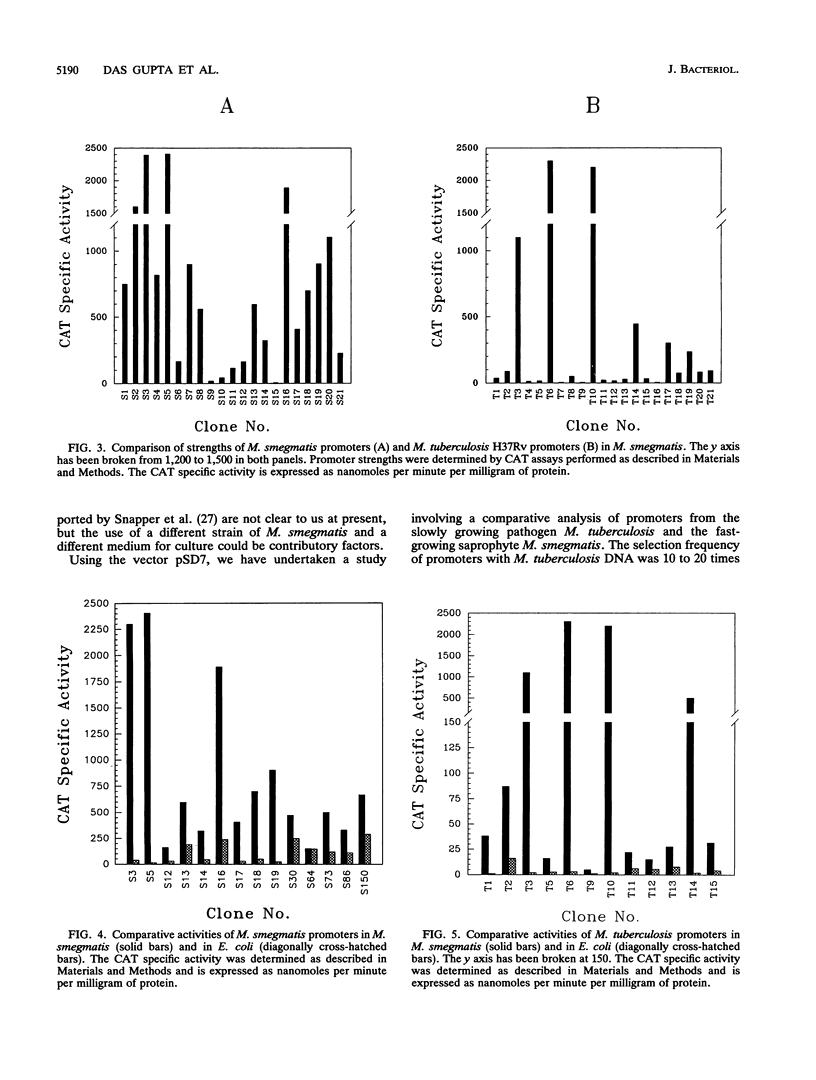
We have constructed a promoter selection vector for mycobacteria to analyze the sequences involved in mycobacterial transcriptional regulation. The vector pSD7 contains extrachromosomal origins of replication from Escherichia coli as well as from Mycobacterium fortuitum and a kanamycin resistance gene for positive selection in mycobacteria. The promoterless chloramphenicol acetyltransferase (CAT) reporter gene has been used to detect mycobacterial promoter elements in a homologous environment and to quantify their relative strengths. Using pSD7, we have isolated 125 promoter clones from the slowly growing pathogen Mycobacterium tuberculosis H37Rv and 350 clones from the fast-growing saprophyte Mycobacterium smegmatis. The promoters exhibited a wide range of strengths, as indicated by their corresponding CAT reporter activities (5 to 2,500 nmol/min/mg of protein). However, while most of the M. smegmatis promoters supported relatively higher CAT activities ranging from 100 to 2,500 nmol/min/mg of protein, a majority of those from M. tuberculosis supported CAT activities ranging from 5 to only about 100 nmol/min/mg of protein. Our results indicate that stronger promoters occur less frequently in the case of M. tuberculosis compared with M. smegmatis. To assess the extent of divergence of mycobacterial promoters vis-à-vis those of E. coli, the CAT activities supported by the promoters in E. coli were measured and compared with their corresponding activities in mycobacteria. Most of the mycobacterial promoter elements functioned poorly in E. coli. The homologous selection system that we have developed has thus enabled the identification of mycobacterial promoters that apparently function optimally only in a native environment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldovini A., Young R. A. Humoral and cell-mediated immune responses to live recombinant BCG-HIV vaccines. Nature. 1991 Jun 6;351(6326):479–482. doi: 10.1038/351479a0. [DOI] [PubMed] [Google Scholar]
- Barletta R. G., Kim D. D., Snapper S. B., Bloom B. R., Jacobs W. R., Jr Identification of expression signals of the mycobacteriophages Bxb1, L1 and TM4 using the Escherichia-Mycobacterium shuttle plasmids pYUB75 and pYUB76 designed to create translational fusions to the lacZ gene. J Gen Microbiol. 1992 Jan;138(1):23–30. doi: 10.1099/00221287-138-1-23. [DOI] [PubMed] [Google Scholar]
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- Chaisson R. E., Slutkin G. Tuberculosis and human immunodeficiency virus infection. J Infect Dis. 1989 Jan;159(1):96–100. doi: 10.1093/infdis/159.1.96. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Curtiss J. E., Jacobs W. R., Docherty M. A., Ritchie L. R., Curtiss R., 3rd Molecular analysis of DNA and construction of genomic libraries of Mycobacterium leprae. J Bacteriol. 1985 Mar;161(3):1093–1102. doi: 10.1128/jb.161.3.1093-1102.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culliton B. J. Drug-resistant TB may bring epidemic. Nature. 1992 Apr 9;356(6369):473–473. doi: 10.1038/356473a0. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange J. M. Recent developments in molecular biology of mycobacteria. Bull Int Union Tuberc Lung Dis. 1990 Jun-Sep;65(2-3):19–23. [PubMed] [Google Scholar]
- Griffiths E. Environmental regulation of bacterial virulence--implications for vaccine design and production. Trends Biotechnol. 1991 Sep;9(9):309–315. doi: 10.1016/0167-7799(91)90101-m. [DOI] [PubMed] [Google Scholar]
- Hinshelwood S., Stoker N. G. Cloning of mycobacterial histidine synthesis genes by complementation of a Mycobacterium smegmatis auxotroph. Mol Microbiol. 1992 Oct;6(19):2887–2895. doi: 10.1111/j.1365-2958.1992.tb01468.x. [DOI] [PubMed] [Google Scholar]
- Husson R. N., James B. E., Young R. A. Gene replacement and expression of foreign DNA in mycobacteria. J Bacteriol. 1990 Feb;172(2):519–524. doi: 10.1128/jb.172.2.519-524.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs W. R., Docherty M. A., Curtiss R., 3rd, Clark-Curtiss J. E. Expression of Mycobacterium leprae genes from a Streptococcus mutans promoter in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1926–1930. doi: 10.1073/pnas.83.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda S., Yabu K. Purification and some properties of beta-lactamase from Mycobacterium smegmatis. Microbiol Immunol. 1983;27(2):191–193. doi: 10.1111/j.1348-0421.1983.tb03583.x. [DOI] [PubMed] [Google Scholar]
- Kieser T., Moss M. T., Dale J. W., Hopwood D. A. Cloning and expression of Mycobacterium bovis BCG DNA in "Streptomyces lividans". J Bacteriol. 1986 Oct;168(1):72–80. doi: 10.1128/jb.168.1.72-80.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labidi A., David H. L., Roulland-Dussoix D. Restriction endonuclease mapping and cloning of Mycobacterium fortuitum var. fortuitum plasmid pAL5000. Ann Inst Pasteur Microbiol. 1985 Sep-Oct;136B(2):209–215. doi: 10.1016/s0769-2609(85)80045-4. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranes M. G., Rauzier J., Lagranderie M., Gheorghiu M., Gicquel B. Functional analysis of pAL5000, a plasmid from Mycobacterium fortuitum: construction of a "mini" mycobacterium-Escherichia coli shuttle vector. J Bacteriol. 1990 May;172(5):2793–2797. doi: 10.1128/jb.172.5.2793-2797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirakova T. D., Bardarov S. S., Kriakov J. I., Markov K. I. Molecular cloning of mycobacterial promoters in Escherichia coli. FEMS Microbiol Lett. 1989 May;50(1-2):153–156. doi: 10.1016/0378-1097(89)90476-x. [DOI] [PubMed] [Google Scholar]
- Snapper S. B., Lugosi L., Jekkel A., Melton R. E., Kieser T., Bloom B. R., Jacobs W. R., Jr Lysogeny and transformation in mycobacteria: stable expression of foreign genes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6987–6991. doi: 10.1073/pnas.85.18.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snapper S. B., Melton R. E., Mustafa S., Kieser T., Jacobs W. R., Jr Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990 Nov;4(11):1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- Storz G., Tartaglia L. A., Farr S. B., Ames B. N. Bacterial defenses against oxidative stress. Trends Genet. 1990 Nov;6(11):363–368. doi: 10.1016/0168-9525(90)90278-e. [DOI] [PubMed] [Google Scholar]
- Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H., Hatfull G. F. New use of BCG for recombinant vaccines. Nature. 1991 Jun 6;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Thole J. E., Dauwerse H. G., Das P. K., Groothuis D. G., Schouls L. M., van Embden J. D. Cloning of Mycobacterium bovis BCG DNA and expression of antigens in Escherichia coli. Infect Immun. 1985 Dec;50(3):800–806. doi: 10.1128/iai.50.3.800-806.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]




