Abstract
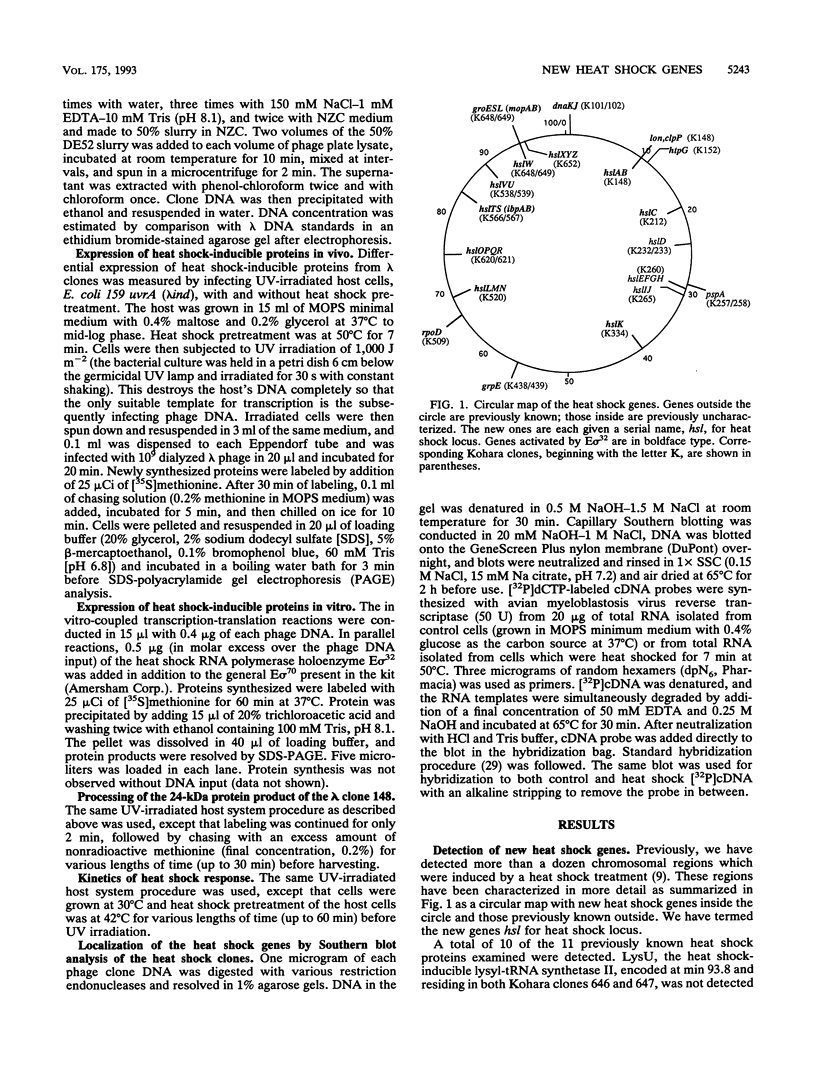
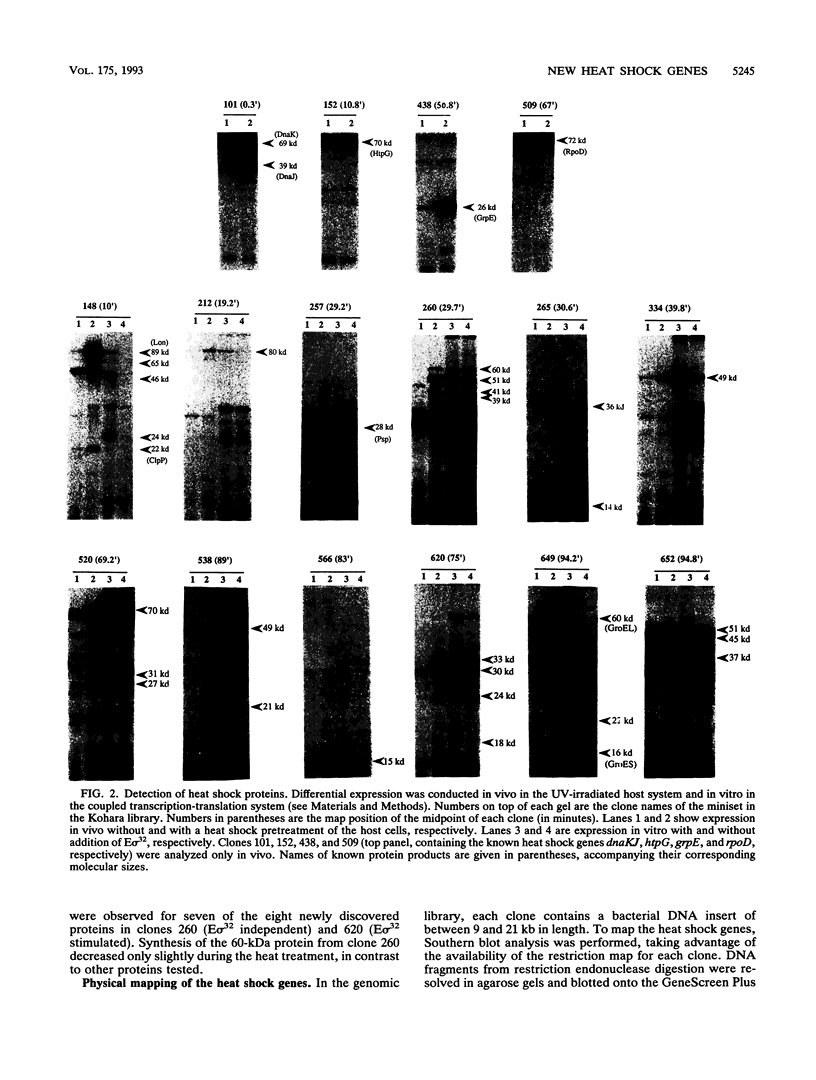
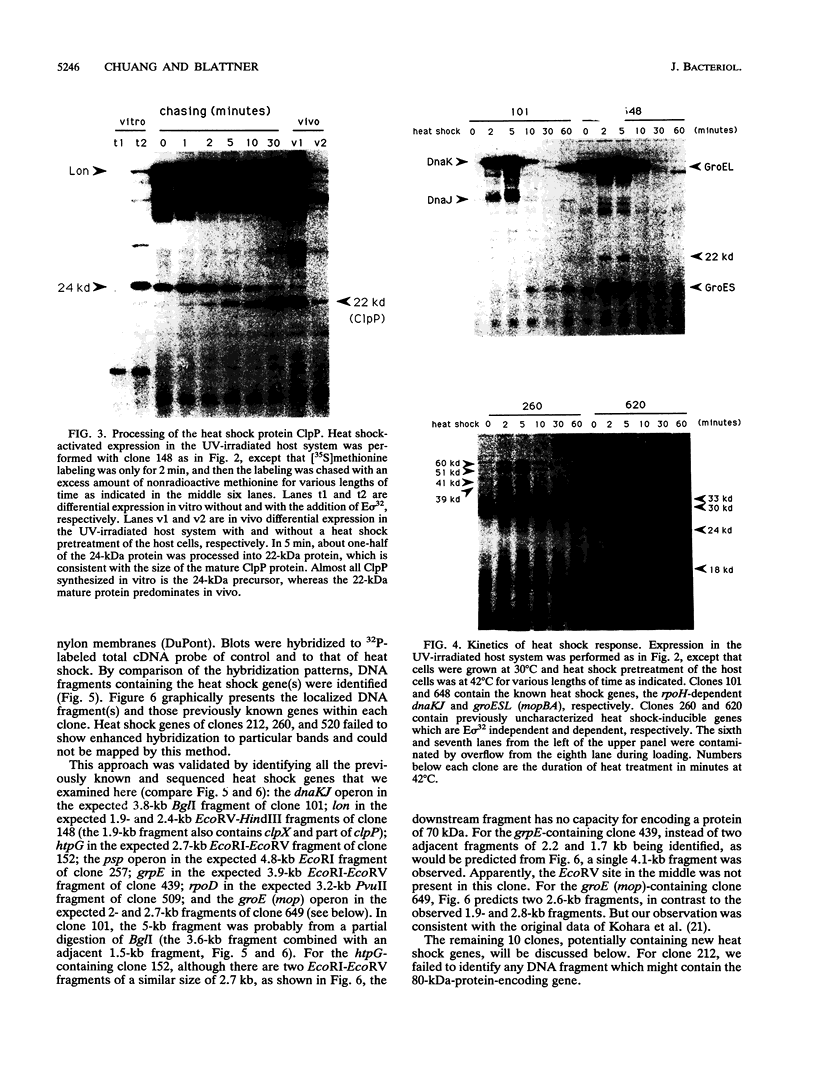
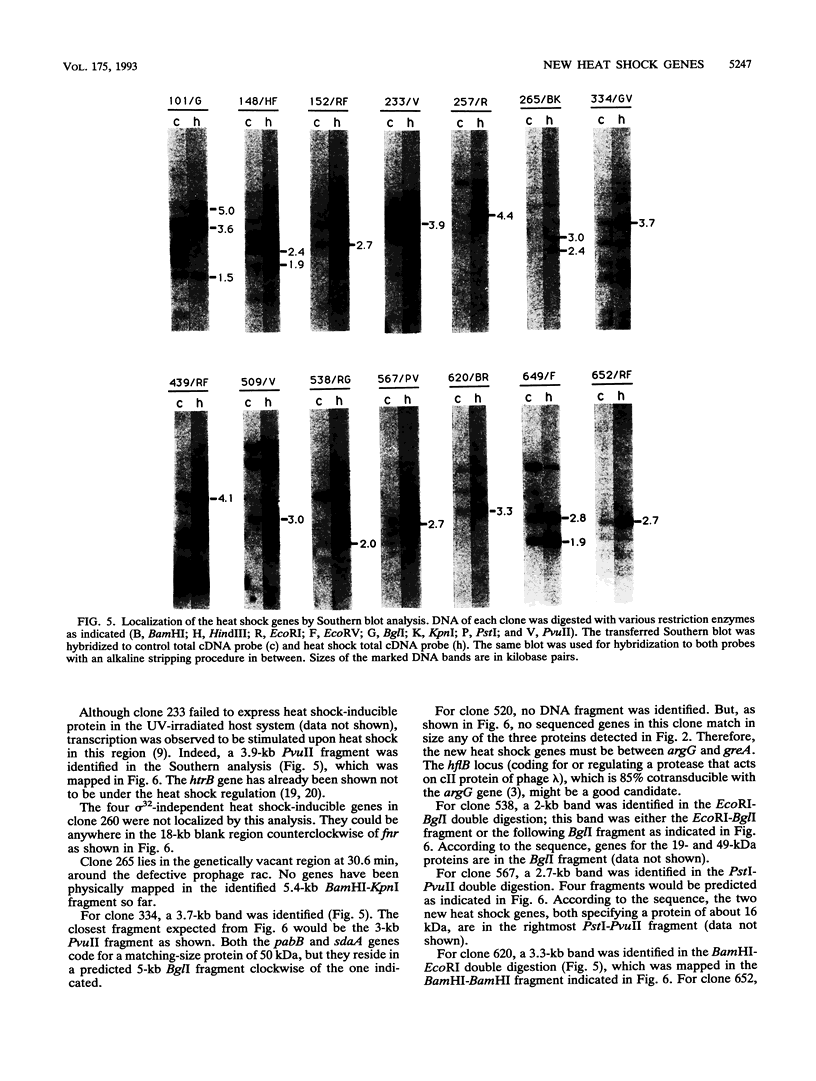
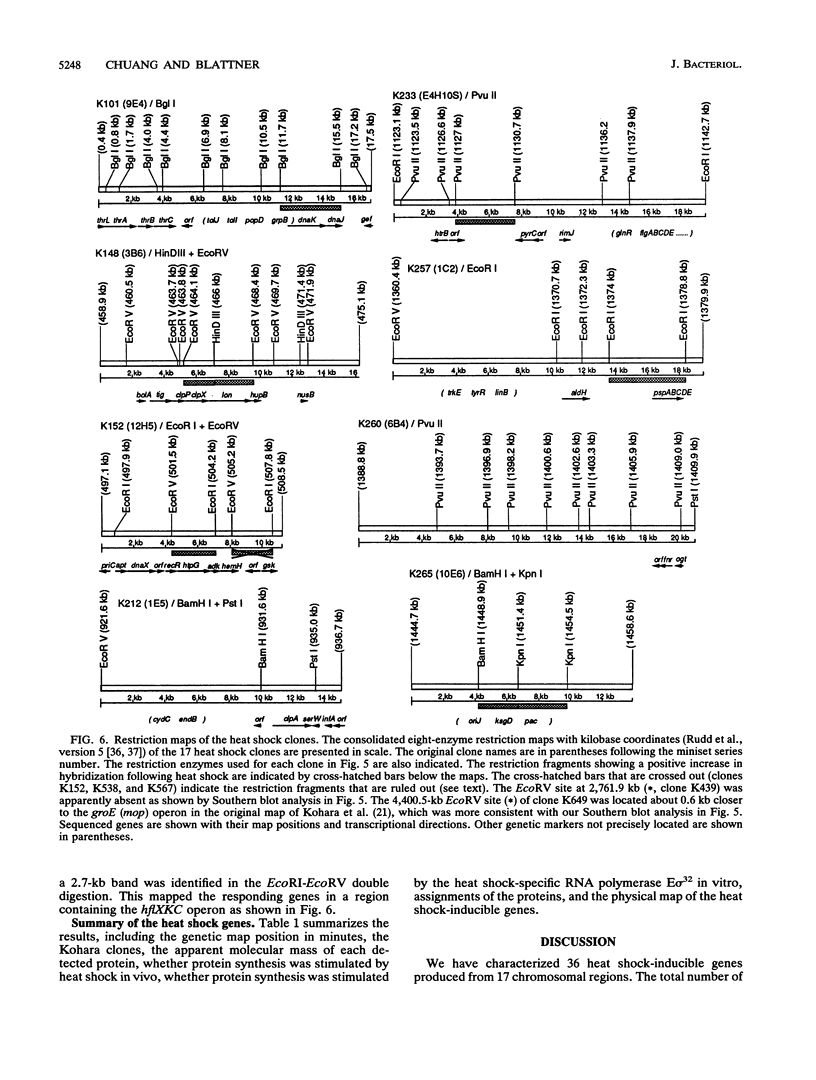
Most organisms respond to heat by substantial alteration of the pattern of gene expression. This has been particularly well studied with Escherichia coli although the response has by no means been completely characterized. Here we report the characterization of 26 new heat shock genes of E. coli, termed hsl, discovered by global transcription analysis with an overlapping lambda clone bank. We have measured the molecular weights of the corresponding heat shock proteins and mapped each of them to within a few kilobases on the E. coli genome. In vitro, 16 of them can be activated by the E sigma 32 RNA polymerase, which specifically transcribes heat shock genes. In vivo expression kinetics of seven of eight examined new proteins were found to be similar to those of the four most studied heat shock proteins, DnaK, DnaJ, GroEL (MopA), and GroES (MopB). In the course of this work, we confirmed that the catalytic subunit of the ATP-dependent Clp protease (also known as Ti protease), ClpP, is derived from a larger precursor protein. Possible assignments of some of the hsl genes to known proteins are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. P., Polazzi J. O., Gierse J. K., Easton A. M. Two novel heat shock genes encoding proteins produced in response to heterologous protein expression in Escherichia coli. J Bacteriol. 1992 Nov;174(21):6938–6947. doi: 10.1128/jb.174.21.6938-6947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F., Herskowitz I. Identification of polypeptides encoded by an Escherichia coli locus (hflA) that governs the lysis-lysogeny decision of bacteriophage lambda. J Bacteriol. 1987 Sep;169(9):4076–4085. doi: 10.1128/jb.169.9.4076-4085.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F., Hoyt M. A., McFarlane L., Echols H., Herskowitz I. hflB, a new Escherichia coli locus regulating lysogeny and the level of bacteriophage lambda cII protein. J Mol Biol. 1986 Jan 20;187(2):213–224. doi: 10.1016/0022-2836(86)90229-9. [DOI] [PubMed] [Google Scholar]
- Bardwell J. C., Craig E. A. Eukaryotic Mr 83,000 heat shock protein has a homologue in Escherichia coli. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5177–5181. doi: 10.1073/pnas.84.15.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette J. L., Russel M., Weiner L., Model P. Phage shock protein, a stress protein of Escherichia coli. Proc Natl Acad Sci U S A. 1990 Feb;87(3):862–866. doi: 10.1073/pnas.87.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette J. L., Weiner L., Ripmaster T. L., Model P. Characterization and sequence of the Escherichia coli stress-induced psp operon. J Mol Biol. 1991 Jul 5;220(1):35–48. doi: 10.1016/0022-2836(91)90379-k. [DOI] [PubMed] [Google Scholar]
- Cheng H. H., Muhlrad P. J., Hoyt M. A., Echols H. Cleavage of the cII protein of phage lambda by purified HflA protease: control of the switch between lysis and lysogeny. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7882–7886. doi: 10.1073/pnas.85.21.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang S. E., Daniels D. L., Blattner F. R. Global regulation of gene expression in Escherichia coli. J Bacteriol. 1993 Apr;175(7):2026–2036. doi: 10.1128/jb.175.7.2026-2036.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Gross C. A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991 Apr;16(4):135–140. doi: 10.1016/0968-0004(91)90055-z. [DOI] [PubMed] [Google Scholar]
- Erickson J. W., Gross C. A. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 1989 Sep;3(9):1462–1471. doi: 10.1101/gad.3.9.1462. [DOI] [PubMed] [Google Scholar]
- Freedman L. P., Zengel J. M., Lindahl L. Genetic dissection of stringent control and nutritional shift-up response of the Escherichia coli S10 ribosomal protein operon. J Mol Biol. 1985 Oct 20;185(4):701–712. doi: 10.1016/0022-2836(85)90055-5. [DOI] [PubMed] [Google Scholar]
- Gamer J., Bujard H., Bukau B. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor sigma 32. Cell. 1992 May 29;69(5):833–842. doi: 10.1016/0092-8674(92)90294-m. [DOI] [PubMed] [Google Scholar]
- Grossman A. D., Taylor W. E., Burton Z. F., Burgess R. R., Gross C. A. Stringent response in Escherichia coli induces expression of heat shock proteins. J Mol Biol. 1985 Nov 20;186(2):357–365. doi: 10.1016/0022-2836(85)90110-x. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Tsui L. Role of the host in virus assembly: cloning of the Escherichia coli groE gene and identification of its protein product. Proc Natl Acad Sci U S A. 1978 Jan;75(1):136–139. doi: 10.1073/pnas.75.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B. J., Woo K. M., Goldberg A. L., Chung C. H. Protease Ti, a new ATP-dependent protease in Escherichia coli, contains protein-activated ATPase and proteolytic functions in distinct subunits. J Biol Chem. 1988 Jun 25;263(18):8727–8734. [PubMed] [Google Scholar]
- Karow M., Fayet O., Cegielska A., Ziegelhoffer T., Georgopoulos C. Isolation and characterization of the Escherichia coli htrB gene, whose product is essential for bacterial viability above 33 degrees C in rich media. J Bacteriol. 1991 Jan;173(2):741–750. doi: 10.1128/jb.173.2.741-750.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow M., Georgopoulos C. Sequencing, mutational analysis, and transcriptional regulation of the Escherichia coli htrB gene. Mol Microbiol. 1991 Sep;5(9):2285–2292. doi: 10.1111/j.1365-2958.1991.tb02159.x. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kroh H. E., Simon L. D. Increased ATP-dependent proteolytic activity in lon-deficient Escherichia coli strains lacking the DnaK protein. J Bacteriol. 1991 Apr;173(8):2691–2695. doi: 10.1128/jb.173.8.2691-2695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroh H. E., Simon L. D. The ClpP component of Clp protease is the sigma 32-dependent heat shock protein F21.5. J Bacteriol. 1990 Oct;172(10):6026–6034. doi: 10.1128/jb.172.10.6026-6034.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner C. G., Inouye M. Pleiotropic changes resulting from depletion of Era, an essential GTP-binding protein in Escherichia coli. Mol Microbiol. 1991 Apr;5(4):951–957. doi: 10.1111/j.1365-2958.1991.tb00770.x. [DOI] [PubMed] [Google Scholar]
- Liberek K., Galitski T. P., Zylicz M., Georgopoulos C. The DnaK chaperone modulates the heat shock response of Escherichia coli by binding to the sigma 32 transcription factor. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3516–3520. doi: 10.1073/pnas.89.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., Fayet O., Baird L., Georgopoulos C. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J Bacteriol. 1989 Mar;171(3):1574–1584. doi: 10.1128/jb.171.3.1574-1584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinska B., King J., Ang D., Georgopoulos C. Sequence analysis and transcriptional regulation of the Escherichia coli grpE gene, encoding a heat shock protein. Nucleic Acids Res. 1988 Aug 11;16(15):7545–7562. doi: 10.1093/nar/16.15.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackow E. R., Chang F. N. Correlation between RNA synthesis and ppGpp content in Escherichia coli during temperature shifts. Mol Gen Genet. 1983;192(1-2):5–9. doi: 10.1007/BF00327639. [DOI] [PubMed] [Google Scholar]
- Matthews R. G., Neidhardt F. C. Abnormal induction of heat shock proteins in an Escherichia coli mutant deficient in adenosylmethionine synthetase activity. J Bacteriol. 1988 Apr;170(4):1582–1588. doi: 10.1128/jb.170.4.1582-1588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi M. R., Clark W. P., Katayama Y., Rudikoff S., Pumphrey J., Bowers B., Gottesman S. Sequence and structure of Clp P, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J Biol Chem. 1990 Jul 25;265(21):12536–12545. [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina S., Georgopoulos C. A new Escherichia coli heat shock gene, htrC, whose product is essential for viability only at high temperatures. J Bacteriol. 1990 Jun;172(6):3417–3426. doi: 10.1128/jb.172.6.3417-3426.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina S., Georgopoulos C. The htrM gene, whose product is essential for Escherichia coli viability only at elevated temperatures, is identical to the rfaD gene. Nucleic Acids Res. 1991 Jul 25;19(14):3811–3819. doi: 10.1093/nar/19.14.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd K. E., Miller W., Werner C., Ostell J., Tolstoshev C., Satterfield S. G. Mapping sequenced E.coli genes by computer: software, strategies and examples. Nucleic Acids Res. 1991 Feb 11;19(3):637–647. doi: 10.1093/nar/19.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- Tobias J. W., Shrader T. E., Rocap G., Varshavsky A. The N-end rule in bacteria. Science. 1991 Nov 29;254(5036):1374–1377. doi: 10.1126/science.1962196. [DOI] [PubMed] [Google Scholar]
- Weiner L., Brissette J. L., Model P. Stress-induced expression of the Escherichia coli phage shock protein operon is dependent on sigma 54 and modulated by positive and negative feedback mechanisms. Genes Dev. 1991 Oct;5(10):1912–1923. doi: 10.1101/gad.5.10.1912. [DOI] [PubMed] [Google Scholar]